Validation of the InterTAK Diagnostic Score for Differentiating Takotsubo Syndrome from Acute Coronary Syndrome in a Middle Eastern Population
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Patient Selection
- Transient left ventricular systolic and diastolic dysfunction with wall motion abnormalities, extending beyond a single epicardial vascular distribution.
- Absence of obstructive coronary artery disease (<50% stenosis) or angiographic evidence of acute plaque rupture
- New electrocardiographic abnormalities or modest elevation of cardiac troponin (<10× upper limit of normal)
- Absence of pheochromocytoma or myocarditis
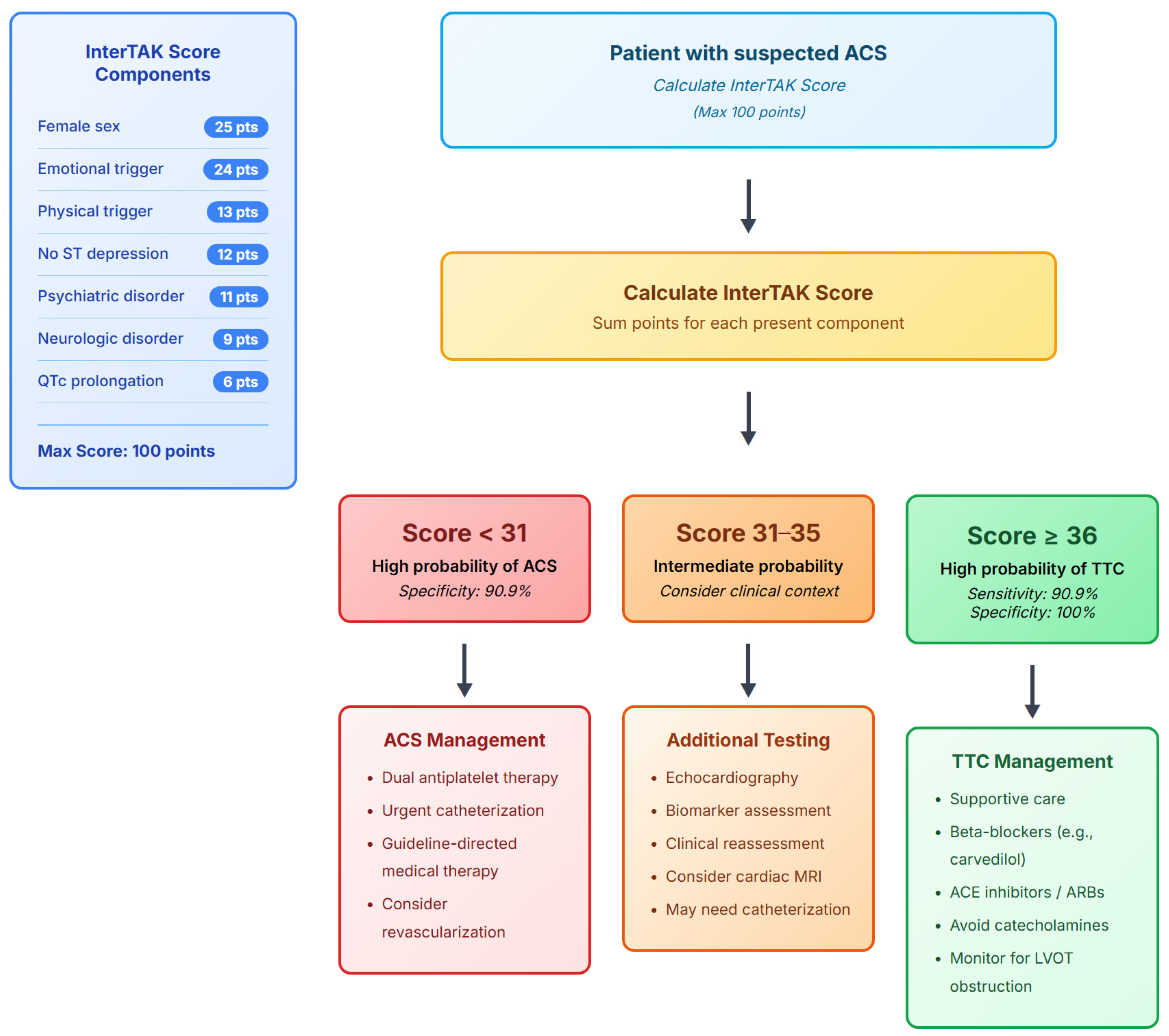
2.3. InterTAK Score Calculation
- Female sex: 25 points.
- Emotional trigger: 24 points (defined as acute emotional stress within 48 h).
- Physical trigger: 13 points (defined as acute physical stress including surgery, trauma, or acute medical illness).
- Absence of ST-segment depression (except aVR lead): 12 points.
- History of diagnosed psychiatric disorders: 11 points (documented diagnosis as per Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition).
- Physician-diagnosed Neurologic disorders: 9 points (including stroke, seizure, or neurodegenerative disease).
- QTc prolongation: 6 points (calculated using the Bazett formula and considered prolonged if >450 milliseconds in males and >470 milliseconds in females.
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. InterTAK Score Components
- Emotional triggers: 54.5% in TS vs. 0% in ACS (p < 0.001); which included bereavement in 3 cases, family conflict in 2, and financial stress in 1.
- Physical triggers: 54.5% in TS vs. 0% in ACS (p < 0.001), which included post-surgical complications in 4 cases and acute medical illness in 2.
- QTc prolongation: 63.6% in TS vs. 26.9% in ACS (p = 0.042)
- Mean QTc interval: 471.2 ± 48.6 ms in TS vs. 419.1 ± 34.5 ms in ACS (p = 0.006), which represented a 52 ms difference with potential arrhythmogenic implications.
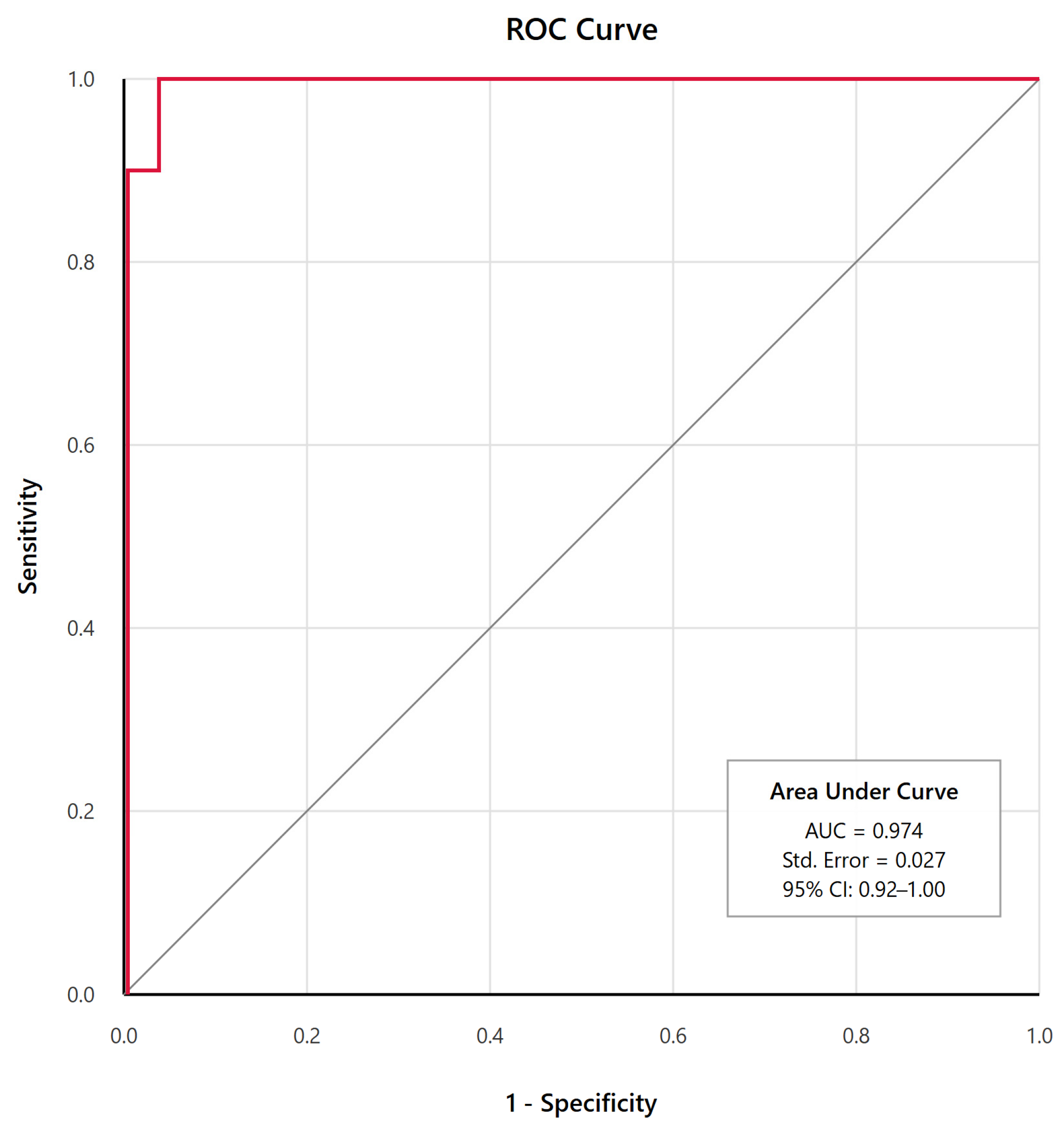
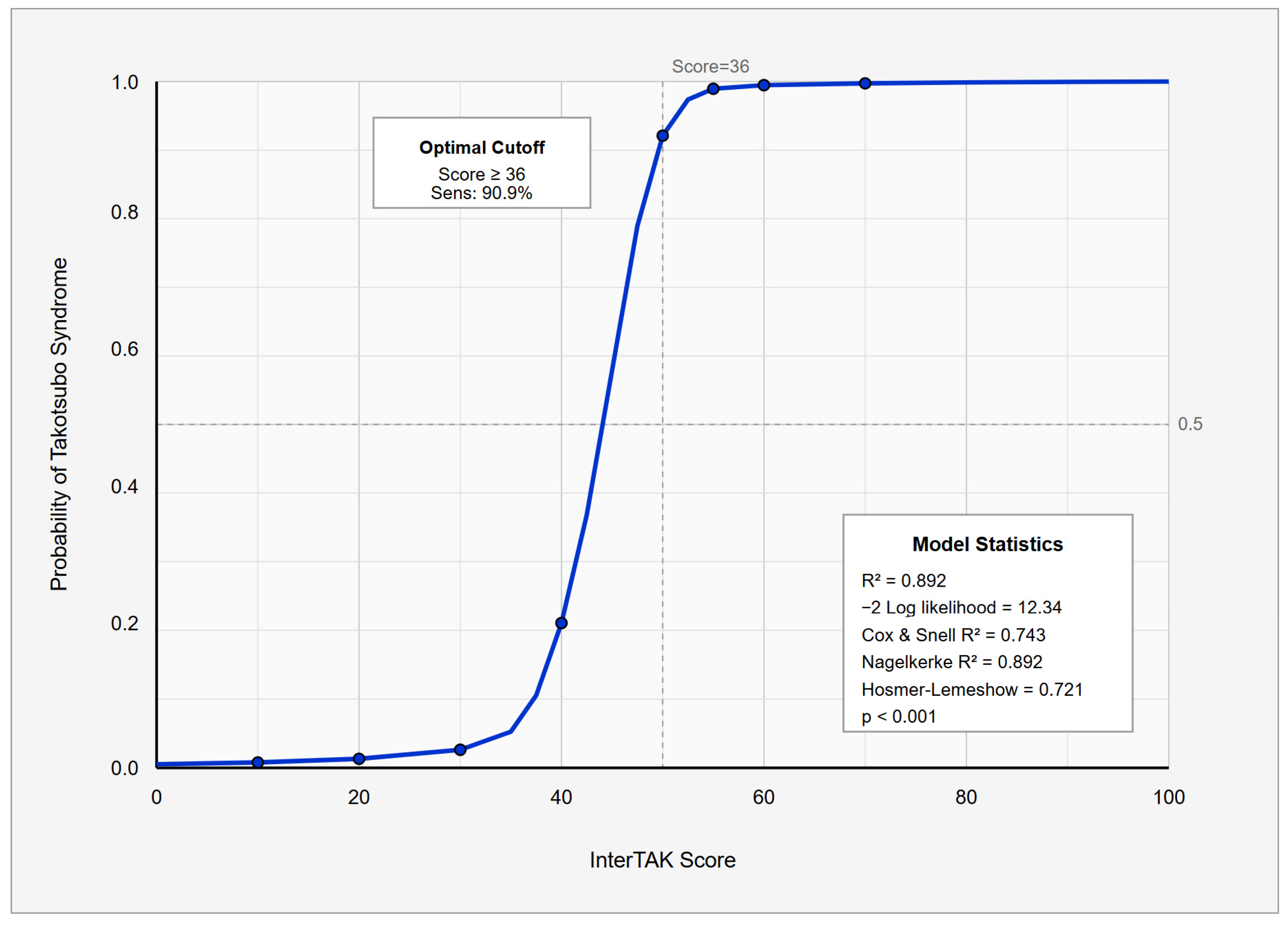
3.3. Diagnostic Performance
3.4. Clinical Outcomes
4. Discussion
4.1. Clinical Implications
4.2. Limitations
4.3. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghadri, J.R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International expert consensus document on Takotsubo syndrome (part I): Clinical characteristics, diagnostic criteria, and pathophysiology. Eur. Heart J. 2018, 39, 2032–2046. [Google Scholar] [CrossRef] [PubMed]
- Matta, A.; Delmas, C.; Campelo-Parada, F.; Lhermusier, T.; Bouisset, F.; Elbaz, M.; Nader, V.; Blanco, S.; Roncalli, J.; Carrié, D. Takotsubo cardiomyopathy. Rev. Cardiovasc. Med. 2022, 23, 38. [Google Scholar] [CrossRef] [PubMed]
- Lüscher, T.F.; Templin, C. Is takotsubo syndrome a microvascular acute coronary syndrome? Towards of a new definition. Eur. Heart J. 2016, 37, 2816–2820. [Google Scholar] [CrossRef]
- Redfors, B.; Jha, S.; Thorleifsson, S.; Jernberg, T.; Angerås, O.; Frobert, O.; Petursson, P.; Tornvall, P.; Sarno, G.; Ekenbäck, C.; et al. Short- and long-term clinical outcomes for patients with takotsubo syndrome and patients with myocardial infarction: A report from the Swedish coronary angiography and angioplasty registry. J. Am. Heart Assoc. 2021, 10, e017290. [Google Scholar] [CrossRef]
- Paur, H.; Wright, P.T.; Sikkel, M.B.; Tranter, M.H.; Mansfield, C.; O’Gara, P.; Stuckey, D.J.; Nikolaev, V.O.; Diakonov, I.; Pannell, L.; et al. High levels of circulating epinephrine trigger apical cardiodepression in a β2-adrenergic receptor/Gi-dependent manner: A new model of Takotsubo cardiomyopathy. Circulation 2012, 126, 697–706. [Google Scholar] [CrossRef]
- Ghadri, J.R.; Kato, K.; Cammann, V.L.; Gili, S.; Jurisic, S.; Di Vece, D.; Candreva, A.; Ding, K.J.; Micek, J.; Szawan, K.A.; et al. Long-term prognosis of patients with takotsubo syndrome. J. Am. Coll. Cardiol. 2018, 72, 874–882. [Google Scholar] [CrossRef]
- Ghadri, J.R.; Cammann, V.L.; Jurisic, S.; Seifert, B.; Napp, L.C.; Diekmann, J.; Bataiosu, D.R.; D’Ascenzo, F.; Ding, K.J.; Sarcon, A.; et al. A novel clinical score (InterTAK Diagnostic Score) to differentiate takotsubo syndrome from acute coronary syndrome: Results from the International Takotsubo Registry. Eur. J. Heart Fail. 2017, 19, 1036–1042. [Google Scholar] [CrossRef]
- Samul-Jastrzębska, J.; Roik, M.; Wretowski, D.; Łabyk, A.; Ślubowska, A.; Bizoń, A.; Paczyńska, M.; Kurnicka, K.; Pruszczyk, P.; Ciurzyński, M. Evaluation of the InterTAK Diagnostic Score in differentiating Takotsubo syndrome from acute coronary syndrome: A single center experience. Cardiol. J. 2021, 28, 416–422. [Google Scholar] [CrossRef]
- Templin, C.; Napp, L.C.; Ghadri, J.R. Takotsubo syndrome: Underdiagnosed, underestimated, but understood? J. Am. Coll. Cardiol. 2016, 67, 1937–1940. [Google Scholar] [CrossRef]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems (10th Revision). Available online: https://icd.who.int/browse10/2019/en (accessed on 24 September 2025).
- Prasad, A.; Lerman, A.; Rihal, C.S. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. Am. Heart J. 2008, 155, 408–417. [Google Scholar] [CrossRef]
- Madhavan, M.; Prasad, A. Proposed Mayo Clinic criteria for the diagnosis of Tako-Tsubo cardiomyopathy and long-term prognosis. Herz 2010, 35, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.G.; Morrow, R.H.; Ross, D.A. Field Trials of Health Interventions: A Toolbox, 3rd ed.; OUP Oxford: Oxford, UK, 2015. [Google Scholar]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Bossone, E.; Schneider, B.; Sechtem, U.; Citro, R.; Underwood, S.R.; Sheppard, M.N.; Figtree, G.A.; Parodi, G.; Akashi, Y.J.; et al. Current state of knowledge on Takotsubo syndrome: A Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Silverio, A.; Parodi, G.; Scudiero, F.; Bossone, E.; Di Maio, M.; Vriz, O.; Bellino, M.; Zito, C.; Provenza, G.; Radano, I.; et al. Beta-blockers are associated with better long-term survival in patients with Takotsubo syndrome. Heart 2022, 108, 1369–1376. [Google Scholar] [CrossRef]
- Boland, T.A.; Lee, V.H.; Bleck, T.P. Stress-induced cardiomyopathy. Crit. Care Med. 2015, 43, 686–693. [Google Scholar] [CrossRef]
- Fröhlich, G.M.; Schoch, B.; Schmid, F.; Keller, P.; Sudano, I.; Lüscher, T.F.; Noll, G.; Ruschitzka, F.; Enseleit, F. Takotsubo cardiomyopathy has a unique cardiac biomarker profile: NT-proBNP/myoglobin and NT-proBNP/troponin T ratios for the differential diagnosis of acute coronary syndromes and stress induced cardiomyopathy. Int. J. Cardiol. 2012, 154, 328–332. [Google Scholar] [CrossRef]
- Lau, C.; Chiu, S.; Nayak, R.; Lin, B.; Lee, M.S. Survival and risk of recurrence of takotsubo syndrome. Heart 2021, 107, 1160–1166. [Google Scholar] [CrossRef]
- Pant, S.; Deshmukh, A.; Mehta, K.; Badheka, A.O.; Tuliani, T.; Patel, N.J.; Dabhadkar, K.; Prasad, A.; Paydak, H. Burden of arrhythmias in patients with Takotsubo cardiomyopathy (apical ballooning syndrome). Int. J. Cardiol. 2013, 170, 64–68. [Google Scholar] [CrossRef]
- Perazzolo Marra, M.; Zorzi, A.; Corbetti, F.; De Lazzari, M.; Migliore, F.; Tona, F.; Tarantini, G.; Iliceto, S.; Corrado, D. Apicobasal gradient of left ventricular myocardial edema underlies transient T-wave inversion and QT interval prolongation (Wellens’ ECG pattern) in Tako-Tsubo cardiomyopathy. Heart Rhythm 2013, 10, 70–77. [Google Scholar] [CrossRef]
- Chong, T.K.; Chen, J.; Lyu, L.; Wei, Y.; Liu, Y.; Wu, L.; Tao, Y.; Jiang, L.; Sun, Z.; Li, D.; et al. Clinical characteristics and outcome correlates of Chinese patients with takotsubo syndrome: Results from the first Chinese takotsubo syndrome registry. Int. J. Cardiol. 2023, 387, 131129. [Google Scholar] [CrossRef]
- Banerjee, S.; Monteleone, P.; Novak, S. Catheterization laboratory activity-based costing. Circ. Cardiovasc. Interv. 2021, 14, e010228. [Google Scholar] [CrossRef]

| Characteristic | Takotsubo Syndrome (n = 11) | Acute Coronary Syndrome (n = 26) | p-Value |
|---|---|---|---|
| Age, y | 53.4 ± 14.1 | 54.6 ± 11.0 | 0.78 |
| Female sex, n (%) | 8 (72.7) | 6 (23.1) | 0.007 * |
| Emirati nationality, n (%) | 4 (36.4) | 8 (30.8) | 0.51 |
| Length of hospital stay, d | 5.8 ± 3.4 | 3.4 ± 2.0 | 0.52 |
| Clinical presentation | |||
| Chest pain, n (%) | 11 (100) | 26 (100) | 1.00 |
| Dyspnea, n (%) | 7 (63.6) | 15 (57.7) | 0.74 |
| Electrocardiographic findings | |||
| ST-segment elevation, n (%) | 4 (36.4) | 10 (38.5) | 0.91 |
| T-wave inversion, n (%) | 7 (63.6) | 14 (53.8) | 0.58 |
| Absence of ST depression, n (%) | 7 (63.6) | 10 (38.5) | 0.15 |
| QTc prolongation, n (%) | 7 (63.6) | 7 (26.9) | 0.042 * |
| QTc interval, ms | 471.2 ± 48.6 | 419.1 ± 34.5 | 0.006 * |
| InterTAK Score Component | Points | Takotsubo Syndrome (n = 11) | Acute Coronary Syndrome (n = 26) | p-Value |
|---|---|---|---|---|
| Female sex | 25 | 8 (72.7) | 6 (23.1) | 0.007 * |
| Emotional trigger | 24 | 6 (54.5) | 0 (0) | <0.001 * |
| Physical trigger | 13 | 6 (54.5) | 0 (0) | <0.001 * |
| Absence of ST depression † | 12 | 7 (63.6) | 10 (38.5) | 0.15 |
| Psychiatric disorders | 11 | 0 (0) | 0 (0) | 1.00 |
| Neurologic disorders | 9 | 2 (18.2) | 3 (11.5) | 0.47 |
| QTc prolongation | 6 | 7 (63.6) | 7 (26.9) | 0.042 * |
| Total InterTAK score | 100 | 49.1 ± 14.8 | 13.0 ± 9.3 | <0.001 * |
| Cutoff Score | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Correctly Classified (%) |
|---|---|---|---|---|---|
| ≥31 | 90.9 | 84.6 | 71.4 | 95.7 | 86.5 |
| ≥36 | 90.9 | 100.0 | 100.0 | 96.3 | 97.3 |
| ≥40 | 81.8 | 100.0 | 100.0 | 92.9 | 94.6 |
| ≥45 | 72.7 | 100.0 | 100.0 | 89.7 | 91.9 |
| ≥50 | 45.5 | 100.0 | 100.0 | 81.3 | 83.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamil, G.; Al Shamisi, A.; AlShamsi, F.; Agha, A. Validation of the InterTAK Diagnostic Score for Differentiating Takotsubo Syndrome from Acute Coronary Syndrome in a Middle Eastern Population. J. Clin. Med. 2025, 14, 7806. https://doi.org/10.3390/jcm14217806
Jamil G, Al Shamisi A, AlShamsi F, Agha A. Validation of the InterTAK Diagnostic Score for Differentiating Takotsubo Syndrome from Acute Coronary Syndrome in a Middle Eastern Population. Journal of Clinical Medicine. 2025; 14(21):7806. https://doi.org/10.3390/jcm14217806
Chicago/Turabian StyleJamil, Gohar, Ali Al Shamisi, Fayez AlShamsi, and Adnan Agha. 2025. "Validation of the InterTAK Diagnostic Score for Differentiating Takotsubo Syndrome from Acute Coronary Syndrome in a Middle Eastern Population" Journal of Clinical Medicine 14, no. 21: 7806. https://doi.org/10.3390/jcm14217806
APA StyleJamil, G., Al Shamisi, A., AlShamsi, F., & Agha, A. (2025). Validation of the InterTAK Diagnostic Score for Differentiating Takotsubo Syndrome from Acute Coronary Syndrome in a Middle Eastern Population. Journal of Clinical Medicine, 14(21), 7806. https://doi.org/10.3390/jcm14217806






