Abstract
Background/Objectives: The only intravenous P2Y12 inhibitor used in the management of acute and chronic coronary syndromes is cangrelor. Previous studies have demonstrated the efficacy of cangrelor. However, limited real-world data are available for cangrelor usage. This study aimed to investigate the use of cangrelor in large-volume percutaneous coronary intervention (PCI) centers in Türkiye, and examine the indications, patient characteristics, bleeding, and ischemic events. The efficacy and safety of cangrelor in this high-risk group were evaluated. Methods: This study was conducted retrospectively in 14 high-volume centers in Türkiye with extensive cangrelor experience. Cangrelor indications, patient clinical characteristics, periprocedural and postprocedural treatments, in-hospital and follow-up bleeding, ischemic events, and mortality were analyzed. Results: This study recruited 411 patients (mean age: 63.8 ± 12.7 years; 76% male). The most common conditions in which cangrelor is used in Türkiye are cardiogenic shock, intubation and nausea/vomiting, where P2Y12 cannot be used adequately due to impaired oral intake. The incidence rate of any bleeding within 48 h was 6.4% (n = 26), with major bleeding accounting for 1.7% of all cases (n = 7). The bleeding rates were similar between patients aged <75 years and those aged ≥75 years (6.0% vs. 8.8%, p = 0.326), as well as between patients with chronic kidney disease (CKD) and those without CKD (6.3% vs. 7.9%, p = 0.600). Conclusions: This is the first multicenter, large-cohort study to examine cangrelor use in Türkiye, providing real-world evidence for the efficacy and safety in high-risk patients with complex clinical features and lesion characteristics.
1. Introduction
Acute coronary syndrome (ACS) is a leading cause of mortality and disability worldwide [1]. According to the current guideline recommendations, early percutaneous coronary intervention (PCI) and stent implantation can aid in mitigating mortality and heart failure rates [2,3]. However, the antiplatelet activities of aspirin and P2Y12 inhibitors must be adequate before PCI to prevent early stent thrombosis, reinfarction, and mortality. The efficacy of commonly used P2Y12 inhibitors (clopidogrel, ticagrelor, and prasugrel) is delayed due to gastrointestinal absorption. Additionally, the effectiveness of these inhibitors may be suboptimal under certain conditions [4,5]. Hypotension, nausea/vomiting, intubation, delayed gastric passage, impaired intestinal perfusion, hypothermia, and opioid use, which are frequently observed in patients with ACS, can limit the efficacy and use of oral P2Y12 inhibitors [6,7,8]. Additionally, 10–15% of patients with ACS may require urgent coronary artery bypass grafting (CABG) post-angiography. The prolonged time required for the activity of oral P2Y12 inhibitors can increase bleeding risk in these patients [5].
The efficacy of cangrelor, an intravenous P2Y12 inhibitor with rapid onset and offset of action [8], has been compared with that of clopidogrel in patients with ACS and chronic coronary syndrome (CCS) undergoing PCI [9,10,11]. Current guidelines recommend cangrelor use with a class 2b indication in patients with ACS who have not received oral P2Y12 inhibitors [2,12]. In Türkiye, cangrelor use in adult patients undergoing PCI who have not received oral P2Y12 inhibitors and in whom oral P2Y12 therapy is not feasible or desirable is prescribed based on the Ministry of Health regulations, which have limited its use to patients with cardiogenic shock or cardiac arrest who are unconscious or intubated or cannot be fitted with a nasogastric tube [13]. This patient group indicates high-risk patients.
This study aimed to evaluate cangrelor use, indications, patient characteristics, bleeding, and ischemic events in large-volume PCI centers in Türkiye.
2. Methods
Data for this study were collected between 20 January 2025 and 1 April 2025, at 14 high-volume PCI centers in Türkiye with established 24/7 cangrelor programs. This study is a retrospective study and the data include past five years usage data of cangrelor. All patients who used cangrelor in the centers participating in the study were included in the study.
In this study, 411 patients diagnosed with ACS or CCS who received cangrelor according to approved indications before PCI were included. Patients with non-obstructive coronary arteries, such as myocardial infarction with non-obstructive coronary arteries or Takotsubo syndrome, were excluded [14,15,16]. Data on cangrelor indications, baseline and clinical characteristics, periprocedural details, in-hospital outcomes, and follow-up bleeding, ischemic events, and mortality were collected. Based on the European Society of Cardiology guidelines, patients were categorized into the following groups: ST-elevation myocardial infarction (STEMI), non-ST-elevation myocardial infarction (NSTEMI), unstable angina (UA), and CCS groups [2,3]. Stent thrombosis was defined according to Academic Research Consortium (ARC) criteria as definite or probable events confirmed angiographically or clinically [17].
Bleeding events were assessed at 48 h and 1 month and classified using the Bleeding Academic Research Consortium (BARC) and Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) criteria.
BARC Classification: Type 1: Bleeding not requiring unplanned tests, hospitalization, or treatment; Type 2: Bleeding requiring non-surgical medical intervention, hospitalization, or increased care; Type 3a: Overt bleeding with 3–5 g/dL hemoglobin drop or transfusion requirement; Type 3b: Overt bleeding with ≥5 g/dL hemoglobin drop, cardiac tamponade, or requiring surgical intervention (excluding dental, nasal, skin, or hemorrhoidal bleeding) or intravenous vasoactive agents; Type 3c: Intracranial or intraspinal bleeding or vision-impairing intraocular bleeding; Type 4: CABG-related bleeding, including perioperative intracranial bleeding, reoperation after sternotomy closure, or significant transfusion requirements; Type 5a: Probable fatal bleeding without autopsy/imaging confirmation; Type 5b: Definite fatal bleeding confirmed using autopsy/imaging. Bleeding events with types 1–2 and types ≥ 3 were considered minor and major events, respectively [18].
GUSTO Classification: Severe/life-threatening: Intracerebral hemorrhage or bleeding causing hemodynamic compromise requiring treatment; Moderate: Requiring transfusion but not causing hemodynamic compromise; Mild: Bleeding not meeting the above criteria [19].
Chronic kidney disease (CKD) was defined as a glomerular filtration rate of <60 mL/min (calculated using the CKD-EPI formula) [20]. Contrast-associated acute kidney injury (CA-AKI) was defined according to KDIGO criteria as an increase in serum creatinine ≥ 0.3 mg/dL within 48 h or ≥1.5 × baseline within 7 days after contrast exposure [21]. Patients receiving chronic dialysis were excluded from CA-AKI analyses. All numeric variables were verified for consistency across centers before analysis, and data audits were performed to ensure accuracy of procedural and outcome variables. In-hospital data were obtained from patient records, while long-term follow-up data were collected from hospital records, telephone interviews, and the national healthcare system (e-nabız) [22]. For survival analysis, the time-to-event variable was defined as the interval (in months) from the index PCI to death or censoring. Death times were obtained from follow-up records; in-hospital deaths without a recorded follow-up duration were assigned survival times equal to the length of hospitalization expressed in months. Patients alive at last contact were censored at their follow-up time, truncated at 12 months. Kaplan–Meier survival curves were generated for each CAD category, and intergroup differences were evaluated using a center-stratified log-rank test. This study complied with the guidelines of the Declaration of Helsinki and was approved by the Trakya University School of Medicine ethics committee (TUTF-GOBAEK-2024/560).
3. Statistical Analysis
Continuous variables were summarized as mean ± standard deviation (SD) or median [interquartile range, IQR], depending on distribution. Categorical variables were reported as frequency and percentage. Incidence rates were calculated with 95% confidence intervals using the Wilson method. Bleeding rates according to BARC and GUSTO classifications were summarized by coronary artery disease type (STEMI, NSTEMI, UA, CCS). Time windows (48 h and a 30-day window with ±2-day allowance) were defined relative to the first occurrence of an event. Kaplan–Meier curves were used to estimate 12-month major adverse cardiac event (MACE)-free survival and overall survival, and groups were compared using the log-rank test; stratified log-rank tests were applied when center imbalance was suspected. Event-free patients at follow-up were right-censored. Missingness was evaluated for each variable; predictors included in the multivariable logistic regression model (age, hypertension, diabetes mellitus, CKD, Killip class, P2Y12 inhibitor type, and gender) had <1% missingness, and variables with >30% missingness were excluded. Multivariable logistic regression was used to identify independent predictors of 48 h bleeding, and results were expressed as odds ratios (OR) with 95% confidence intervals. A two-sided p < 0.05 was considered statistically significant. All analyses were performed using R version 4.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
4. Results
Of the 411 patients included in this study, the median age of patients in the STEMI, NSTEMI, UA, and CCS groups was 64, 70, 66, and 59 years, respectively. Patients in the STEMI group exhibited advanced Killip classes, increased heart rates, and decreased systolic and diastolic blood pressures at presentation. Hypertension prevalence was high in all groups. Patients in the STEMI and NSTEMI groups exhibited increased prevalence of CKD and diabetes, respectively. The lowest median ejection fraction was observed in the STEMI group (Table 1).

Table 1.
Demographic and clinical characteristics of the study participants.
Cangrelor use was most common in cases of cardiogenic shock, intubation, and nausea/vomiting due to oral intake impairment. The most common major lesion was located in the left anterior descending artery. In the study cohort, 59% of patients had two or more coronary lesions. The femoral approach was used in 72% of cases, while 95% of patients received drug-eluting stents, predominantly everolimus-eluting stents. All patients received aspirin and heparin. Clopidogrel was the most commonly used P2Y12 inhibitor (57%). P2Y12 was started immediately after the end of cangrelor in all cases. Median stent diameter was 3.0 mm, total stent length was 34.3 ± 19.7 mm, median procedure duration was 39 min (IQR 26–55), contrast volume was 150 mL (IQR 110–220), and cangrelor infusion duration was 2 h (IQR 2–2) (Table 2).

Table 2.
Detailed characteristics of percutaneous coronary intervention.
In-hospital events included inotrope requirement (40%), in-hospital mortality (31%), contrast-induced nephropathy (14%), and transfusion requirement (3.9%). The incidences of inotrope use and mortality were significantly high in the STEMI group. Other in-hospital events were similar between the groups.
The incidence rate of any bleeding events within 48 h was 6.4% (n = 26). The major bleeding rate was 1.7% (n = 7), which was not significantly different between the groups. The most common bleeding sites were vascular access and the urinary system. Most major bleeds were located in the gastrointestinal system. Among patients with gastrointestinal bleeding, 57% (n = 4) were being treated with glycoprotein IIb/IIIa inhibitors. Allergic reactions or adverse events requiring cangrelor discontinuation were not noted (Table 3 and Table 4).

Table 3.
Detailed description of the observed bleeding events.

Table 4.
Distribution of Bleeding Events According to BARC and GUSTO Classifications at 48 h and 1 Month, Stratified by ACS Subtype.
The number of bleeding events within 48 h in the ticagrelor/prasugrel-treated group was non-significantly higher than that in the clopidogrel-treated group (p = 0.227 for BARC, p = 0.307 for GUSTO). After age stratification, the bleeding rates in patients treated with BARC aged <75 years and ≥75 years were 6.0% and 8.8%, respectively. Meanwhile, the bleeding rates in patients treated with GUSTO aged <75 years and ≥75 years were 5.6% and 8.8%, respectively (relative risk (RR): 0.97; p = 0.326). Among patients treated with BARC, the bleeding rates in the CKD and non-CKD groups were 6.3% and 7.9% (CKD), respectively. Meanwhile, the bleeding rates in the non-CKD and CKD groups were 6.0% and 7.9%, respectively, among patients treated with GUSTO (RR = 0.98, p = 0.600).
No significant differences in 12-month overall survival were observed between CAD groups (STEMI, NSTEMI, UA, and CCS; log-rank p = 0.392, stratified by center) (Figure 1). At 12 months, Kaplan–Meier estimated survival was 54.6% (95% CI 48.8–61.1) for STEMI, 63.3% (95% CI 54.0–74.1) for NSTEMI, 90.0% (95% CI 73.2–100) for UA, and 97.0% (95% CI 91.3–100) for CCS, with 35, 13, 3, and 12 patients remaining at risk, respectively. Advanced age (≥75 years) and CKD were not associated with bleeding risk within 48 h, despite slightly increased rates in these subgroups.
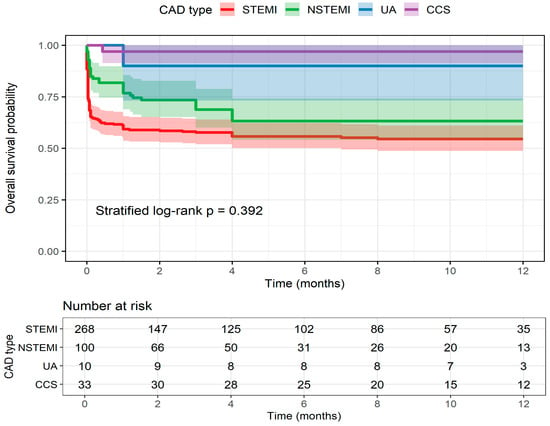
Figure 1.
Kaplan–Meier curves showing 12-month overall survival stratified by coronary artery disease (CAD) subtype: ST-elevation myocardial infarction (STEMI), non-ST-elevation myocardial infarction (NSTEMI), unstable angina (UA), and chronic coronary syndrome (CCS). Shaded areas represent 95% confidence intervals. The table below displays the number of patients at risk over time. Differences between groups were assessed using a center-stratified log-rank test.
Age was an independent and significant predictor of 48 h bleeding (odds ratio [OR]: 1.04; 95% CI: 1.01–1.08; p = 0.018), indicating that bleeding risk increases with age. The bleeding risk in patients treated with potent P2Y12 inhibitors (ticagrelor/prasugrel) was higher than that in patients treated with clopidogrel (OR: 2.17; p = 0.064). However, the wide CI and borderline p-value suggest the need for confirmation in larger samples. Hypertension was associated with decreased bleeding risk (OR: 0.32; p = 0.007). Cardiogenic shock (Killip 4), diabetes, CKD, and female sex were not significantly associated with 48 h bleeding (Table 5).

Table 5.
Determinants of 48 h bleeding.
The incidence of major ischemic events (myocardial infarction, revascularization, and stent thrombosis) within 48 h and 1 month was 4.2%. Definite stent thrombosis occurred in 9 patients (2.2%) within the first month after PCI, with no additional cases through 12 months. Although ischemic event rates were lower in the ticagrelor/prasugrel group than in the clopidogrel group (RR = 0.75 and 0.76, p = 0.770), the difference did not reach statistical significance.
5. Discussion
Cangrelor, which is the only intravenous P2Y12 inhibitor, is critical for ACS management [23,24]. The CHAMPION trials established the efficacy of cangrelor. However, real-world data on the efficacy of cangrelor are limited. In Türkiye, cangrelor use is restricted to high-risk patients due to reimbursement regulations. The most common conditions for cangrelor are cardiogenic shock and intubation due to impaired oral intake. The high prevalence of inotrope use and in-hospital mortality in the study cohort indicates that cangrelor is used in very high-risk patients in Türkiye. This multicenter, real-world study provides valuable insights into cangrelor use in high-risk populations, supporting recent discussions on its role in managing cardiogenic shock [25,26,27].
The mean age, male predominance, and smoking prevalence of the study cohort are consistent with the characteristics of previous ACS studies in Türkiye. However, this study reported increased incidence rates of hypertension and diabetes when compared with previous studies [28,29]. Compared with international studies, the study cohort exhibited a high burden of risk factors, especially smoking and diabetes [30,31,32].
Cangrelor is most frequently preferred worldwide in patients who do not receive oral P2Y12 due to its rapid effect during the transition to these drugs [33]. However, it can also be used for perioperative P2Y12 bridging [34]. These data are not available in our study because there is no approval in Türkiye. Switching patients using Cangrelor to oral P2Y12 inhibitors can be done immediately after cangrelor discontinuation, as in our study. However, clopidogrel can be started 30 min before discontinuing cangrelor [35].
This study reported low incidence rates of bleeding within 48 h. In particular, the number of bleeding events in patients treated with BARC was ≥3 (1.7%) even though more than half of these patients received glycoprotein IIb/IIIa inhibitors. The bleeding rates were low at 1 month and were comparable between STEMI and other groups. These results are consistent with those of the multicenter ARCANGELO study in Italy, which reported similar bleeding rates in 324 patients [36,37]. These findings suggest the need for avoiding glycoprotein IIb/IIIa inhibitors in cangrelor-treated patients and implementing gastrointestinal protective strategies, especially in those with a history of gastrointestinal bleeding. Subgroup analyses from the CHAMPION trials reported similar ischemic outcomes but reported increased bleeding risk with glycoprotein IIb/IIIa inhibitors [38,39].
The ARCANGELO study, which investigated cangrelor in P2Y12-naïve ACS patients, reported that the 1-month MACE rate was 1.4%. The majority of events occurred in the first 48 h, with a 1.0% MACE rate in the first 48 h. Cangrelor was initiated for emergency reasons in 89% of the study population, and the rates of patients with cardiogenic shock and cardiac arrest were quite low [36,37]. In our study, 40% required inotropes, and approximately one-third experienced in-hospital mortality. Bleeding rates in our study, which was higher risk than the patients in the ARCANGELO study, were similar to this study. These results indicate that cangrelor is safe in high-risk patients.
Clopidogrel was used as a P2Y12 inhibitor in more than half of the patients in this study, which is contrary to guideline recommendations. The TURKMI study in Türkiye also reported clopidogrel as the most commonly used P2Y12 inhibitor in ACS. This may be due to the need for additional conditions for the use of prasugrel and ticagrelor in Türkiye (approved only in troponin-positive ACS patients), physician preference, or the high risk of bleeding in these patients [28]. The bleeding or ischemic events were not significantly different between clopidogrel-treated and ticagrelor/prasugrel-treated groups in the early period (48 h–1 month). However, clopidogrel was associated with increased bleeding tendency, while ticagrelor/prasugrel was associated with increased ischemic event tendency. These findings must be confirmed in studies with a large sample size.
The bleeding rates were similar in patients aged ≥75 years and those with CKD, which was consistent with the findings of previous studies examining the safety of cangrelor in these groups [40]. Age and potent P2Y12 inhibitor use were significant bleeding predictors, emphasizing the need for gastrointestinal protection, avoidance of glycoprotein IIb/IIIa inhibitors, heparin dose adjustment, and radial access in elderly patients and those on potent P2Y12 inhibitors [41,42]. Hypertension was not a bleeding predictor, which may be due to confounding factors, such as medication choice or clinical management [43].
Given the limited number of bleeding events (n = 26), the multivariate logistic regression model included seven predictors (age, hypertension, diabetes mellitus, CKD, Killip class, P2Y12 inhibitor type, and sex). This event-to-variable ratio is below the conventional threshold for robust logistic regression, and therefore, the results should be interpreted with caution. The model findings should be viewed as exploratory and hypothesis-generating rather than confirmatory.
In this study, the femoral approach was used in the majority of cases. While current literature recommends the radial approach, the femoral approach is still frequently preferred in our country due to its ease of access and time savings, especially in patients with cardiogenic shock [44]. Although, in the CHAMPION PHOENIX cohort, bleeding rates were lower in the radial intervention group, our study shows that cangrelor is safe to use in patients undergoing the femoral approach [45]. Approximately half of the patients had two or more lesions, and 14% developed contrast-induced nephropathy, suggesting the complexity of PCI procedures in this cohort. These findings are consistent with those of recent studies examining the efficacy and safety of cangrelor in complex PCI [46,47,48,49]. Allergic reactions or adverse events requiring cangrelor discontinuation were not observed, further supporting its safety [36,50]. The majority of our study population consists of patients with cardiogenic shock and cardiac arrest. In these patients, oral intake and gastrointestinal absorption are impaired, and the risk of bleeding also increases, and intravenous ASA and cangrelor with parenteral antiplatelet strategy should be considered as the best way to provide timely DAPT [49].
The multicenter, real-world design of this study is a significant strength. However, the retrospective nature of this study and the restriction of cangrelor use to high-risk patients due to reimbursement policies limit the generalizability of results to the broad CAD population. Large-scale studies must be performed with all CAD groups to validate these findings. The lack of standardized angiographic data such as clot burden and lesion scoring is also a limitation. Therewithal, the retrospective design reduces the level of evidence of the study data because there is no randomized comparison of cangrelor with any other drug.
6. Conclusions
This is the first multicenter, large-cohort study on cangrelor use in Türkiye. The findings of this study indicate the efficacy and safety of cangrelor use in high-risk patients with complex clinical and lesion characteristics. Although the bleeding rates were low, glycoprotein IIb/IIIa inhibitors should be avoided, and gastrointestinal protective strategies must be implemented. Cangrelor was safe in the following patient groups: age ≥ 75 years, CKD, and undergoing femoral access. These results suggest that cangrelor use should be expanded in countries with health policy restrictions, such as Türkiye.
Author Contributions
Conceptualization, S.A., A.B.Ç., S.S.Y. and İ.G.; methodology, S.A., M.V.Y. and Ç.Y.; software, S.K. and D.K.; validation, H.F. and Ş.Ç.; formal analysis, S.K., B.Ç. and C.S.; investigation, F.A., M.Ç. and İ.G. resources, M.Ç., Ş.Ç. and D.K.; data curation, F.A., S.Y., M.K., C.S. and H.F.; writing—original draft preparation, S.A., A.B.Ç. and M.K. writing—review and editing, S.A., C.S. and S.Y.; visualization, S.A., S.S.Y. and İ.G.; supervision, M.V.Y. and C.S.; project administration, S.S.Y., B.Ç. and Ç.Y.; funding acquisition, S.A. and S.S.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Trakya University School of Medicine (TUTF-GOBAEK-2024/560, date of approval 20 January 2025).
Informed Consent Statement
Patient consent was waived due to retrospective study.
Data Availability Statement
The datasets analyzed or generated during the current study are available from the corresponding author on reasonable request. S.A has full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Acknowledgments
The authors would like to thank Gökhan Alıcı and Ahmet Karaduman for their support in data collection. The language and editing of this article were provided by Chiesi Türkiye as part of its unconditional scientific support. Chiesi Türkiye did not contribute to the content of the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826, Erratum in Eur. Heart J. 2024, 45, 1145. https://doi.org/10.1093/eurheartj/ehad870. [Google Scholar] [CrossRef] [PubMed]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537, Erratum in Eur. Heart J. 2025, 46, 1565. https://doi.org/10.1093/eurheartj/ehaf079. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.-P.; Costa, F.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2018, 39, 213–260. [Google Scholar] [CrossRef]
- Fox, K.A.; Mehta, S.R.; Peters, R.; Zhao, F.; Lakkis, N.; Gersh, B.J.; Yusuf, S. Clopidogrel in Unstable angina to prevent Recurrent ischemic Events Trial. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: The Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004, 110, 1202–1208. [Google Scholar] [CrossRef]
- Forsberg, J.; Bedard, E.; Mahmoud, S.H. Bioavailability of Orally Administered Drugs in Critically Ill Patients. J. Pharm. Pract. 2023, 36, 967–979. [Google Scholar] [CrossRef]
- Tavenier, A.H.; Hermanides, R.S.; Ottervanger, J.P.; Tolsma, R.; van Beurden, A.; Slingerland, R.J.; Ter Horst, P.G.J.; Gosselink, A.T.M.; Dambrink, J.E.; van Leeuwen, M.A.H.; et al. Impact of opioids on P2Y12 receptor inhibition in patients with ST-elevation myocardial infarction who are pre-treated with crushed ticagrelor: Opioids aNd crushed Ticagrelor In Myocardial infarction Evaluation (ON-TIME 3) trial. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 4–12. [Google Scholar] [CrossRef]
- Kordis, P.; Berden, J.; Mikuz, U.; Noc, M. Immediate Platelet Inhibition Strategy for Comatose Out-of-Hospital Cardiac Arrest Survivors Undergoing Percutaneous Coronary Intervention and Mild Therapeutic Hypothermia. J. Clin. Med. 2024, 13, 2121. [Google Scholar] [CrossRef]
- Harrington, R.A.; Stone, G.W.; McNulty, S.; White, H.D.; Lincoff, A.M.; Gibson, C.M.; Pollack, C.V.J.; Montalescot, G.; Mahaffey, K.W.; Kleiman, N.S.; et al. Platelet inhibition with cangrelor in patients undergoing PCI. N. Engl. J. Med. 2009, 361, 2318–2329. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Lincoff, A.M.; Gibson, C.M.; Stone, G.W.; McNulty, S.; Montalescot, G.; Kleiman, N.S.; Goodman, S.G.; White, H.D.; Mahaffey, K.W.; et al. Intravenous platelet blockade with cangrelor during PCI. N. Engl. J. Med. 2009, 361, 2330–2341. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Stone, G.W.; Mahaffey, K.W.; Gibson, C.M.; Steg, P.G.; Hamm, C.W.; Price, M.J.; Leonardi, S.; Gallup, D.; Bramucci, E.; et al. Effect of platelet in hibition with cangrelor during PCI on ischemic events. N. Engl. J. Med. 2013, 368, 1303–1313. [Google Scholar] [CrossRef]
- Rao, S.V.; O’Donoghue, M.L.; Ruel, M.; Rab, T.; Tamis-Holland, J.E.; Alexander, J.H.; Baber, U.; Baker, H.; Cohen, M.G.; Cruz-Ruiz, M.; et al. 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients with Acute Coronary Syndromes: A report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation 2025, 151, e771–e862. [Google Scholar] [CrossRef]
- Available online: https://www.sgk.gov.tr/Duyuru/Detay/20230316-Degisiklik-Tebligi-Islenmis-Guncel-2013-SUT-2023-06-05-03-02-46 (accessed on 14 November 2025).
- Pasupathy, S.; Tavella, R.; McRae, S.; Beltrame, J.F. Myocardial Infarction With Non-obstructive Coronary Arteries—Diagnosis and Management. Eur. Cardiol. 2015, 10, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, H.; Gibson, C.M. MINOCA: Myocardial infarction no obstructive coronary artery disease. Am. Heart J. Plus 2023, 33, 100312. [Google Scholar] [CrossRef] [PubMed]
- Yalta, K.; Madias, J.E.; Kounis, N.G.; Y-Hassan, S.; Polovina, M.; Altay, S.; Mebazaa, A.; Yilmaz, M.B.; Lopatin, Y.; Mamas, M.A.; et al. Takotsubo Syndrome: An International Expert Consensus Report on Practical Challenges and Specific Conditions (Part-2: Specific Entities, Risk Stratification and Challenges After Recovery). Balkan Med. J. 2024, 41, 442–457. [Google Scholar] [CrossRef] [PubMed]
- Cutlip, D.E.; Windecker, S.; Mehran, R.; Boam, A.; Cohen, D.J.; van Es, G.A.; Steg, P.G.; Morel, M.A.; Mauri, L.; Vranckx, P.; et al. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation 2007, 115, 2344–2351. [Google Scholar] [CrossRef]
- Wells, G.A.; Elliott, J.; Kelly, S.; Bai, Z.; Boucher, M.; Skidmore, B. Bleeding Classification System Definitions. 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK542934/ (accessed on 25 November 2021).
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized bleeding definitions for cardiovascular clinical trials. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Levey, A.S.; Levin, A.; Kellum, J.A. Definition and classification of kidney diseases. Am. J. Kidney Dis. 2013, 61, 686–688. [Google Scholar] [CrossRef]
- Birinci, Ş. A Digital Opportunity for Patients to Manage Their Health: Turkey National Personal Health Record System (The e-Nabız). Balkan Med. J. 2023, 40, 215–221. [Google Scholar] [CrossRef]
- Akers, W.S.; Oh, J.J.; Oestreich, J.H.; Ferraris, S.; Wethington, M.; Steinhubl, S.R. Pharmacokinetics and pharmacodynamics of a bolus and infusion of cangrelor: A direct, parenteral P2Y12 receptor antagonist. J. Clin. Pharmacol. 2010, 50, 27–35. [Google Scholar] [CrossRef]
- Kubica, J.; Adamski, P.; Dobrzycki, S.; Gajda, R.; Gąsior, M.; Gierlotka, M.; Jaguszewski, M.; Legutko, J.; Lesiak, M.; Navarese, E.P.; et al. Cangrelor—Expanding therapeutic options in patients with acute coronary syndrome. Cardiol. J. 2024, 31, 133–146. [Google Scholar] [CrossRef]
- Bennar, W.; Garin, D.; Zhi, Y.; Schaffner, C.; Pittet, T.; Attinger, A.; Togni, M.; Cuculi, F.; Cook, S.; Bossard, M.; et al. Safety and Efficacy of Cangrelor in Acute Myocardial Infarction-Related Cardiogenic Shock with and Without Cardiac Arrest. Catheter. Cardiovasc. Interv. 2025, 106, 2395–2409. [Google Scholar] [CrossRef]
- Rollini, F.; Franchi, F. Cangrelor in Patients with Cardiogenic Shock or Cardiac Arrest. JACC Cardiovasc. Interv. 2025, 18, 863–865. [Google Scholar] [CrossRef] [PubMed]
- Ferlini, M.; Raone, L.; Bendotti, S.; Currao, A.; Primi, R.; Bongiorno, A.; Fava, C.; Dall’Oglio, L.; Adamo, M.; Ghiraldin, D.; et al. Cangrelor in Patients Undergoing Percutaneous Coronary Intervention After Out-of-Hospital Cardiac Arrest. J. Clin. Med. 2025, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Erol, M.K.; Kayıkçıoğlu, M.; Kılıçkap, M.; Arın, C.B.; Kurt, I.H.; Aktaş, I.; Güneş, Y.; Özkan, E.; Şen, T.; Ince, O.; et al. Baseline clinical characteristics and patient profile of the TURKMI registry: Results of a nation-wide acute myocardial infarction registry in Turkey. Anatol. J. Cardiol. 2020, 24, 43–53. [Google Scholar] [CrossRef]
- Tokgözoğlu, L.; Kayıkçıoğlu, M.; Altay, S.; Aydoğdu, S.; Barçın, C.; Bostan, C.; Çakmak, H.A.; Çatakoğlu, A.B.; Emet, S.; Ergene, O.; et al. EUROASPIRE-IV: Avrupa Kardiyoloji Derneği’nin koroner arter hastalarında yaşam tarzı, risk faktörleri ve tedavi yaklaşımı üzerine çalışması: Türkiye verileri [EUROASPIRE-IV: European Society of Cardiology study of lifestyle, risk factors, and treatment approaches in patients with coronary artery disease: Data from Turkey]. Turk Kardiyol. Dern. Ars. 2017, 45, 134–144. [Google Scholar]
- Schiele, F.; Gale, C.P.; Simon, T.; Fox, K.A.A.; Bueno, H.; Lettino, M.; Tubaro, M.; Puymirat, E.; Ferrières, J.; Meneveau, N.; et al. Assessment of Quality Indicators for Acute Myocardial Infarction in the FAST-MI (French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction) Registries. Circ. Cardiovasc. Qual. Outcomes 2017, 10, 003336. [Google Scholar] [CrossRef]
- Pitta, S.R.; Grzybowski, M.; Welch, R.D.; Frederick, P.D.; Wahl, R.; Zalenski, R.J. ST-segment depression on the initial electrocardiogram in acute myocardial infarction-prognostic significance and its effect on short-term mortality: A report from the National Registry of Myocardial Infarction (NRMI-2, 3, 4). Am. J. Cardiol. 2005, 95, 843–848. [Google Scholar] [CrossRef]
- Wilkinson, C.; Weston, C.; Timmis, A.; Quinn, T.; Keys, A.; Gale, C.P. The Myocardial Ischaemia National Audit Project (MINAP). Eur. Heart J. Qual. Care Clin. Outcomes 2020, 6, 19–22. [Google Scholar] [CrossRef] [PubMed]
- De Sio, V.; Gragnano, F.; Cesaro, A.; Moscarella, E.; Guarnaccia, N.; Capolongo, A.; Maddaluna, P.; Verde, G.; Acerbo, V.; Scherillo, G.; et al. Cangrelor in percutaneous coronary interventions: Advances in evidence, clinical applications, and future directions. Expert Rev. Cardiovasc. Ther. 2025, 23, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Salgia, A.; Krueger, C.K.; Gillette, M.A. Perioperative Antiplatelet Bridging with Cangrelor: A Cohort Study and Narrative Review. Ann. Pharmacother. 2023, 57, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Angiolillo, D.J.; Rollini, F.; Storey, R.F.; Bhatt, D.L.; James, S.; Schneider, D.J.; Sibbing, D.; So, D.Y.F.; Trenk, D.; Alexopoulos, D.; et al. International Expert Consensus on Switching Platelet P2Y12 Receptor-Inhibiting Therapies. Circulation 2017, 136, 1955–1975. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.; Calabrò, P.; Chirillo, F.; Rolfo, C.; Menozzi, A.; Capranzano, P.; Menichelli, M.; Nicolini, E.; Mauro, C.; Trani, C.; et al. Use of cangrelor in patients with acute coronary syndromes undergoing percutaneous coronary intervention: Study design and interim analysis of the ARCANGELO study. Clin. Cardiol. 2022, 45, 913–920. [Google Scholar] [CrossRef]
- De Luca, L.; Calabrò, P.; Capranzano, P.; Di Mario, C.; Chirillo, F.; Rolfo, C.; Menozzi, A.; Menichelli, M.; Bolognese, L.; Musumeci, G. Safety of cangrelor and transition to oral P2Y12 inhibitors in patients undergoing percutaneous coronary intervention: The ARCANGELO study. Eur. Heart J. Open 2023, 3, oead076. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Harrington, R.A.; Stone, G.W.; Deliargyris, E.N.; Steg, P.G.; Gibson, C.M.; Hamm, C.W.; Price, M.J.; Menozzi, A.; Prats, J.; et al. Evaluation of Ischemic and Bleeding Risks Associated with 2 Parenteral Antiplatelet Strategies Comparing Cangrelor with Glycoprotein IIb/IIIa Inhibitors: An Exploratory Analysis from the CHAMPION Trials. JAMA Cardiol. 2017, 2, 127–135. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Harrington, R.A.; Stone, G.W.; Deliargyris, E.N.; Steg, P.G.; Gibson, C.M.; Hamm, C.W.; Price, M.J.; Menozzi, A.; Prats, J.; et al. Cangrelor with and Without Glycoprotein IIb/IIIa Inhibitors in Patients Undergoing Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2017, 69, 176–185. [Google Scholar] [CrossRef]
- Capranzano, P.; Calabrò, P.; Musumeci, G.; Mario, C.D.; Chirillo, F.; Rolfo, C.; Menozzi, A.; Menichelli, M.; Maffeo, D.; Talanas, G.; et al. Use of Cangrelor in Older Patients: Findings from the itAlian pRospective Study on CANGrELOr Study. Am. J. Cardiol. 2025, 240, 31–37. [Google Scholar] [CrossRef]
- Capodanno, D.; Bhatt, D.L.; Gibson, C.M.; James, S.; Kimura, T.; Mehran, R.; Rao, S.V.; Steg, P.G.; Urban, P.; Valgimigli, M.; et al. Bleeding avoidance strategies in percutaneous coronary intervention. Nat. Rev. Cardiol. 2022, 19, 117–132. [Google Scholar] [CrossRef]
- Nanna, M.G.; Sutton, N.R.; Kochar, A.; Rymer, J.A.; Lowenstern, A.M.; Gackenbach, G.; Hummel, S.L.; Goyal, P.; Rich, M.W.; Kirkpatrick, J.N.; et al. Assessment and Management of Older Adults Undergoing PCI, Part 1: A JACC: Advances Expert Panel. JACC Adv. 2023, 2, 100389. [Google Scholar] [CrossRef]
- De Luca, G.; Nardin, M.; Algowhary, M.; Uguz, B.; Oliveira, D.C.; Ganyukov, V.; Zimbakov, Z.; Cercek, M.; Jensen, L.O.; Loh, P.H.; et al. Impact of hypertension on mortality in patients with ST-Elevation myocardial infarction undergoing primary angioplasty: Insights from the international multicenter ISACS-STEMI registry. J. Hypertens. 2025, 43, 246–254. [Google Scholar] [CrossRef]
- Akkuş, O.; Yadsıbaş, R.; Demirkıran, R.F.; Elitaş, V.; Bekler, Ö.; Şen, F.; Binokay, H.; Akkuş, G.; Okuyan, E. Changes in Acute Coronary Syndrome Clinic after the Devastating Earthquake in Türkiye. Anatol. J. Cardiol. 2024, 28, 446–453. [Google Scholar] [CrossRef]
- Gutierrez, J.A.; Harrington, R.A.; Blankenship, J.C.; Stone, G.W.; Steg, P.G.; Gibson, C.M.; Hamm, C.W.; Price, M.J.; Généreux, P.; Prats, J.; et al. The effect of cangrelor and access site on ischaemic and bleeding events: Insights from CHAMPION PHOENIX. Eur. Heart J. 2016, 37, 1122–1130. [Google Scholar] [CrossRef]
- Uzun, F.; Güner, A.; Çizgici, A.Y.; Çiloğlu, K.; Aktürk, I.F. A Novel Modified Mini-Crush Technique for Complex Coronary Bifurcation Lesions: Controlled Balloon-Crush. Balkan Med. J. 2025, 42, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.J.; Okamoto, N.; Murphy, J.; Vengrenyuk, Y.; Sharma, S.K.; Kini, A.S. Management of calcified coronary artery bifurcation lesions. Catheter. Cardiovasc. Interv. 2021, 97, 1407–1416. [Google Scholar] [CrossRef]
- Şahin, A.; Doğan, Z.; Çinçin, A.; Gürel, E.; Sünbül, M.; Sayar, N. Stent Implantation Results in Long Lesions and Small Coronary Vessels. Inter. Cardio. Pers. 2025, 1, 6–12. [Google Scholar] [CrossRef]
- Zeymer, U.; Geisler, T.; Westermann, D.; Huber, K. The role of cangrelor in acute and high-risk PCI settings. Eur. Heart J. Cardiovasc. Pharmacother. 2025, 11, 540–551. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.; Steg, P.G.; Bhatt, D.L.; Capodanno, D.; Angiolillo, D.J. Cangrelor: Clinical Data, Contemporary Use, and Future Perspectives. J. Am. Heart Assoc. 2021, 10, e022125. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).