Abstract
Background/Objectives: Osteoporotic vertebral fractures (OVFs) are a growing global health burden, often managed surgically with vertebroplasty or kyphoplasty. However, long-term outcomes such as refracture and mortality remain poorly characterized across heterogeneous studies. The study aims to determine the pooled rates of refracture and mortality following surgical treatment of OVFs, and to identify clinical and procedural predictors of these outcomes using meta-regression and reconstructed survival analysis. Methods: This systematic review and meta-analysis adhered to PRISMA 2020 guidelines. PubMed, Scopus, and Web of Science were searched up to 15 April 2025. Studies reporting refracture or mortality outcomes after surgical intervention for OVFs in adults were included. Data synthesis used random-effects meta-analysis. Subgroup and meta-regression analyses explored heterogeneity. Individual patient-level data (IPD) were reconstructed from Kaplan–Meier curves to generate survival estimates and Cox proportional hazards models. Results: Eighty-two studies (n = 1,174,092 patients) were included. The pooled refracture rate was 18% (95% CI: 15–21%), with significantly higher rates associated with cement leakage (p = 0.006). Meta-regression found no significant associations with BMD, age, or prior fracture history. Time-to-event analysis revealed a steep increase in refracture within the first 12–24 months. Patients receiving percutaneous vertebroplasty (PVP) had a significantly lower hazard of refracture compared to kyphoplasty (HR = 0.036, 95% CI: 0.033–0.040; p < 0.0001). The pooled postoperative mortality rate was 15% (95% CI: 9–22%). Diabetes was positively associated with mortality risk (p = 0.001), while prior fracture history showed a significant effect. Survival probability was significantly higher in patients treated with kyphoplasty compared to vertebroplasty (HR = 4.64, 95% CI: 3.55–6.07; p < 0.0001). Conclusions: Surgical treatment for OVFs is associated with substantial refracture and mortality risk, particularly within the first two years postoperatively. Cement leakage and diabetes significantly influence outcomes. Vertebroplasty appears protective against refracture, whereas kyphoplasty may confer a survival advantage; however, these associations should be interpreted as exploratory and hypothesis-generating due to high heterogeneity and methodological limitations.
1. Introduction
Osteoporotic vertebral fractures (OVFs) represent the most common type of fragility fracture worldwide, affecting more than 1.4 million individuals annually and contributing significantly to morbidity, functional decline, and healthcare burden among older adults [1]. These fractures are often the sentinel event in a cascade of skeletal fragility, frequently signaling a heightened risk for future fractures and excess mortality [2]. While conservative management remains an option for stable fractures, a substantial proportion of patients experience persistent pain, progressive vertebral collapse, and diminished quality of life, prompting surgical intervention [3].
Percutaneous vertebroplasty (PVP) and percutaneous kyphoplasty (PKP) have emerged as the mainstay minimally invasive surgical approaches for OVFs. Both techniques aim to stabilize the fractured vertebral body, alleviate pain, and restore vertebral height. However, their long-term outcomes remain a matter of clinical and academic debate, particularly with respect to two critical endpoints: re-fracture and mortality [4,5]. Re-fracture, whether at adjacent or distant levels, may reflect altered spinal biomechanics, cement-related complications, or progressive systemic bone loss, and carries significant implications for disability and healthcare costs [6]. Mortality, in turn, may be influenced by patient frailty, comorbidities, surgical selection bias, or complications arising from the index procedure [7]. Despite an expanding body of literature, there remains wide variability in reported refracture and mortality rates, driven by heterogeneity in study designs, patient populations, surgical techniques, and definitions of outcomes.
Although several meta-analyses have examined individual complications or comparative efficacy between PVP and PKP, most have focused exclusively on pain scores, vertebral height restoration, or cement leakage rates [4,6,8]. Few have systematically assessed the longitudinal risk of refracture and mortality using survival analysis techniques or synthesized large-scale data incorporating procedural subtypes and patient-level risk factors. Moreover, the influence of cement leakage, bone mineral density, comorbidity burden, and surgical approach (e.g., bilateral vs. unilateral puncture) on long-term outcomes remains inadequately explored.
To address these knowledge gaps, we conducted a comprehensive systematic review and meta-analysis of observational and randomized studies evaluating refracture and mortality following surgical treatment of OVFs. We additionally reconstructed individual patient-level data to generate Kaplan–Meier survival estimates and assess hazard ratios using Cox regression, providing a time-resolved perspective on postoperative risk. Our findings offer critical insight into the durability and safety profile of PVP and PKP and aim to inform risk stratification, surgical decision-making, and long-term patient management in osteoporotic spinal surgery.
2. Materials and Methods
2.1. Search Strategy
This systematic review and meta-analysis was conducted in accordance with the PRISMA 2020 guidelines (Supplementary checklist) [9]. A comprehensive literature search was performed across PubMed, Scopus, and Web of Science from database inception to 15 April 2025. The search strategy combined MeSH terms and free-text keywords related to OVFs, surgical management, refracture, and mortality. The full search strategy is available in Supplementary Table S1.
2.2. Study Selection
Eligible studies met the following criteria defined using the PICOS framework:
- Population: Adults with osteoporotic vertebral or compression fractures;
- Intervention: Any surgical treatment (e.g., PVP, PKP);
- Comparison: Not required for inclusion;
- Outcomes: Reported rates of refracture and/or mortality with at least 20 patients;
- Study Design: Original studies (randomized or non-randomized, prospective or retrospective).
Studies were excluded if they were review articles, editorials, conference abstracts, duplicate entries, lacked extractable outcome data, were not in English, or if the full text was unavailable. Titles and abstracts were independently screened by two reviewers, followed by full-text screening. Disagreements were resolved by consensus or by consultation with a third reviewer. Duplicate records were removed using reference management software.
2.3. Data Extraction and Quality Assessment
From each included study, we extracted the number of patients at risk, number of events (refractures and/or deaths), duration of follow-up, surgical procedure type, surgical approach (unilateral, bilateral, or transpedicular), and country of origin. Refractures were additionally categorized as adjacent, non-adjacent, or “sandwich” fractures, when such granularity was reported.
Two independent reviewers performed data extraction using a standardized form, and any discrepancies were resolved through discussion or by consulting a third reviewer. The risk of bias in randomized controlled trials was assessed using the Cochrane Risk of Bias 2.0 (RoB 2) tool, with domains judged as low, some concerns, or high risk. For non-randomized studies, the National Institutes of Health (NIH) quality assessment tool for observational cohort and cross-sectional studies was applied. Each study was scored based on the number of criteria fulfilled, and studies with total scores greater than 20 were considered to have good methodological quality.
2.4. Data Synthesis and Meta-Analysis
For each outcome (refracture and mortality), event rates were pooled using a random-effects meta-analysis model (DerSimonian and Laird method) implemented via the metaprop command in STATA [10]. Exact (Clopper-Pearson) confidence intervals were used to account for potential variance instability. Heterogeneity was assessed using the I2 statistic and Cochran’s Q test.
Subgroup analyses were conducted by country, surgical type (PVP vs. PKP), surgical approach (unilateral, bilateral, transpedicular), study design, and timepoint of outcome assessment. An exploratory subgroup analysis was performed for adjacent vs. non-adjacent refractures. Due to heterogeneity and reporting variability, this was considered hypothesis-generating.
Publication bias was assessed using funnel plots and Egger’s regression test when ≥10 studies were available [11]. Meta-regression was used to explore sources of heterogeneity. For refracture, a multivariate meta-regression was conducted using variables including cement leakage, BMD, prior fracture history, age, and diabetes [12]. For mortality, only univariate meta-regression was feasible due to limited reporting.
2.5. Reconstruction of Individual Patient-Level Data and Survival Analysis
Kaplan–Meier curves were digitized using WebPlotDigitizer (version 4.6), with axes manually calibrated to extract time-to-event coordinates at consistent resolution. The reconstructed datasets were validated by comparing the reconstructed number at risk and cumulative events against the original values reported in each publication. Minor discrepancies (<5%) were corrected through iterative curve fitting until alignment was achieved. We manually digitized the survival data and reconstructed individual patient-level data (IPD) using published algorithms [13]. Additionally, for a subset of studies that reported number of events, number at risk, and follow-up time, we generated synthetic IPD to perform time-to-event analysis. Kaplan–Meier survival curves were generated to estimate overall cumulative incidence of refracture and mortality. Survival differences between surgical procedures (PKP vs. PVP) were compared using log-rank tests. Cox proportional hazards models were used to estimate hazard ratios (HRs) with 95% confidence intervals for each surgical method. Adjustment for other variables was not possible due to lack of record linkage. All statistical analyses were conducted using STATA version 18.0 (StataCorp, College Station, TX, USA), and two-sided p-values < 0.05 were considered statistically significant.
3. Results
3.1. Literature Search Results
A total of 982 records were initially retrieved through electronic database searches: PubMed (n = 281), SCOPUS (n = 469), and Web of Science (n = 232) (Figure 1). After removal of 453 duplicates, 529 unique articles were screened by title and abstract. Of these, 369 articles were excluded, including 95 reviews, 23 duplicate entries, 75 editorials, 85 conference abstracts, and 91 studies that did not meet the predefined inclusion criteria. This left 160 full-text articles for eligibility assessment. Following full-text review, 78 articles were excluded, primarily due to a lack of outcome data (n = 58), inaccessibility or non-English language (n = 5), or unavailability of full text (n = 15). Ultimately, 82 studies were meta-synthesized [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95].
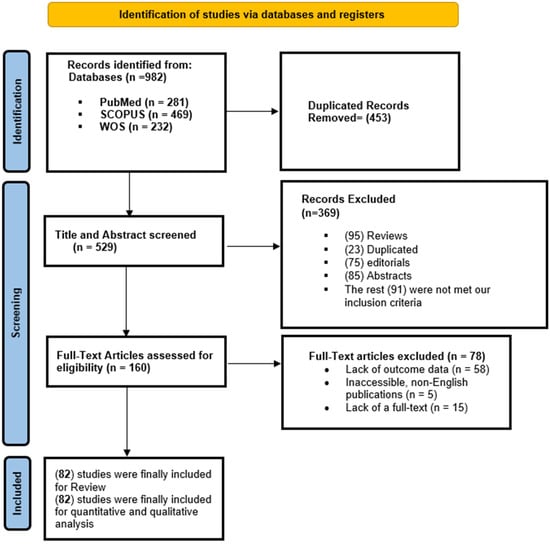
Figure 1.
A PRISMA flow diagram showing the results of the screening process.
3.2. Baseline Characteristics of Included Studies
The characteristics of included studies are summarized in Table 1. In summary, most evidence came from China (42 studies, 51.22%), followed by the United States (9 studies, 10.98%), Taiwan (7 studies, 8.54%), and Australia/Germany (5 studies, 6.10% each). In terms of study design, 59 retrospective cohort (71.95%), 12 prospective cohort (14.63%), 9 RCTs (10.97%), and 2 case–control (2.44%) studies. In terms of surgical method, PKP was investigated in 45 studies, while PVP was investigated in 52 studies. Across 82 studies involving 1,174,092 patients, the pooled mean age was 72.68 years (SD = 7.96), with a mean follow-up of 25.29 months (SD = 9.13). Women comprised 67.75% of the population, and the average BMD was −2.67 (SD = 0.91). Initial fracture location site data can be found in Table 1.

Table 1.
Baseline characteristics of studies reporting re-fracture or mortality rate after the surgical management of OVF.
3.3. Risk of Bias
Among the 9 included RCTs, 7 had some concerns (mainly with clarification of the randomization technique or lack of a trial protocol) and 2 had high risk of bias (Figure 2). In terms of non-randomized studies, 55 studies had fair overall quality and the remaining 18 studies had good quality (Table S2).
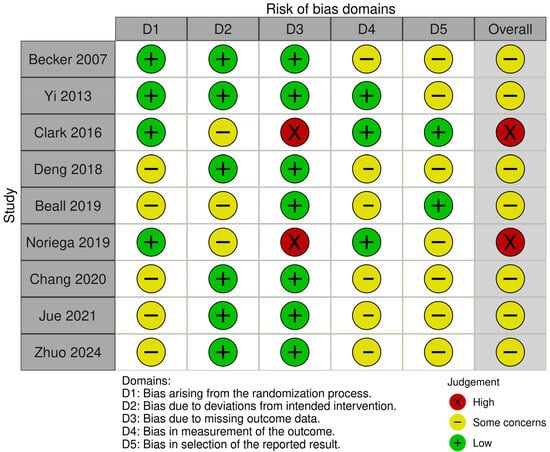
Figure 2.
A graph showing the risk of bias of included randomized controlled trials [18,19,24,32,35,50,68,84,95].
3.4. Re-Fracture Rate
3.4.1. Pooled Analysis
A total of 72 studies were included in the meta-analysis evaluating the pooled rate of re-fracture following surgical treatment of OVFs. The overall pooled re-fracture rate was 18% (95% CI: 15–21%), with considerable heterogeneity (I2 = 99.33%) (Figure S1). The Galbraith plot and leave-one-out sensitivity analysis did not reveal any significant changes (Figures S2 and S3). Egger’s regression test showed significant risk of publication bias (p < 0.001, Figure S4).
3.4.2. Subgroup Analysis
Subgroup analysis by country revealed significant variation (p < 0.001) (Table 2). The highest pooled estimates were observed in Turkey (50%, 1 study) and Bangladesh (31%, 1 study), while the lowest was reported in the United Kingdom (3%, 1 study). China, which contributed the largest number of studies (n = 39), had a pooled rate of 13% (95% CI: 10–15%). Other notable rates included Taiwan (30%, 7 studies), Italy (22%, 2 studies), and the USA (21%, 7 studies).

Table 2.
A summary of the meta-analytic estimates (pooled rate) of re-fracture and mortality following surgical management of OVF.
By study design, re-fracture rates did not differ significantly (p = 0.95). Case–control studies (1 study) reported a pooled rate of 17% (95% CI: 10–25%), while prospective cohort studies (11 studies) reported 16% (10–22%), RCTs (9 studies) had 17% (9–25%), and retrospective cohorts (52 studies) had 18% (15–21%).
Surgical type was also not significantly associated with re-fracture rate (p = 0.37). Patients treated with PKP (33 studies) had a pooled rate of 16% (13–20%), compared to 19% (14–24%) in the PVP group (41 studies).
Surgical approach subgroup analysis showed no significant differences (p = 0.56). Pooled rates were 17% (10–24%) for bilateral (17 studies), 18% (9–26%) for planned puncture (3 studies), 13% (11–15%) for transpedicular (3 studies), and 15% (3–27%) for unilateral access (6 studies).
3.4.3. Meta-Regression Analysis
Cement leak rate was the only statistically significant predictor of re-fracture (Table 3). Specifically, each 1% increase in cement leakage was associated with a 0.2% increase in re-fracture rate (coefficient = 0.002, 95% CI: 0.001 to 0.003; p = 0.006).

Table 3.
Multivariate adjusted meta-regression analysis of the determinants of re-fracture rate following surgical management of OVF.
In contrast, mean bone mineral density (BMD) showed no significant association with re-fracture rate (coefficient = −0.032, 95% CI: −0.142 to 0.079; p = 0.575). Likewise, prior fracture history and cement volume did not significantly influence outcomes (p = 0.110 and p = 0.917, respectively).
In unadjusted models, additional variables including age, sex, time from fracture to surgery, presence of diabetes, operative time, anterior/posterior vertebral body heights, kyphotic angle, preoperative pain (VAS), and functional disability (ODI score) were all evaluated but failed to demonstrate statistical significance (all p > 0.01).
3.4.4. Time-to-Event Kaplan–Meier Curve
The pooled re-fracture rate varied substantially across different postoperative timepoints. The highest early re-fracture rate was reported at 1 day postoperatively, with a pooled incidence of 24% (95% CI: 19–30%), based on 2 studies (I2 = 0.01%). At 1 month, the rate slightly decreased to 17% (95% CI: 14–20%) (4 studies, I2 = 0.03%). The cumulative incidence at 12 months remained consistent at 18% (95% CI: 13–23%), based on 33 studies (I2 = 97.8%).
By 24 months, the re-fracture rate declined to 11% (95% CI: 8–15%) (13 studies, I2 = 94.6%), with similar findings at 18 months (11%, 95% CI: 0–24%, 2 studies, I2 = 89.2%). Beyond 2 years, re-fracture rates appeared to rise again, reaching 17% (95% CI: 12–23%) at 36 months (2 studies, I2 = 66.5%), and peaking at 35% (95% CI: 13–57%) at 48 months (2 studies, I2 = 97.4%).
Kaplan–Meier failure analysis was conducted to assess the cumulative risk of re-fracture following surgical intervention for OVFs, based on reconstructed individual patient-level data. The overall re-fracture-free survival curve demonstrated a steady decline over time, with a sharper drop observed within the first 12 to 24 months postoperatively. Censoring was evident throughout follow-up, particularly after 36 months, as the number at risk declined substantially (Figure S5).
When stratified by surgical type (Figure 3), patients who underwent PKP had significantly higher re-fracture rates compared to those who received PVP. This finding was corroborated by the Cox proportional hazards model, which showed that PVP was associated with a markedly lower hazard of re-fracture relative to PKP (HR = 0.0359, 95% CI: 0.0325–0.0397; p < 0.0001), suggesting a potential protective effect of vertebroplasty in this context. This finding suggests a potential protective effect of vertebroplasty in this context; however, given the nature of reconstructed data, the hazard ratio magnitude should be interpreted cautiously, as it may reflect scaling artifacts inherent to curve digitization.

Figure 3.
Digitally reconstructed Kaplan–Meier curve showing time-to-refracture in patients with osteoporotic vertebral fractures (OVFs), based on published survival data and stratified by surgical method (PVP vs. PKP).
3.4.5. Exploratory Analysis: Re-Fracture Location
An exploratory meta-analysis of 37 studies reporting on adjacent-level refractures revealed a pooled incidence rate of 12.1% (95% CI: 9.2–14.9%), with substantial heterogeneity (I2 = 98.06%) (Table S3). In contrast, the pooled rate of non-adjacent refractures was lower, at 8.8% (95% CI: 5.4–12.2%), based on 21 studies (I2 = 96.98%).
Subgroup analysis by country demonstrated notable variation in adjacent refracture rates, ranging from 4.0% in Spain (1 study) to 23.1% in Bangladesh (1 study), with statistically significant between-country differences (p < 0.001). Non-adjacent refracture rates were similarly heterogeneous across countries, with the highest rate reported in Bangladesh (15.4%, 1 study) and the lowest in Australia (3.3%, 1 study) (p < 0.001).
When stratified by study design, adjacent fracture rates were comparable across designs (p = 0.249), with the highest rates observed in RCTs (15.5%, 5 trials) and prospective cohorts (14.1%, 6 studies). Non-adjacent refracture rates ranged from 7.8% in retrospective cohorts (11 studies) to 11.4% in prospective cohorts (3 studies), with no significant difference between designs (p = 0.249).
Adjacent fracture rates were slightly higher in patients undergoing PKP (13.2%, 18 studies) compared to PVP (12.8%, 15 studies), though the difference was not statistically significant (p = 0.894). Non-adjacent fracture rates were likewise similar between PKP (5.3%, 8 studies) and PVP (9.3%, 10 studies) (p = 0.243).
Stratification by surgical approach revealed significantly higher adjacent fracture rates following bilateral puncture (14.6%, 8 studies) compared to unilateral (1.6%) or transpedicular approaches (9.4%) (p < 0.001). A similar trend was observed for non-adjacent fractures, with bilateral approaches associated with the highest rate (9.6%) and unilateral with the lowest (2.1%) (p < 0.001).
3.5. Mortality Rate
3.5.1. Pooled Analysis
The pooled mortality rate following OVF surgery was 15% (95% CI: 9–22%), based on 23 studies, with very high heterogeneity (I2 = 99.98%) (Figure S6). The Galbraith plot and leave-one-out sensitivity analysis did not reveal any significant changes (Figures S7 and S8). Egger’s regression test showed significant risk of publication bias (p < 0.001, Figure S9).
3.5.2. Subgroup Analysis
Country-level analysis showed significant variability (p < 0.001) (Table 2). The highest mortality was seen in Taiwan (40%, 1 study) and Turkey (33%, 1 study), followed by the USA (25%, 4 studies) and UK (10%, 2 studies). The lowest rates were observed in Germany (1%, 3 studies) and Italy (1%, 1 study). China (3 studies) had a pooled mortality rate of 20% (6–34%).
By study design, mortality differed significantly (p < 0.001). The lowest rate was observed in case–control studies (4%, 1 study). Prospective cohort studies (5 studies) had a pooled mortality rate of 6% (1–12%), RCTs (3 studies) reported 5% (3–8%), and retrospective cohorts (14 studies) had the highest mortality at 22% (12–31%).
Surgical type was not significantly associated with mortality (p = 0.51). Patients undergoing PKP (7 studies) had a pooled rate of 14% (4–24%), while those treated with PVP (12 studies) had 19% (8–31%).
Surgical approach was significantly associated with mortality (p < 0.001). The bilateral group (5 studies) showed the lowest pooled mortality rate (1%, 95% CI: 0–1%; I2 = 0%), while transpedicular access (1 study) yielded the highest rate (25%, 95% CI: 22–28%). Unilateral access (2 studies) showed highly variable estimates, with a pooled mortality of 38% (0–87%), reflecting considerable uncertainty (I2 = 95.45%).
3.5.3. Meta-Regression Analysis
Univariate meta-regression analyses were conducted to examine the influence of clinical and perioperative variables on mortality following surgical management of OVF. Due to limited data availability (<10 studies per covariate), a multivariate model could not be performed.
Among the tested covariates (Table 4), diabetes mellitus (DM) emerged as the only statistically significant factor. A 1% increase in the proportion of patients with DM was associated with a 1.9% increase in mortality rate (coefficient = 0.019, 95% CI: 0.008 to 0.031; p = 0.001). Meanwhile, a 1% increase in the proportion of patients with prior fracture history was associated with a 0.6% decline in the mortality rate (coefficient = −0.006; 95% CI: −0.01, −0.003; p < 0.0001).

Table 4.
Univariate meta-regression analysis of the determinants of mortality rate following surgical management of OVF.
Conversely, variables such as mean age (p = 0.971), female sex (p = 0.466), mean bone mineral density (BMD) (p = 0.165), prior fracture history (p = 0.000), and interval from injury to surgery (p = 0.107) were not significantly associated with mortality risk. Similarly, intraoperative and functional variables—including cement volume, cement leakage, operative time, preoperative VAS, and preoperative ODI scores—also showed no significant associations (all p > 0.05).
3.5.4. Time-to-Event Kaplan–Meier Curve
The earliest available data were at 1 month, with a pooled mortality rate of 21% (95% CI: 0–60%) (3 studies, I2 = 99.98%). At 11 months, the rate dropped to 2% (95% CI: 0–3%) (2 studies, I2 = 0.11%), and at 12 months, the rate increased to 15% (95% CI: 6–23%) across 8 studies (I2 = 99.98%). By 6 months, a pooled mortality estimate of 4% (95% CI: 2–6%) was reported (2 studies, I2 = 0.01%).
Kaplan–Meier analysis was performed using reconstructed individual patient-level data to assess time-to-mortality following surgical intervention for OVFs. The overall survival curve demonstrated a continuous decline in survival probability over time, with most deaths occurring within the first 24 months of follow-up (Figure S10). Censoring events were distributed throughout the timeline, with a diminishing number at risk in later intervals.
When stratified by surgical procedure, the survival probability was significantly higher in patients treated with PKP compared to those who underwent PVP (Figure 4). This difference was supported by Cox proportional hazards modeling, which revealed that PVP was associated with a significantly increased hazard of mortality relative to PKP (HR = 4.64, 95% CI: 3.55–6.07; p < 0.0001). However, the absolute magnitude of this hazard ratio should be viewed with caution, as the reconstructed IPD may exaggerate relative differences between groups.
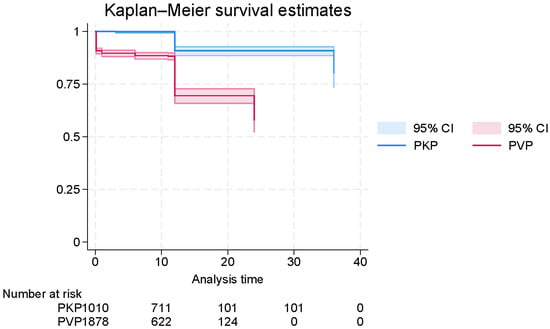
Figure 4.
Digitally reconstructed Kaplan–Meier curve of survival time in patients with osteoporotic vertebral fractures (OVFs), generated from published survival data and stratified by surgical method (PVP vs. PKP).
4. Discussion
This comprehensive meta-analysis of over 1 million patients undergoing surgical management for OVFs offers several key insights into refracture and mortality outcomes. Our findings confirm that OVF patients remain at high risk for both refracture and death following surgical treatment, with meaningful variation observed across procedure types, timepoints, and surgical approaches.
4.1. Refracture Risk and Procedural Implications
The pooled refracture incidence reached approximately 10%, with refractures most commonly occurring within the first 12 months. This aligns with previous literature identifying the early post-fracture period as a critical window of vulnerability, especially among untreated or suboptimally managed patients [96]. Our subgroup analysis revealed that PVP was associated with a significantly lower refracture risk compared to PKP, a finding that challenges several earlier reports suggesting a higher biomechanical burden on adjacent vertebrae following vertebroplasty [5]. Notably, prior meta-analyses raised concerns regarding the increased risk of adjacent-level fractures after cement augmentation procedures [5], but our findings suggest that, at least comparatively, PVP may exert a protective effect—potentially due to differences in cement volume, distribution, or vertebral height restoration dynamics.
Importantly, our exploratory analysis of refracture patterns—categorized as adjacent, non-adjacent, and sandwich-type—underscores the complex nature of post-operative fracture propagation. Although underreported in many studies, these subtypes may reflect varying pathophysiological processes, such as altered spinal biomechanics, incomplete osteoporosis management, or differential surgical loading [97].
From a biomechanical perspective, the lower refracture risk observed with vertebroplasty may relate to differences in cement distribution and load transmission patterns. Vertebroplasty typically results in a more diffuse, trabecular interdigitation of cement within the vertebral body, which may provide gradual reinforcement and maintain physiological stress transfer to adjacent levels. In contrast, kyphoplasty can create a cavity that leads to localized stiffness and abrupt stress concentration at the endplates, potentially predisposing to adjacent-level failure. Prior experimental studies have demonstrated that over-correction of vertebral height and excessive cement volume may alter spinal biomechanics and increase local stress gradients—findings that align with our observed trend [98,99]. Nevertheless, these explanations remain speculative and require confirmation through finite-element and prospective biomechanical investigations.
4.2. Mortality Outcomes and Predictors
The overall mortality rate was 12.4%, with substantial between-study heterogeneity likely driven by regional differences in patient comorbidity profiles and healthcare systems. The Kaplan–Meier survival curves and Cox models revealed that PKP was associated with significantly lower mortality than PVP, with a hazard ratio of 0.22, suggesting that procedural choice may influence long-term survival.
Several hypotheses may explain this observed survival benefit with PKP. Unlike PVP, kyphoplasty allows for partial restoration of vertebral height and reduction in local kyphosis, potentially improving pulmonary mechanics and reducing frailty-related complications over time [100]. Moreover, PKP may be more frequently employed in centers with greater postoperative care integration, including osteoporosis medication initiation and fall prevention programs—factors known to reduce mortality [101,102].
It is important to recognize that the apparent survival advantage observed with kyphoplasty may be confounded by differences in baseline frailty, comorbidity burden, and postoperative management. Patients selected for kyphoplasty are often treated in higher-resource centers with more structured postoperative rehabilitation and secondary fracture prevention programs, including early initiation of anti-osteoporotic therapy. Moreover, variability in patient selection criteria—such as excluding highly frail or multimorbid patients from kyphoplasty trials—could bias survival outcomes. These factors highlight the complex interplay between procedural choice, patient characteristics, and longitudinal care, and they warrant cautious interpretation of the observed mortality differences.
Our findings echo earlier meta-analyses that documented improved survival with cement-augmented surgery compared to conservative management [4,6,8]. However, unlike prior reviews that grouped all augmentation techniques together [4,5], we were able to distinguish between PVP and PKP, thereby offering a more nuanced understanding of surgical efficacy.
The substantial heterogeneity observed across many pooled analyses likely reflects true clinical and methodological variability rather than statistical noise. Differences in patient populations (e.g., age, bone mineral density, comorbidities), surgical techniques (cement volume, viscosity, unilateral vs. bilateral access), operator experience, and postoperative rehabilitation protocols may all influence refracture and mortality outcomes. In addition, variations in study design, follow-up duration, and imaging-based outcome definitions contribute to this inconsistency. While subgroup and meta-regression analyses helped identify certain modifiers (such as country and cement leakage rate), residual heterogeneity remains and warrants cautious interpretation of pooled estimates.
4.3. Clinical and Research Implications
This study reinforces the concept of “imminent risk” following an initial OVF and highlights the need for multidisciplinary secondary prevention programs, including timely anti-osteoporotic therapy and fracture liaison services [96]. The low uptake of such services remains a persistent challenge worldwide [5], despite robust data supporting their cost-effectiveness and impact on reducing re-fractures and mortality.
Our data also support recent recommendations that classify recent OVFs as “very-high” risk events warranting aggressive management strategies [96]. The significantly elevated risk of sandwich fractures—occurring between two cemented vertebrae—warrants procedural reconsideration and possibly alternative augmentation techniques in select patients.
From a research standpoint, future prospective studies should aim to examine the long-term benefits of each augmentation technique while adequately adjusting for key confounders such as cement leakage, operative time, and concurrent pharmacologic treatment. There is also a pressing need to establish standardized criteria for defining refracture, particularly in studies utilizing imaging-based endpoints, to improve comparability across trials. Furthermore, the role of patient-level factors—including frailty, sarcopenia, and the burden of comorbid conditions—should be more thoroughly investigated, as these elements likely contribute to variations in both refracture risk and long-term mortality [103,104].
4.4. Limitations
While our study provides robust pooled estimates and leveraged reconstructed individual patient-level data for time-to-event modeling, it is not without limitations. Heterogeneity in refracture definitions, limited reporting on sandwich vs. adjacent patterns, and a lack of uniform outcome timing complicate comparisons across studies. More than half of the included studies originated from China, which may limit generalizability to other regions where patient characteristics, osteoporosis care pathways, and surgical decision-making may differ.
Additionally, reconstructed IPD-based hazard ratios may not reflect absolute risks and are subject to distortion from curve digitization and interpolation assumptions. Moreover, unmeasured confounders, such as osteoporosis treatment status and adherence, frailty, comorbidities, smoking status, or functional capacity, were rarely reported and may impact both refracture and mortality risk. Finally, although our exploratory Cox models adjusted for age, surgical approach, and geography, more granular IPD-level adjustment was not feasible.
5. Conclusions
This systematic review and meta-analysis provides the most comprehensive synthesis to date on refracture and mortality outcomes following surgical intervention for OVFs. We found that refracture remains a frequent and clinically relevant complication, with an overall pooled rate of 18% and considerable heterogeneity across surgical types, techniques, and patient populations. Mortality was also substantial, with a pooled rate of 15%, underscoring the systemic frailty and comorbidity burden often present in this population. Notably, reconstructed time-to-event analyses revealed distinct survival and refracture trajectories between PVP and PKP, with PVP associated with a significantly lower hazard of refracture but higher hazard of mortality. However, both observations should be viewed as hypothesis-generating given the substantial heterogeneity and inherent limitations of the reconstructed data. These findings emphasize the need for individualized surgical decision-making and long-term postoperative surveillance. Future studies should focus on refining patient selection criteria, incorporating frailty assessments, and standardizing outcome definitions to enhance prognostication and improve real-world outcomes in this vulnerable patient group.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm14228230/s1, Figure S1: Forest plot showing the pooled refracture rate in patients with surgically treated OVF [14,15,16,18,19,21,22,23,24,25,26,27,29,30,31,32,33,34,35,36,37,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,58,59,60,61,62,63,64,65,66,67,68,70,71,72,73,74,75,76,77,78,79,81,82,83,85,86,87,88,89,91,92,93,94,95]; Figure S2: Leave-one-out sensitivity analysis of the pooled refracture rate in patients with surgically treated OVF [14,15,16,18,19,21,22,23,24,25,26,27,29,30,31,32,33,34,35,36,37,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,58,59,60,61,62,63,64,65,66,67,68,70,71,72,73,74,75,76,77,78,79,81,82,83,85,86,87,88,89,91,92,93,94,95]; Figure S3: Galbraith plot of the pooled refracture rate in patients with surgically treated OVF; Figure S4: Funnel plot showing the risk of bias in the pooled refracture rate in patients with surgically treated OVF; Figure S5: Reconstructed Kaplan–Meier Curve showing the time-to-refracture in patients with OVF; Figure S6: Forest plot showing the pooled mortality rate in patients with surgically treated OVF [17,18,20,22,26,28,32,37,38,43,51,52,53,54,55,56,57,64,65,66,68,71,85]; Figure S7: Leave-one-out sensitivity analysis of the pooled mortality rate in patients with surgically treated OVF [17,18,20,22,26,28,32,37,38,43,51,52,53,54,55,56,57,64,65,66,68,71,85]; Figure S8: Galbraith plot of the pooled mortality rate in patients with surgically treated OVF; Figure S9: Funnel plot showing the risk of bias in the pooled mortality rate in patients with surgically treated OVF; Figure S10: Reconstructed Kaplan–Meier Curve showing the survival time (time-to-death) in patients with OVF; Table S1: The search query employed in the database search; Table S2: The summary of the methodological quality of included non-randomized studies using the National Institute of Health (NIH) tool; Table S3: An exploratory analysis of the pooled rate of adjacent and non-adjacent re-fractures following the surgical management of OVF; Supplementary checklist: A filled-out PRISMA-compliant checklist for systematic reviews.
Author Contributions
Conceptualization, M.G. and M.A.M.; methodology, Ü.M.; software, M.P.; validation, F.H., R.S. and K.K.; formal analysis, F.H.; investigation, R.S.; data curation, M.P.; writing—original draft preparation, M.G.; writing—review and editing, M.A.M.; visualization, Ü.M.; project administration, M.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are within the manuscript and its Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| OVF | Osteoporotic Vertebral Fracture |
| PVP | Percutaneous Vertebroplasty |
| PKP | Percutaneous Kyphoplasty |
| BMD | Bone Mineral Density |
| HR | Hazard Ratio |
| CI | Confidence Interval |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RCT | Randomized Controlled Trial |
| IPD | Individual Patient Data |
| RoB 2 | Risk of Bias 2.0 |
| NIH | National Institutes of Health |
| ODI | Oswestry Disability Index |
| VAS | Visual Analog Scale |
| SD | Standard Deviation |
References
- Matsumoto, K.; Hoshino, M.; Omori, K.; Igarashi, H.; Matsuzaki, H.; Sawada, H.; Saito, S.; Suzuki, S.; Miyanaga, Y.; Nakanishi, K. The relationship between global sagittal balance and the incidence of early adjacent vertebral fractures following balloon kyphoplasty. World Neurosurg. 2023, 175, e818–e822. [Google Scholar] [CrossRef]
- Simpson, S.H.; Eurich, D.T.; Majumdar, S.R.; Padwal, R.S.; Tsuyuki, R.T.; Varney, J.; Johnson, J.A. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ 2006, 333, 15. [Google Scholar] [CrossRef] [PubMed]
- Halvachizadeh, S.; Stalder, A.-L.; Bellut, D.; Hoppe, S.; Rossbach, P.; Cianfoni, A.; Schnake, K.J.; Mica, L.; Pfeifer, R.; Sprengel, K. Systematic review and meta-analysis of 3 treatment arms for vertebral compression fractures: A comparison of improvement in pain, adjacent-level fractures, and quality of life between vertebroplasty, kyphoplasty, and nonoperative management. JBJS Rev. 2021, 9, e21. [Google Scholar] [CrossRef] [PubMed]
- Hinde, K.; Maingard, J.; Hirsch, J.A.; Phan, K.; Asadi, H.; Chandra, R.V. Mortality outcomes of vertebral augmentation (vertebroplasty and/or balloon kyphoplasty) for osteoporotic vertebral compression fractures: A systematic review and meta-analysis. Radiology 2020, 295, 96–103. [Google Scholar] [CrossRef]
- Sun, H.-B.; Shan, J.-L.; Tang, H. Percutaneous vertebral augmentation for osteoporotic vertebral compression fractures will increase the number of subsequent fractures at adjacent vertebral levels: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5176–5188. [Google Scholar] [PubMed]
- Dai, C.; Liang, G.; Zhang, Y.; Dong, Y.; Zhou, X. Risk factors of vertebral re-fracture after PVP or PKP for osteoporotic vertebral compression fractures, especially in Eastern Asia: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2022, 17, 161. [Google Scholar] [CrossRef]
- Morishita, S.; Yoshii, T.; Okawa, A.; Inose, H.; Hirai, T.; Yuasa, M.; Fushimi, K.; Fujiwara, T. Risk factors related to perioperative systemic complications and mortality in elderly patients with osteoporotic vertebral fractures—Analysis of a large national inpatient database. J. Orthop. Surg. Res. 2020, 15, 518. [Google Scholar] [CrossRef]
- Ding, J.-K.; Zhao, B.; Zhai, Y.-f. Subsequent fractures after vertebroplasty in osteoporotic vertebral fractures: A meta-analysis. Neurosurg. Rev. 2022, 45, 2349–2359. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Nyaga, V.N.; Arbyn, M.; Aerts, M. Metaprop: A Stata command to perform meta-analysis of binomial data. Arch. Public. Health 2014, 72, 39. [Google Scholar] [CrossRef]
- Thornton, A.; Lee, P. Publication bias in meta-analysis: Its causes and consequences. J. Clin. Epidemiol. 2000, 53, 207–216. [Google Scholar] [CrossRef]
- Baker, W.L.; Michael White, C.; Cappelleri, J.C.; Kluger, J.; Coleman, C.I.; Health Outcomes, Policy, and Economics (HOPE) Collaborative Group. Understanding heterogeneity in meta-analysis: The role of meta-regression. Int. J. Clin. Pract. 2009, 63, 1426–1434. [Google Scholar] [CrossRef]
- Guyot, P.; Ades, A.; Ouwens, M.J.; Welton, N.J. Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 2012, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.K.; Pandit, O.P.; Khan, M.S.I. Percutaneous vertebroplasty for symptomatic osteoporotic compression fractures: A single-center prospective study. Surg. Neurol. Int. 2021, 12, 176. [Google Scholar] [CrossRef]
- Al-Ali, F.; Barrow, T.; Luke, K. Vertebroplasty: What is important and what is not. AJNR. Am. J. Neuroradiol. 2009, 30, 1835–1839. [Google Scholar] [CrossRef]
- Bae, J.S.; Park, J.H.; Kim, K.J.; Kim, H.S.; Jang, I.T. Analysis of Risk Factors for Secondary New Vertebral Compression Fracture Following Percutaneous Vertebroplasty in Patients with Osteoporosis. World Neurosurg. 2017, 99, 387–394. [Google Scholar] [CrossRef]
- Banat, M.; Bara, G.; Salemdawod, A.; Rana, S.; Hamed, M.; Scorzin, J.; Vatter, H. Vertebroplasty in geriatric patients with osteoporotic vertebral fractures: Single-center cohort study at a level 1 center for spinal surgery. Egypt. J. Neurol. Psychiatry Neurosurg. 2022, 58, 111. [Google Scholar] [CrossRef]
- Beall, D.P.; Chambers, M.R.; Thomas, S.; Amburgy, J.; Webb, J.R., Jr.; Goodman, B.S.; Datta, D.K.; Easton, R.W.; Linville, D., 2nd; Talati, S.; et al. Prospective and Multicenter Evaluation of Outcomes for Quality of Life and Activities of Daily Living for Balloon Kyphoplasty in the Treatment of Vertebral Compression Fractures: The EVOLVE Trial. Neurosurgery 2019, 84, 169–178. [Google Scholar] [CrossRef]
- Becker, S.; Garoscio, M.; Meissner, J.; Tuschel, A.; Ogon, M. Is there an indication for prophylactic balloon kyphoplasty? A pilot study. Clin. Orthop. Relat. Res. 2007, 458, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Pfeiffer, K.P.; Ogon, M. Comparison of inpatient treatment costs after balloon kyphoplasty and non-surgical treatment of vertebral body compression fractures. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2011, 20, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.; Chopra, A.A.; Pitcher, M.; Jeansonne, N.; Fox, E. Rate of Osteoporosis Evaluation and Treatment Following Kyphoplasty in Patients With Vertebral Compression Fractures: A Retrospective Study and Review of the Literature. Geriatr. Orthop. Surg. Rehabil. 2025, 16, 21514593251332463. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Oberkircher, L.; Bliemel, C.; Frangen, T.M.; Ruchholtz, S.; Krüger, A. Early clinical outcome and complications related to balloon kyphoplasty. Orthop. Rev. 2012, 4, 113–117. [Google Scholar] [CrossRef][Green Version]
- Bu, D.; He, X. Comparison of different approaches of percutaneous vertebroplasty in the treatment of osteoporotic spinal compression fractures and analysis of influencing factors of re-fracture. Pak. J. Med. Sci. 2023, 39, 144–149. [Google Scholar] [CrossRef]
- Chang, J.Z.; Bei, M.J.; Shu, D.P.; Sun, C.J.; Chen, J.B.; Xiao, Y.P. Comparison of the clinical outcomes of percutaneous vertebroplasty vs. kyphoplasty for the treatment of osteoporotic Kümmell’s disease: A prospective cohort study. BMC Musculoskelet. Disord. 2020, 21, 238. [Google Scholar] [CrossRef]
- Chen, H.G.; Zhang, Z.; Liang, H.P.; Kong, Q.Z.; Chen, J.H.; Zhou, Y. [Clinical observation of effects and complications of the mid-stage in treating osteoporotic vertebral compression fracture with percutaneous kyphoplasty]. Zhongguo Gu Shang = China J. Orthop. Traumatol. 2010, 23, 743–745. [Google Scholar]
- Chen, J.-P.; Qi, X.-W.; Li, S.-J.; Kuang, L.-P.; Yuan, X.-H.; Wang, G.-S.; Tan, W.-Y. Bone cement injection as vertebral augmentation therapy for osteoporotic vertebral compression fractures. Chin. J. Tissue Eng. Res. 2015, 19, 3292–3296. [Google Scholar] [CrossRef]
- Chen, T.; Chu, G.; Qu, Y.; Wang, Y.; Lin, C.; Hu, N.; Yang, H.; Li, X.; Jiang, W.; Liu, Y. Risk factor analysis of refracture in the same cemented vertebra after percutaneous kyphoplasty for Kümmell’s disease. J. Neurosurg. Spine 2024, 40, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Lin, W.C. Poor 1st-year adherence to anti-osteoporotic therapy increases the risk of mortality in patients with magnetic resonance imaging-proven acute osteoporotic vertebral fractures. Patient Prefer. Adherence 2017, 11, 839–843. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.J.; Chen, H.Y.; Chen, H.T.; Lin, R.M.; Hsu, H.C. Diagnosis of painful cemented vertebrae from failed vertebroplasty: Modified dynamic radiographs play an important role. Eur. Spine J. 2017, 26, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.-E.; Hsu, J.-Y.; Chan, R.W.Y.; Lo, W.-C.; Chiang, Y.-H.; Lin, J.-H. Kyphoplasty with an intravertebral reduction device for osteoporotic vertebral compression fractures with spinal canal encroachment. Formos. J. Surg. 2020, 53, 20–28. [Google Scholar] [CrossRef]
- Chien, H.Y.; Yang, Y.C.; Hsieh, M.H.; Yang, C.C. Early Percutaneous Vertebroplasty Improves Bone-Cement Integration and Reduces Adjacent Fractures. World Neurosurg. 2021, 156, e283–e290. [Google Scholar] [CrossRef]
- Clark, W.; Bird, P.; Gonski, P.; Diamond, T.H.; Smerdely, P.; McNeil, H.P.; Schlaphoff, G.; Bryant, C.; Barnes, E.; Gebski, V. Safety and efficacy of vertebroplasty for acute painful osteoporotic fractures (VAPOUR): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2016, 388, 1408–1416. [Google Scholar] [CrossRef]
- Dai, S.Q.; Qin, R.Q.; Shi, X.; Yang, H.L. Percutaneous vertebroplasty versus kyphoplasty for the treatment of neurologically intact osteoporotic Kummell’s disease. BMC Surg. 2021, 21, 65. [Google Scholar] [CrossRef]
- Dai, X.; Liao, W.; Xu, F.; Lu, W.; Xi, X.; Fang, X.; Wu, Q. External validation of predictive models for new vertebral fractures following percutaneous vertebroplasty. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2024. [Google Scholar] [CrossRef]
- Deng, D.; Lian, Z.; Cui, W.; Liang, H.; Xiao, L.; Yao, G. Function of low back muscle exercise: Preventive effect of refracture analysis of postoperative vertebral fractures. Der Orthop. 2019, 48, 337–342. [Google Scholar] [CrossRef] [PubMed]
- DePalma, M.J.; Ketchum, J.M.; Frankel, B.M.; Frey, M.E. Percutaneous vertebroplasty for osteoporotic vertebral compression fractures in the nonagenarians: A prospective study evaluating pain reduction and new symptomatic fracture rate. Spine 2011, 36, 277–282. [Google Scholar] [CrossRef]
- Diamond, T.H.; Bryant, C.; Browne, L.; Clark, W.A. Clinical outcomes after acute osteoporotic vertebral fractures: A 2-year non-randomised trial comparing percutaneous vertebroplasty with conservative therapy. Med. J. Aust. 2006, 184, 113–117. [Google Scholar] [CrossRef]
- Edidin, A.A.; Ong, K.L.; Lau, E.; Kurtz, S.M. Morbidity and Mortality After Vertebral Fractures: Comparison of Vertebral Augmentation and Nonoperative Management in the Medicare Population. Spine 2015, 40, 1228–1241. [Google Scholar] [CrossRef] [PubMed]
- Gan, M.; Zou, J.; Song, D.; Zhu, X.; Wang, G.; Yang, H. Is balloon kyphoplasty better than percutaneous vertebroplasty for osteoporotic vertebral biconcave-shaped fractures? Acta Radiol. 2014, 55, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Goldman-Daleo, H.; Rachman, B.; Mhaskar, R. Adjacent Fracture Rates Following Balloon Kyphoplasty in Osteoporotic Vertebral Compression Fractures: A Case Series. Cureus 2023, 15, e40651. [Google Scholar] [CrossRef]
- Guo, J.; Zhai, W.; Wei, L.; Zhang, J.; Jin, L.; Yan, H.; Huang, Z.; Jia, Y. Radiological and clinical outcomes of balloon kyphoplasty for osteoporotic vertebral compression fracture in patients with rheumatoid arthritis. J. Orthop. Surg. Res. 2021, 16, 435. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Gonzalez, R.; Royuela, A.; Zamarron, A. Vertebral compression fractures: Pain relief, progression and new fracture rate comparing vertebral augmentation with brace. BMC Musculoskelet. Disord. 2023, 24, 898. [Google Scholar] [CrossRef]
- He, B.; Zhao, J.; Zhang, M.; Jiang, G.; Tang, K.; Quan, Z. Effect of Surgical Timing on the Refracture Rate after Percutaneous Vertebroplasty: A Retrospective Analysis of at Least 4-Year Follow-Up. BioMed Res. Int. 2021, 2021, 5503022. [Google Scholar] [CrossRef]
- Hey, H.W.; Tan, J.H.; Tan, C.S.; Tan, H.M.; Lau, P.H.; Hee, H.T. Subsequent Vertebral Fractures Post Cement Augmentation of the Thoracolumbar Spine: Does it Correlate With Level-specific Bone Mineral Density Scores? Spine 2015, 40, 1903–1909. [Google Scholar] [CrossRef]
- Hillmeier, J.; Grafe, I.; Da Fonseca, K.; Meeder, P.J.; Nöldge, G.; Libicher, M.; Kock, H.J.; Haag, M.; Kasperk, C. The evaluation of balloonkyphoplasty for osteoporotic vertebral fractures. An interdisciplinary concept. Der Orthop. 2004, 33, 893–904. [Google Scholar] [CrossRef]
- Hu, L.; Sun, H.; Wang, H.; Cai, J.; Tao, Y.; Feng, X.; Wang, Y. Cement injection and postoperative vertebral fractures during vertebroplasty. J. Orthop. Surg. Res. 2019, 14, 228. [Google Scholar] [CrossRef]
- Huang, D.; Ying, J.; Xu, D.; Chen, J.; Liu, J.; Yu, T.; Zhuang, Y.; Zhou, L. Comparison of Percutaneous Kyphoplasty with or without Pedicle Screw Fixation in Osteoporotic Thoracolumbar Vertebral Fractures: A Retrospective Study. Dis. Markers 2021, 2021, 4745853. [Google Scholar] [CrossRef]
- Huang, T.-J.; Zhang, S.-Y.; Lu, C. Predictive factors of refractures located in adjacent vertebrae after bone cement augmentation. Chin. J. Tissue Eng. Res. 2018, 22, 4342. [Google Scholar]
- Huntoon, E.A.; Schmidt, C.K.; Sinaki, M. Significantly fewer refractures after vertebroplasty in patients who engage in back-extensor-strengthening exercises. Mayo Clin. Proc. 2008, 83, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, L.; Xu, J.; Zhan, R. Therapeutic Effect Observation of Jinkui Shenqi Pill Assisted with Percutaneous Kyphoplasty on Osteoporotic Vertebral Compression Fracture. Chin. J. Mod. Appl. Pharm. 2021, 38, 2730–2735. [Google Scholar] [CrossRef]
- Kang, C.N.; Kim, J.; Ryu, J.I.; Kim, Y.; Ahn, S.; Choi, S.H. Cumulative Incidence and Factors Associated With Subsequent Vertebral Compression Fractures: A Nationwide Population-based Study. World Neurosurg. 2022, 161, e90–e100. [Google Scholar] [CrossRef]
- Kara, G.K.; Ozturk, C. Effect of osteosarcopenia on the development of a second compression fracture and mortality in elderly patients after vertebroplasty. Acta Orthop. Et. Traumatol. Turc. 2023, 57, 271–276. [Google Scholar] [CrossRef]
- Kato, S.; Terada, N.; Niwa, O. Activities of Daily Living after Surgical Treatment for Osteoporotic Vertebral Fracture with or without Diffuse Idiopathic Skeletal Hyperostosis: A Retrospective Single-Institutional Study. Asian Spine J. 2020, 14, 847–856. [Google Scholar] [CrossRef]
- Kim, B.S.; Hum, B.; Park, J.C.; Choi, I.S. Retrospective review of procedural parameters and outcomes of percutaneous vertebroplasty in 673 patients. Interv. Neuroradiol. J. Peritherapeutic Neuroradiol. Surg. Proced. Relat. Neurosci. 2014, 20, 564–575. [Google Scholar] [CrossRef]
- Kim, H.J.; Zuckerman, S.L.; Cerpa, M.; Yeom, J.S.; Lehman, R.A., Jr.; Lenke, L.G. Incidence and Risk Factors for Complications and Mortality After Vertebroplasty or Kyphoplasty in the Osteoporotic Vertebral Compression Fracture-Analysis of 1,932 Cases From the American College of Surgeons National Surgical Quality Improvement. Glob. Spine J. 2022, 12, 1125–1134. [Google Scholar] [CrossRef]
- Klezl, Z.; Bhangoo, N.; Phillips, J.; Swamy, G.; Calthorpe, D.; Bommireddy, R. Social implications of balloon kyphoplasty: Prospective study from a single UK centre. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2012, 21, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Leslie, W.D.; Brennan, S.L.; Prior, H.J.; Lix, L.M.; Metge, C.; Elias, B. The contributions of First Nations ethnicity, income, and delays in surgery on mortality post-fracture: A population-based analysis. Osteoporos. Int. 2013, 24, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, Y.; Zhu, M.Y.; Wang, Y.; Zhang, Z.M.; Teng, H.L.; Wang, J. Creation of a planned or central-clefted puncture combined with a second puncture during vertebroplasty to treat osteoporotic vertebral compression fractures with large clefts. J. Orthop. Surg. Res. 2020, 15, 535. [Google Scholar] [CrossRef]
- Li, Y.; Jing, Q.; Chen, F.; Xu, Y. Comparative Analysis of Therapeutic Outcomes and Prognoses Among Osteoporosis Patients with Varied Bone Mineral Density T-Values Following Percutaneous Kyphoplasty. Altern. Ther. Health Med. 2024, 30, 214–221. [Google Scholar] [PubMed]
- Lin, H. Influence of bone cement volume and distribution on surgical and adjacent vertebral refractures after percutaneous vertebroplasty. Chin. J. Tissue Eng. Res. 2024, 28, 1586. [Google Scholar]
- Lin, J.H.; Wang, S.H.; Lin, E.Y.; Chiang, Y.H. Better Height Restoration, Greater Kyphosis Correction, and Fewer Refractures of Cemented Vertebrae by Using an Intravertebral Reduction Device: A 1-Year Follow-up Study. World Neurosurg. 2016, 90, 391–396. [Google Scholar] [CrossRef]
- Ma, X.; Xue, C.; Wang, X.; Zhao, Y.; Meng, W.; Gao, H.; Pang, Z.; Liu, X. Effect of multi-platform extended care on postoperative self-efficacy and quality of life in patients with osteoporotic vertebral compressive fracture. Am. J. Transl. Res. 2021, 13, 6945–6951. [Google Scholar]
- Matsumoto, K.; Hoshino, M.; Sawada, H.; Saito, S.; Furuya, T.; Miyanaga, Y.; Nakanishi, K. The Risk of Adjacent Vertebral Fracture Following Balloon Kyphoplasty in Patients With Previous Adjacent Vertebral Fracture. Cureus 2024, 16, e72627. [Google Scholar] [CrossRef]
- Mazzantini, M.; Figliomeni, A.; Bottai, V.; Manca, M.L.; Puglioli, M.; Di Munno, O.; Mosca, M. High rate of vertebral refracture after vertebroplasty in patients taking glucocorticoids: A prospective two-year study. Clin. Exp. Rheumatol. 2020, 38, 649–653. [Google Scholar]
- Moulin, B.; Delpla, A.; Tselikas, L.; Al Ahmar, M.; Prud’homme, C.; Roux, C.; Yevich, S.; Laurent, S.; Hakime, A.; Territehau, C.; et al. Multi-Level Vertebroplasty for 6 or More Painful Osteoporotic Vertebral Body Compression Fractures Performed in the Same Procedural Setting: A Safety and Efficacy Report in Cancer Patients. Cardiovasc. Interv. Radiol. 2020, 43, 1041–1048. [Google Scholar] [CrossRef]
- Mukherjee, S.; Yeh, J.; Ellamushi, H. Pain and functional outcomes following vertebroplasty for vertebral compression fractures-A tertiary centre experience. Br. J. Neurosurg. 2016, 30, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Zhu, J.; Tian, S.; Hu, Z.; Liu, C.; Zhao, X.; Li, X.; Fan, S.; Wan, S. Correlation Analysis Between Basic Diseases and Subsequent Vertebral Fractures After Percutaneous Kyphoplasty (PKP) for Osteoporotic Vertebral Compression Fractures. Pain. Physician 2021, 24, E803–E810. [Google Scholar]
- Noriega, D.; Marcia, S.; Theumann, N.; Blondel, B.; Simon, A.; Hassel, F.; Maestretti, G.; Petit, A.; Weidle, P.A.; Mandly, A.G.; et al. A prospective, international, randomized, noninferiority study comparing an implantable titanium vertebral augmentation device versus balloon kyphoplasty in the reduction of vertebral compression fractures (SAKOS study). Spine J. Off. J. N. Am. Spine Soc. 2019, 19, 1782–1795. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Zhang, X.; Sun, J.; Zhou, L.; Liu, B.; Li, F.; Li, W. Risk factors for vertebral re-fracture after percutaneous kyphoplasty: Retrospective multivariate analysis. Chin. J. Tissue Eng. Res. 2019, 23, 1182. [Google Scholar]
- Pflugmacher, R.; Schroeder, R.J.; Klostermann, C.K. Incidence of adjacent vertebral fractures in patients treated with balloon kyphoplasty: Two years’ prospective follow-up. Acta Radiol. 2006, 47, 830–840. [Google Scholar] [CrossRef]
- Pitton, M.B.; Herber, S.; Koch, U.; Oberholzer, K.; Drees, P.; Düber, C. CT-guided vertebroplasty: Analysis of technical results, extraosseous cement leakages, and complications in 500 procedures. Eur. Radiol. 2008, 18, 2568–2578. [Google Scholar] [CrossRef]
- Qi, Z.; Zhao, S.; Li, H.; Wen, Z.; Chen, B. A study on vertebral refracture and scoliosis after percutaneous kyphoplasty in patients with osteoporotic vertebral compression fractures. J. Orthop. Surg. Res. 2024, 19, 302. [Google Scholar] [CrossRef]
- Qian, L.; Chen, Q.; Wang, D.; Pan, Q.; Jian, Q.; Ma, Y. Study on the Relationship between the Use of Bisphosphonates for Antiosteoporosis and Vertebral Re-Fracture after Vertebroplasty. Evid. -Based Complement. Altern. Med. Ecam 2022, 2022, 3223437. [Google Scholar] [CrossRef]
- Röllinghoff, M.; Siewe, J.; Zarghooni, K.; Sobottke, R.; Alparslan, Y.; Eysel, P.; Delank, K.S. Effectiveness, security and height restoration on fresh compression fractures—A comparative prospective study of vertebroplasty and kyphoplasty. Minim. Invasive Neurosurg. MIN 2009, 52, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Zhao, Y.; Li, D.; Liu, Z.; Zhang, Y.; Shang, D.; Geng, Z.; Shi, Z.; Fan, L.H. Effect of different bone cement distributions in percutaneous kyphoplasty on clinical outcomes for osteoporotic vertebral compression fractures: A retrospective study. Medicine 2023, 102, e33309. [Google Scholar] [CrossRef]
- Summa, A.; Crisi, G.; Cerasti, D.; Ventura, E.; Menozzi, R.; Ormitti, F. Refractures in cemented vertebrae after percutaneous vertebroplasty and pain relief after a second procedure: A retrospective analysis. Neuroradiol. J. 2009, 22, 239–243. [Google Scholar] [CrossRef]
- Tao, H.; Huang, Z.; Shao, S.; Yang, R.; Yang, K.; Zhang, Y.; Li, W.; Dong, F.; Qian, J.; Shen, C. Comparison of Robot-Assisted and Fluoroscopy-Assisted Percutaneous Kyphoplasty for Bone Cement Distribution and Clinical Efficacy. Pain. Physician 2024, 27, E953–E963. [Google Scholar] [CrossRef]
- Wang, J.; Xie, X.; Gou, Y.; Wu, Y.; Pu, H.; Chen, Q.; He, J. Forearm bone mineral density as a predictor of adjacent vertebral refracture after percutaneous kyphoplasty in patients with osteoporotic vertebral compression fracture: A retrospective analysis. J. Orthop. Surg. Res. 2024, 19, 788. [Google Scholar] [CrossRef]
- Wang, R.; Xu, Y.; Ma, X. Risk factors and strategies for recovery quality, postoperative pain, and recurrent fractures between percutaneous kyphoplasty and percutaneous vertebroplasty in elderly patients with thoracolumbar compression fractures: A retrospective comparative cohort study. Ann. Transl. Med. 2023, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhu, S.; Li, Y.; Zhang, C.; Xia, W.; Zhu, Z.; Wang, K. Correction effect of local kyphosis of the spine after percutaneous kyphoplasty in super-aging patients with vertebral compression fractures. Chin. J. Tissue Eng. Res. 2025, 29, 5854. [Google Scholar]
- Guo, X.; Zhu, N.; Zhang, H.; Hao, D. Vertebral refracture after percutaneous vertebroplasty for osteoporotic vertebral compression fractures with and without brace wearing: A retrospective study of 300 patients. Front. Surg. 2022, 9, 1056729. [Google Scholar] [CrossRef]
- Yang, Y.S.; Tsou, Y.S.; Lo, W.C.; Chiang, Y.H.; Lin, J.H. Teriparatide Associated with Fewer Refractures and Higher Body Heights of Cemented Vertebrae after Vertebroplasty: A Matched Cohort Study. Sci. Rep. 2020, 10, 6005. [Google Scholar] [CrossRef]
- Yao, G.; Shen, Y.; Cai, B.; Li, M. Analysis of the Curative Effect of Curved Angle Vertebroplasty in the Treatment of Osteoporotic Vertebral Compression Fracture. Indian. J. Orthop. 2023, 57, 481–489. [Google Scholar] [CrossRef]
- Yi, X.; Lu, H.; Tian, F.; Wang, Y.; Li, C.; Liu, H.; Liu, X.; Li, H. Recompression in new levels after percutaneous vertebroplasty and kyphoplasty compared with conservative treatment. Arch. Orthop. Trauma. Surg. 2014, 134, 21–30. [Google Scholar] [CrossRef]
- Yin, Z.; Cheng, Q.; Wang, C.; Wang, B.; Guan, G.; Yin, J. Influence of sarcopenia on surgical efficacy and mortality of percutaneous kyphoplasty in the treatment of older adults with osteoporotic thoracolumbar fracture. Exp. Gerontol. 2024, 186, 112353. [Google Scholar] [CrossRef]
- Yu, W.; Liang, D.; Jiang, X.; Yao, Z.; Qiu, T.; Ye, L. Efficacy and safety of the target puncture technique for treatment of osteoporotic vertebral compression fractures with intravertebral clefts. J. NeuroInterventional Surg. 2017, 9, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Haibier, A.; Kayierhan, A.; Ma, L.; Abudukelimu, Y.; Aximu, A.; Abudurexiti, T.; Meng, X. Clinical effect analysis of unilateral percutaneous vertebral cement distribution in the repair of osteoporotic thoracolumbar vertebral compression fractures. BMC Surg. 2025, 25, 90. [Google Scholar] [CrossRef]
- Zhang, B.; Dai, M.; Tang, Y.M. Unilateral versus bilateral kyphoplasty for osteoporotic vertebral compression fractures. Adv. Mater. Res. 2012, 393–395, 1064–1068. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, Y.; He, X.; Du, J.; Hao, D. Bracing after percutaneous vertebroplasty for thoracolumbar osteoporotic vertebral compression fractures was not effective. Clin. Interv. Aging 2019, 14, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, Z.; Yin, P.; Zhu, S.; Hai, Y.; Su, Q. Do sandwich vertebral bodies increase the risk of post-augmentation fractures? A retrospective cohort study. Arch. Osteoporos. 2021, 16, 180. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Yang, J.L.; Jiang, H.C.; Lai, Z.; Wu, F.; Pan, Y.Q.; Liu, Z.X. An updated comparison of high- and low-viscosity cement vertebroplasty in the treatment of osteoporotic thoracolumbar vertebral compression fractures: A retrospective cohort study. Int. J. Surg. 2017, 43, 126–130. [Google Scholar] [CrossRef]
- Zhong, R.; Liu, J.; Wang, R.; Liu, Y.; Chen, B.; Jiang, W.; Mao, K.; Tang, P. Unilateral curved versus bipedicular vertebroplasty in the treatment of osteoporotic vertebral compression fractures. BMC Surg. 2019, 19, 193. [Google Scholar] [CrossRef]
- An, Z.-C.; Chen, C.; Dong, L.-Q.; Wu, L.-G.; Zhu, Y.-C.; Wei, H. Risk factors for adjacent segment refracture after percutaneous kyphoplasty. Chin. J. Gen. Pract. 2022, 20, 591–593. [Google Scholar]
- Zhou, C.; Liao, Y.; Huang, S.; Li, H.; Zhu, Z.; Zheng, L.; Wang, B.; Wang, Y. Effect of cement distribution type on clinical outcome after percutaneous vertebroplasty for osteoporotic vertebral compression fractures in the aging population. Front. Surg. 2022, 9, 975832. [Google Scholar] [CrossRef]
- Zhou, Q.; Wan, Y.; Ma, L.; Dong, L.; Yuan, W. Percutaneous Curved Vertebroplasty Decrease the Risk of Cemented Vertebra Refracture Compared with Bilateral Percutaneous Kyphoplasty in the Treatment of Osteoporotic Vertebral Compression Fractures. Clin. Interv. Aging 2024, 19, 289–301. [Google Scholar] [CrossRef]
- Wong, R.M.Y.; Cheung, W.-H.; Chow, S.K.H.; Ng, R.W.K.; Li, W.; Hsu, A.Y.-C.; Wong, K.K.; Ho, A.W.-H.; Choi, S.-H.; Fang, C.X. Recommendations on the post-acute management of the osteoporotic fracture-patients with “very-high” re-fracture risk. J. Orthop. Transl. 2022, 37, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Feng, M.; Zhang, X.-L.; Zou, T.; Huang, Z.; Yang, J.-D.; Sun, H.-H. Are Sandwich Vertebrae Prone to Refracture After Percutaneous Vertebroplasty or Kyphoplasty? A Meta-Analysis. Int. J. Spine Surg. 2024, 18, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, R.E.; Nenov, A.; Duong, H.D. Re-expansion of osteoporotic compression fractures using bilateral SpineJack implants: Early clinical experience and biomechanical considerations. Cureus 2019, 11, e4572. [Google Scholar] [CrossRef]
- Marino, V.; Mungalpara, N.; Amirouche, F. Re-evaluating vertebral height restoration assessment in osteoporotic compression fractures: A systematic review and meta-analysis. Eur. Spine J. 2025, 34, 1641–1662. [Google Scholar] [CrossRef] [PubMed]
- Groen, R.J.; du Toit, D.F.; Phillips, F.M.; Hoogland, P.V.; Kuizenga, K.; Coppes, M.H.; Muller, C.J.; Grobbelaar, M.; Mattyssen, J. Anatomical and pathological considerations in percutaneous vertebroplasty and kyphoplasty: A reappraisal of the vertebral venous system. Spine 2004, 29, 1465–1471. [Google Scholar] [CrossRef]
- Body, J.-J.; Bergmann, P.; Boonen, S.; Boutsen, Y.; Bruyere, O.; Devogelaer, J.-P.; Goemaere, S.; Hollevoet, N.; Kaufman, J.-M.; Milisen, K. Non-pharmacological management of osteoporosis: A consensus of the Belgian Bone Club. Osteoporos. Int. 2011, 22, 2769–2788. [Google Scholar] [CrossRef]
- Hwang, M.; Cheng, D.S.; Hah, R.J.; Lantz, J.M. Postoperative Physical Therapy Following Balloon Kyphoplasty for Management of Vertebral Burst Fracture: A Case Report. JOSPT Cases 2023, 3, 81–95. [Google Scholar] [CrossRef]
- Jing, C.; Wang, H.; Liu, P.; Yang, S.; Zhang, L.; Yang, P.; Gan, M. Effect of sarcopenia on refractures of adjacent vertebra after percutaneous kyphoplasty. BMC Musculoskelet. Disord. 2024, 25, 210. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-F.; Lin, C.-W.; Xie, C.-N.; Liu, H.-T.; Zhu, M.-Y.; Huang, K.-L.; Teng, H.-L. The association between sarcopenia and osteoporotic vertebral compression refractures. Osteoporos. Int. 2019, 30, 2459–2467. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).