A New Direction in Endometrial Cancer Therapy—PD-1 and PD-L1 Immune Checkpoint Inhibitors—Where Will It Take Us?
Abstract
1. Introduction
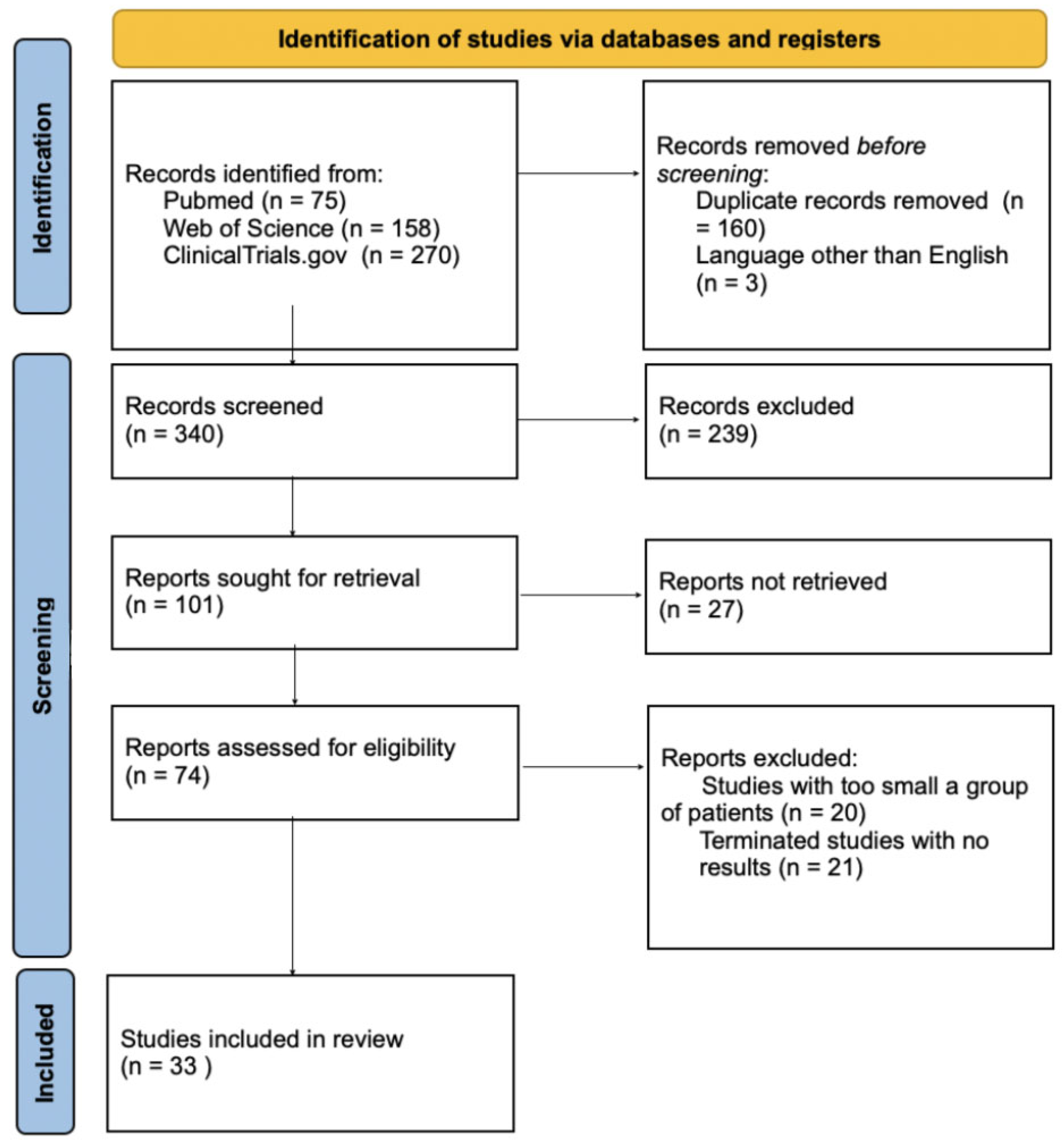
2. Material and Methods
3. Results
3.1. ICIs
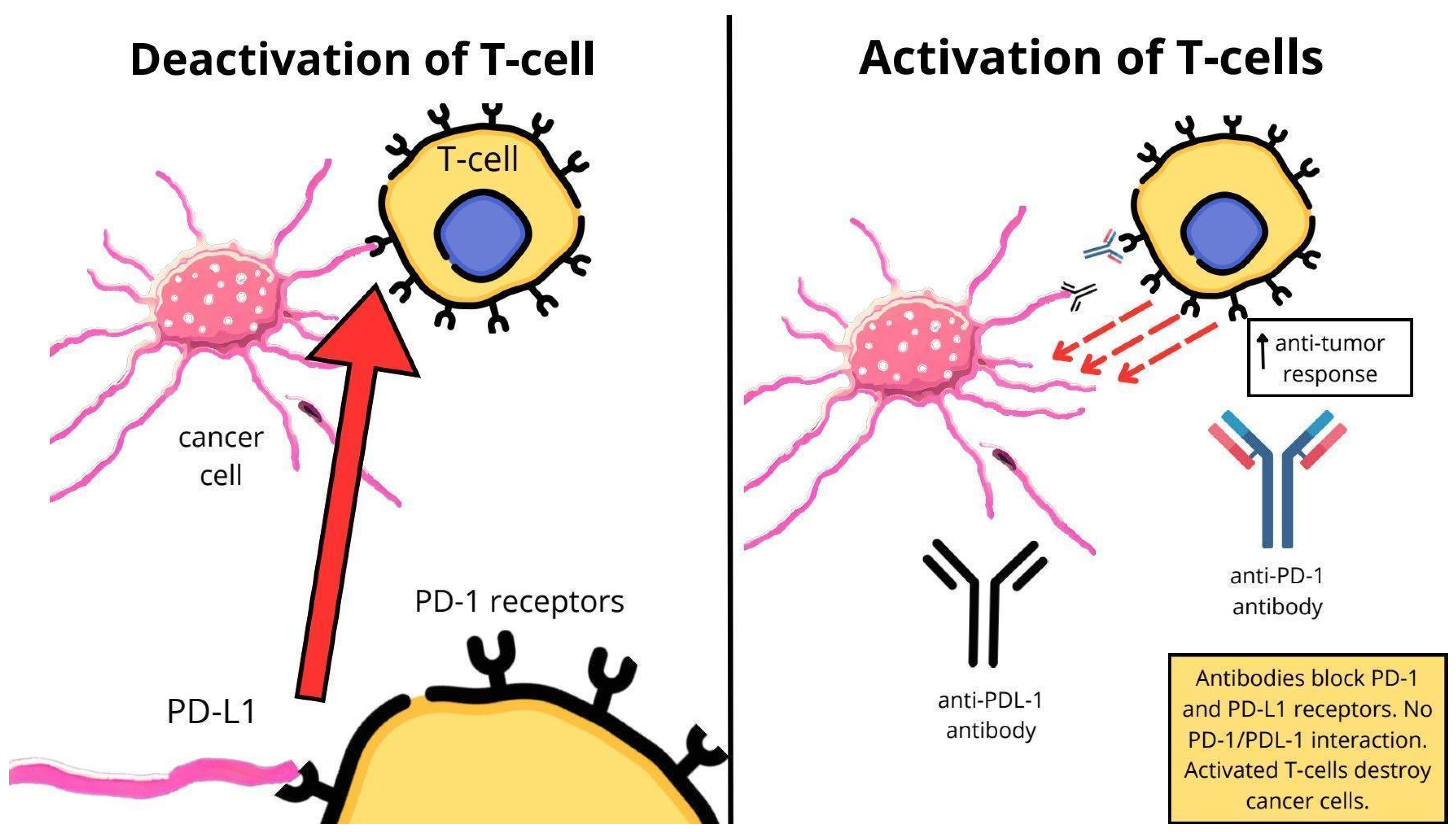
3.2. Mechanism of Action of ICIs
3.3. ICIs in Monotherapy
3.4. ICIs and Chemotherapy
3.5. ICIs and Multikinase Inhibitors
3.6. ICIs and PARP Inhibitors
3.7. The Efficiency of Therapy and the Type of ICIs Used
3.7.1. Pembrolizumab
3.7.2. Atezolizumab
3.7.3. Dostarlimab
3.7.4. Nivolumab
3.7.5. Durvalumab
3.7.6. Avelumab
3.8. Resistance to Immunotherapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PRISMA | Preferred Reporting Items For Systematic Reviews And Meta-Analyses |
| EC | Endometrial Cancer |
| PFS | Progression-free survival |
| OS | Overall Survival |
| ORR | Objective Response Rate |
| DFS | Disease-free survival |
| TRAE | Treatment-related adverse event |
| AE | Adverse event |
| i.v. | intravenous |
References
- Wan, X.; Huang, J.; Huang, L.; Wang, Y.; Fu, Y.; Jin, X.; Huang, Z.; Xiong, J. Effectiveness and Safety of PD-1/PD-L1 Inhibitors Monotherapy in Patients with Endometrial Cancer. Discov. Oncol. 2024, 15, 168. [Google Scholar] [CrossRef]
- Yen, T.-T.; Wang, T.-L.; Fader, A.N.; Shih, I.-M.; Gaillard, S. Molecular Classification and Emerging Targeted Therapy in Endometrial Cancer. Int. J. Gynecol. Pathol. 2020, 39, 26–35. [Google Scholar] [CrossRef]
- Makker, V.; MacKay, H.; Ray-Coquard, I.; Levine, D.A.; Westin, S.N.; Aoki, D.; Oaknin, A. Endometrial Cancer. Nat. Rev. Dis. Primer 2021, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, H.; Chelariu-Raicu, A.; Slomovitz, B.M. Immunotherapy in Endometrial Cancer. Int. J. Gynecol. Cancer 2023, 33, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N.; Endometrial Cancer Staging Subcommittee, FIGO Women’s Cancer Committee. FIGO Staging of Endometrial Cancer: 2023. Int. J. Gynecol. Obstet. 2023, 162, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Tung, H.-J.; Huang, H.-J.; Lai, C.-H. Adjuvant and Post-Surgical Treatment in Endometrial Cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2022, 78, 52–63. [Google Scholar] [CrossRef]
- Oaknin, A.; Bosse, T.J.; Creutzberg, C.L.; Giornelli, G.; Harter, P.; Joly, F.; Lorusso, D.; Marth, C.; Makker, V.; Mirza, M.R.; et al. Endometrial Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2022, 33, 860–877. [Google Scholar] [CrossRef]
- Van Den Heerik, A.S.V.M.; Horeweg, N.; De Boer, S.M.; Bosse, T.; Creutzberg, C.L. Adjuvant Therapy for Endometrial Cancer in the Era of Molecular Classification: Radiotherapy, Chemoradiation and Novel Targets for Therapy. Int. J. Gynecol. Cancer 2021, 31, 594–604. [Google Scholar] [CrossRef]
- Eskander, R.N.; Sill, M.W.; Beffa, L.; Moore, R.G.; Hope, J.M.; Musa, F.B.; Mannel, R.; Shahin, M.S.; Cantuaria, G.H.; Girda, E.; et al. Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2159–2170. [Google Scholar] [CrossRef]
- Makker, V.; Colombo, N.; Herráez, A.C.; Monk, B.J.; Mackay, H.; Santin, A.D.; Miller, D.S.; Moore, R.G.; Baron-Hay, S.; Ray-Coquard, I.; et al. Lenvatinib Plus Pembrolizumab in Previously Treated Advanced Endometrial Cancer: Updated Efficacy and Safety From the Randomized Phase III Study 309/KEYNOTE-775. J. Clin. Oncol. 2023, 41, 2904–2910. [Google Scholar] [CrossRef]
- Miller, D.S.; Filiaci, V.L.; Mannel, R.S.; Cohn, D.E.; Matsumoto, T.; Tewari, K.S.; DiSilvestro, P.; Pearl, M.L.; Argenta, P.A.; Powell, M.A.; et al. Carboplatin and Paclitaxel for Advanced Endometrial Cancer: Final Overall Survival and Adverse Event Analysis of a Phase III Trial (NRG Oncology/GOG0209). J. Clin. Oncol. 2020, 38, 3841–3850. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Roudko, V.; Bozkus, C.C.; Orfanelli, T.; McClain, C.B.; Carr, C.; O’Donnell, T.; Chakraborty, L.; Samstein, R.; Huang, K.; Blank, S.V.; et al. Shared Immunogenic Poly-Epitope Frameshift Mutations in Microsatellite Unstable Tumors. Cell 2020, 183, 1634–1649.e17. [Google Scholar] [CrossRef]
- Cosgrove, C.M.; Cohn, D.E.; Hampel, H.; Frankel, W.L.; Jones, D.; McElroy, J.P.; Suarez, A.A.; Zhao, W.; Chen, W.; Salani, R.; et al. Epigenetic Silencing of MLH1 in Endometrial Cancers Is Associated with Larger Tumor Volume, Increased Rate of Lymph Node Positivity and Reduced Recurrence-Free Survival. Gynecol. Oncol. 2017, 146, 588–595. [Google Scholar] [CrossRef] [PubMed]
- McMeekin, D.S.; Tritchler, D.L.; Cohn, D.E.; Mutch, D.G.; Lankes, H.A.; Geller, M.A.; Powell, M.A.; Backes, F.J.; Landrum, L.M.; Zaino, R.; et al. Clinicopathologic Significance of Mismatch Repair Defects in Endometrial Cancer: An NRG Oncology/Gynecologic Oncology Group Study. J. Clin. Oncol. 2016, 34, 3062–3068. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Ma, X.; Fischer, J.V.; Sun, C.; Kong, B.; Zhang, Q. Immunotherapy in Endometrial Cancer: Rationale, Practice and Perspectives. Biomark. Res. 2021, 9, 49. [Google Scholar] [CrossRef]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. Mech. Dis. 2021, 16, 223–249. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, S.F.; Bao, W. Molecular Subtypes of Endometrial Cancer: Implications for Adjuvant Treatment Strategies. Int. J. Gynecol. Obstet. 2024, 164, 436–459. [Google Scholar] [CrossRef]
- Karpel, H.C.; Slomovitz, B.; Coleman, R.L.; Pothuri, B. Treatment Options for Molecular Subtypes of Endometrial Cancer in 2023. Curr. Opin. Obstet. Gynecol. 2023, 35, 270–278. [Google Scholar] [CrossRef]
- Pavelescu, L.A.; Enache, R.M.; Roşu, O.A.; Profir, M.; Creţoiu, S.M.; Gaspar, B.S. Predictive Biomarkers and Resistance Mechanisms of Checkpoint Inhibitors in Malignant Solid Tumors. Int. J. Mol. Sci. 2024, 25, 9659. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Lee, S.; Cho, H.-W.; Min, K.-J.; Hong, J.-H.; Song, J.-Y.; Lee, J.-K.; Lee, N.-W. Application of Immune Checkpoint Inhibitors in Gynecological Cancers: What Do Gynecologists Need to Know before Using Immune Checkpoint Inhibitors? Int. J. Mol. Sci. 2023, 24, 974. [Google Scholar] [CrossRef] [PubMed]
- Grywalska, E.; Sobstyl, M.; Putowski, L.; Roliński, J. Current Possibilities of Gynecologic Cancer Treatment with the Use of Immune Checkpoint Inhibitors. Int. J. Mol. Sci. 2019, 20, 4705. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, D.M.; Bariani, G.M.; Cassier, P.A.; Marabelle, A.; Hansen, A.R.; De Jesus Acosta, A.; Miller, W.H.; Safra, T.; Italiano, A.; Mileshkin, L.; et al. Pembrolizumab in Patients with Microsatellite Instability–High Advanced Endometrial Cancer: Results From the KEYNOTE-158 Study. J. Clin. Oncol. 2022, 40, 752–761. [Google Scholar] [CrossRef]
- Martinez-Cannon, B.A.; Colombo, I. The Evolving Role of Immune Checkpoint Inhibitors in Cervical and Endometrial Cancer. Cancer Drug Resist. 2024, 7, 23. [Google Scholar] [CrossRef]
- Oaknin, A.; Pothuri, B.; Gilbert, L.; Sabatier, R.; Brown, J.; Ghamande, S.; Mathews, C.; O’Malley, D.M.; Kristeleit, R.; Boni, V.; et al. Safety, Efficacy, and Biomarker Analyses of Dostarlimab in Patients with Endometrial Cancer: Interim Results of the Phase I GARNET Study. Clin. Cancer Res. 2023, 29, 4564–4574. [Google Scholar] [CrossRef]
- Mirza, M.R.; Chase, D.M.; Slomovitz, B.M.; dePont Christensen, R.; Novák, Z.; Black, D.; Gilbert, L.; Sharma, S.; Valabrega, G.; Landrum, L.M.; et al. Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2145–2158. [Google Scholar] [CrossRef]
- Colombo, N.; Biagioli, E.; Harano, K.; Galli, F.; Hudson, E.; Antill, Y.; Choi, C.H.; Rabaglio, M.; Marmé, F.; Marth, C.; et al. Atezolizumab and Chemotherapy for Advanced or Recurrent Endometrial Cancer (AtTEnd): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2024, 25, 1135–1146. [Google Scholar] [CrossRef]
- Makker, V.; Taylor, M.H.; Aghajanian, C.; Oaknin, A.; Mier, J.; Cohn, A.L.; Romeo, M.; Bratos, R.; Brose, M.S.; DiSimone, C.; et al. Lenvatinib Plus Pembrolizumab in Patients with Advanced Endometrial Cancer. J. Clin. Oncol. 2020, 38, 2981–2992. [Google Scholar] [CrossRef]
- Miller, R.E.; Lewis, A.J.; Powell, M.E. PARP Inhibitors and Immunotherapy in Ovarian and Endometrial Cancers. Br. J. Radiol. 2021, 94, 20210002. [Google Scholar] [CrossRef]
- Post, C.C.B.; Westermann, A.M.; Bosse, T.; Creutzberg, C.L.; Kroep, J.R. PARP and PD-1/PD-L1 Checkpoint Inhibition in Recurrent or Metastatic Endometrial Cancer. Crit. Rev. Oncol. Hematol. 2020, 152, 102973. [Google Scholar] [CrossRef]
- Westin, S.N.; Moore, K.; Chon, H.S.; Lee, J.-Y.; Thomes Pepin, J.; Sundborg, M.; Shai, A.; De La Garza, J.; Nishio, S.; Gold, M.A.; et al. Durvalumab Plus Carboplatin/Paclitaxel Followed by Maintenance Durvalumab With or Without Olaparib as First-Line Treatment for Advanced Endometrial Cancer: The Phase III DUO-E Trial. J. Clin. Oncol. 2024, 42, 283–299. [Google Scholar] [CrossRef]
- Stark, M.C.; Joubert, A.M.; Visagie, M.H. Molecular Farming of Pembrolizumab and Nivolumab. Int. J. Mol. Sci. 2023, 24, 10045. [Google Scholar] [CrossRef] [PubMed]
- Kwok, G.; Yau, T.C.C.; Chiu, J.W.; Tse, E.; Kwong, Y.-L. Pembrolizumab (Keytruda). Hum. Vaccines Immunother. 2016, 12, 2777–2789. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulos, P.A.; Gockley, A.A.; Xiong, N.; Krasner, C.; Horowitz, N.; Campos, S.; Wright, A.A.; Liu, J.F.; Shea, M.; Yeku, O.; et al. Evaluation of Treatment with Talazoparib and Avelumab in Patients with Recurrent Mismatch Repair Proficient Endometrial Cancer. JAMA Oncol. 2022, 8, 1317. [Google Scholar] [CrossRef] [PubMed]
- Makker, V.; Colombo, N.; Casado Herráez, A.; Santin, A.D.; Colomba, E.; Miller, D.S.; Fujiwara, K.; Pignata, S.; Baron-Hay, S.; Ray-Coquard, I.; et al. Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N. Engl. J. Med. 2022, 386, 437–448. [Google Scholar] [CrossRef]
- Van Gorp, T.; Cibula, D.; Lv, W.; Backes, F.; Ortaç, F.; Hasegawa, K.; Lindemann, K.; Savarese, A.; Laenen, A.; Kim, Y.M.; et al. ENGOT-En11/GOG-3053/KEYNOTE-B21: A Randomised, Double-Blind, Phase III Study of Pembrolizumab or Placebo plus Adjuvant Chemotherapy with or without Radiotherapy in Patients with Newly Diagnosed, High-Risk Endometrial Cancer. Ann. Oncol. 2024, 35, 968–980. [Google Scholar] [CrossRef]
- Yan, G.; Du, Y.; Zhang, H.; Yan, J.; Liu, Y.; Ban, Z.; Guo, Y.-Z.; Zeng, X. Efficacy and Safety of Lenvatinib plus Pembrolizumab in Patients with Advanced and Recurrent Endometrial Cancer: A Systematic Review and Meta-Analysis. Front. Immunol. 2024, 15, 1404669. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; Von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus Docetaxel in Patients with Previously Treated Non-Small-Cell Lung Cancer (OAK): A Phase 3, Open-Label, Multicentre Randomised Controlled Trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Azad, N.S.; Gray, R.J.; Overman, M.J.; Schoenfeld, J.D.; Mitchell, E.P.; Zwiebel, J.A.; Sharon, E.; Streicher, H.; Li, S.; McShane, L.M.; et al. Nivolumab Is Effective in Mismatch Repair–Deficient Noncolorectal Cancers: Results From Arm Z1D—A Subprotocol of the NCI-MATCH (EAY131) Study. J. Clin. Oncol. 2020, 38, 214–222. [Google Scholar] [CrossRef]
- Lheureux, S.; Matei, D.E.; Konstantinopoulos, P.A.; Wang, B.X.; Gadalla, R.; Block, M.S.; Jewell, A.; Gaillard, S.L.; McHale, M.; McCourt, C.; et al. Translational Randomized Phase II Trial of Cabozantinib in Combination with Nivolumab in Advanced, Recurrent, or Metastatic Endometrial Cancer. J. Immunother. Cancer 2022, 10, e004233. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M.M.; Doria, E.R.; Konner, J.; Lichtman, S.; Zhou, Q.; Iasonos, A.; Sarasohn, D.; Troso-Sandoval, T.; Friedman, C.; O’Cearbhaill, R.; et al. Durvalumab with or without Tremelimumab in Patients with Persistent or Recurrent Endometrial Cancer or Endometrial Carcinosarcoma: A Randomized Open-Label Phase 2 Study. Gynecol. Oncol. 2023, 169, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Post, C.C.B.; Westermann, A.M.; Boere, I.A.; Witteveen, P.O.; Ottevanger, P.B.; Sonke, G.S.; Lalisang, R.I.; Putter, H.; Meershoek-Klein Kranenbarg, E.; Braak, J.P.B.M.; et al. Efficacy and Safety of Durvalumab with Olaparib in Metastatic or Recurrent Endometrial Cancer (Phase II DOMEC Trial). Gynecol. Oncol. 2022, 165, 223–229. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Luo, W.; Liu, J.F.; Gulhan, D.C.; Krasner, C.; Ishizuka, J.J.; Gockley, A.A.; Buss, M.; Growdon, W.B.; Crowe, H.; et al. Phase II Study of Avelumab in Patients with Mismatch Repair Deficient and Mismatch Repair Proficient Recurrent/Persistent Endometrial Cancer. J. Clin. Oncol. 2019, 37, 2786–2794. [Google Scholar] [CrossRef]
- Bai, R.; Chen, N.; Li, L.; Du, N.; Bai, L.; Lv, Z.; Tian, H.; Cui, J. Mechanisms of Cancer Resistance to Immunotherapy. Front. Oncol. 2020, 10, 1290. [Google Scholar] [CrossRef]
- Bellone, S.; Roque, D.M.; Siegel, E.R.; Buza, N.; Hui, P.; Bonazzoli, E.; Guglielmi, A.; Zammataro, L.; Nagarkatti, N.; Zaidi, S.; et al. A Phase 2 Evaluation of Pembrolizumab for Recurrent Lynch-like versus Sporadic Endometrial Cancers with Microsatellite Instability. Cancer 2022, 128, 1206–1218. [Google Scholar] [CrossRef]
| Author of the Study | Year of Publication | Medicine and Dose | Number of Patients | Adverse Events | Median PFS |
|---|---|---|---|---|---|
| O’ Malley D.M. et al. [24] | 2022 | 200 mg of pembrolizumab i.v. every 3 weeks for 35 cycles | 79 | pruritus, fatigue, diarrhea, hypothyroidism, hyperthyroidism | 13.1 months |
| Oaknin A. et al. [26] | 2023 | 500 mg of dostarlimab every 3 weeks for 4 cycles, next 1000 mg of dostarlimab every 6 weeks | 299 (cohort A1 [dMMR/MSI-H] = 143, cohort A2 [MMRp/MSS] = 156) | fatigue, diarrhea, nausea, hypothyroidism, arthralgia, increased AST and ALT, anemia | A1 cohort = 6 months A2 cohort = 2.7 months |
| Author of the Study | Year of Publication | Medicine and Dose | Number of Patients | Adverse Events | Median PFS |
|---|---|---|---|---|---|
| Mirza M.R. et al. [27] | 2023 | 500 mg of dostarlimab (or placebo) i.v. in combination with 5 mg/mL/min carboplatin and 175 mg/per square meter of body-surface area i.v. paclitaxel every 3 weeks for the first 6 cycles, next 1000 mg of dostarlimab (or placebo) i.v. every 6 weeks for up to 3 years | 494 (245—dostarlimab group; 249—placebo group) | nausea, alopecia, fatigue, maculopapular rash | dostarlimab group = 11.8 months placebo group = 7.9 months |
| Eskander R.N. et al. [9] | 2023 | 200 mg of pembrolizumab (or placebo) i.v. in a 30 min infusion every 3 weeks in combination with chemotherapy, next 400 mg of pembrolizumab (or placebo) i.v. in a 30 min infusion every 6 weeks | 816 (225—EC dMMR, 591—EC pMMR) | fatigue, peripheral sensory neuropathy, anemia, nausea, constipation, diarrhea | pMMR cohort: -pembrolizumab group = 13.1 months -placebo group = 8.7 months 12-month PFS in dMMR cohort: -pembrolizumab group = 74% -placebo group = 38% |
| Colombo N. et al. [28] | 2024 | 1200 mg of atezolizumab (or placebo) i.v. in combination with chemotherapy on day 1 every 21 days for 6–8 cycles, next 1200 mg of atezolizumab (or placebo) on day 1 every 21 days until disease progression or unacceptable toxicity | 549 (360—atezolizumab group, 189—placebo group) | neutropenia, anemia, leukopenia, thrombocytopenia, febrile neutropenia, diarrhea, pneumonia, hypopituitarism | atezolizumab group = 10.1 months, placebo group = 8.9 months |
| Author of the Study | Year of Publication | Medicine and Dose | Number of Patients | Adverse Events | Median PFS |
|---|---|---|---|---|---|
| Makker V. et al. [29] | 2020 | 20 mg of lenvatinib orally once a day in combination with 200 mg of pembrolizumab i.v. once every 3 weeks in 3-week cycles | 108 | hypertension, diarrhea, fatigue, decreased appetite, hypothyroidism, nausea, stomatitis, decreased appetite, dysphonia, arthralgia | 7.4 months |
| Makker V. et al. [10] | 2023 | A1: 20 mg of lenvatinib orally once a day in combination with 200 mg of pembrolizumab i.v. once every 3 weeks in 3-week cycles A2: doxorubicin 60 mg/m2 i.v. once every 3 weeks or paclitaxel 80 mg/m2 i.v. once a week | 827 (416-chemotherapy group [A1], 411-lenvatinib and pembrolizumab group [A2]) | hypertension, anemia, hypothyroidism, diarrhea, nausea, decreased appetite, vomiting, fatigue, arthralgia, proteinuria, constipation, headache | A1 group = 7.3 months A2 group = 3.8 months |
| Monoclonal Antibody | Pembrolizumab | Atezolizumab | Dostarlimab | Nivolumab | Durvalumab | Avelumab |
|---|---|---|---|---|---|---|
| All available trials * | 72 | 12 | 17 | 24 | 15 | 4 |
| Trials with results | 12 | 0 | 0 | 5 | 2 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mielnicka, N.; Dutka, M.; Kułak, K.; Kułak, A.; Tarkowski, R. A New Direction in Endometrial Cancer Therapy—PD-1 and PD-L1 Immune Checkpoint Inhibitors—Where Will It Take Us? J. Clin. Med. 2025, 14, 8366. https://doi.org/10.3390/jcm14238366
Mielnicka N, Dutka M, Kułak K, Kułak A, Tarkowski R. A New Direction in Endometrial Cancer Therapy—PD-1 and PD-L1 Immune Checkpoint Inhibitors—Where Will It Take Us? Journal of Clinical Medicine. 2025; 14(23):8366. https://doi.org/10.3390/jcm14238366
Chicago/Turabian StyleMielnicka, Natalia, Martyna Dutka, Krzysztof Kułak, Anna Kułak, and Rafał Tarkowski. 2025. "A New Direction in Endometrial Cancer Therapy—PD-1 and PD-L1 Immune Checkpoint Inhibitors—Where Will It Take Us?" Journal of Clinical Medicine 14, no. 23: 8366. https://doi.org/10.3390/jcm14238366
APA StyleMielnicka, N., Dutka, M., Kułak, K., Kułak, A., & Tarkowski, R. (2025). A New Direction in Endometrial Cancer Therapy—PD-1 and PD-L1 Immune Checkpoint Inhibitors—Where Will It Take Us? Journal of Clinical Medicine, 14(23), 8366. https://doi.org/10.3390/jcm14238366





