Specific Trends in Blood Utilization During the COVID-19 Pandemic: A Retrospective Analysis of a Hungarian Clinical Centre
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
2.2. Data Source and Preparation
2.3. Descriptive Statistics
2.4. Case-Based Generalized Additive Models
2.5. Model Interpretation
2.6. Temporal Trend Visualization
3. Results
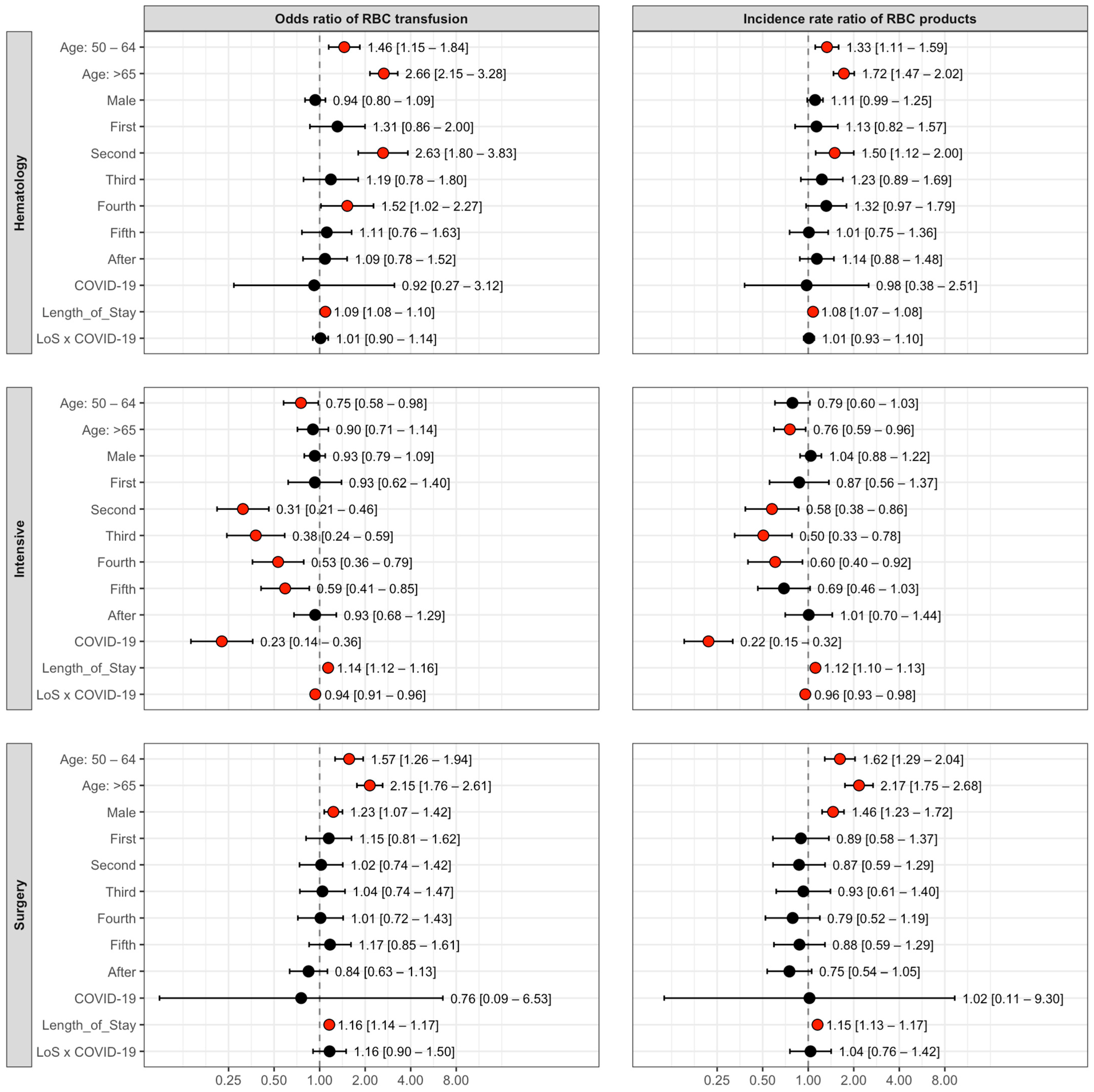
3.1. GAM Results by Clinic
3.2. Visual Trend Analysis of Weekly Active Inpatients, Transfused Patients, and the Ratio of Patients Transfused
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | Confidence Interval |
| COVID-19 | Coronavirus Disease 2019 |
| GAM | Generalized additive model |
| ICD-10 | International Classification of Diseases, Tenth Revision |
| ICU | Intensive care unit |
| IQR | Interquartile range |
| IRR | Incidence rate ratio |
| OR | Odds ratio |
| PBM | Patient Blood Management |
| RBC | Red blood cell |
| WHO | World Health Organization |
References
- Sakr, Y.; Reinhart, K.; Lobo, S.; Esser, E.; Knuepfer, S.; Barz, D.; Settmacher, U.; Bauer, M. Anemia and Blood Transfusion in a Surgical Intensive Care Unit. Crit. Care 2010, 14, R92. [Google Scholar] [CrossRef]
- Coccolini, F.; Shander, A.; Ceresoli, M.; Moore, E.; Tian, B.; Parini, D.; Sartelli, M.; Sakakushev, B.; Doklestich, K.; Abu-Zidan, F.; et al. Strategies to Prevent Blood Loss and Reduce Transfusion in Emergency General Surgery, WSES-AAST Consensus Paper. World J. Emerg. Surg. 2024, 19, 26. [Google Scholar] [CrossRef]
- Shore-Lesserson, L.; Vela-Cantos, F.; Manspeizer, H.E.; Deperio, M.; Ergin, M.A.; Francis, S. Thromboelastography-Guided Transfusion Algorithm Reduces Transfusions in Complex Cardiac Surgery. Anesth. Analg. 1999, 88, 312–319. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Y.; Feng, Z.; Yan, F.; Hei, F.; Liu, J.; Long, C.; Zhao, J. Perioperative Monitoring of Thromboelastograph on Blood Protection and Recovery for Severely Cyanotic Patients Undergoing Complex Cardiac Surgery. Artif. Organs 2010, 34, 955–960. [Google Scholar] [CrossRef]
- Lelubre, C.; Vincent, J.-L. Red Blood Cell Transfusion in the Critically Ill Patient. Ann. Intensive Care 2011, 1, 43. [Google Scholar] [CrossRef]
- Cheng, B.H.W.; Chan, K.Y.; Au, H.Y.; Sham, M.M.K.; Li, C.W. Intensive Palliative Care for Patients With Hematological Cancer Dying in Hospice. Am. J. Hosp. Palliat. Care 2013, 32, 221–225. [Google Scholar] [CrossRef]
- Van Der Linden, P.; Kimbimbi, P.; Daper, A.; Trenchant, A.; De Boelpaepe, C.; Jacobs, D.; De Hert, S.; Defrance, P.; Simoens, G. A Standardized Multidisciplinary Approach Reduces the Use of Allogeneic Blood Products in Patients Undergoing Cardiac Surgery. Can. J. Anesth. 2001, 48, 894–901. [Google Scholar] [CrossRef]
- Shander, A.; Perelman, S.; Javidroozi, M.; Lobel, G.; Puzio, T. From Bloodless Surgery to Patient Blood Management. Mt. Sinai J. Med. 2012, 79, 56–65. [Google Scholar] [CrossRef]
- Klein, A.; Agarwal, S.; Cholley, B.; Fassl, J.; Griffin, M.; Kaakinen, T.; Paulus, P.; Rex, S.; Siegemund, M.; Van Saet, A. A Review of European Guidelines for Patient Blood Management with a Particular Emphasis on Antifibrinolytic Drug Administration for Cardiac Surgery. J. Clin. Anesth. 2022, 78, 110654. [Google Scholar] [CrossRef]
- Theusinger, O.M.; Felix, C.; Spahn, D.R. Strategies to Reduce the Use of Blood Products. Curr. Opin. Anaesthesiol. 2012, 25, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Gammon, R.R.; Bocquet, C.; Radhakrishnan Nair, A.; Nalezinski, S.; Gupta, G.K.; Lamba, D.S.; Dubey, R.; Jindal, A.; Mangwana, S.; Hinrichsen, C. Patient Blood Management and Its Role in Supporting Blood Supply. J. Blood Med. 2023, 14, 595–611. [Google Scholar] [CrossRef]
- Sadana, D.; Pratzer, A.; Frank, S.M.; Auron, M.; Volpicelli, F.M.; Saag, H.S.; Adler, N.; Scher, L.J. Promoting High-Value Practice by Reducing Unnecessary Transfusions With a Patient Blood Management Program. JAMA Intern. Med. 2017, 178, 116. [Google Scholar] [CrossRef]
- Goodnough, L.T.; Hollenhorst, M.A. Clinical Decision Support and Improved Blood Use in Patient Blood Management. Hematology 2019, 2019, 577–582. [Google Scholar] [CrossRef]
- Butcher, A.; Richards, T. Cornerstones of Patient Blood Management in Surgery. Transfus. Med. 2017, 28, 150–157. [Google Scholar] [CrossRef]
- Mehra, T.; Dutkowski, P.; Volbracht, J.; Manz, M.G.; Holubec, T.; Moos, R.M.; Wanner, G.; Seifert, B.; Spahn, D.R.; Bravo-Reiter, S. Implementation of a Patient Blood Management Monitoring and Feedback Program Significantly Reduces Transfusions and Costs. Transfusion 2015, 55, 2807–2815. [Google Scholar] [CrossRef]
- Lau, Y.-Y.; Dulebenets, M.A.; Yip, H.-T.; Tang, Y.-M. Healthcare Supply Chain Management under COVID-19 Settings: The Existing Practices in Hong Kong and the United States. Healthcare 2022, 10, 1549. [Google Scholar] [CrossRef]
- Parveen, S.; Mahbub, M.S.; Nahar, N.; Morshed, K.A.M.; Rahman, N.; Evana, E.T.; Islam, N.; Miah, A.S.M.J. The Impact of COVID-19 on Healthcare Services in Bangladesh: A Qualitative Study on Healthcare Providers’ Perspectives. J. Prev. Med. Public Health 2024, 57, 356–369. [Google Scholar] [CrossRef]
- Søvold, L.E.; Münter, L.; Kousoulis, A.A.; Naslund, J.A.; Saxena, S.; Qoronfleh, M.W.; Grobler, C. Prioritizing the Mental Health and Well-Being of Healthcare Workers: An Urgent Global Public Health Priority. Front. Public Health 2021, 9, 679397. [Google Scholar] [CrossRef]
- Miskeen, E.; Karar, H.K.; Eljack, T.B.; Yahia, A.I.O. The Impact of COVID-19 Pandemic on Blood Transfusion Services: A Perspective from Health Professionals and Donors. J. Multidiscip. Healthc. 2021, 14, 3063–3071. [Google Scholar] [CrossRef]
- Hu, Q.; Pan, L.; Han, W.; Hu, W.; Zheng, Y. Association Between Concerns About COVID-19 Infection and Blood Donation Intention: Cross-Sectional Survey Study Through a Mobile Communication Platform. J. Med. Internet Res. 2023, 25, e46588. [Google Scholar] [CrossRef]
- Wang, Y.; Han, W.; Hu, W.; Wang, C.; Zheng, X.; Pan, L.; Zhou, H.; Liu, Y. Impact of COVID-19 on Blood Centres in Zhejiang Province China. Vox Sang. 2020, 115, 502–506. [Google Scholar] [CrossRef]
- Shokouhifar, M.; Ranjbarimesan, M. Multivariate Time-Series Blood Donation/Demand Forecasting for Resilient Supply Chain Management during COVID-19 Pandemic. Clean. Logist. Supply Chain. 2022, 5, 100078. [Google Scholar] [CrossRef]
- Sachdev, S.; Singh, L.; Dhawan, H.K.; Kishore, K.; Grover, S.; Sharma, R.R.; Hans, R.; Lamba, D.S. Exploration of COVID-19 Related Fears Deterring from Blood Donation in India. ISBT Sci. Ser. 2021, 16, 147–157. [Google Scholar] [CrossRef]
- Al-Riyami, A.Z.; Vermeulen, M.; Meshi, A.; Pandey, H.C.; Lee, C.K.; Bengtsson, J.; Torres, O.W.; Van Den Berg, K.; Schulze, T.J.; Dubey, R.; et al. International Society of Blood Transfusion Survey of Experiences of Blood Banks and Transfusion Services during the COVID-19 Pandemic. Vox Sang. 2022, 117, 822–830. [Google Scholar] [CrossRef]
- Uzzoli, A. COVID-19 Epidemic Waves in Hungary and Their Regional Distribution. Zb. Rad. Departmana Za Geogr. Turiz. I Hotel. 2025, 54, 34–47. [Google Scholar] [CrossRef]
- Moletta, L.; Pierobon, E.S.; Capovilla, G.; Costantini, M.; Salvador, R.; Merigliano, S.; Valmasoni, M. International Guidelines and Recommendations for Surgery during Covid-19 Pandemic: A Systematic Review. Int. J. Surg. 2020, 79, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Kennedy, K.; Jiménez-Martín, A.; Moreno-Jiménez, G.; Tenorio, M.; Vallés Carboneras, A.; Jiménez-Chillón, C.; Luna, A.; López-Jiménez, F.J.; García García, I.; Sánchez-Tornero, A. Transfusion Support in COVID-19 Patients: Impact on Hospital Blood Component Supply during the Outbreak. Transfusion 2020, 61, 361–367. [Google Scholar] [CrossRef]
- Coccolini, F.; Perrone, G.; Chiarugi, M.; Di Marzo, F.; Ansaloni, L.; Scandroglio, I.; Marini, P.; Zago, M.; De Paolis, P.; Forfori, F.; et al. Surgery in COVID-19 Patients: Operational Directives. World J. Emerg. Surg. 2020, 15, 25. [Google Scholar] [CrossRef]
- Stroth, L.C.; Jahns, F.; Bode, B.; Stender, M.; Schmidt, M.; Baschnegger, H.; Epstein, N.; Sandmeyer, B.; Nau, C. Workforce Strategies during the First Wave of the COVID-19 Pandemic: A Retrospective Online Survey at Intensive Care Units in Germany. BMC Health Serv. Res. 2024, 24, 407. [Google Scholar] [CrossRef]
- Roubinian, N.H.; Murphy, E.L.; Gottschall, J.L.; Triulzi, D.J.; Escobar, G.J.; Carson, J.L.; Wu, Y.; Swain, B.E.; Gardner, M.N.; Kleinman, S.H.; et al. Trends in Red Blood Cell Transfusion and 30-Day Mortality among Hospitalized Patients. Transfusion 2014, 54, 2678–2686. [Google Scholar] [CrossRef]
- Dongelmans, D.A.; De Lange, D.W.; Bussel, B.; Arbous, M.S.; Bakhshi-Raiez, F.; De Keizer, N.F.; Brinkman, S.; Termorshuizen, F.; Author_Id, N. Characteristics and Outcome of COVID-19 Patients Admitted to the ICU: A Nationwide Cohort Study on the Comparison between the First and the Consecutive Upsurges of the Second Wave of the COVID-19 Pandemic in the Netherlands. Ann. Intensive Care 2022, 12, 5. [Google Scholar] [CrossRef]
- Abu-Ismail, L.; Taha, M.J.J.; Abuawwad, M.T.; Al-Bustanji, Y.; Al-Shami, K.; Nashwan, A.; Yassin, M. COVID-19 and Anemia: What Do We Know So Far? Hemoglobin 2023, 47, 122–129. [Google Scholar] [CrossRef]
- Bergamaschi, G.; Cambiè, G.; Iadarola, C.; Savioli, J.; Codega, S.; Pace, L.; Melazzini, F.; Soriano, S.; Ferrari, S.; Salvi, L.; et al. Anemia in Patients with Covid-19: Pathogenesis and Clinical Significance. Clin. Exp. Med. 2021, 21, 239–246. [Google Scholar] [CrossRef]
- Douglas, I.S.; Mehta, A.; Mansoori, J. Policy Proposals for Mitigating Intensive Care Unit Strain: Insights from the COVID-19 Pandemic. Ann. Am. Thorac. Soc. 2024, 21, 1633–1642. [Google Scholar] [CrossRef]
- Delabranche, X.; Bertrand, F.; Mertes, P.; Tran Ba Loc, P.; Kientz, D.; Gachet, C.; Humbrecht, C.; Levy, F.; Sirlin, F.; Roche, A.; et al. Impact of COVID-19 and Lockdown Regarding Blood Transfusion. Transfusion 2021, 61, 2327–2335. [Google Scholar] [CrossRef]
- Barriteau, C.M.; Hartman, K.; Ramsey, G.; Bochey, P.; Sumugod, R.; Lindholm, P.F. Blood Transfusion Utilization in Hospitalized COVID-19 Patients. Transfusion 2020, 60, 1919–1923. [Google Scholar] [CrossRef]
- Romani, G.; Massaro, M.; Cobianchi, L.; Barach, P.; Barcellini, A.; Lucà, R.; Ferrara, M.; Modenese, M.; Ricciardi, W.; Dal Mas, F. Population Health Strategies to Support Hospital and Intensive Care Unit Resiliency During the COVID-19 Pandemic: The Italian Experience. Popul. Health Manag. 2020, 24, 174–181. [Google Scholar] [CrossRef]
- Tyrrell, C.S.B.; Narula, A.A.; Broadbent, J.; Mcgrath, B.; Abdul Pari, A.A.; Lupton, M.; Thomas-Meyer, M.; Mavrodaris, A.; Ahmed, A.; Gentry, S.V.; et al. Managing Intensive Care Admissions When There Are Not Enough Beds during the COVID-19 Pandemic: A Systematic Review. Thorax 2021, 76, 302–312. [Google Scholar] [CrossRef]
- Mccabe, R.; Brazeau, N.F.; Knock, E.; Whittles, L.K.; Ghani, A.C.; Hauck, K.; Walker, P.G.; Schmit, N.; Watson, O.J.; Whittaker, C.; et al. Modelling Intensive Care Unit Capacity under Different Epidemiological Scenarios of the COVID-19 Pandemic in Three Western European Countries. Int. J. Epidemiol. 2021, 50, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.D.; Ingram, M.; Paterson, J.; Danjoux, G.R.; Berry, S.K.; Pokhrel, S.; Brand, J.W.; Monkhouse, D. Peri-Operative COVID-19 Infection in Urgent Elective Surgery during a Pandemic Surge Period: A Retrospective Observational Cohort Study. Anaesthesia 2020, 75, 1596–1604. [Google Scholar] [CrossRef]
- Shih, C.-L.; Huang, H.-T.; Lee, T.-C.; Hsu, C.-H.; Chen, C.-H.; Huang, P.-J. Impact of the COVID-19 Pandemic and Its Related Psychological Effect on Orthopedic Surgeries Conducted in Different Types of Hospitals in Taiwan. J. Orthop. Surg. 2021, 29, 230949902199607. [Google Scholar] [CrossRef]
- Jakobsen, C.-J.; Tang, M.; Mortensen, P.E.; Ryhammer, P.K.; Andreasen, J.J. Transfusion of Blood during Cardiac Surgery Is Associated with Higher Long-Term Mortality in Low-Risk Patients. Eur. J. Cardio-Thorac. Surg. 2012, 42, 114–120. [Google Scholar] [CrossRef]
- Siegal, D.M.; Belley-Côté, E.P.; Tsang, J.L.Y.; D’Aragon, F.; Connolly, S.J.; Hill, S.; Marfo, G.; Al-Hazzani, W.; Arnold, D.M.; Lee, S.F.; et al. Small-Volume Blood Collection Tubes to Reduce Transfusions in Intensive Care. JAMA 2023, 330, 1872. [Google Scholar] [CrossRef] [PubMed]
- Riley, W.; Mccullough, J.; Love, K. Public Policy Impact of the COVID-19 Pandemic on Blood Supply in the United States. Am. J. Public Health 2021, 111, 860–866. [Google Scholar] [CrossRef]
- Oláh, Z.; Babik, B.; Matusovits, A.; Gál, J.; Fülesdi, B. Principles of the Perioperative Patient Blood Management. Orvosi Hetil. 2020, 161, 1554–1568. [Google Scholar] [CrossRef]
- Horváth, K.K.; Baróti-Tóth, K.; Marton, I.; Fődi, É.; Lázár, M. A Vértakarékos Betegellátás Bevezetésének Szükségessége És Lehetőségei, Valamint a Pandémia Hatása a Regionális Vérellátás Helyzetére. Hematológia–Transzfuziológia 2022, 54, 205–215. [Google Scholar] [CrossRef]
- Babik, B.; Matusovits, A.; Fazakas, J.; Fülesdi, B.; Gál, J. Perioperative Patient Blood Management: Common Risk, Common Tasks, Common Responsibility. Orvosi Hetil. 2020, 161, 1545–1553. [Google Scholar] [CrossRef]
- Oláh, Z.; Deli, T.; Mühl, D. Obstetrical Aspects of the National Blood Donation and Blood Saving Program. Orvosi Hetil. 2020, 161, 1588–1598. [Google Scholar] [CrossRef]
- Retteghy, T.A. A Vértakarékos Betegellátás a Perioperatív Szakban. Hematológia−Transzfuziológia 2018, 51, 194–203. [Google Scholar] [CrossRef]
- Smudla, A.; Fülesdi, B.; Gál, J.; Matusovits, A.; Babik, B.; Fazakas, J. National Blood Donation and Blood Saving Program in Hungary. Further Steps Are Required to Improve Patient Safety. Orvosi Hetil. 2020, 161, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Koszta, G.; Sira, G.T.; Béczy, K. Patient Blood Management in Cardiac Anesthesia. Orvosi Hetil. 2020, 161, 1579–1587. [Google Scholar] [CrossRef]
- Lu, W.; Yazer, M.; Li, N.; Ziman, A.; Wendel, S.; Tang, H.; Tsang, H.; Titlestad, K.; Thibodeaux, S.R.; Shih, A.W.; et al. Hospital red blood cell and platelet supply and utilization from March to December of the first year of the COVID-19 pandemic: The BEST collaborative study. Transfusion 2022, 62, 1559–1570. [Google Scholar] [CrossRef]
- Senapati, J.; Aggarwal, M.; Louis, L.; Mirza, S.A.; Kumar, P.; Dhawan, R.; Dass, J.; Vishwanathan, G.K.; Pandey, H.C.; Coshic, P.; et al. Transfusion Practices during the COVID-19 Pandemic: An Experience from a Hematology Daycare in India. Transfus. Apher. Sci. 2020, 60, 103025. [Google Scholar] [CrossRef]
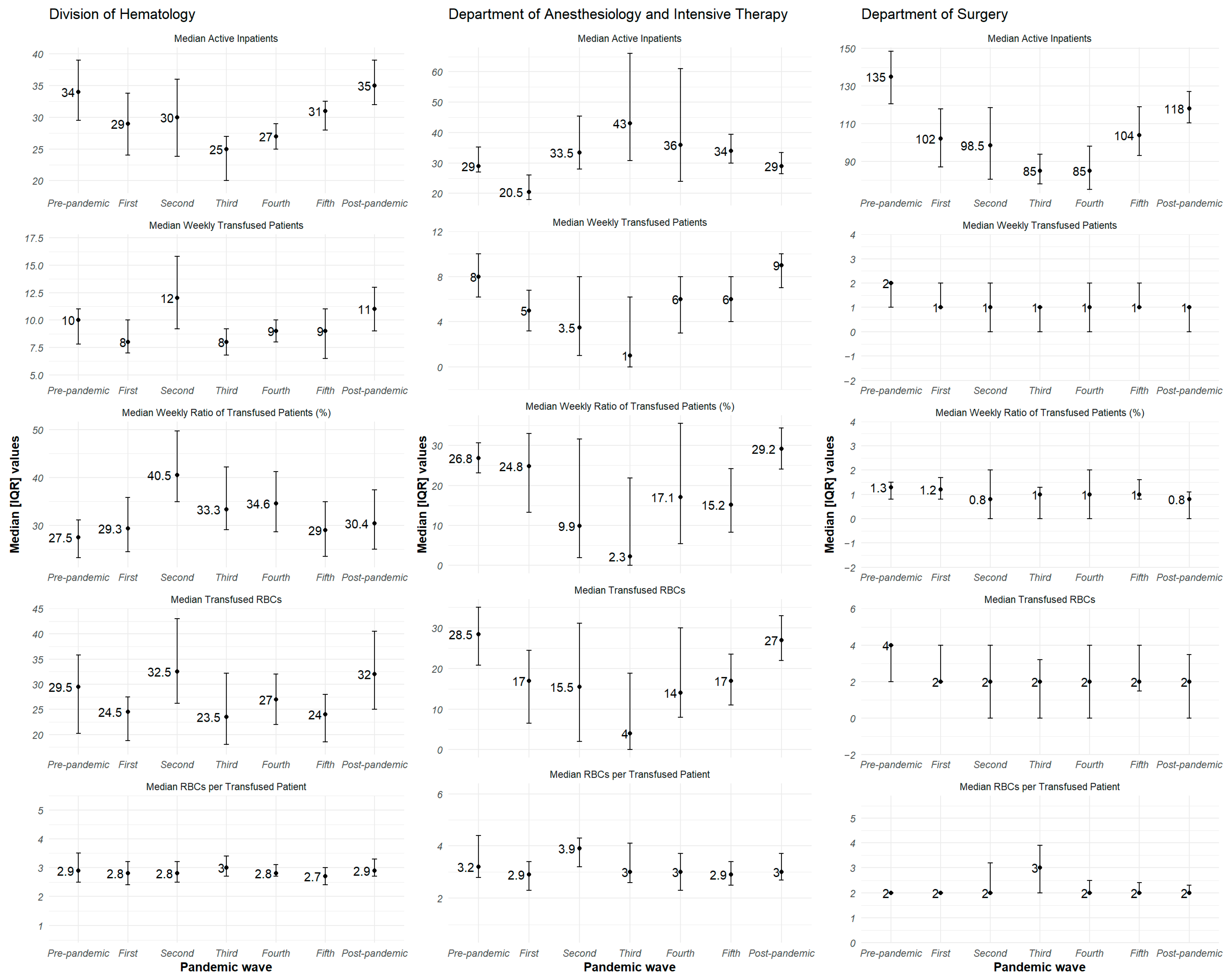

| Active Inpatients 1 | Transfused Patients 1 | Transfused Blood Product 1 | Product Per Transfused Patient 3 | Ratio of Transfused Patients 2 | |
|---|---|---|---|---|---|
| Division of Hematology | 31.0 [26.0–36.0] | 29.0 [23.0–37.0] | 10.0 [8.0–12.0] | 2.9 [2.6–3.2] | 32.2 [26.6–40.0] |
| Department of Anesthesiology and Intensive Therapy | 31.0 [27.0–39.0] | 25.0 [13.0–34.0] | 8.0 [4.0–10.0] | 3.0 [2.5–3.7] | 27.2 [13.0–35.6] |
| Department of Surgery | 105.0 [88.0–121.0] | 14.0 [9.0–18.0] | 5.0 [4.0–7.0] | 2.5 [2.0–2.8] | 5.1 [3.8–6.7] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pál, S.; Solymár, M.; Réger, B.; Alizadeh, H.; Vereczkei, A.; Kiss, T.; Faust, Z. Specific Trends in Blood Utilization During the COVID-19 Pandemic: A Retrospective Analysis of a Hungarian Clinical Centre. J. Clin. Med. 2025, 14, 7943. https://doi.org/10.3390/jcm14227943
Pál S, Solymár M, Réger B, Alizadeh H, Vereczkei A, Kiss T, Faust Z. Specific Trends in Blood Utilization During the COVID-19 Pandemic: A Retrospective Analysis of a Hungarian Clinical Centre. Journal of Clinical Medicine. 2025; 14(22):7943. https://doi.org/10.3390/jcm14227943
Chicago/Turabian StylePál, Sándor, Margit Solymár, Barbara Réger, Hussain Alizadeh, András Vereczkei, Tamás Kiss, and Zsuzsanna Faust. 2025. "Specific Trends in Blood Utilization During the COVID-19 Pandemic: A Retrospective Analysis of a Hungarian Clinical Centre" Journal of Clinical Medicine 14, no. 22: 7943. https://doi.org/10.3390/jcm14227943
APA StylePál, S., Solymár, M., Réger, B., Alizadeh, H., Vereczkei, A., Kiss, T., & Faust, Z. (2025). Specific Trends in Blood Utilization During the COVID-19 Pandemic: A Retrospective Analysis of a Hungarian Clinical Centre. Journal of Clinical Medicine, 14(22), 7943. https://doi.org/10.3390/jcm14227943





