The Impact of Non-Coding RNA on Inflammation and Airway Remodeling in Asthma Related to Obesity: State-of-the-Art and Therapeutic Perspectives
Abstract
1. Introduction
2. Classes of Non-Coding RNAs
3. Inflammation
4. Airway Remodeling
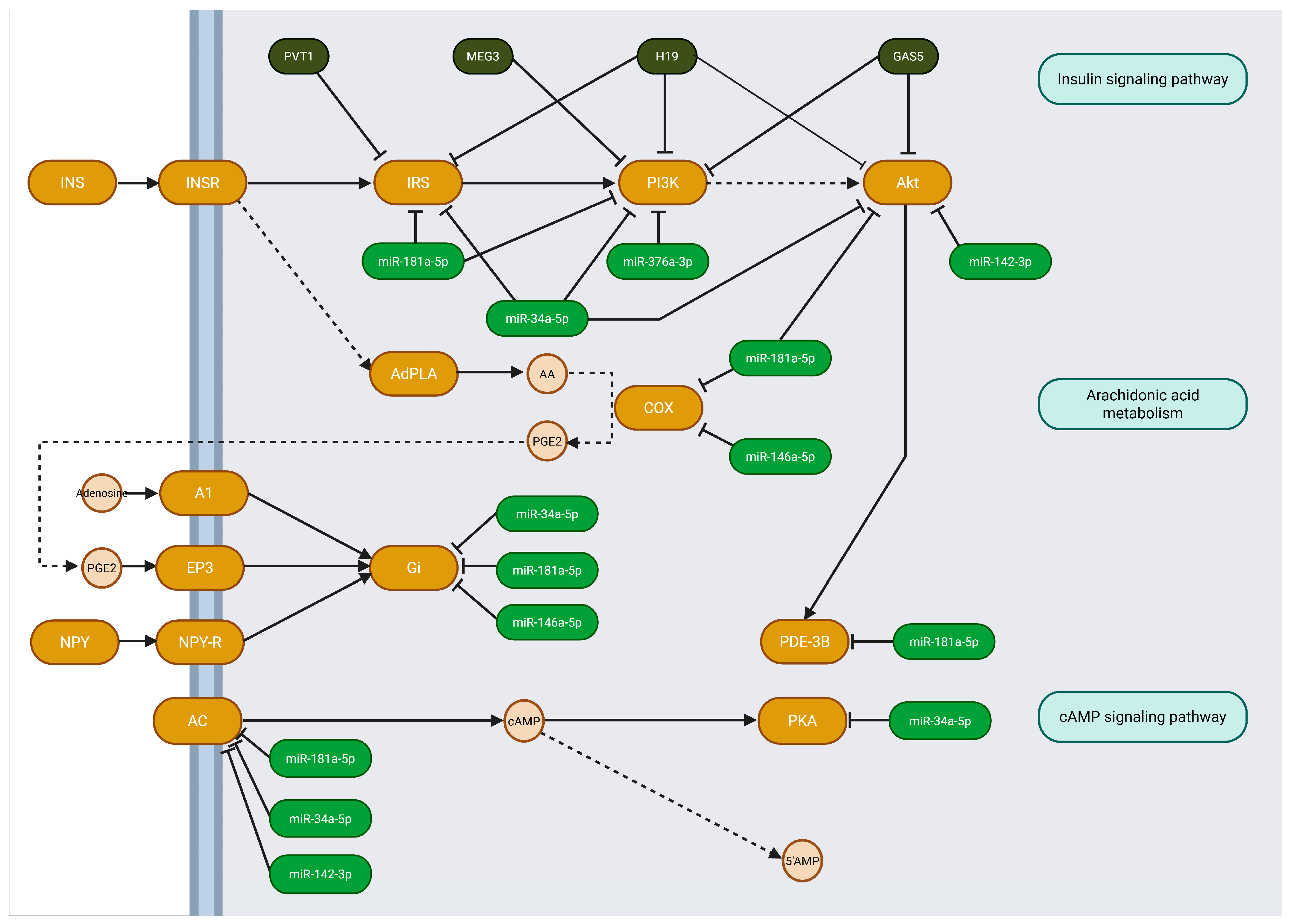
5. Identifying the Pathways of Selected ncRNA—In Silico Analysis
6. Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asthma. Available online: https://www.who.int/news-room/fact-sheets/detail/asthma (accessed on 2 January 2025).
- Yuan, L.; Tao, J.; Wang, J.; She, W.; Zou, Y.; Li, R.; Ma, Y.; Sun, C.; Bi, S.; Wei, S.; et al. Global, Regional, National Burden of Asthma from 1990 to 2021, with Projections of Incidence to 2050: A Systematic Analysis of the Global Burden of Disease Study 2021. EClinicalMedicine 2025, 80, 103051. [Google Scholar] [CrossRef]
- Ojo, R.O.; Okobi, O.E.; Ezeamii, P.C.; Ezeamii, V.C.; Nwachukwu, E.U.; Gebeyehu, Y.H.; Okobi, E.; David, A.B.; Akinsola, Z. Epidemiology of Current Asthma in Children Under 18: A Two-Decade Overview Using National Center for Health Statistics (NCHS) Data. Cureus 2023, 15, e49229. [Google Scholar] [CrossRef] [PubMed]
- Enilari, O.; Sinha, S. The Global Impact of Asthma in Adult Populations. Ann. Glob. Health 2019, 85, 2. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Li, H. T-Helper Cells and Their Cytokines in Pathogenesis and Treatment of Asthma. Front. Immunol. 2023, 14, 1149203. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, C.; Tulic, M.K.; Hamid, Q. Airway Remodelling in Asthma: From Benchside to Clinical Practice. Can. Respir. J. J. Can. Thorac. Soc. 2010, 17, e85. [Google Scholar] [CrossRef]
- Kim, Y.; Moonie, S.; Yoo, J.W.; Chung, T.-H. Class III Obesity as a Risk Factor for Persistent Asthma. Respir. Care 2024, 70, 100–107. [Google Scholar] [CrossRef]
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 11 August 2025).
- Farzan, S.; Coyle, T.; Coscia, G.; Rebaza, A.; Santiago, M. Clinical Characteristics and Management Strategies for Adult Obese Asthma Patients. J. Asthma Allergy 2022, 15, 673–689. [Google Scholar] [CrossRef]
- Scott, H.A.; Ng, S.H.M.; McLoughlin, R.F.; Valkenborghs, S.R.; Nair, P.; Brown, A.C.; Carroll, O.R.; Horvat, J.C.; Wood, L.G. Effect of Obesity on Airway and Systemic Inflammation in Adults with Asthma: A Systematic Review and Meta-Analysis. Thorax 2023, 78, 957–965. [Google Scholar] [CrossRef]
- Zhou, B.; Yang, H.; Yang, C.; Bao, Y.L.; Yang, S.M.; Liu, J.; Xiao, Y.F. Translation of Noncoding RNAs and Cancer. Cancer Lett. 2021, 497, 89–99. [Google Scholar] [CrossRef]
- Cable, J.; Heard, E.; Hirose, T.; Prasanth, K.V.; Chen, L.L.; Henninger, J.E.; Quinodoz, S.A.; Spector, D.L.; Diermeier, S.D.; Porman, A.M.; et al. Noncoding RNAs: Biology and Applications-a Keystone Symposia Report. Ann. N. Y. Acad. Sci. 2021, 1506, 118–141. [Google Scholar] [CrossRef]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An Overview of MicroRNAs: Biology, Functions, Therapeutics, and Analysis Methods. J. Cell Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Sengar, R.S. Biogenesis and Mechanisms of MicroRNA-Mediated Gene Regulation. Biotechnol. Bioeng. 2022, 119, 685–692. [Google Scholar] [CrossRef]
- Rupaimoole, R.; Slack, F.J. MicroRNA Therapeutics: Towards a New Era for the Management of Cancer and Other Diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef]
- Friedman, R.C.; Farh, K.K.H.; Burge, C.B.; Bartel, D.P. Most Mammalian MRNAs Are Conserved Targets of MicroRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene Regulation by Long Non-Coding RNAs and Its Biological Functions. Nat. Rev. Mol. Cell Biol. 2020, 22, 96. [Google Scholar] [CrossRef]
- Tsagakis, I.; Douka, K.; Birds, I.; Aspden, J.L. Long Non-Coding RNAs in Development and Disease: Conservation to Mechanisms. J. Pathol. 2020, 250, 480–495. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.Y.; Cai, Z.R.; Liu, J.; Wang, D.S.; Ju, H.Q.; Xu, R.H. Circular RNA: Metabolism, Functions and Interactions with Proteins. Mol. Cancer 2020, 19, 172. [Google Scholar] [CrossRef]
- Bantulà, M.; Roca-Ferrer, J.; Arismendi, E.; Picado, C. Asthma and Obesity: Two Diseases on the Rise and Bridged by Inflammation. J. Clin. Med. 2021, 10, 169. [Google Scholar] [CrossRef]
- Sideleva, O.; Suratt, B.T.; Black, K.E.; Tharp, W.G.; Pratley, R.E.; Forgione, P.; Dienz, O.; Irvin, C.G.; Dixon, A.E. Obesity and Asthma: An Inflammatory Disease of Adipose Tissue Not the Airway. Am. J. Respir. Crit. Care Med. 2012, 186, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Leija-Martínez, J.J.; Guzmán-Martín, C.A.; González-Ramírez, J.; Giacoman-Martínez, A.; Del-Río-Navarro, B.E.; Romero-Nava, R.; Villafaña, S.; Flores-Saenz, J.L.; Sánchez-Muñoz, F.; Huang, F. Whole Blood Expression Levels of Long Noncoding RNAs: HOTAIRM1, GAS5, MZF1-AS1, and OIP5-AS1 as Biomarkers in Adolescents with Obesity-Related Asthma. Int. J. Mol. Sci. 2023, 24, 6481. [Google Scholar] [CrossRef]
- Wang, Z.; Song, Y.; Jiang, J.; Piao, Y.; Li, L.; Bai, Q.; Xu, C.; Liu, H.; Li, L.; Piao, H.; et al. MicroRNA-182-5p Attenuates Asthmatic Airway Inflammation by Targeting NOX4. Front. Immunol. 2022, 13, 853848. [Google Scholar] [CrossRef]
- Pan, Y.; Hui, X.; Chong Hoo, R.L.; Ye, D.; Cheung Chan, C.Y.; Feng, T.; Wang, Y.; Ling Lam, K.S.; Xu, A. Adipocyte-Secreted Exosomal MicroRNA-34a Inhibits M2 Macrophage Polarization to Promote Obesity-Induced Adipose Inflammation. J. Clin. Invest. 2019, 129, 834–849. [Google Scholar] [CrossRef] [PubMed]
- Harman-Boehm, I.; Blüher, M.; Redel, H.; Sion-Vardy, N.; Ovadia, S.; Avinoach, E.; Shai, I.; Klöting, N.; Stumvoll, M.; Bashan, N.; et al. Macrophage Infiltration into Omental versus Subcutaneous Fat across Different Populations: Effect of Regional Adiposity and the Comorbidities of Obesity. J. Clin. Endocrinol. Metab. 2007, 92, 2240–2247. [Google Scholar] [CrossRef]
- Gil-Martínez, M.; Lorente-Sorolla, C.; Rodrigo-Muñoz, J.M.; Naharro, S.; García-de Castro, Z.; Sastre, J.; Valverde-Monge, M.; Quirce, S.; Caballero, M.L.; Olaguibel, J.M.; et al. Obese Asthma Phenotype Is Associated with Hsa-MiR-26a-1-3p and Hsa-MiR-376a-3p Modulating the IGF Axis. Int. J. Mol. Sci. 2023, 24, 11620. [Google Scholar] [CrossRef]
- Zarrati, M.; Salehi, E.; Razmpoosh, E.; Shoormasti, R.S.; Hosseinzadeh-attar, M.J.; Shidfar, F. Relationship between Leptin Concentration and Body Fat with Peripheral Blood Mononuclear Cells Cytokines among Obese and Overweight Adults. Ir. J. Med. Sci. 2017, 186, 133–142. [Google Scholar] [CrossRef]
- Qiu, Y.Y.; Wu, Y.; Lin, M.J.; Bian, T.; Xiao, Y.L.; Qin, C. LncRNA-MEG3 Functions as a Competing Endogenous RNA to Regulate Treg/Th17 Balance in Patients with Asthma by Targeting MicroRNA-17/ RORγt. Biomed. Pharmacother. 2019, 111, 386–394. [Google Scholar] [CrossRef]
- Lischka, J.; Schanzer, A.; Hojreh, A.; Ba-Ssalamah, A.; de Gier, C.; Valent, I.; Item, C.B.; Greber-Platzer, S.; Zeyda, M. Circulating MicroRNAs 34a, 122, and 192 Are Linked to Obesity-Associated Inflammation and Metabolic Disease in Pediatric Patients. Int. J. Obes. 2021, 45, 1763–1772. [Google Scholar] [CrossRef]
- Han, Y.B.; Tian, M.; Wang, R.N.; Guo, D.H.; Zhang, D.D.; Liu, L. LncRNA SNHG14/MiR-497a-5p/BACE1 Axis Modulates Obesity-Induced Adipocyte Inflammation and Endoplasmic Reticulum Stress. J. Biochem. Mol. Toxicol. 2023, 37, e23343. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, M.; Song, J.; Zeng, Y.; Xia, S.; Chen, C.; Jin, M.; Song, Y. The Circular RNA CircTXNRD1 Promoted Ambient Particulate Matter-Induced Inflammation in Human Bronchial Epithelial Cells by Regulating MiR-892a/COX-2 Axis. Chemosphere 2022, 286, 131614. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Y.; Gu, H.; Zhang, J.; Tang, H.; Rong, Q.; Gu, L.; Pan, J.; Zhao, D.; Liu, F. LncRNA-AK149641 Associated with Airway Inflammation in an OVA-Induced Asthma Mouse Model. J. Bioenerg. Biomembr. 2020, 52, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Simpson, L.J.; Patel, S.; Bhakta, N.R.; Choy, D.F.; Brightbill, H.D.; Ren, X.; Wang, Y.; Pua, H.H.; Baumjohann, D.; Montoya, M.M.; et al. A MicroRNA Upregulated in Asthma Airway T Cells Promotes TH2 Cytokine Production. Nat. Immunol. 2014, 15, 1162–1170. [Google Scholar] [CrossRef]
- Maes, T.; Cobos, F.A.; Schleich, F.; Sorbello, V.; Henket, M.; De Preter, K.; Bracke, K.R.; Conickx, G.; Mesnil, C.; Vandesompele, J.; et al. Asthma Inflammatory Phenotypes Show Differential MicroRNA Expression in Sputum. J. Allergy Clin. Immunol. 2016, 137, 1433–1446. [Google Scholar] [CrossRef]
- Chung, S.; Lee, Y.G.; Karpurapu, M.; Englert, J.A.; Ballinger, M.N.; Davis, I.C.; Park, G.Y.; Christman, J.W. Depletion of MicroRNA-451 in Response to Allergen Exposure Accentuates Asthmatic Inflammation by Regulating Sirtuin2. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 318, L921–L930. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, Q.; Hao, J.; Wang, J.; Wang, C. LncRNA PVT1 Exacerbates the Inflammation and Cell-Barrier Injury during Asthma by Regulating MiR-149. J. Biochem. Mol. Toxicol. 2020, 34, e22563. [Google Scholar] [CrossRef]
- Chen, Y.; He, S.D.; Li, X.D.; Hu, Z.L.; Zhang, C.; Xu, F. Long Noncoding RNA Atlas of the Inflammation Caused by Asthma in Mice. Arch. Pharm. Res. 2020, 43, 421–432. [Google Scholar] [CrossRef]
- Yuan, Y.; He, Y.; Wasti, B.; Duan, W.; Jia, J.; Chen, Z.; Xiang, X.; Zeng, Q. LncRNA CRNDE Affects Th17/IL-17A and Inhibits Epithelial-Mesenchymal Transition in Lung Epithelial Cells Reducing Asthma Signs. Oxid. Med. Cell. Longev. 2023, 2023, 2092184. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; He, S.; Yang, X.; Chen, X.; Zhao, S.; Wang, J. Circular RNA DHTKD1 Targets MiR-338-3p/ETS1 Axis to Regulate the Inflammatory Response in Human Bronchial Epithelial Cells. Exp. Ther. Med. 2023, 26, 316. [Google Scholar] [CrossRef]
- Xia, L.; Wang, X.; Liu, L.; Fu, J.; Xiao, W.; Liang, Q.; Han, X.; Huang, S.; Sun, L.; Gao, Y.; et al. Lnc-BAZ2B Promotes M2 Macrophage Activation and Inflammation in Children with Asthma through Stabilizing BAZ2B Pre-MRNA. J. Allergy Clin. Immunol. 2021, 147, 921–932.e9. [Google Scholar] [CrossRef] [PubMed]
- Pei, W.; Zhang, Y.; Li, X.; Luo, M.; Chen, T.; Zhang, M.; Zhong, M.; Lv, K. LncRNA AK085865 Depletion Ameliorates Asthmatic Airway Inflammation by Modulating Macrophage Polarization. Int. Immunopharmacol. 2020, 83, 106450. [Google Scholar] [CrossRef]
- Li, Q.; Lu, L.; Li, X.; Lu, S. Long Non-Coding RNA NKILA Alleviates Airway Inflammation in Asthmatic Mice by Promoting M2 Macrophage Polarization and Inhibiting the NF-ΚB Pathway. Biochem. Biophys. Res. Commun. 2021, 571, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Karkeni, E.; Astier, J.; Tourniaire, F.; El Abed, M.; Romier, B.; Gouranton, E.; Wan, L.; Borel, P.; Salles, J.; Walrand, S.; et al. Obesity-Associated Inflammation Induces MicroRNA-155 Expression in Adipocytes and Adipose Tissue: Outcome on Adipocyte Function. J. Clin. Endocrinol. Metab. 2016, 101, 1615–1626. [Google Scholar] [CrossRef]
- Miranda, K.; Mehrpouya-Bahrami, P.; Nagarkatti, P.S.; Nagarkatti, M. Cannabinoid Receptor 1 Blockade Attenuates Obesity and Adipose Tissue Type 1 Inflammation Through MiR-30e-5p Regulation of Delta-Like-4 in Macrophages and Consequently Downregulation of Th1 Cells. Front. Immunol. 2019, 10, 1049. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, M.; Ma, Y.; Peng, Y. LncRNA TUG1 Reduces Inflammation and Enhances Insulin Sensitivity in White Adipose Tissue by Regulating MiR-204/SIRT1 Axis in Obesity Mice. Mol. Cell Biochem. 2020, 475, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, H.; Cao, K.; Zhai, Z.; Wang, Z.; Yang, L.; Han, J. Transcriptome of Visceral Adipose Tissue Identifies an Inflammation-Related CeRNA Network That Regulates Obesity. Mol. Cell Biochem. 2022, 477, 1095–1106. [Google Scholar] [CrossRef]
- Patra, D.; Roy, S.; Arora, L.; Kabeer, S.W.; Singh, S.; Dey, U.; Banerjee, D.; Sinha, A.; Dasgupta, S.; Tikoo, K.; et al. MiR-210-3p Promotes Obesity-Induced Adipose Tissue Inflammation and Insulin Resistance by Targeting SOCS1-Mediated NF-ΚB Pathway. Diabetes 2023, 72, 375–388. [Google Scholar] [CrossRef]
- Bestepe, F.; Pal-Ghosh, R.; Fritsche, C.; Lakhotiya, K.; Smolgovsky, S.; Weston, J.; Stepanian, A.; Alvarado, F.; Catalano, P.; O’tierney-Ginn, P.F.; et al. 354-OR: Attenuation of Obesity-Induced Vascular Inflammation by MicroRNA-485 in Endothelial Cells. Diabetes 2023, 72, 354. [Google Scholar] [CrossRef]
- Daneshmoghadam, J.; Omidifar, A.; Akbari Dilmaghani, N.; Karimi, Z.; Emamgholipour, S.; Shanaki, M. The Gene Expression of Long Non-Coding RNAs (LncRNAs): MEG3 and H19 in Adipose Tissues from Obese Women and Its Association with Insulin Resistance and Obesity Indices. J. Clin. Lab. Anal. 2021, 35, e23741. [Google Scholar] [CrossRef]
- Tanwar, V.S.; Reddy, M.A.; Das, S.; Samara, V.A.; Abdollahi, M.; Dey, S.; Malek, V.; Ganguly, R.; Stapleton, K.; Lanting, L.; et al. Palmitic Acid-Induced Long Noncoding RNA PARAIL Regulates Inflammation via Interaction With RNA-Binding Protein ELAVL1 in Monocytes and Macrophages. Arter. Thromb. Vasc. Biol. 2023, 43, 1157–1175. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Wang, Y.; Liu, F.; Fan, X.; Shi, C.; Su, X.; Tan, M.; Yang, Y.; Lin, B.; et al. LncRNA LINK-A Remodels Tissue Inflammatory Microenvironments to Promote Obesity. Adv. Sci. (Weinh) 2024, 11, 2303341. [Google Scholar] [CrossRef] [PubMed]
- Mirra, D.; Cione, E.; Spaziano, G.; Esposito, R.; Sorgenti, M.; Granato, E.; Cerqua, I.; Muraca, L.; Iovino, P.; Gallelli, L.; et al. Circulating MicroRNAs Expression Profile in Lung Inflammation: A Preliminary Study. J. Clin. Med. 2022, 11, 5446. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Sun, Q.; Roth, M. Immunologic and Non-Immunologic Mechanisms Leading to Airway Remodeling in Asthma. Int. J. Mol. Sci. 2020, 21, 757. [Google Scholar] [CrossRef]
- Ritchie, A.I.; Jackson, D.J.; Edwards, M.R.; Johnston, S.L. Airway Epithelial Orchestration of Innate Immune Function in Response to Virus Infection. A Focus on Asthma. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. S1), S55–S63. [Google Scholar] [CrossRef]
- Prakash, Y.S. Emerging Concepts in Smooth Muscle Contributions to Airway Structure and Function: Implications for Health and Disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 311, L1113–L1140. [Google Scholar] [CrossRef]
- McDonald, D.M. Angiogenesis and Remodeling of Airway Vasculature in Chronic Inflammation. Am. J. Respir. Crit. Care Med. 2001, 164, S39–S45. [Google Scholar] [CrossRef] [PubMed]
- Guida, G.; Riccio, A.M. Immune Induction of Airway Remodeling. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2019; Volume 46. [Google Scholar] [CrossRef]
- Pillai, P.; Corrigan, C.J.; Ying, S. Airway Epithelium in Atopic and Nonatopic Asthma: Similarities and Differences. ISRN Allergy 2011, 2011, 195846. [Google Scholar] [CrossRef] [PubMed]
- Shahana, S.; Björnsson, E.; Lúdvíksdóttir, D.; Janson, C.; Nettelbladt, O.; Venge, P.; Roomans, G.M.; Amin, K.; Ahlander, A.; Boman, G.; et al. Ultrastructure of Bronchial Biopsies from Patients with Allergic and Non-Allergic Asthma. Respir. Med. 2005, 99, 429–443. [Google Scholar] [CrossRef]
- Shailesh, H.; Bhat, A.A.; Janahi, I.A. Obesity-Associated Non-T2 Mechanisms in Obese Asthmatic Individuals. Biomedicines 2023, 11, 2797. [Google Scholar] [CrossRef]
- Shailesh, H.; Janahi, I.A. Role of Obesity in Inflammation and Remodeling of Asthmatic Airway. Life 2022, 12, 948. [Google Scholar] [CrossRef] [PubMed]
- Palma, G.; Sorice, G.P.; Genchi, V.A.; Giordano, F.; Caccioppoli, C.; D’oria, R.; Marrano, N.; Biondi, G.; Giorgino, F.; Perrini, S. Adipose Tissue Inflammation and Pulmonary Dysfunction in Obesity. Int. J. Mol. Sci. 2022, 23, 7349. [Google Scholar] [CrossRef]
- Holgate, S.T.; Wenzel, S.; Postma, D.S.; Weiss, S.T.; Renz, H.; Sly, P.D. Asthma. Nat. Rev. Dis. Primers 2015, 1, 15025. [Google Scholar] [CrossRef]
- Yang, Z.C.; Qu, Z.H.; Yi, M.J.; Shan, Y.C.; Ran, N.; Xu, L.; Liu, X.J. MiR-448-5p Inhibits TGF-Β1-Induced Epithelial-Mesenchymal Transition and Pulmonary Fibrosis by Targeting Six1 in Asthma. J. Cell Physiol. 2019, 234, 8804–8814. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, Q.; Shang, Y. MiRNA-451a Inhibits Airway Remodeling by Targeting Cadherin 11 in an Allergic Asthma Model of Neonatal Mice. Int. Immunopharmacol. 2020, 83, 106440. [Google Scholar] [CrossRef] [PubMed]
- Boulet, L.P. Airway Remodeling in Asthma: Update on Mechanisms and Therapeutic Approaches. Curr. Opin. Pulm. Med. 2018, 24, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Prakash, Y.S. Airway Smooth Muscle in Airway Reactivity and Remodeling: What Have We Learned? Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L912–L933. [Google Scholar] [CrossRef]
- Huang, J.; Wang, F.H.; Wang, L.; Li, Y.; Lu, J.; Chen, J.Y. LncRNA MALAT1 Promotes Proliferation and Migration of Airway Smooth Muscle Cells in Asthma by Downregulating MicroRNA-216a. Saudi J. Biol. Sci. 2021, 28, 4124–4131. [Google Scholar] [CrossRef]
- Huang, W.; Yu, C.; Liang, S.; Wu, H.; Zhou, Z.; Liu, A.; Cai, S. Long Non-Coding RNA TUG1 Promotes Airway Remodeling and Mucus Production in Asthmatic Mice through the MicroRNA-181b/HMGB1 Axis. Int. Immunopharmacol. 2021, 94, 107488. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Chen, M.; Ouyang, L.; Wang, Q.; Guo, Y.; Huang, L.; Jiang, S. MiR-142-5p and MiR-130a-3p Regulate Pulmonary Macrophage Polarization and Asthma Airway Remodeling. Immunol. Cell Biol. 2020, 98, 715–725. [Google Scholar] [CrossRef]
- Lin, J.; Feng, X.; Zhang, J. Circular RNA CircHIPK3 Modulates the Proliferation of Airway Smooth Muscle Cells by MiR-326/STIM1 Axis. Life Sci. 2020, 255, 117835. [Google Scholar] [CrossRef]
- Huang, J.Q.; Wang, F.; Wang, L.T.; Li, Y.M.; Lu, J.L.; Chen, J.Y. Circular RNA ERBB2 Contributes to Proliferation and Migration of Airway Smooth Muscle Cells via MiR-98-5p/IGF1R Signaling in Asthma. J. Asthma Allergy 2021, 14, 1197–1207. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X. Long Noncoding RNA Antisense Noncoding RNA in the INK4 Locus Inhibition Alleviates Airway Remodeling in Asthma through the Regulation of the MicroRNA-7-5p/Early Growth Response Factor 3 Axis. Immun. Inflamm. Dis. 2023, 11, e823. [Google Scholar] [CrossRef]
- Austin, P.J.; Tsitsiou, E.; Boardman, C.; Jones, S.W.; Lindsay, M.A.; Adcock, I.M.; Chung, K.F.; Perry, M.M. Transcriptional Profiling Identifies the Long Noncoding RNA Plasmacytoma Variant Translocation (PVT1) as a Novel Regulator of the Asthmatic Phenotype in Human Airway Smooth Muscle. J. Allergy Clin. Immunol. 2017, 139, 780–789. [Google Scholar] [CrossRef]
- Bartel, S.; Carraro, G.; Alessandrini, F.; Krauss-Etschmann, S.; Ricciardolo, F.L.M.; Bellusci, S. MiR-142-3p Is Associated with Aberrant WNT Signaling during Airway Remodeling in Asthma. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 315, L328–L333. [Google Scholar] [CrossRef]
- Lou, L.; Tian, M.; Chang, J.; Li, F.; Zhang, G. MiRNA-192-5p Attenuates Airway Remodeling and Autophagy in Asthma by Targeting MMP-16 and ATG7. Biomed. Pharmacother. 2020, 122, 109692. [Google Scholar] [CrossRef]
- Quan, L.; Ren, G.; Liu, L.; Huang, W.; Li, M. Circular RNA Circ_0002594 Regulates PDGF-BB-Induced Proliferation and Migration of Human Airway Smooth Muscle Cells via Sponging MiR-139-5p/TRIM8 in Asthma. Autoimmunity 2022, 55, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Circ_0000029 Interacts with the MiR-576-5p/KCNA1 Axis to Hamper the Development of Pediatric Asthma in an Asthma-Like in Vitro Assessment. Available online: http://www.annclinlabsci.org/content/53/2/200.abstract (accessed on 31 December 2024).
- Chen, D.; Wu, W.; Yi, L.; Feng, Y.; Chang, C.; Chen, S.; Gao, J.; Chen, G.; Zhen, G. A Potential CircRNA-MiRNA-MRNA Regulatory Network in Asthmatic Airway Epithelial Cells Identified by Integrated Analysis of Microarray Datasets. Front. Mol. Biosci. 2021, 8, 703307. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and Integrative Analysis of Large Gene Lists Using DAVID Bioinformatics Resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Alhamwe, B.A.; Potaczek, D.P.; Miethe, S.; Alhamdan, F.; Hintz, L.; Magomedov, A.; Garn, H. Extracellular Vesicles and Asthma-More Than Just a Co-Existence. Int. J. Mol. Sci. 2021, 22, 4984. [Google Scholar] [CrossRef]
- Cañas, J.A.; Rodrigo-Muñoz, J.M.; Gil-Martínez, M.; Sastre, B.; Del Pozo, V. Exosomes: A Key Piece in Asthmatic Inflammation. Int. J. Mol. Sci. 2021, 22, 963. [Google Scholar] [CrossRef]
- Srinivasan, A.; Sundar, I.K. Recent Updates on the Role of Extracellular Vesicles in the Pathogenesis of Allergic Asthma. Extracell. Vesicles Circ. Nucl. Acids 2021, 2, 127–147. [Google Scholar] [CrossRef]
- Pua, H.H.; Happ, H.C.; Gray, C.J.; Mar, D.J.; Chiou, N.T.; Hesse, L.E.; Ansel, K.M. Increased Hematopoietic Extracellular RNAs and Vesicles in the Lung during Allergic Airway Responses. Cell Rep. 2019, 26, 933–944.e4. [Google Scholar] [CrossRef]
- Bartel, S.; La Grutta, S.; Cilluffo, G.; Perconti, G.; Bongiovanni, A.; Giallongo, A.; Behrends, J.; Kruppa, J.; Hermann, S.; Chiang, D.; et al. Human Airway Epithelial Extracellular Vesicle MiRNA Signature Is Altered upon Asthma Development. Allergy 2020, 75, 346–356. [Google Scholar] [CrossRef]
- Zhao, M.; Juanjuan, L.; Weijia, F.; Jing, X.; Qiuhua, H.; Hua, Z.; Fuhe, L.; Hao, P. Expression Levels of MicroRNA-125b in Serum Exosomes of Patients with Asthma of Different Severity and Its Diagnostic Significance. Curr. Drug Metab. 2019, 20, 781–784. [Google Scholar] [CrossRef]
- Zhao, M.; Li, Y.-P.; Geng, X.-R.; Zhao, M.; Ma, S.-B.; Yang, Y.-H.; Deng, Z.-H.; Luo, L.-M.; Pan, X.-Q. Expression Level of MiRNA-126 in Serum Exosomes of Allergic Asthma Patients and Lung Tissues of Asthmatic Mice. Curr. Drug Metab. 2019, 20, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo-Muñoz, J.M.; Cañas, J.A.; Sastre, B.; Rego, N.; Greif, G.; Rial, M.; Mínguez, P.; Mahíllo-Fernández, I.; Fernández-Nieto, M.; Mora, I.; et al. Asthma Diagnosis Using Integrated Analysis of Eosinophil MicroRNAs. Allergy 2019, 74, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Bahmer, T.; Krauss-Etschmann, S.; Buschmann, D.; Behrends, J.; Watz, H.; Kirsten, A.M.; Pedersen, F.; Waschki, B.; Fuchs, O.; Pfaffl, M.W.; et al. RNA-Seq-Based Profiling of Extracellular Vesicles in Plasma Reveals a Potential Role of MiR-122-5p in Asthma. Allergy 2021, 76, 366–371. [Google Scholar] [CrossRef] [PubMed]
| Disease | miRNA | lncRNA | circRNA | References | |||
|---|---|---|---|---|---|---|---|
| Up | Down | Up | Down | Up | Down | ||
| Asthma | let-7e-5p | miR-135b-5p | AK085865 | 4930597A21Rik | circDHTKD1 | - | miRNA: |
| miR-142-3p | miR-149 | AK149641 | Gm13372 | circTXNRD1 | [23,28,31,33,34,35,36,37,38,39] | ||
| miR-19a | miR-17 | CRNDE | Gm17501 | LncRNA: | |||
| miR-223-3p | miR-182-5p | Gm11529 | H19 | [28,32,36,37,38,40,41,42] | |||
| miR-629-3p | miR-29a-3p | lnc-BAZ2B | PVT1 | CircRNA: | |||
| miR-338-3p | MEG3 | [31,39] | |||||
| miR-451 | NKILA | ||||||
| miR-892a | |||||||
| Obesity | miR-122 | miR-197-5p | LINK-A | H19 | - | circNTRK2 | miRNA: |
| miR-155 | miR-497a-5p | MEG3 | PARAIL | circORC5 | [24,29,30,43,44,45,46,47,48] | ||
| miR-192 | miR-760 | SNHG14 | TUG1 | LncRNA: | |||
| miR-204 | [30,45,49,50,51] | ||||||
| miR-210-3p | CircRNA: [46] | ||||||
| miR-30e-5p | |||||||
| miR-34a | |||||||
| miR-34a | |||||||
| miR-485-5p | |||||||
| miR-378a-3p | |||||||
| Obesity-related Asthma | miR-26a-1-3p | miR-146a-5p | GAS5 | OIP5-AS1 | - | - | miRNA: |
| miR-376a-3p | miR-181a-5p | HOTAIRM1 | [26,52] | ||||
| miR-34a-5p | MZF1-AS1 | LncRNA: | |||||
| [22] | |||||||
| Disease | miRNA | lncRNA | circRNA | References | |||
|---|---|---|---|---|---|---|---|
| Up | Down | Up | Down | Up | Down | ||
| Asthma | miR-142-3p | miR-145 | PVT1 | - | circHIPK3 | circ_000002 | miRNA |
| miR-142-5p | miR-448-5p | MALAT1 | circERBB2 | ||||
| miR-576-5p | miR-192-5p | TUG1 | circ_000259 | [64,65,68,69,70,73,75,76,77,78] | |||
| miR-130a-3p | ANRIL | ||||||
| miR-451a | lncRNA: | ||||||
| miR-216a | |||||||
| miR-98-5p | [68,69,73,74] | ||||||
| miR-181b | CircRNA: | ||||||
| miR-139-5p | [71,72,77,78,79] | ||||||
| miR-7-5p | |||||||
| Pathway | Genes | Fold Enrichment | FDR |
|---|---|---|---|
| SHC-related events triggered by IGF1R | NRAS, IGF2, GRB2, KRAS, IGF1, SOS1, HRAS, IGF1R | 4.0133 | 0.0041 |
| Regulation of cellular response to insulin stimulus | NCOA1, NCOA2, BGLAP, CUL3, USO1, KBTBD2, PPARG, ATP2B1 | 3.7422 | 0.0337 |
| Positive regulation of fat cell differentiation | CDS1, CEBPB, CREBL2, HTR2A, STK4, MEDAG, ZFP36L1, WIF1, AKT1, LMO1, XBP1, LMO3, ZBTB16, SIRT6, TMEM64, AXIN2, BMP2, SFRP1, CREB1, KLF5, CARM1, ID2, ASXL2, SNAI2, PPARG, WDFY2 | 2.1718 | 0.0041 |
| IGF1R signaling cascade | KLB, IRS1, PDE3B, IRS2, PIK3R2, PIK3CB, PIK3R1, FGF1, FGF2, IGF1R, FGF7, NRAS, THEM4, HRAS, PDPK1, IGF2, GAB1, FRS2, PTPN11, IGF1, PIK3CA, GRB2, KRAS, SOS1, FGF10 | 2.0066 | 0.0039 |
| Signaling by Type 1 Insulin-like Growth Factor 1 Receptor (IGF1R) | KLB, IRS1, PDE3B, IRS2, PIK3R2, PIK3CB, PIK3R1, FGF1, FGF2, IGF1R, FGF7, NRAS, THEM4, HRAS, PDPK1, IGF2, GAB1, FRS2, PTPN11, IGF1, PIK3CA, GRB2, KRAS, SOS1, FGF10 | 1.9673 | 0.0051 |
| Insulin receptor signalling cascade | KLB, IRS1, PDE3B, IRS2, PIK3R2, PIK3CB, PIK3R1, FGF1, FGF2, FGF7, NRAS, GRB10, THEM4, MAPK1, HRAS, MAPK3, PDPK1, GAB1, FRS2, PTPN11, PIK3CA, GRB2, KRAS, SOS1, FGF10 | 1.9295 | 0.0069 |
| Negative regulation of fat cell differentiation | YAP1, SMAD2, WWTR1, TGFB1, JAG1, SMAD3, SORT1, WNT3A, ADIPOQ, RORA, SIRT1, TNF, FOXO1, ZFP36L2, VEGFA, BMP2, IL6, DDIT3, ID4, E2F1, ENPP1, WNT1, BMAL1, TRIB2 | 1.9028 | 0.0387 |
| Response to glucose | PFKFB2, CDKN1B, ILDR2, IRS2, RASAL2, ELAVL1, THBS1, ACVR1C, SGCB, CASP3, HNF4A, SESN2, GLUL, ZBED3, SREBF1, TCF7L2, EGR1, PRKCB, ADIPOQ, ACVR2B, RPS6KB1, TXNIP, COL6A3, SELENOT, VAMP2, SIDT2 | 1.9003 | 0.0274 |
| Fat-cell differentiation | RNASEL, STEAP4, CBY1, FITM2, FOXO1, C1QTNF3, BCL2L13, CCND1, SOX8, TBL1X, WNT1, ZBTB7A, SREBF1, OSBPL8, TCF7L2, EGR2, NEGR1, WNT3A, HMGA2, ARID5B, INHBB, SENP2, KLF4, PIAS1, NR4A2, CLIP3, NR4A3, ID4, CNTN2, PLCB1, ATF5, TRIM32 | 1.6819 | 0.0505 |
| Regulation of lipolysis in adipocytes | IRS1, PDE3B, GNAI3, PIK3R3, ADCY2, PIK3R2, IRS2, ADCY1, PIK3CB, PIK3R1, PTGS2, GNAI1, TSHR, ADCY5, GNAI2, ADCY9, PIK3CA, AKT3, GNAS, AKT1, PRKACB, MGLL, PRKG1 | 1.5495 | 0.0351 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kachel, M.; Langwiński, W.; Szczepankiewicz, A. The Impact of Non-Coding RNA on Inflammation and Airway Remodeling in Asthma Related to Obesity: State-of-the-Art and Therapeutic Perspectives. J. Clin. Med. 2025, 14, 7161. https://doi.org/10.3390/jcm14207161
Kachel M, Langwiński W, Szczepankiewicz A. The Impact of Non-Coding RNA on Inflammation and Airway Remodeling in Asthma Related to Obesity: State-of-the-Art and Therapeutic Perspectives. Journal of Clinical Medicine. 2025; 14(20):7161. https://doi.org/10.3390/jcm14207161
Chicago/Turabian StyleKachel, Maria, Wojciech Langwiński, and Aleksandra Szczepankiewicz. 2025. "The Impact of Non-Coding RNA on Inflammation and Airway Remodeling in Asthma Related to Obesity: State-of-the-Art and Therapeutic Perspectives" Journal of Clinical Medicine 14, no. 20: 7161. https://doi.org/10.3390/jcm14207161
APA StyleKachel, M., Langwiński, W., & Szczepankiewicz, A. (2025). The Impact of Non-Coding RNA on Inflammation and Airway Remodeling in Asthma Related to Obesity: State-of-the-Art and Therapeutic Perspectives. Journal of Clinical Medicine, 14(20), 7161. https://doi.org/10.3390/jcm14207161







