Abstract
Background: Evidence guiding secondary repair of persistent full-thickness macular holes (FTMHs) remains limited and heterogeneous. Temporal arcuate relaxing retinotomy has been described as a salvage maneuver intended to increase temporal retinal compliance, yet functional safety data are scarce. We report consecutive real-world outcomes of temporal arcuate relaxing retinotomy for persistent FTMHs after failed standard repair(s). Methods: Retrospective consecutive case series of patients with persistent FTMH after ≥1 pars plana vitrectomy (PPV) with internal limiting membrane (ILM) peeling, treated with repeat PPV and temporal arcuate relaxing retinotomy. Outcomes included OCT (Optical Coherence Tomography)-confirmed closure after gas absorption and best-corrected visual acuity (BCVA, logMAR), ellipsoid zone (EZ) status, retinotomy-site morphology on OCT/fundus autofluorescence (FAF), and safety/functional outcomes (systematic scotoma symptom inquiry; Humphrey visual field testing when feasible). Exact binomial 95% confidence intervals (CI) were calculated for proportions. Results: Nine eyes (median age 70 years; range 55–76) underwent temporal arcuate relaxing retinotomy for persistent FTMH. Minimum linear diameter ranged 412–1037 µm (median 613 µm). OCT-confirmed closure was achieved in 7/9 eyes (77.8%; 95% CI 40.0–97.2) at a mean follow-up of 5.9 months (range 2–12). BCVA improved in 8/9 eyes (88.9%; 95% CI 51.8–99.7); mean BCVA improved from 1.26 ± 0.51 logMAR pre-operatively to 0.61 ± 0.18 logMAR at last follow-up (mean change −0.64 logMAR; Wilcoxon signed-rank test p = 0.011). As a sensitivity analysis, the paired t-test yielded p = 0.008. Humphrey visual fields were obtained in 6/9 eyes; one patient reported a new paracentral nasal scotoma, which was subjectively well tolerated. Conclusions: In this small consecutive series, temporal arcuate relaxing retinotomy was associated with a 78% closure rate and mean BCVA improvement in eyes with persistent FTMH after failed standard repair(s), with limited symptomatic scotoma reporting in those assessed. Given the retrospective design, small cohort, and incomplete standardized functional testing, larger comparative studies with uniform functional endpoints (microperimetry, RNFL/GCL metrics, and systematic perimetry) are needed to define patient selection, reproducibility, and relative performance versus contemporary salvage options.
1. Introduction
Pars plana vitrectomy (PPV) combined with internal limiting membrane (ILM) peeling and intraocular gas tamponade remains the standard surgical treatment for full-thickness macular holes (FTMHs), with reported closure rates commonly ranging from approximately 80% to near 100% depending on case mix and hole size [1,2,3,4,5,6]. Despite these favorable outcomes, anatomical failure still occurs, and macular holes may either remain open after the initial attempt or reopen after a period of documented closure [6,7]. FTMHs that do not close after primary surgery are commonly described as persistent (or refractory), whereas those that reopen after at least four weeks of confirmed closure are considered recurrent [6,8].
Secondary repair is challenging for several reasons. Residual ILM may be limited or absent, reducing feasibility of repeat peeling or flap techniques, and reoperation generally carries lower anatomical success than primary surgery [9]. Nevertheless, reintervention can be justified because meaningful anatomical and functional gains remain possible and the natural course of an open hole may involve enlargement with progressive atrophy and visual deterioration [10,11,12,13].
A broad range of surgical strategies has been proposed, including revision PPV with extension of ILM peeling [14], biologic adjuvants such as autologous plasma/platelet products [15], practical secondary-repair approaches [16], subretinal fluid injection [17], fluid–gas exchange [18], retinal massage [19], microdrain techniques [12,20], macular buckling in selected settings [21], and tissue “plug” approaches such as autologous neurosensory retinal free flap (autologous retinal transplant) [22], human amniotic membrane grafting [23], ILM free flap transplantation [24], and lens capsular flap transplantation [25,26]. Evidence remains largely retrospective and heterogeneous, with limited head-to-head comparisons and inconsistent standardized reporting of functional safety outcomes [7,27].
Relaxing retinotomy represents a distinct salvage approach that aims to increase local retinal compliance via a controlled full-thickness incision adjacent to the hole, thereby reducing constraining tangential forces and facilitating edge approximation [6,28,29,30,31,32]. In 2013, Charles et al. described a temporal arcuate full-thickness retinotomy placed adjacent to the macular hole for large holes that remained open after standard surgery [29]. The rationale for performing a temporal arcuate retinotomy is to increase retinal elasticity beyond that achievable with ILM peeling alone, thereby allowing the temporal retinal bridge to relax and shift into a position that promotes macular hole closure [6,29]. The temporal location is intended to align with axonal orientation near the horizontal raphe and potentially reduce risk of papillomacular bundle injury and symptomatic nasal visual field defects [29]. A related but anatomically different approach, nasal parafoveal retinotomy, has also been reported and includes discussion of scotoma symptoms and perimetry findings [30,33].
In this retrospective case series, we aimed to assess both anatomical closure rates and functional outcomes following temporal arcuate relaxing retinotomy for the management of persistent FTMHs. The present study does not propose a new procedure. It provides a consecutive case series describing real-world outcomes, with explicit reporting of postoperative assessment and available functional safety data.
2. Material and Methods
This retrospective, single-center interventional case series assessed the effectiveness of temporal arcuate relaxing retinotomy in eyes with persistent full-thickness macular holes (FTMHs). Eligible patients were recruited at Beauregard Hospital (Aosta, Italy) between November 2022 and April 2024.
Informed consent was obtained from all participants for the surgical procedure and for the use of anonymized clinical data for research purposes, as temporal arcuate relaxing retinotomy represents an established salvage option for persistent/refractory full-thickness macular holes; for this retrospective analysis of anonymized data, formal Ethics Committee approval was not required according to local institutional policy, and the study was conducted in accordance with the principles of the Declaration of Helsinki.
The data presented in this study are available from the corresponding author upon reasonable request; the data are not publicly available due to privacy and institutional restrictions.
Inclusion criteria included patients with persistent FTMH after one or more prior PPV with ILM peeling (with or without ILM flap).
Exclusion criteria were (1) clinically significant macular comorbidity likely to confound outcomes (advanced diabetic retinopathy, retinal vein occlusion with macular sequelae, end-stage age-related macular degeneration); (2) inability to comply with postoperative face-down positioning. Because relaxing retinotomy approaches may be associated with postoperative scotomas and potential neuroretinal consequences, advanced glaucoma was considered a relative contraindication in clinical decision-making consistent with previous reports [30].
Baseline data collected included demographics (age, sex), laterality, macular hole etiology, previous repair attempts, and lens status. FTMH size was assessed with spectral-domain OCT (Spectralis; Heidelberg Engineering). Minimum linear diameter (MLD) was defined as the smallest aperture diameter across macular scans, and basal diameter as the maximal width at the level of the retinal pigment epithelium when available. OCT assessment (closure type and ellipsoid zone status) was performed by a single experienced grader using predefined criteria.
BCVA was recorded and converted to logMAR for analysis.
All procedures were performed by a single surgeon (LV) using 23-gauge or 25-gauge transconjunctival PPV (Constellation, Alcon) under 3D visualization (NGENUITY, Alcon). Residual ILM near the intended retinotomy area was stained using viewILM dye (Alchimia, Italy). If residual ILM was present, an extended circumferential peel was performed and documented (yes/no), consistent with revision strategies [14].
A full-thickness temporal arcuate (semicircular) relaxing retinotomy was created using vertical or horizontal scissors, positioned approximately one hole diameter temporal to the macular hole edge and centered along the temporal horizontal meridian (Figure 1), consistent with Charles’ technique [29]. Fluid–air exchange and tamponade were then performed using sulphur hexafluoride (SF6) or perfluoropropane (C3F8); silicone oil was used in selected circumstances based on clinical considerations. Patients were instructed to maintain face-down positioning for four days after surgery (unless otherwise clinically indicated).
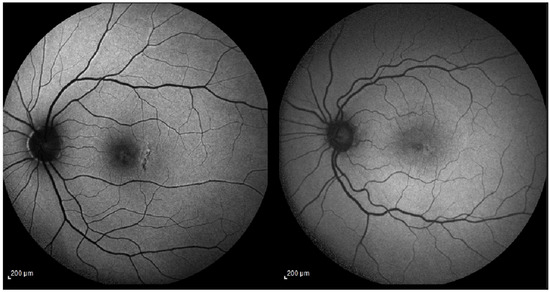
Figure 1.
(Left) (patient 2): Fundus autofluorescence (AF) shows a well-defined area of retinal pigment epithelium (RPE) alteration temporal to the fovea, corresponding to the arcuate full-thickness relaxing retinotomy (one hole diameter temporal to the macular hole edge). (Right) (patient 1): AF demonstrates minimal RPE disturbance at the temporal retinotomy site, with almost imperceptible changes. Abbreviations: AF, autofluorescence; RPE, retinal pigment epithelium.
Postoperative follow-up visits were scheduled at day 1, week 1, and at 1, 3, 6, and 12 months after surgery, and thereafter as clinically indicated. Starting at postoperative month 1, patients were systematically asked at each visit about newly developed scotomas or subjective visual field disturbances, reflecting concerns described in prior retinotomy series. Automated perimetry (Humphrey Field Analyzer 3, HFA3; Carl Zeiss Meditec) was offered after the secondary procedure when feasible with the 30-2 program (SITA Standard), monocularly in the operated eye. Reliability indices (fixation losses, false-positive and false-negative responses) were recorded and considered when interpreting results. Standardized microperimetry and peripapillary RNFL/GCL thickness metrics were not routinely available for all patients in this retrospective series and were not used as endpoints.
The primary outcome was OCT-confirmed anatomical closure of the FTMH after tamponade absorption and at final follow-up. Secondary outcomes included (1) change in BCVA (logMAR) from baseline to last follow-up; (2) EZ integrity in eyes achieving closure; (3) retinotomy-site morphology on OCT and FAF; (4) functional safety outcomes including patient-reported scotoma and HVF findings when available.
Anatomical outcome was graded on OCT as type 1 closure (complete foveal tissue continuity without a neurosensory defect) or type 2 closure (flattened edges with a persistent foveal defect). In this study, ‘closure’ refers to type 1 closure.
Descriptive statistics were used (mean ± SD, median and range). Proportions were reported with exact binomial 95% CI. Pre- vs. postoperative BCVA was primarily compared using the Wilcoxon signed-rank test; p < 0.05 was considered statistically significant. Given the sample size, results are presented as hypothesis-generating. Given the small sample size, changes in BCVA were primarily analyzed using the Wilcoxon signed-rank test. A paired t-test was additionally computed as a sensitivity analysis.
3. Results
Nine consecutive eyes from nine patients were included (seven male, two female), with a median age of 70 years (range 55–76). Etiology was idiopathic in six eyes, traumatic in two eyes (patients 5 and 9), and post-retinal detachment repair in one eye (patient 4). Two traumatic cases were long-standing (≥6 months), including one documented for more than four years (patient 9). All eyes were pseudophakic at baseline.
FTMH MLD ranged from 412 to 1037 µm (median 613 µm). All eyes had previously undergone at least one PPV with ILM peeling (with or without ILM flap) without closure, consistent with persistent/refractory FTMH status [6,7,8,9].
All eyes underwent repeat PPV and temporal arcuate relaxing retinotomy. Residual ILM at the retinotomy site was documented in 4/9 eyes, in which an extended circumferential ILM peel was performed. Tamponade agents included SF6 (4/9), C3F8 (4/9), and silicone oil (1/9).
After surgery, all patients adhered to a face-down positioning regimen for at least 4 days. A summary of baseline characteristics, macular hole features, surgical details, and postoperative outcomes is provided in Table 1.

Table 1.
Baseline characteristics, surgical details, and postoperative outcomes of eyes undergoing temporal arcuate relaxing retinotomy for macular hole.
OCT-confirmed closure was achieved in 7/9 eyes (77.8%; exact 95% CI 40.0–97.2). Mean follow-up was 5.9 months (range 2–12) (Figure 2). Among closed holes, EZ restoration was complete or partial (granular) in 4 eyes. Retinotomy-site imaging (OCT through the retinotomy and FAF when available) did not show signs of pathology at the incision site in the cases with documentation.
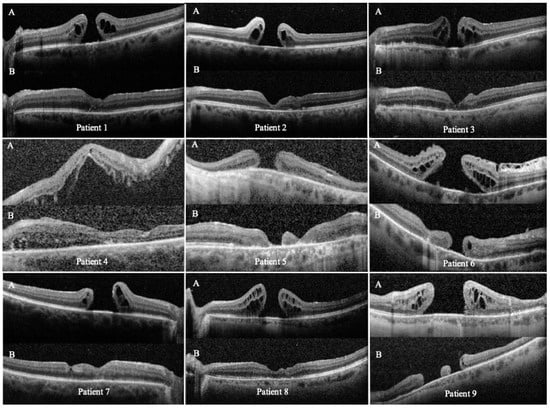
Figure 2.
Preoperative (A) and postoperative (B) OCT scans of the nine eyes that underwent temporal arcuate relaxing retinotomy for persistent full-thickness macular hole (FTMH) repair. Each panel shows cross-sectional scans through the fovea and the temporal arcuate retinotomy site. Abbreviations: OCT, optical coherence tomography; FTMH, full-thickness macular hole.
BCVA improved in 8/9 eyes (88.9%; exact 95% CI 51.8–99.7). Mean BCVA improved from 1.26 ± 0.51 logMAR at baseline to 0.61 ± 0.18 logMAR at last follow-up (Wilcoxon signed-rank test, p = 0.011). As a sensitivity analysis, the paired t-test yielded p = 0.008 (Figure 3).
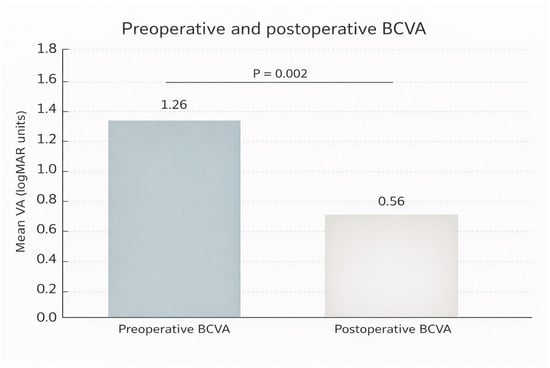
Figure 3.
Preoperative and postoperative best-corrected visual acuity (BCVA; mean ± SE, logMAR) in nine eyes undergoing temporal arcuate relaxing retinotomy for repair of persistent full-thickness macular hole (FTMH). Abbreviations: VA, visual acuity; BCVA, best-corrected visual acuity; logMAR, logarithm of the minimum angle of resolution.
One patient reported a new paracentral nasal scotoma that was subjectively well tolerated. This patient experienced better functionality and quality of life with a postoperative paracentral scotoma than with a central scotoma due to FTMH.
HVF testing was obtained in 6/9 eyes (Figure 4). Four tested eyes showed no scotomas. One tested eye demonstrated a superotemporal scotoma considered consistent with pre-existing chorioretinal atrophy. Three eyes did not undergo HVF testing.
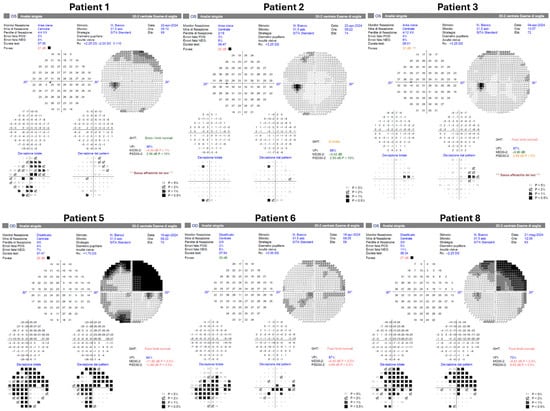
Figure 4.
Postoperative Humphrey visual field testing was successfully performed in six patients using the HFA3 perimeter (Carl Zeiss Meditec, Dublin, CA, USA). Patients 4, 7, and 9 did not undergo postoperative visual field examination.
Two eyes did not close at last follow-up: one idiopathic persistent hole with short follow-up of 2 months (patient 6) and one (patient 9) very chronic traumatic hole (documented > 4 years). Additional structural features (including basal diameter and edge configuration) were not consistently available for all cases.
The temporal arcuate relaxing retinotomy successfully enabled closure even in one very large, long-standing FTMH (patient 5, expanded case study ahead).
Expanded Case Study
Patient 5 was a 70-year-old man with a long-standing traumatic right-eye FTMH following severe blunt trauma two decades before presentation. The FTMH was chronic (lasting more than 1 year), measuring 1037 µm in aperture diameter, with flat borders and inferonasal chorioretinal atrophy. Several years ago, this traumatized eye had already undergone a PPV with ILM peeling, without achieving FTMH closure.
We performed a 23-G pars plana vitrectomy with extended ILM peeling and a temporal relaxing retinotomy to attempt to close this large macular hole, followed by gas tamponade (SF6) and 7 days of face-down positioning.
At follow-up, the FTMH achieved complete anatomic closure, with BCVA improving from counting fingers at 30 cm to 20/100. The retinotomy site remained open, but the most significant finding is that the macular hole completely closed, confirming the partial slippage of the retina to close the hole, considering the notable inferonasal chorioretinal atrophy (Figure 5).
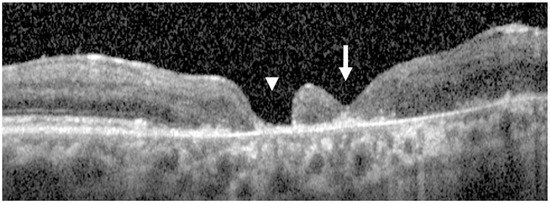
Figure 5.
Postoperative OCT scans of patient 5. The white arrow indicates the completely closed macular hole, while the arrowhead marks the persistently open retinotomy site. Despite the open retinotomy and marked inferonasal chorioretinal atrophy, partial retinal slippage resulted in complete macular hole closure.
4. Discussion
In this small consecutive series of persistent/refractory FTMH after failed PPV/ILM peeling-based repair(s), temporal arcuate relaxing retinotomy combined with repeat PPV achieved OCT-confirmed closure in 78% of eyes and was associated with significant BCVA improvement. Available reports on retinotomy-based salvage for persistent/refractory macular holes are limited and heterogeneous; a concise comparison of the main published retinotomy approaches and reported outcomes is provided in Table 2 [28,29,30,31,32].

Table 2.
Literature comparison of retinotomy-based salvage approaches for persistent/refractory macular holes.
A structured symptom inquiry and HVF testing when feasible identified limited symptomatic scotoma reporting in those assessed. These findings align with prior reports suggesting that relaxing retinotomy can facilitate closure in selected difficult cases, while overall evidence remains limited and heterogeneous [6,28,29,30].
The reported outcomes of currently available techniques suggest that reoperation can be justified in recurrent or refractory FTMHs. However, head-to-head comparisons across procedures remain difficult because randomized controlled trials and large prospective comparative studies are scarce [7]. Accordingly, the best operative strategy remains uncertain, and no standardized management pathway for recurrent or refractory full-thickness macular holes has been established [7].
This study does not claim technical novelty. Its contribution is incremental: consecutive real-world outcomes in a defined salvage population and transparent reporting of postoperative assessment and available functional safety findings, addressing a recognized limitation of the current literature on secondary repair strategies [7,27].
Persistent holes after ILM peeling-based repair likely reflect insufficient retinal compliance and ongoing tangential forces that prevent stable edge approximation [6,27]. A controlled full-thickness arcuate incision temporal to the hole is intended to increase local compliance and allow temporal retinal mobility beyond that achievable with ILM peeling alone, thereby facilitating closure [29]. The temporal arcuate design along the horizontal raphe was proposed to reduce the likelihood of severing major axonal trajectories and to limit the risk of symptomatic nasal field defects compared with approaches closer to the papillomacular bundle [29]. While gliotic responses after retinal injury have been proposed as a contributor to closure, mechanistic inference remains speculative without dedicated biomarkers and standardized structure–function correlation [28].
Two eyes did not close. One was a very chronic traumatic hole documented for more than four years. Chronicity and atrophic substrate are recognized challenges in secondary repair and may limit the biomechanical and biological capacity for edge re-approximation [7,10,11,12,27]. The second non-closure case had only two months of follow-up, raising the possibility that the observation window was insufficient to capture delayed closure. Given the small cohort, these findings are descriptive and do not identify predictors; however, they underscore the need for future studies to standardize reporting of chronicity, basal diameter, hole configuration, and timing of closure assessment [9,14].
Relaxing retinotomy is inherently tissue-sacrificing and may be associated with RPE disturbance at the incision site and postoperative scotomas [6,30]. In Tsipursky et al.’s series of nasal parafoveal retinotomy, scotomas were reported and objective perimetry demonstrated defects in a subset, though functional impact was generally outweighed by improvements in central vision [20]. In the present series, symptom inquiry was systematic from postoperative month 2 onward and HVF was performed in two-thirds of eyes; one patient reported a new paracentral scotoma that was well tolerated.
Nonetheless, the lack of uniform microperimetry and RNFL/GCL metrics limits conclusions regarding neuroretinal safety, and larger prospective studies should incorporate standardized functional endpoints [30]. Careful selection is advisable in eyes with significant macular comorbidity or advanced glaucoma, where additional neuroretinal compromise may be clinically consequential [30].
Secondary repair strategies include revision PPV with ILM peeling extension [14], biologic augmentation such as autologous plasma products [15], subretinal fluid injection [17], fluid–gas exchange [18], and tissue plug/graft approaches such as autologous retinal transplant [22], lens capsule flap transplantation [25,26], ILM free flap transplantation [24], and amniotic membrane grafting [23]. Tissue plug techniques may be favored for very large or chronically persistent holes when ILM is exhausted, while compliance-based maneuvers (including relaxing retinotomy) may be considered when the surgeon aims to promote closure without introducing graft material into the defect, preserving the option of subsequent plug strategies if needed [22,25,27]. This series does not compare approaches and does not support superiority claims.
This study is limited by its retrospective design, small cohort, heterogeneous tamponade selection, and variable follow-up intervals. A control group was not available, precluding comparison against other salvage techniques [7,27]. OCT grading was not performed by masked independent graders, and inter-observer variability could not be assessed. Functional testing was not standardized across all eyes; microperimetry and RNFL/GCL thickness were not routinely obtained, limiting safety inference [30]. Quantitative measures of retinotomy size and precise morphology were not systematically recorded and should be incorporated into future studies to improve reproducibility and to explore structure–function correlations.
5. Conclusions
In this small consecutive series, temporal arcuate relaxing retinotomy as part of repeat PPV was associated with a 78% closure rate and significant BCVA improvement in persistent FTMHs after failed standard repair(s). Larger comparative studies with standardized imaging-derived retinotomy metrics and uniform functional endpoints (perimetry, microperimetry, RNFL/GCL analyses) are needed to define reproducibility, patient selection, and relative performance versus contemporary salvage options [7,17,22,25,27,30].
Author Contributions
Conceptualization, L.V.; Methodology, L.V. and A.V.; Data curation, E.M. and A.V.; Writing—original draft, E.M. and A.V.; Writing—review and editing, G.D.S.; Supervision, G.D.S. and M.R. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Institutional Review Board Statement
Ethical review and approval were waived for this study because it is a retrospective analysis of anonymized data from patients undergoing standard-of-care surgery, according to the policy of our institution. The study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s). Data from this study were presented in part at the International Edition of XXIII Congress Italian Society of Vitreoretinal Surgery, Trieste, Italy, 8–10 June 2023. Luca Ventre and Erik Mus are co-first authors for this study. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
References
- Abdelkader, E.; Lois, N. Internal limiting membrane peeling in vitreo-retinal surgery. Surv. Ophthalmol. 2008, 53, 368–396. [Google Scholar] [CrossRef]
- Ch’ng, S.W.; Patton, N.; Ahmed, M.; Ivanova, T.; Baumann, C.; Charles, S.; Jalil, A. The Manchester Large Macular Hole Study: Is it Time to Reclassify Large Macular Holes? Am. J. Ophthalmol. 2018, 195, 36–42. [Google Scholar] [CrossRef] [PubMed]
- la Cour, M.; Friis, J. Macular holes: Classification, epidemiology, natural history and treatment. Acta Ophthalmol. Scand. 2002, 80, 579–587. [Google Scholar] [CrossRef]
- Lois, N.; Burr, J.; Norrie, J.; Vale, L.; Cook, J.; McDonald, A.; Boachie, C.; Ternent, L.; McPherson, G. Full-thickness Macular Hole and Internal Limiting Membrane Peeling Study (FILMS) Group. Internal limiting membrane peeling versus no peeling for idiopathic full-thickness macular hole: A pragmatic randomized controlled trial. Investig. Opthalmology Vis. Sci. 2011, 52, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Steel, D.H.; Donachie, P.H.J.; Aylward, G.W.; Laidlaw, D.A.; Williamson, T.H.; Yorston, D. Factors affecting anatomical and visual outcome after macular hole surgery: Findings from a large prospective UK cohort. Eye 2021, 35, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Ventre, L.; Mus, E.; Maradei, F.; Imparato, R.; Pintore, G.; Parisi, G.; Marolo, P.; Reibaldi, M. Relaxing Retinotomy in Recurrent and Refractory Full-Thickness Macular Holes: The State of the Art. Life 2023, 13, 1844. [Google Scholar] [CrossRef]
- Romano, M.R.; Rossi, T.; Borgia, A.; Catania, F.; Sorrentino, T.; Ferrara, M. Management of refractory and recurrent macular holes: A comprehensive review. Surv. Ophthalmol. 2022, 67, 908–931. [Google Scholar] [CrossRef]
- Abbey, A.M.; Van Laere, L.; Shah, A.R.; Hassan, T.S. Recurrent Macular Holes in The Era of Small-Gauge Vitrectomy: A Review of Incidence, Risk Factors, and Outcomes. Retina 2017, 37, 921–924. [Google Scholar] [CrossRef]
- Hillenkamp, J.; Kraus, J.; Framme, C.; Jackson, T.L.; Roider, J.; Gabel, V.-P.; Sachs, H.G. Retreatment of full-thickness macular hole: Predictive value of optical coherence tomography. Br. J. Ophthalmol. 2007, 91, 1445–1449. [Google Scholar] [CrossRef]
- Reid, G.A.; Mcdonagh, N.; Wright, D.M.; Yek, J.T.O.; Essex, R.W.; Lois, N. First Failed Macular Hole Surgery or Reopening of a Previously Closed Hole: Do We Gain by Reoperating?—A Systematic Review and Meta-analysis. Retina 2020, 40, 1–15. [Google Scholar] [CrossRef]
- Valldeperas, X.; Wong, D. Is it worth reoperating on macular holes? Ophthalmology 2008, 115, 158–163. [Google Scholar] [CrossRef]
- Maguire, M.J.M.; Steel, D.H.M.; Yorston, D.F.; Hind, J.F.; El-Faouri, M.F.; Jalil, A.F.; Tyagi, P.F.; Wickham, L.F.; Laidlaw, A.H.M. Outcome of Revision Procedures for Failed Primary Macular Hole Surgery. Retina 2021, 41, 1389–1395. [Google Scholar] [CrossRef]
- D’Souza, M.J.J.; Chaudhary, V.; Devenyi, R.; Kertes, P.J.; Lam, W.C. Re-operation of idiopathic full-thickness macular holes after initial surgery with internal limiting membrane peel. Br. J. Ophthalmol. 2011, 95, 1564–1567. [Google Scholar] [CrossRef]
- Baumann, C.M.; El-Faouri, M.M.; Ivanova, T.; Patton, N.M.C.; Ch’Ng, S.W.F.; Dhawahir-Scala, F.F.; Jalil, A.M. Manchester Revisional Macular Hole Study: Predictive Value of Optical Coherence Tomography Parameters on Outcomes of Repeat Vitrectomy, Extension of Internal Limiting Membrane Peel, and Gas Tamponade for Persistent Macular Holes. Retina 2021, 41, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, M.S.; Mora Cantallops, A.; Virgili, G.; Govetto, A. Long-term results of autologous plasma as adjuvant to pars plana vitrectomy in the treatment of high myopic full-thickness macular holes. Eur. J. Ophthalmol. 2021, 31, 2612–2620. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.L.; Kaiser, P.K. Surgical Management of Recurrent and Persistent Macular Holes: A Practical Approach. Ophthalmol. Ther. 2021, 10, 1137–1153. [Google Scholar] [CrossRef]
- Meyer, C.H.; Szurman, P.; Haritoglou, C.; Maier, M.; Wolf, A.; Lytvynchuk, L.; Priglinger, S.; Hillenkamp, J.; Wachtlin, J.; Becker, M.; et al. Application of subretinal fluid to close refractory full thickness macular holes: Treatment strategies and primary outcome: Apostel study. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 2151–2161. [Google Scholar] [CrossRef]
- Rao, X.; Wang, N.K.; Chen, Y.P.; Hwang, Y.S.; Chuang, L.H.; Liu, I.C.; Chen, K.J.; Wu, W.C.; Lai, C.C. Outcomes of outpatient fluid-gas exchange for open macular hole after vitrectomy. Am. J. Ophthalmol. 2013, 156, 326–333.e1. [Google Scholar] [CrossRef]
- Wang, H.; Ji, M.; Di, R.; Qi, Y.; Pei, C.; Gao, S.; Liu, S.W.; Xie, A.M.; Cheng, Y.H. Parafoveal retinal massage combined with autologous blood cover in the management of giant, persistent or recurrent macular holes. Int. J. Ophthalmol. 2020, 13, 1773–1779. [Google Scholar] [CrossRef]
- Hejsek, L.; Dusova, J.; Stepanov, A.; Rozsival, P. Re-operation of idiopathic macular hole after failed initial surgery. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2014, 158, 596–599. [Google Scholar] [CrossRef]
- Mura, M.; Iannetta, D.; Buschini, E.; De Smet, M.D. T-shaped macular buckling combined with 25G pars plana vitrectomy for macular hole, macular schisis, and macular detachment in highly myopic eyes. Br. J. Ophthalmol. 2017, 101, 383–388. [Google Scholar] [CrossRef]
- Grewal, D.S.; Charles, S.; Parolini, B.; Kadonosono, K.; Mahmoud, T.H. Autologous Retinal Transplant for Refractory Macular Holes: Multicenter International Collaborative Study Group. Ophthalmology 2019, 126, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, S.; Caporossi, T.; Tartaro, R.; Finocchio, L.; Franco, F.; Barca, F.; Giansanti, F. A Human Amniotic Membrane Plug to Promote Retinal Breaks Repair and Recurrent Macular Hole Closure. Retina 2019, 39, S95–S103. [Google Scholar] [CrossRef]
- Morizane, Y.; Shiraga, F.; Kimura, S.; Hosokawa, M.; Shiode, Y.; Kawata, T.; Hosogi, M.; Shirakata, Y.; Okanouchi, T. Autologous transplantation of the internal limiting membrane for refractory macular holes. Am. J. Ophthalmol. 2014, 157, 861–869.e1. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.N.; Yang, C.M. Lens Capsular Flap Transplantation in The Management of Refractory Macular Hole From Multiple Etiologies. Retina 2016, 36, 163–170. [Google Scholar] [CrossRef]
- Peng, J.; Chen, C.; Zhang, H.; Zhang, L.; Liu, J.; Ren, J.; Zhao, P. Long-Term Surgical Outcomes of Lens Capsular Flap Transplantation in the Management of Refractory Macular Hole. Retina 2021, 41, 726–734. [Google Scholar] [CrossRef]
- Tam, A.L.C.; Yan, P.; Gan, N.Y.; Lam, W.C. The Current Surgical Management of Large, Recurrent, or Persistent Macular Holes. Retina 2018, 38, 1263–1275. [Google Scholar] [CrossRef]
- Reis, R.; Ferreira, N.; Meireles, A. Management of Stage IV Macular Holes: When Standard Surgery Fails. Case Rep. Ophthalmol. 2012, 3, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Charles, S.; Randolph, J.C.; Neekhra, A.; Salisbury, C.D.; Littlejohn, N.; Calzada, J.I. Arcuate retinotomy for the repair of large macular holes. Ophthalmic Surg. Lasers Imaging 2013, 44, 69–72. [Google Scholar] [CrossRef]
- Tsipursky, M.S.; Byun, M.; Jager, R.D.; Sheth, V.S. Anatomical and Functional Outcomes of Relaxing Parafoveal Nasal Retinotomy for Refractory Macular Hole Repair. J. Vitr. Dis. 2021, 5, 479–487. [Google Scholar] [CrossRef]
- Knight, D.; Yu, J.J.; Adrean, S.D. Relaxing Nasal Retinotomy Technique for Closure of a Macular Hole that Reopened after Primary Vitrectomy. Retin. Cases Brief. Rep. 2021, 15, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Karacorlu, M.; Sayman Muslubas, I.; Hocaoglu, M.; Arf, S.; Ersoz, M.G. Double Arcuate Relaxing Retinotomy for a Large Macular Hole. Retin. Cases Brief Rep. 2019, 13, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Tsipursky, M. Creation of Paracentral Retinotomy to Facilitate Closure of Persistent Macular Holes. In Proceedings of the American Society of Retina Specialists 35th Annual Meeting, Boston, MA, USA, 11–15 August 2017. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.