Aortic Valve Replacement vs. Balloon-Expandable and Self-Expandable Transcatheter Implantation in Low-Risk Patients
Abstract
1. Introduction
2. Method
2.1. Patient Population and Study Design
2.2. Operative Technique
2.3. Endpoints and Definitions
2.4. Statistical Analysis
3. Results
3.1. Overall Population
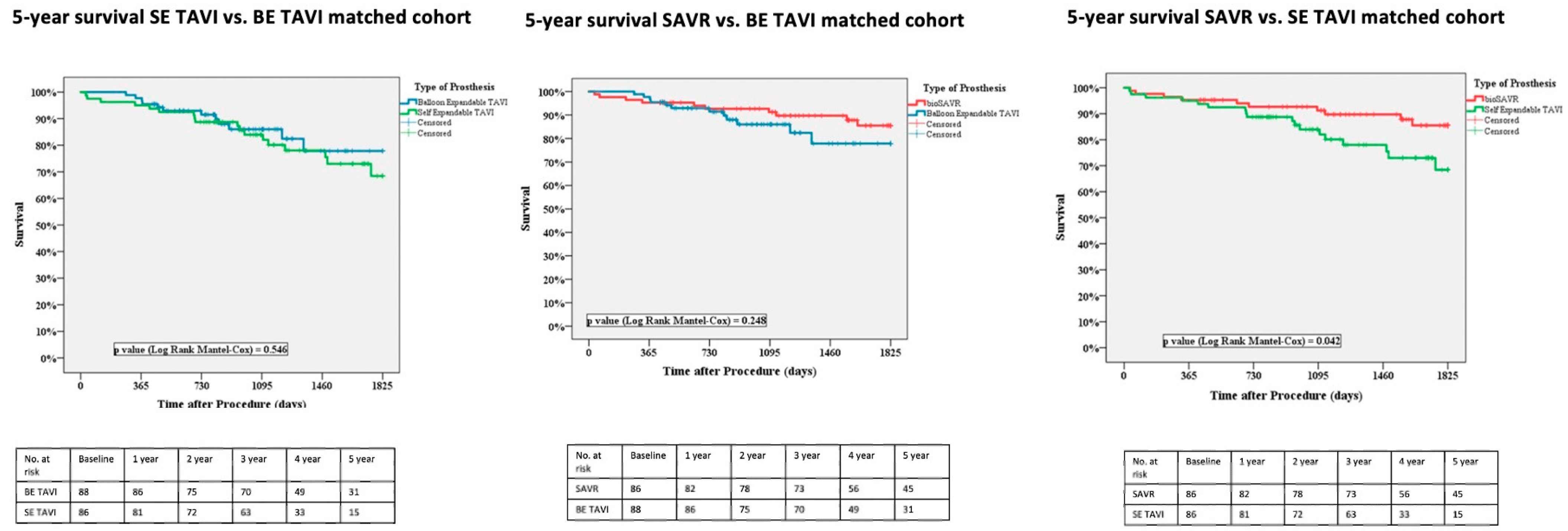
3.2. Matched Population
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Pibarot, P.; Hahn, R.T.; Genereux, P.; Kodali, S.K.; Kapadia, S.R.; Cohen, D.J.; Pocock, S.J.; et al. Transcatheter Aortic-Valve Replacement in Low-Risk Patients at Five Years. N. Engl. J. Med. 2023, 389, 1949–1960. [Google Scholar] [CrossRef]
- Forrest, J.K.; Deeb, G.M.; Yakubov, S.J.; Gada, H.; Mumtaz, M.A.; Ramlawi, B.; Teirstein, P.S.; DeFrain, M.; Muppala, M.; Rutkin, B.J.; et al. 3-Year Outcomes After Transcatheter or Surgical Aortic Valve Replacement in Low-Risk Patients With Aortic Stenosis. J. Am. Coll. Cardiol. 2023, 8, 1663–1674. [Google Scholar] [CrossRef]
- Rotman, O.M.; Bianchi, M.; Ghosh, R.P.; Kovarovic, B.; Bluestein, D. Principles of TAVR valve design, modelling, and testing. Expert. Rev. Med. Devices 2018, 15, 771–791. [Google Scholar] [CrossRef]
- Wang, B.; Mei, Z.; Ge, X.; Li, Y.; Zhou, Q.; Meng, X.; An, G. Comparison of outcomes of self-expanding versus balloon-expandable valves for transcatheter aortic valve replacement: A meta-analysis of randomized and propensity-matched studies. BMC Cardiovasc. Disord. 2023, 23, 382. [Google Scholar] [CrossRef]
- Vollenbroich, R.; Wenaweser, P.; Macht, A.; Stortecky, S.; Praz, F.; Rothenbühler, M.; Roost, E.; Hunziker, L.; Räber, L.; Windecker, S.; et al. Long-term outcomes with balloon-expandable and self-expandable prostheses in patients undergoing transfemoral transcatheter aortic valve implantation for severe aortic stenosis. Int. J. Cardiol. 2019, 290, 45–51. [Google Scholar] [CrossRef]
- Vincent, F.; Ternacle, J.; Delhaye, C.; Auffret, V.; Debry, N.; Manigold, T.; Cosenza, A.; Amabile, N.; Lhermusier, T.; Porouchani, S.; et al. Balloon-Expandable Versus Self-Expanding Transcatheter Aortic Valve Replacement: A Propensity-Matched Comparison From the FRANCE-TAVI Registry. Circulation 2020, 141, 243–259. [Google Scholar] [CrossRef]
- Martinsson, A.; Nielsen, S.J.; Milojevic, M.; Redfors, B.; Omerovic, E.; Tønnessen, T.; Gudbjartsson, T.; Dellgren, G.; Jeppsson, A. Life Expectancy After Surgical Aortic Valve Replacement. J. Am. Coll. Cardiol. 2021, 78, 2147–2157. [Google Scholar] [CrossRef]
- Francica, A.; Benvegnù, L.; Biagio, L.S.; Tropea, I.; Luciani, G.B.; Faggian, G.; Onorati, F. Ten-year clinical and echocardiographic follow-up of third-generation biological prostheses in the aortic position. J. Thorac. Cardiovasc. Surg. 2024, 167, 1705–1713.e8. [Google Scholar] [CrossRef]
- Nashef, S.A.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardiothorac. Surg. 2012, 41, 734–744. [Google Scholar] [CrossRef]
- O’BRien, S.M.; Shahian, D.M.; Filardo, G.; Ferraris, V.A.; Haan, C.K.; Rich, J.B.; Normand, S.-L.T.; DeLong, E.R.; Shewan, C.M.; Dokholyan, R.S.; et al. Society of Thoracic Surgeons Quality Measurement Task Force. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: Part 2-isolated valve surgery. Ann. Thorac. Surg. 2009, 88 (Suppl. 1), S23–S42. [Google Scholar] [CrossRef]
- Lodo, V.; Italiano, E.G.; Weltert, L.; Zingarelli, E.; Pietropaolo, C.; Buono, G.; Centofanti, P. Transcatheter aortic valve implantation versus surgery in low-risk patients: In-hospital and mid-term outcomes. Interdiscip. Cardiovasc. Thorac. Surg. 2025, 40, ivaf103. [Google Scholar] [CrossRef]
- Lodo, V.; Italiano, E.G.; Weltert, L.; Zingarelli, E.; Perrucci, C.; Pietropaolo, C.; Buono, G.; Centofanti, P. The influence of gender on outcomes following transcatheter aortic valve implantation. Front. Cardiovasc. Med. 2024, 11, 1417430. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Eckardt, K.-U.; Tsukamoto, Y.; Levin, A.; Coresh, J.; Rossert, J.; Zeeuw, D.D.; Hostetter, T.H.; Lameire, N.; Eknoyan, G. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005, 67, 2089–2100. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; ESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef]
- Praz, F.; Borger, M.A.; Lanz, J.; Marin-Cuartas, M.; Abreu, A.; Adamo, M.; Rocca, B. ESC/EACTS Scientific Document Group. 2025 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2025, 29, ehaf194. [Google Scholar]
- Glaser, N.; Persson, M.; Jackson, V.; Holzmann, M.J.; Franco-Cereceda, A.; Sartipy, U. Loss in Life Expectancy After Surgical Aortic Valve Replacement: SWEDEHEART Study. J. Am. Coll. Cardiol. 2019, 74, 26–33. [Google Scholar] [CrossRef]
- Foroutan, F.; Guyatt, G.H.; O’bRien, K.; Bain, E.; Stein, M.; Bhagra, S.; Sit, D.; Kamran, R.; Chang, Y.; Devji, T.; et al. Prognosis after surgical replacement with a bioprosthetic aortic valve in patients with severe symptomatic aortic stenosis: Systematic review of observational studies. BMJ 2016, 28, 354. [Google Scholar] [CrossRef]
- Søndergaard, L.; Ihlemann, N.; Capodanno, D.; Jørgensen, T.H.; Nissen, H.; Kjeldsen, B.J.; Chang, Y.; Steinbrüchel, D.A.; Olsen, P.S.; Petronio, A.S.; et al. Durability of Transcatheter and Surgical Bioprosthetic Aortic Valves in Patients at Lower Surgical Risk. J. Am. Coll. Cardiol. 2019, 73, 546–553. [Google Scholar] [CrossRef]
- Barbanti, M.; Costa, G.; Zappulla, P.; Todaro, D.; Picci, A.; Rapisarda, G.; Di Simone, E.; Sicuso, R.; Buccheri, S.; Gulino, S.; et al. Incidence of Long-Term Structural Valve Dysfunction and Bioprosthetic Valve Failure After Transcatheter Aortic Valve Replacement. J. Am. Heart Assoc. 2018, 7, e008440. [Google Scholar] [CrossRef]
- Thyregod, H.G.H.; Jørgensen, T.H.; Ihlemann, N.; Steinbrüchel, D.A.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; De Backer, O.; Olsen, P.S.; Søndergaard, L. Transcatheter or surgical aortic valve implantation: 10-year outcomes of the NOTION trial. Eur. Heart J. 2024, 45, 1116–1124. [Google Scholar] [CrossRef]
- Fukuhara, S.; Shiomi, S.; Yang, B.; Kim, K.; Bolling, S.F.; Haft, J.; Tang, P.; Pagani, F.; Prager, R.L.; Chetcuti, S.; et al. Early Structural Valve Degeneration of Trifecta Bioprosthesis. Ann. Thorac. Surg. 2020, 109, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Cangut, B.; Schaff, H.V.; Suri, R.M.; Greason, K.L.; Stulak, J.M.; Lahr, B.D.; Michelena, H.I.; Daly, R.C.; Dearani, J.A.; Crestanello, J.A. Excess Reintervention With Mitroflow Prosthesis for Aortic Valve Replacement: Ten-Year Outcomes of a Randomized Trial. Ann. Thorac. Surg. 2023, 115, 949–956. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.; Mehilli, J.; Frerker, C.; Neumann, F.J.; Kurz, T.; Tölg, R.; Richardt, G. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcatheter aortic valve replacement: The CHOICE randomized clinical trial. JAMA 2014, 311, 1503–1514. [Google Scholar] [CrossRef]
- Sá, M.P.B.; Simonato, M.; Eynde, J.V.D.; Cavalcanti, L.R.P.; Alsagheir, A.; Tzani, A.; Fovino, L.N.; Kampaktsis, P.N.; Gallo, M.; Laforgia, P.L.; et al. Balloon versus self-expandable transcatheter aortic valve implantation for bicuspid aortic valve stenosis: A meta-analysis of observational studies. Catheter. Cardiovasc. Interv. 2021, 98, E746–E757. [Google Scholar] [CrossRef]
- Lerman, T.T.; Levi, A.; Kornowski, R. Meta-analysis of short- and long-term clinical outcomes of the self-expanding Evolut R/pro valve versus the balloon-expandable Sapien 3 valve for transcatheter aortic valve implantation. Int. J. Cardiol. 2023, 371, 100–108. [Google Scholar] [CrossRef]
- Thiele, H.; Kurz, T.; Feistritzer, H.-J.; Stachel, G.; Hartung, P.; Eitel, I.; Marquetand, C.; Nef, H.; Doerr, O.; Lauten, A.; et al. Comparison of newer generation self-expandable vs. balloon-expandable valves in transcatheter aortic valve implantation: The randomized SOLVE-TAVI trial. Eur. Heart J. 2020, 41, 1890–1899. [Google Scholar] [CrossRef]
- Bhushan, S.; Huang, X.; Li, Y.; He, S.; Mao, L.; Hong, W.; Xiao, Z. Paravalvular Leak After Transcatheter Aortic Valve Implantation Its Incidence, Diagnosis, Clinical Implications, Prevention, Management, and Future Perspectives: A Review Article. Curr. Probl. Cardiol. 2021, 47, 100957. [Google Scholar] [CrossRef] [PubMed]
- Sá, M.P.; Jacquemyn, X.; Van den Eynde, J.; Tasoudis, P.; Erten, O.; Sicouri, S.; Ramlawi, B. Impact of Paravalvular Leak on Outcomes After Transcatheter Aortic Valve Implantation: Meta-Analysis of Kaplan-Meier-derived Individual Patient Data. Struct. Heart. 2022, 7, 100118. [Google Scholar] [CrossRef] [PubMed]
- Coeman, M.; Kayaert, P.; Philipsen, T.; Calle, S.; Gheeraert, P.; Gevaert, S.; Czapla, J.; Timmers, L.; Van Heuverswyn, F.; De Pooter, J. Different dynamics of new-onset electrocardiographic changes after balloon- and self-expandable transcatheter aortic valve replacement: Implications for prolonged heart rhythm monitoring. J. Electrocardiol. 2020, 59, 68–73. [Google Scholar] [CrossRef]
- Auffret, V.; Puri, R.; Urena, M.; Chamandi, C.; Rodriguez-Gabella, T.; Philippon, F.; Rodes-Cabau, J. Conduction Disturbances After Transcatheter Aortic Valve Replacement: Current Status and Future Perspectives. Circulation 2017, 136, 1049–1069. [Google Scholar] [CrossRef]
- Bendandi, F.; Taglieri, N.; Ciurlanti, L.; Mazzapicchi, A.; Foroni, M.; Lombardi, L.; Palermo, F.; Filice, F.; Ghetti, G.; Bruno, A.G.; et al. Development and validation of the D-PACE scoring system to predict delayed high-grade conduction disturbances after transcatheter aortic valve implantation. EuroIntervention 2025, 21, e119–e129. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.; Ahmed, M.; Brieger, D.; Baer, A.; Hansen, P.; Bhindi, R. The Prognostic Relevance of a New Bundle Branch Block After Transcatheter Aortic Valve Implantation: A Systematic Review and Meta-Analysis. Struct. Heart 2024, 9, 100392. [Google Scholar] [CrossRef] [PubMed]
- Deharo, P.; Bisson, A.; Herbert, J.; Lacour, T.; Saint Etienne, C.; Grammatico-Guillon, L.; Fauchier, L. Impact of Sapien 3 Balloon-Expandable Versus Evolut R Self-Expandable Transcatheter Aortic Valve Implantation in Patients With Aortic Stenosis: Data From a Nationwide Analysis. Circulation 2020, 141, 260–268. [Google Scholar] [CrossRef]
- Jacquemyn, X.; Van den Eynde, J.; Caldonazo, T.; Brown, J.A.; Dokollari, A.; Serna-Gallegos, D.; Clavel, M.A.; Sá, M.P. Late Outcomes After Transcatheter Aortic Valve Implantation with Balloon-Versus Self-Expandable Valves: Meta-Analysis of Reconstructed Time-To-Event Data. Cardiol. Clin. 2024, 42, 373–387. [Google Scholar]
- Yudi, M.B.; Sharma, S.K.; Tang, G.H.; Kini, A. Coronary Angiography and Percutaneous Coronary Intervention After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2018, 71, 1360–1378. [Google Scholar] [CrossRef]
- Ochiai, T.; Chakravarty, T.; Yoon, S.-H.; Kaewkes, D.; Flint, N.; Patel, V.; Mahani, S.; Tiwana, R.; Sekhon, N.; Nakamura, M.; et al. Coronary Access After TAVR. JACC Cardiovasc. Interv. 2020, 13, 693–705. [Google Scholar] [CrossRef]
- Tarantini, G.; Nai Fovino, L.; Belloni, F.; Barbierato, M.; Gallo, F.; Vercellino, M.; Trani, C.; Burzotta, F.; Serra, L.A.; Petronio, A.S.; et al. The Coronary Access After TAVI (CAvEAT) Study: A Prospective Registry of CA After TAVR. JACC Cardiovasc. Interv. 2025, 18, 1571–1583. [Google Scholar] [CrossRef] [PubMed]
| Variables | SAVR (n = 85) | BE TAVI (n = 88) | SE TAVI (n = 80) | p Value |
|---|---|---|---|---|
| Age, median (IQR), years | 78 (76–80) | 79 (77–81) | 79 (78–81) | 0.062 |
| Female, n (%) | 40 (46.5) | 35 (39.8) | 41 (51.2) | 0.323 |
| BMI, mean (SD), kg/m2 | 26.4 (3.3) | 26.5 (4.1) | 26.4 (2.8) | 0.675 |
| Hypertension, n (%) | 79 (91.9) | 81 (92.0) | 78 (97.5) | 0.240 |
| Diabetes, n (%) | 24 (27.9) | 26 (29.4) | 23 (28.7) | 1.000 |
| Dyslipidemia, n (%) | 42 (48.8) | 50 (56.8) | 39 (48.8) | 0.477 |
| Smoke, n (%) | 27 (31.4) | 29 (32.9) | 28 (35) | 0.849 |
| COPD, n (%) | 10 (11.6) | 16 (18.2) | 16 (20.0) | 0.306 |
| PAD, n (%) | 15 (17.4) | 21 (23.9) | 14 (17.5) | 0.475 |
| CKD (eGFR < 60 mL/min), n (%) | 24 (27.9) | 17 (19.3) | 26 (32.5) | 0.142 |
| RRT, n (%) | 1 (1.2) | 0 | 1 (1.2) | 0.497 |
| History of cerebrovascular event, n (%) | 8 (9.3) | 10 (11.4) | 8 (10) | 0.975 |
| History of coronary disease, n (%) | 9 (10.5) | 12 (13.63) | 11 (13.7) | 0.915 |
| RBBB, n (%) | 6 (6.9) | 7 (7.9) | 8 (10) | 0.767 |
| Mean gradient, mean (IQR), mmHg | 48 (44–52) | 46 (42–50) | 46 (41–48) | 0.647 |
| AVA, mean (IQR), cm2 | 0.70 (0.38–0.93) | 0.68 (0.33–0.95) | 0.70 (0.41–0.95) | 0.723 |
| LF-LG, n (%) | 5 (5.8) | 7 (7.9) | 5 (6.2) | 0.998 |
| NYHA III-IV, n (%) | 38 (44.2) | 33 (37.5) | 29 (36.3) | 0.524 |
| History of heart failure, n (%) | 12 (14.0) | 15 (17.0) | 16 (20.0) | 0.583 |
| EF, median (IQR) | 60 (56–65) | 60 (58–65) | 60 (55–65) | 0.715 |
| Euroscore II, median (IQR) | 2.21 (1.56–3.06) | 1.94 (1.51–2.55) | 2.17 (1.63–2.80) | 0.337 |
| STS score, median (IQR) | 2.19 (1.65–2.78) | 1.89 (1.49–2.68) | 2.15 (1.67–2.89) | 0.356 |
| Variables | SAVR (n = 85) | BE TAVI (n = 88) | SE TAVI (n = 80) | p Value |
|---|---|---|---|---|
| AKI, n (%) | 20 (23.3) | 2 (2.3) | 4 (5.0) | <0.001 |
| 2 (2.3) | 0 | 1 (1.2) | 0.089 |
| Stroke, n (%) | 2 (2.3) | 0 | 5 (6.3) | 0.045 |
| MI, n (%) | 2 (2.3) | 2 (2.3) | 2 (2.5) | 0.896 |
| New onset LBBB, n (%) | 0 | 16 (18.2) | 19 (23.8) | <0.001 |
| PM implantation, n (%) | 4 (4.7) | 11 (12.5) | 10 (12.5) | 0.139 |
| PVL (≥mild to moderate), n (%) | 0 | 2 (2.4) | 6 (8.1) | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lodo, V.; Italiano, E.G.; Weltert, L.; Zingarelli, E.; Viscido, C.; Buono, G.; Centofanti, P. Aortic Valve Replacement vs. Balloon-Expandable and Self-Expandable Transcatheter Implantation in Low-Risk Patients. J. Clin. Med. 2025, 14, 8278. https://doi.org/10.3390/jcm14238278
Lodo V, Italiano EG, Weltert L, Zingarelli E, Viscido C, Buono G, Centofanti P. Aortic Valve Replacement vs. Balloon-Expandable and Self-Expandable Transcatheter Implantation in Low-Risk Patients. Journal of Clinical Medicine. 2025; 14(23):8278. https://doi.org/10.3390/jcm14238278
Chicago/Turabian StyleLodo, Vittoria, Enrico Giuseppe Italiano, Luca Weltert, Edoardo Zingarelli, Cristina Viscido, Gabriella Buono, and Paolo Centofanti. 2025. "Aortic Valve Replacement vs. Balloon-Expandable and Self-Expandable Transcatheter Implantation in Low-Risk Patients" Journal of Clinical Medicine 14, no. 23: 8278. https://doi.org/10.3390/jcm14238278
APA StyleLodo, V., Italiano, E. G., Weltert, L., Zingarelli, E., Viscido, C., Buono, G., & Centofanti, P. (2025). Aortic Valve Replacement vs. Balloon-Expandable and Self-Expandable Transcatheter Implantation in Low-Risk Patients. Journal of Clinical Medicine, 14(23), 8278. https://doi.org/10.3390/jcm14238278








