The WHO2SAFE Score: A Predictive Tool for Postoperative Oxygen Requirement After PACU Recovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Ethical Declarations
2.2. Population and Characteristics
2.3. Data Variables
2.4. Postoperative Oxygen Requirement Measurement
2.5. Statistical Analysis
2.6. Predictive Model Development and Internal Validation
2.7. Cut-Off Selection
3. Results
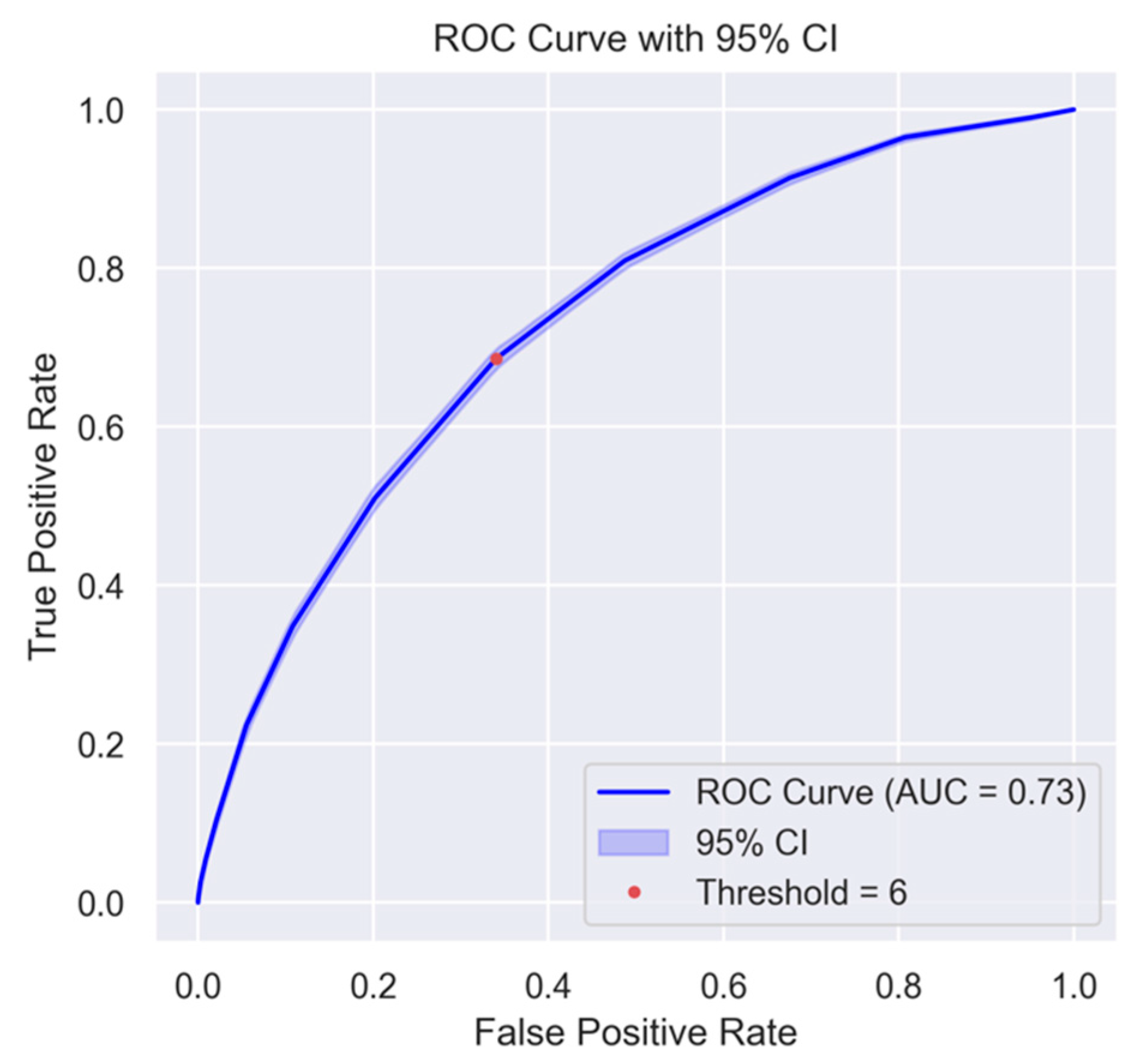
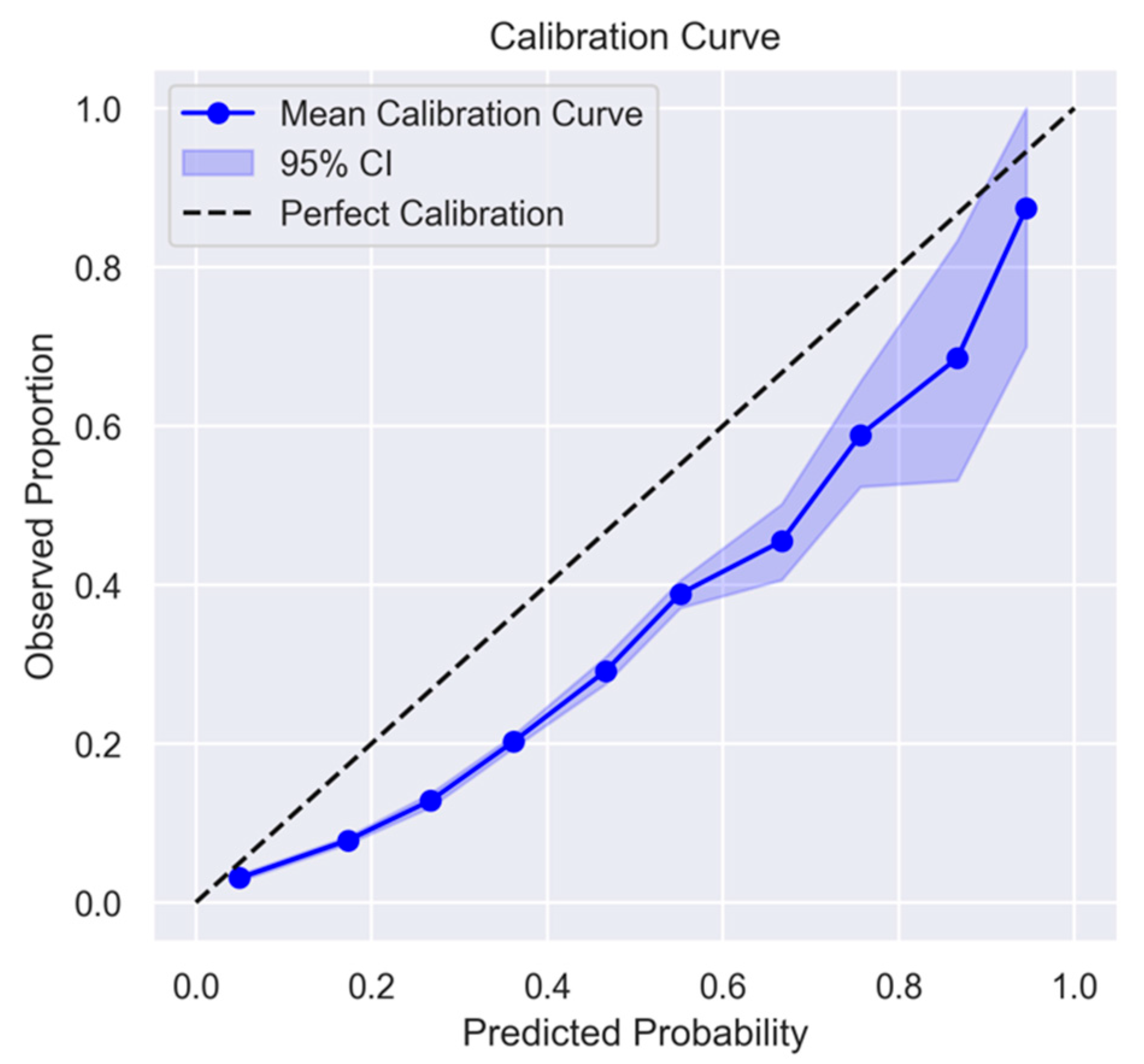
3.1. Development and Validation of WHO2SAFE Score
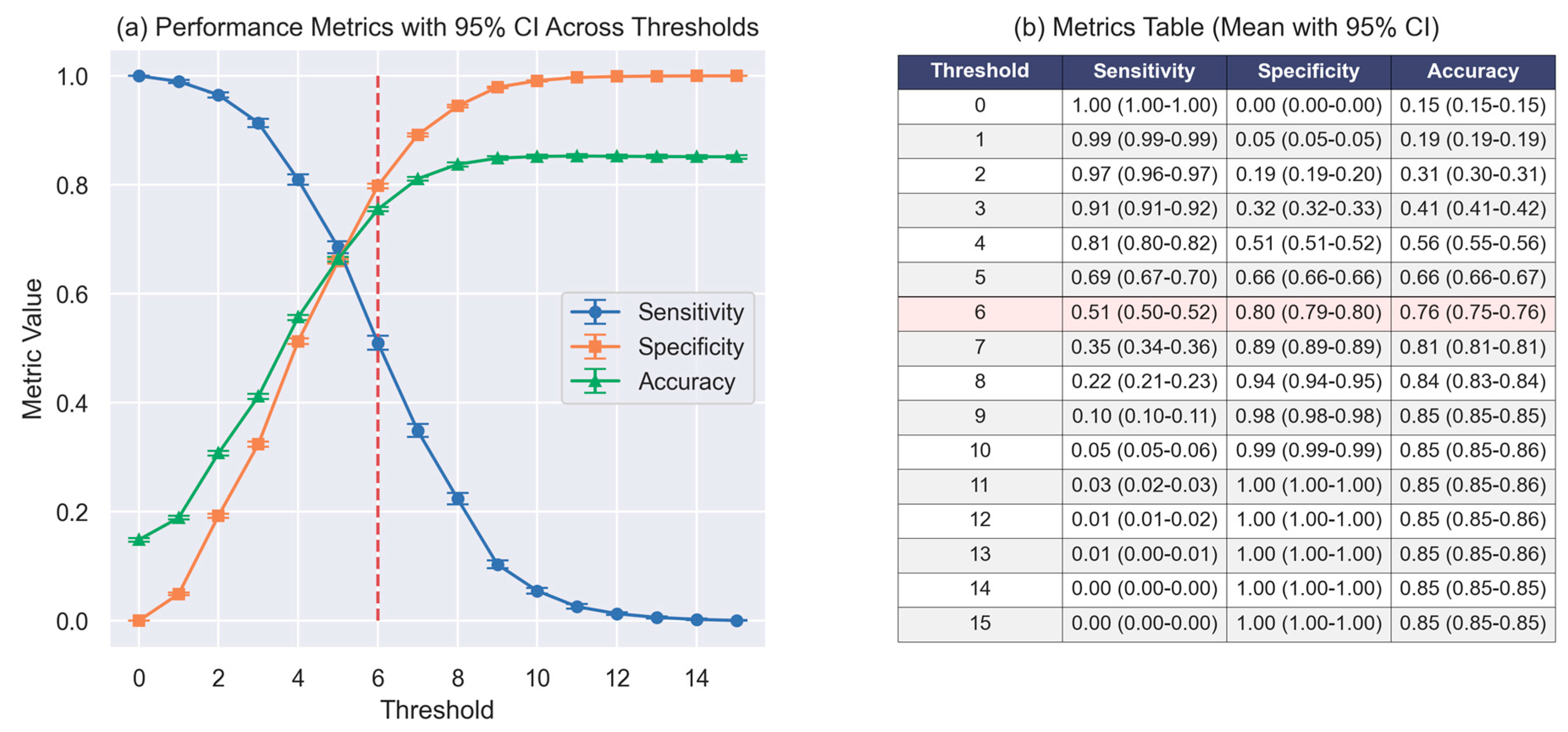
3.2. Cutoff Threshold and Clinical Application
4. Discussion
4.1. Significance of Predictive Factors
4.2. Clinical Implications and Practical Use
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASA | American Society of Anesthesiologists |
| BMI | Body Mass Index |
| EMR | Electronic Medical Record |
| EBUS | Endobronchial Ultrasound |
| FRC | Functional Residual Capacity |
| GA | General Anesthesia |
| HIS | Hospital Information System |
| ICU | Intensive Care Unit |
| IQR | Interquartile Range |
| IOH | Intraoperative Hypotension |
| MAP | Mean Arterial Pressure |
| OR | Odds Ratio |
| OSA | Obstructive Sleep Apnea |
| PACU | Post-Anesthesia Care Unit |
| RA | Regional Anesthesia |
| SpO2 | Peripheral Oxygen Saturation |
| TB | Tuberculosis |
| TIVA | Total Intravenous Anesthesia |
| URI | Upper Respiratory Tract Infection |
References
- Sun, Z.; Sessler, D.I.; Dalton, J.E.; Devereaux, P.J.; Shahinyan, A.; Naylor, A.J.; Hutcherson, M.T.; Finnegan, P.S.; Tandon, V.; Darvish-Kazem, S.; et al. Postoperative Hypoxemia Is Common and Persistent: A Prospective Blinded Observational Study. Anesth. Analg. 2015, 121, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Melesse, D.Y.; Denu, Z.A.; Kassahun, H.G.; Agegnehu, A.F. The incidence of early post-operative hypoxemia and its contributing factors among patients underwent operation under anesthesia at University of Gondar comprehensive and specialized referral hospital, Gondar, North West Ethiopia, 2018. A prospective observational study. Int. J. Surg. Open 2020, 22, 38–46. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E., Jr.; Chung, M.K.; de Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 127, e362–e425. [Google Scholar] [CrossRef]
- Karcz, M.; Papadakos, P.J. Respiratory complications in the postanesthesia care unit: A review of pathophysiological mechanisms. Can. J. Respir. Ther. 2013, 49, 21–29. [Google Scholar]
- Lagier, D.; Zeng, C.; Fernandez-Bustamante, A.; Vidal Melo, M.F. Perioperative Pulmonary Atelectasis: Part II. Clinical Implications. Anesthesiology 2022, 136, 206–236. [Google Scholar]
- Hillman, D.R.; Platt, P.R.; Eastwood, P.R. The upper airway during anaesthesia. BJA Br. J. Anaesth. 2003, 91, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Russell, G.B.; Graybeal, J.M. Hypoxemic Episodes of Patients in a Postanesthesia Care Unit. Chest 1993, 104, 899–903. [Google Scholar] [CrossRef]
- Bang, Y.J.; Park, I.H.; Jeong, H. Frequency and risk factors for failed weaning from supplemental oxygen therapy after general anesthesia at a postanesthesia care unit: A retrospective cohort study. BMC Anesthesiol. 2023, 23, 231. [Google Scholar] [CrossRef]
- Smetana, G.W.; Lawrence, V.A.; Cornell, J.E. Preoperative pulmonary risk stratification for noncardiothoracic surgery: Systematic review for the American College of Physicians. Ann. Intern. Med. 2006, 144, 581–595. [Google Scholar] [CrossRef]
- World Health Organization. The Asia Pacific Perspective: Redefining Obesity and Its Treatment. 2000. Available online: http://www.wpro.who.int/nutrition/documents/Redefining_obesity/en/ (accessed on 1 December 2024).
- Berhanu, M.; Dadi, N.; Mengistu, B.; Muluken, Z.; Tolesa, A.; Tageza, T.; Kalbesa, M.; Tesfaye, G.; Zawdie, B. Magnitude of early postoperative hypoxemia and its associated factors among adult patients who undergo emergency surgery under general anesthesia at Jimma Medical Center, Jimma, Southwest Ethiopia, 2021: A prospective observational study. Perioper. Med. 2023, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhu, L.; Yuan, S.; Lu, S.; Zhang, X. Identifying risk factors for hypoxemia during emergence from anesthesia in patients undergoing robot-assisted laparoscopic radical prostatectomy. J. Robot. Surg. 2024, 18, 200. [Google Scholar] [CrossRef]
- Fernandez-Bustamante, A.; Frendl, G.; Sprung, J.; Kor, D.J.; Subramaniam, B.; Martinez Ruiz, R.; Lee, J.W.; Henderson, W.G.; Moss, A.; Mehdiratta, N.; et al. Postoperative Pulmonary Complications, Early Mortality, and Hospital Stay Following Noncardiothoracic Surgery: A Multicenter Study by the Perioperative Research Network Investigators. JAMA Surg. 2017, 152, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Schouten, L.R.; Helmerhorst, H.J.; Wagenaar, G.T.; Haltenhof, T.; Lutter, R.; Roelofs, J.J.; van Woensel, J.B.; van Kaam, A.H.; Bos, A.P.; Schultz, M.J.; et al. Age-Dependent Changes in the Pulmonary Renin-Angiotensin System Are Associated With Severity of Lung Injury in a Model of Acute Lung Injury in Rats. Crit. Care Med. 2016, 44, e1226–e1235. [Google Scholar] [CrossRef]
- Sharma, G.; Goodwin, J. Effect of aging on respiratory system physiology and immunology. Clin. Interv. Aging 2006, 1, 253–260. [Google Scholar] [CrossRef]
- Yeo, E.-J. Hypoxia and aging. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef]
- Xiong, N.; Nong, Y.; Yi, Y. Meta-analysis of risk factors associated with postoperative hypoxemia in the postanesthesia care unit. Am. J. Transl. Res. 2024, 16, 5787–5796. [Google Scholar] [CrossRef]
- Ji, J.Y.; Chung, J.H.; Kim, N.S.; Seo, Y.H.; Jung, H.S.; Chun, H.R.; Gong, H.Y.; Kim, W.J.; Ahn, J.M.; Park, Y.J. Causes and Treatment of Hypoxia during Total Hip Arthroplasty in Elderly Patients: A Case Report. Int. J. Environ. Res. Public Health 2021, 18, 12931. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; van Diepen, S.; Beekman, R.; Sinha, S.S.; Brusca, S.B.; Alviar, C.L.; Jentzer, J.; Bohula, E.A.; Katz, J.N.; Shahu, A.; et al. Oxygen Supplementation and Hyperoxia in Critically Ill Cardiac Patients: From Pathophysiology to Clinical Practice. JACC Adv. 2022, 1, 100065. [Google Scholar] [CrossRef]
- Dixon, A.E.; Peters, U. The effect of obesity on lung function. Expert Rev. Respir. Med. 2018, 12, 755–767. [Google Scholar] [CrossRef]
- Liu, C.; Chen, M.S.; Yu, H. The relationship between obstructive sleep apnea and obesity hypoventilation syndrome: A systematic review and meta-analysis. Oncotarget 2017, 8, 93168–93178. [Google Scholar] [CrossRef] [PubMed]
- Luna, I.E.; Kehlet, H.; Olsen, R.M.; Wede, H.R.; Hoevsgaard, S.J.; Aasvang, E.K. Hypoxemia following hospital discharge after fast-track hip and knee arthroplasty—A prospective observational study subanalysis. Acta Anaesthesiol. Scand. 2020, 64, 1405–1413. [Google Scholar] [CrossRef]
- Gregory, A.; Stapelfeldt, W.H.; Khanna, A.K.; Smischney, N.J.; Boero, I.J.; Chen, Q.; Stevens, M.; Shaw, A.D. Intraoperative Hypotension Is Associated With Adverse Clinical Outcomes After Noncardiac Surgery. Anesth. Analg. 2021, 132, 1654–1665. [Google Scholar] [CrossRef]
- Harris, R.S.; Winkler, T.; Tgavalekos, N.; Musch, G.; Melo, M.F.; Schroeder, T.; Chang, Y.; Venegas, J.G. Regional pulmonary perfusion, inflation, and ventilation defects in bronchoconstricted patients with asthma. Am. J. Respir. Crit. Care Med. 2006, 174, 245–253. [Google Scholar] [CrossRef]
- Woods, B.D.; Sladen, R.N. Perioperative considerations for the patient with asthma and bronchospasm. Br. J. Anaesth. 2009, 103, i57–i65. [Google Scholar] [CrossRef] [PubMed]
- Maimon, N.; Hanly, P.J. Does snoring intensity correlate with the severity of obstructive sleep apnea? J. Clin. Sleep. Med. 2010, 6, 475–478. [Google Scholar] [CrossRef]
- Ursavaş, A.; Güven, T.; Coskun, F.; Ege, E.; Yılmazlar, A. Association between self reported snoring, STOP questionnaire and postoperative pulmonary complications in patients submitted to ortophaedic surgery. Multidiscip. Respir. Med. 2013, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Clymer, J.W.; Po-Han Chen, B.; Sadeghirad, B.; Ferko, N.C.; Cameron, C.G.; Hinoul, P. Prolonged operative duration is associated with complications: A systematic review and meta-analysis. J. Surg. Res. 2018, 229, 134–144. [Google Scholar] [CrossRef]
- Harvey, B.J.; McElvaney, N.G. Sex differences in airway disease: Estrogen and airway surface liquid dynamics. Biol. Sex Differ. 2024, 15, 56. [Google Scholar] [CrossRef]
- Card, J.W.; Zeldin, D.C. Hormonal influences on lung function and response to environmental agents: Lessons from animal models of respiratory disease. Proc. Am. Thorac. Soc. 2009, 6, 588–595. [Google Scholar] [CrossRef]
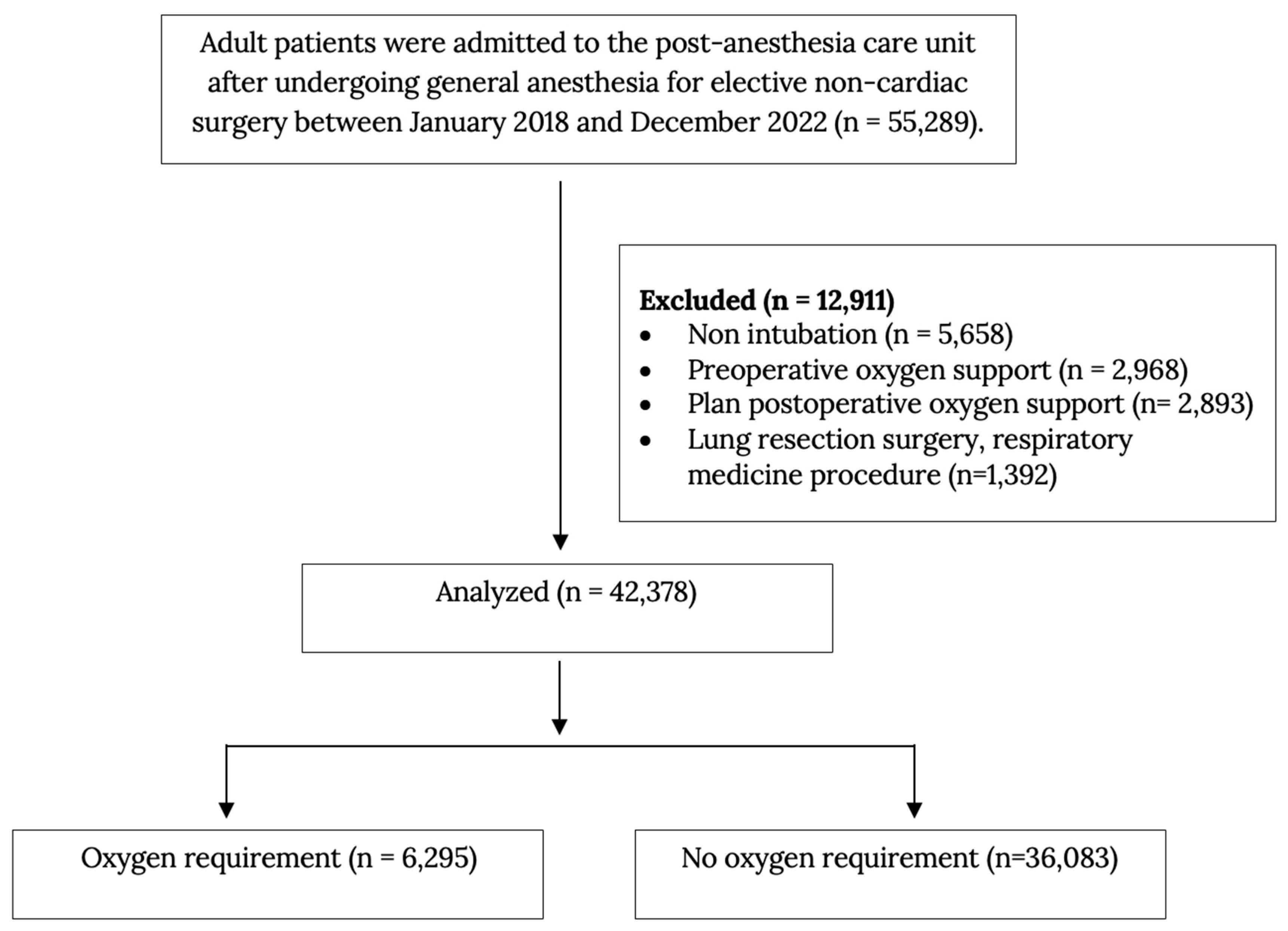

| Variables | Oxygen Requirement (n = 6295) | No Oxygen Requirement (n = 36,083) | p Value |
|---|---|---|---|
| Age (Median [IQR]) | 61 (50, 71) | 51 (38, 64) | <0.001 |
| Female, n (%) | 3777 (60) | 216,721 (60) | 0.938 |
| Smoking, n (%) | 704 (11.2) | 3987 (11) | 0.771 |
| Weight, kg (Median [IQR]) | 64.8 (55.76, 76.2) | 60 (52, 69) | <0.001 |
| Height, cm (Median [IQR]) | 158 (152, 165) | 160 (154, 165) | <0.001 |
| BMI, kg/m2 (Median [IQR]) | 25.8 (22.1, 30.2) | 23.4 (20.7, 26.6) | <0.001 |
| Arrhythmia, n (%) | 581 (9.2) | 2160 (6) | <0.001 |
| Congenital heart disease, n (%) | 17 (0.3) | 78 (0.2) | 0.490 |
| Hypertension, n (%) | 3238 (51.4) | 10,099 (28) | <0.001 |
| Ischemic heart disease, n (%) | 254 (4) | 866 (2.4) | <0.001 |
| Altered of consciousness, n (%) | 23 (0.4) | 575 (1.6) | <0.001 |
| History of pulmonary TB, n (%) | 141 (2.2) | 721 (2) | 0.228 |
| Asthma, n (%) | 213 (3.4) | 791 (2.2) | <0.001 |
| COPD, n (%) | 136 (2.2) | 444 (1.2) | <0.001 |
| URI within 8 weeks, n (%) | 43 (0.7) | 161 (0.4) | 0.016 |
| History of COVID-19, n (%) | 290 (4.6) | 1659 (4.6) | 1.000 |
| Obstructive sleep apnea, n (%) | 1216 (19.3) | 4420 (12.2) | <0.001 |
| ASA classification, n (%) | <0.001 | ||
| 1 | 71 (1.1) | 2583 (7.2) | |
| 2 | 3130 (49.7) | 23,645 (65.5) | |
| 3 | 370 (48.8) | 9458 (26.2) | |
| 4 | 24 (0.4) | 397 (1.1) |
| Variables | Oxygen Requirement (n = 6295) | No oxygen Requirement (n = 36,083) | p Value |
|---|---|---|---|
| IOH, n (%) | 3419 (54.1) | 13,753 (37.9) | <0.001 |
| Intraoperative wheezing, n (%) | 269 (4.3) | 364 (1) | <0.001 |
| Difficult intubation, n (%) | 21 (0.3) | 46 (0.1) | <0.001 |
| Type of anesthesia | 0.002 | ||
| GA, n (%) | 5910 (93.9) | 34,237 (94.9) | |
| GA combine with RA, n (%) | 385 (6.1) | 1846 (5.1) | |
| Position | <0.001 | ||
| Jackknife, n (%) | 4 (0.1) | 41 (0.1) | |
| Kidney position, n (%) | 56 (0.9) | 174 (0.5) | |
| Lateral decubitus, n (%) | 449 (7.1) | 1248 (3.5) | |
| Lithotomy, n (%) | 538 (8.5) | 4647 (12.9) | |
| Prone, n (%) | 302 (4.8) | 1847 (5.1) | |
| Sitting, n (%) | 33 (0.5) | 166 (0.5) | |
| Supine, n (%) | 4913 (78) | 27,960 (77.5) | |
| Duration, min (Median [IQR]) | 175 (115, 260) | 125 (75, 195) | <0.001 |
| Blood loss, mL (Median [IQR]) | 50 (10, 200) | 20 (5, 100) | <0.001 |
| Anesthetic agent | |||
| Sevoflurane, n (%) | 3172 (50.4) | 20,580 (57) | <0.001 |
| Desflurane, n (%) | 2320 (36.9) | 7837 (21.7) | <0.001 |
| TIVA, n (%) | 803 (12.7) | 7666(21.3) | <0.001 |
| Morphine, n (%) | 1127 (17.9) | 6943 (19.2) | 0.013 |
| Fentanyl, n (%) | 5748 (91.3) | 31,450 (87.2) | <0.001 |
| Succinylcholine, n (%) | 1566 (24.9) | 6858 (19) | <0.001 |
| Cisatracurium, n (%) | 5251 (83.4) | 25,401 (70.4) | <0.001 |
| Rocuronium, n (%) | 15 (0.2) | 169 (0.5) | 0.014 |
| Site of operation | <0.001 | ||
| Intracranial, n (%) | 107 (1.7) | 600 (1.7) | |
| Intrathoracic, n (%) | 362 (5.8) | 507 (1.4) | |
| Open upper abdomen, n (%) | 132 (2.1) | 475 (1.3) | |
| Open lower abdomen, n (%) | 1294 (20.5) | 7735 (21.4) | |
| Laparoscopy, n (%) | 1117 (17.7) | 4191 (11.6) | |
| Groin, n (%) | 222 (3.5) | 3034 (8.4) | |
| Neck, n (%) | 223 (3.5) | 954 (2.6) | |
| Ear Nose Throat, n (%) | 853 (13.6) | 4592 (12.7) | |
| Spine, n (%) | 232 (3.7) | 957 (2.7) | |
| Vascular, n (%) | 160 (2.5) | 1672 (4.6) | |
| Extremities, n (%) | 590 (9.4) | 4078 (11.3) | |
| Eye, n (%) | 294 (4.7) | 2352 (6.5) | |
| Breast, n (%) | 413 (6.6) | 3108 (8.6) | |
| Endoscopy, n (%) | 240 (3.8) | 1502 (4.2) | |
| Cystoscopy, n (%) | 56 (0.9) | 326 (0.9) |
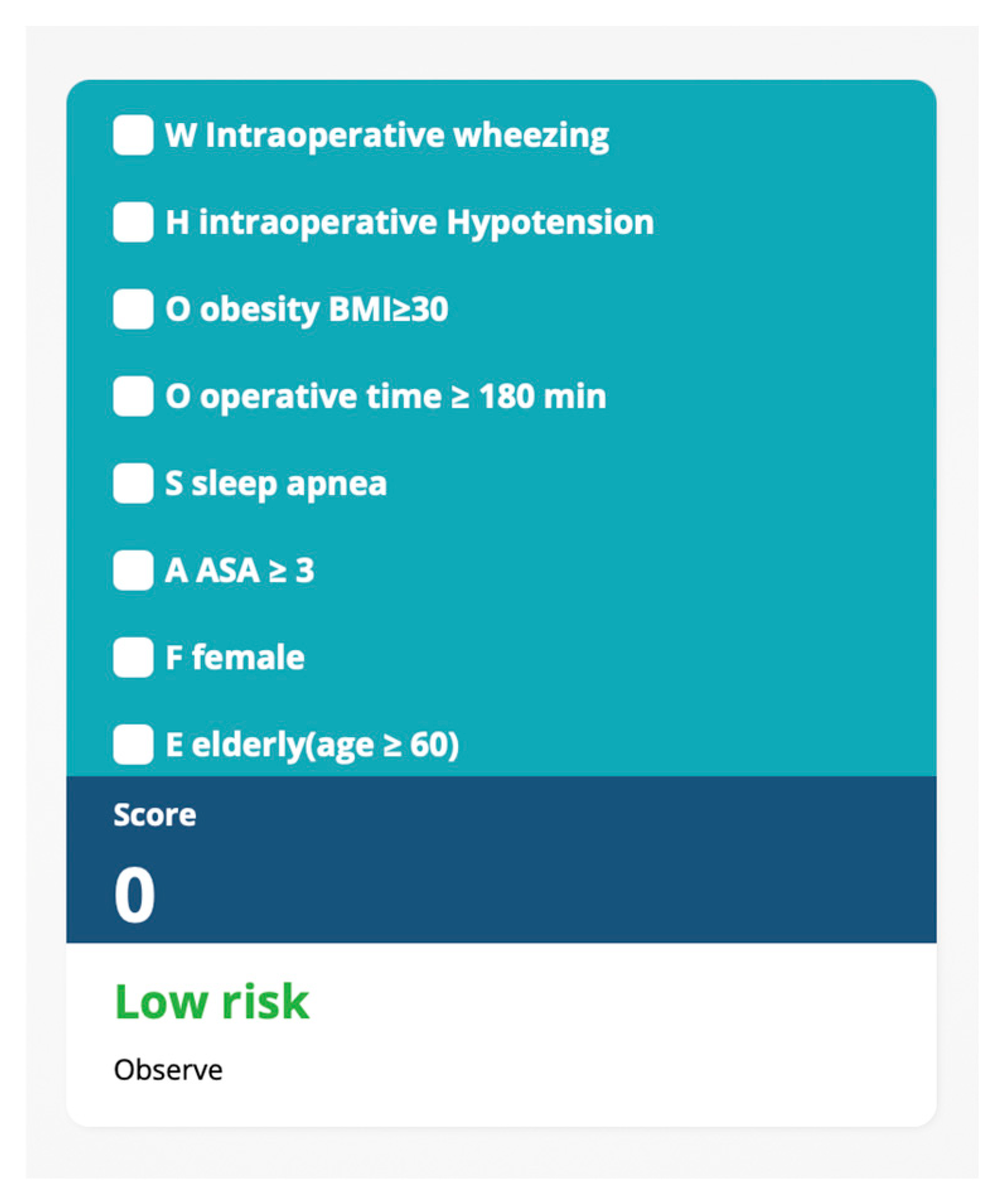
| Mnemonic | Factor | p Value | Adjusted OR (95%CI) | ln (Adjusted OR) | Scores # |
|---|---|---|---|---|---|
| W | Wheezing | <0.001 | 3.17 (2.67, 3.77) | 1.15 | 4 |
| H | Hypotension | <0.001 | 1.38 (1.30, 1.46) | 0.32 | 1 |
| O | Obesity (BMI ≥ 30) | <0.001 | 2.06 (1.94, 2.18) | 0.72 | 2 |
| O | Operative time ≥ 180 min | <0.001 | 2.10 (1.99, 2.23) | 0.74 | 2 |
| S | Sleep Apnea | <0.001 | 1.40 (1.30, 1.51) | 0.34 | 1 |
| A | ASA classification ≥ 3 | <0.001 | 2.13 (2.00, 2.25) | 0.76 | 2 |
| F | Female | <0.001 | 1.20 (1.14, 1.28) | 0.18 | 1 |
| E | Elderly (Age ≥ 60 years) | <0.001 | 2.17 (2.04, 2.30) | 0.77 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sungworawongpana, C.; Chaichulee, S.; Chaochankit, W.; Vichitkunakorn, P.; Gosiyaphant, N.; Thongaek, C.S.; Boonchai, R.; Sutthibenjakul, K.; Tantisarasart, T. The WHO2SAFE Score: A Predictive Tool for Postoperative Oxygen Requirement After PACU Recovery. J. Clin. Med. 2025, 14, 2603. https://doi.org/10.3390/jcm14082603
Sungworawongpana C, Chaichulee S, Chaochankit W, Vichitkunakorn P, Gosiyaphant N, Thongaek CS, Boonchai R, Sutthibenjakul K, Tantisarasart T. The WHO2SAFE Score: A Predictive Tool for Postoperative Oxygen Requirement After PACU Recovery. Journal of Clinical Medicine. 2025; 14(8):2603. https://doi.org/10.3390/jcm14082603
Chicago/Turabian StyleSungworawongpana, Chutida, Sitthichok Chaichulee, Wongsakorn Chaochankit, Polathep Vichitkunakorn, Nachawan Gosiyaphant, Chayaporn Subanphanichkul Thongaek, Ratikorn Boonchai, Karuna Sutthibenjakul, and Thadakorn Tantisarasart. 2025. "The WHO2SAFE Score: A Predictive Tool for Postoperative Oxygen Requirement After PACU Recovery" Journal of Clinical Medicine 14, no. 8: 2603. https://doi.org/10.3390/jcm14082603
APA StyleSungworawongpana, C., Chaichulee, S., Chaochankit, W., Vichitkunakorn, P., Gosiyaphant, N., Thongaek, C. S., Boonchai, R., Sutthibenjakul, K., & Tantisarasart, T. (2025). The WHO2SAFE Score: A Predictive Tool for Postoperative Oxygen Requirement After PACU Recovery. Journal of Clinical Medicine, 14(8), 2603. https://doi.org/10.3390/jcm14082603





