Abstract
Background: New-onset postoperative atrial fibrillation (POAF) is the most common complication after cardiac surgery, occurring approximately in one-third of the patients. This study considered all-comer patients who underwent cardiac surgery to build a predictive model for POAF. Methods: A total of 3467 (Center 1) consecutive patients were used as a derivation cohort to build the model. The POLARIS score was then derived proportionally from the odds ratios obtained following multivariable logistic regression (MLR). The Brier Score, the area under the receiver operating characteristic curve, and the Hosmer–Lemeshow goodness-of-fit test were used to validate the model. Then, 2272 (Center 2) consecutive patients were used as an external validation cohort. Results: In the overall population (n = 5739), POAF occurred in 32.7% of patients. MLR performed in the derivation cohort showed that age, obesity, chronic renal failure, pulmonary hypertension, minimally invasive surgery, and aortic and mitral valve surgery were predictors of POAF. The derived POLARIS score was used to further stratify the population into four risk clusters: low (1.5–3), intermediate (3.5–5), high (5.5–7), and very high (7.5–9), each progressively showing an increase in POAF incidence. This was confirmed in a correlation analysis (Spearman’s rho: 0.636). Conclusions: The POLARIS score is a simple-to-use tool to stratify patients at higher risk of POAF. Precise identification of such patients might be used to implement clinical practice with the introduction of preoperative antiarrhythmic prophylaxis, further reducing the incidence of POAF and, potentially, its clinical sequelae, despite further investigations being warranted to test this model in prospective studies.
1. Introduction
New-onset postoperative atrial fibrillation (POAF) is the most common complication after cardiac surgery, occurring in approximately one-third of patients, but it can peak at 40–50% after valve-related surgery. POAF is variably associated with increased in-hospital morbidity and mortality, as well as prolonged length of hospital stay and excess hospitalization costs [1,2,3,4]. Atrial structural alterations, pericardial effusion and inflammation, peri-atrial adipose tissue metabolic activity, autonomic neuromodulation, and re-entry and ectopic activity in the pulmonary veins area were identified among the main pathophysiological mechanisms underlying POAF [4]. Efforts have been made to build risk models identifying specific groups of patients at high risk of developing POAF [5,6,7]. However, most of these attempts have been performed in a selected population, thus excluding, for example, patients undergoing complex cardiac procedures. Previous risk models for POAF in cardiac surgery were often based on small cohorts or focused exclusively on CABG patients, excluding high-risk valve surgery populations. Even larger cohort studies incorporated postoperative variables, limiting the ability to predict POAF preoperatively and apply prophylactic treatments, particularly in urgent or emergent cases. Therefore, the present study considered all-comer patients who underwent cardiac surgery in order to determine risk factors implied in the development of POAF. We then set up an additive risk calculator for POstoperative atriaL fibrillAtion in caRdIac Surgery (POLARIS Score) according to the derived odds ratios and performed an external validation to test our hypothesis.
2. Materials and Methods
2.1. Study Population
Data from adult patients undergoing cardiac surgery with no history of preoperative atrial tachyarrhythmia were retrospectively retrieved from institutional databases at two different European cardiac centers (ASST Spedali Civili di Brescia, University of Brescia, Italy [Center 1] and Centro Cardiologico Monzino IRCCS, Milano, Italy [Center 2]). We did include all patients who underwent elective, urgent, and emergent procedures, as well as complex procedures (more than 2 or 3 procedures/patient). All data, including demographics and clinical and surgical details, have been prospectively recorded and collected at the time of the hospitalization.
The study protocol was approved by the Ethics Committee at Spedali Civili di Brescia, Brescia, Italy, with number NP4980 (15 October 2021) and waived for patient informed consent. This study was reported according to the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) guidelines [8] (Supplementary Materials). The data that support the findings of this study are available from the corresponding author upon reasonable request.
2.2. Endpoint and Definitions
The primary endpoints of the current study were the incidence and the definition of independent predictors for postoperative AF through regression analysis. The secondary endpoint was to build and test a predictive model of POAF in patients undergoing cardiac surgery.
POAF was defined by the documentation of AF of any duration at any point in the perioperative period before discharge on a rhythm strip or 12-lead electrocardiogram. Body mass index (BMI) was calculated using the formula BMI = weight (kg)/height2 (m2). Obesity was considered above a BMI of 30 kg/m2. The estimated glomerular filtration rate (eGFR) was calculated with the use of the Chronic Kidney Disease Epidemiology Collaboration equation.
2.3. Atrial Fibrillation Pharmacological Management
Patients considered in this study were in sinus rhythm without a history of AF and were not in class I or III antiarrhythmic drug (AAD) regimens, while 45.0% (2581/5739) of patients were in class II (b-blockers) or IV (Ca-antagonists) preoperative antiarrhythmic therapy. This regimen was continued until the day of surgery and then routinely restarted at the same, lower, or increased doses according to the patients’ hemodynamics. During the whole hospitalization period, patients were monitored daily until discharge with ECG telemetry and standard 12-lead ECG in case of clinical suspicion of AF. Unless contraindicated, intravenous/oral amiodarone was the first line of therapy in patients experiencing POAF. In case AAD therapy failed to restore sinus rhythm, patients were discharged with oral amiodarone. The anticoagulation regimen was achieved with warfarin, aiming to target an INR between 2 and 3, and continued for at least 1 month. In case of hemodynamic instability due to the POAF, patients underwent electrical cardioversion. Following 30 days from discharge, patients underwent clinical follow-up, and in case of documentation of sinus rhythm restoration, amiodarone and warfarin were discontinued unless indicated by the index surgical procedure.
2.4. Statistical Analysis
Clinical data were prospectively recorded on Microsoft Excel (Microsoft Corp., Redmond, WA, USA). Statistical analysis was performed using R (R Project for Statistical Computing, Wien, Austria) version 4.2.1, using the “PredictABEL” package version 1.2-4.
Categorical variables were presented as frequency counts and percentages and compared between groups using the Chi-squared test or Fisher exact test, accordingly. After checking the normality of continuous variables with the Kolmogorov–Smirnov test, they were presented as mean and standard deviation if normally distributed and were compared between groups using the Student’s t-test. If they were not normally distributed, median and interquartile ranges were presented and compared between groups using the Mann–Whitney U test. All tests were two-sided, and the alpha level was set at 0.05 for statistical significance.
For the model building, data from Center 1 were used as a derivation dataset, while data from Center 2 were used as external validation. Univariable logistic regression was used for initial variable selection. Only variables with a p-value < 0.1, with clinical significance, and that avoided multicollinearity (e.g., creatinine, eGFR, and CKD) were included in the regression models to avoid overfitting. Multivariable analysis was performed and further refined through stepwise logistic regression with forward and backward selection. Akaike’s Information Criterion (AIC) was used for model comparison. The effect size on variables with POAF was estimated by calculating the odds ratio (OR) and 95% confidence interval (CI). The additive risk score was built following a weighted analysis derived from ORs obtained from the multivariable logistic regression from Center 1. Thereafter, the risk factors were ranked from the “strongest” to the “weakest”, and a value ranging from 2 to 0.5 was assigned to each risk factor proportionally in order to obtain the POLARIS score (Table S1). Then, this score was applied and calculated into the validation dataset (Center 2) for each patient. The Brier Score was calculated to measure the accuracy of probabilistic predictions. Model discrimination was evaluated by using the area under the receiver operating characteristic (ROC) curve. The model was calibrated using the Hosmer–Lemeshow goodness-of-fit test.
Afterward, the predictive ability was calculated as percentage proportions between the number of POAF patients and the total number of patients in each POLARIS score group separately for the two centers; the correlation between the two series of percentages was then calculated using Spearman’s rho coefficient.
3. Results
A total of 5739 patients were included in the present study: 3467 from Center 1 and 2272 from Center 2. The flowchart for patient selection can be seen in Figure 1. Patients’ baseline characteristics are depicted in Table 1, while Table 2 and Table 3 summarize operative details and postoperative complications. In the overall population, the median age was 66 years (IQR: 56–73.40), and men were 70.7% (4055/5739). Postoperative AF occurred in 32.7% of patients (1874/5739). Patients suffering from postoperative AF were further analyzed and compared to those who did not experience such complications. The analysis of the two subpopulations showed that postoperative AF was associated with more comorbid patients, as reported in Table 4. They were older (p < 0.001) and suffered more from systemic (p < 0.001) and pulmonary arterial hypertension (p < 0.001), chronic kidney disease (p < 0.001), dyslipidemia (p = 0.011), and peripheral artery disease (p = 0.002).
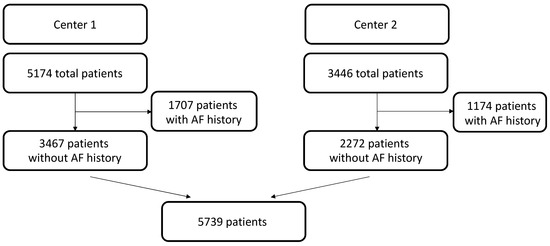
Figure 1.
Patient selection flowchart. Institutional databases at two different European cardiac centers (ASST Spedali Civili di Brescia, University of Brescia, Italy [Center 1] (n = 5174) and Centro Cardiologico Monzino IRCCS, Milano, Italy [Center 2] (n = 3446) were retrieved. Patient selection excluded history of AF. AF = atrial fibrillation.

Table 1.
Patients’ baseline demographics.

Table 2.
Intraoperative outcomes.

Table 3.
Postoperative outcomes.

Table 4.
Patients’ demographics and perioperative outcomes according to the occurrence of postoperative AF.
Intraoperatively, postoperative AF patients underwent more mitral (p < 0.001), tricuspid (p = 0.011), and aortic (p < 0.001) valve surgery and fewer minimally invasive procedures (p < 0.001). Postoperatively, besides higher AF episodes, these patients suffered more from blood transfusions (p < 0.001), perioperative CVA (p < 0.001), need of tracheostomy (p = 0.019), severe GI complications (p < 0.001), and septicemia (p = 0.001). However, 30-day mortality did not significantly differ between the two groups (p = 0.227).
The type of cardioplegia influenced POAF rates. Cold cardioplegias showed higher rates of POAF compared to off-pump surgery and warm cardioplegia (Table S2). At regression analysis these results were confirmed (Table S3).
Derivation and Validation Cohorts
For the derivation cohort, significant variables (p < 0.1) from the univariable regression were selected for the multivariable analysis. From the multivariable regression, significant variables were further analyzed through stepwise regression (Table 5). The AIC of the multivariable regression and after the stepwise regression were 3827 and 3820.1, respectively. The final OR with 95%CI is depicted in Table 5. For the predictors that were considered in the final model, the Brier score was 0.1833. Figures S1 and S2 show the population distribution based on the presence of POAF and the predictiveness curve. Discrimination analysis showed an AUC of the ROC of 0.655 (95%CI: 0.634–0.675)—Figure S3. The Hosmer–Lemeshow goodness-of-fit test for model calibration yielded a p-value of 0.878 (Figure A1). Table S1 shows the points of the POLARIS score based on the final ORs for each variable. Of note, age is also a well-known risk factor for stroke in AF patients [9], and 65 years of age was taken as the threshold for age grouping [10].

Table 5.
Multivariable and stepwise regression.
Table 6 depicts patients’ distribution in the derivation, validation, and overall groups for each POLARIS score value and the prevalence of POAF stratified accordingly, Central Picture. A correlation analysis was conducted between the two centers to evaluate POAF percent by POLARIS score. The Spearman’s rho was 0.636. In the validation cohort, the Brier score was 0.2348, the AUC of the ROC was 0.635 (95%CI: 0.61–0.66), and the Hosmer–Lemeshow goodness-of-fit test p-value was 0.5543 (Figure S4 and Figure A2, respectively). The predictiveness curve is shown in Figure S5.

Table 6.
POAF and POLARIS score distribution in the derivation, validation groups, and total population.
A correlation between the present predictive score and the CHA2DS2-VASc score was performed to strengthen the validity of POLARIS. The regression analysis reported an estimate of 0.248 ± 0.013 (p < 0.001), thus suggesting a positive correlation between the two.
4. Discussion
Atrial fibrillation remains the most frequent complication in patients undergoing cardiac surgery, increasing the incidence of perioperative morbidity and mortality and reflecting a prolonged length of stay and increase in hospitalization costs. Different risk factors have been identified, and efforts were mainly directed towards the prediction and prevention of AF in surgical patients [4]. Pharmacological prophylaxes showed promising results, although, in non-selected patients, the incidence of adverse events could overcome the potential benefits, exposing low-risk patients to unnecessary toxicity and costs [11,12,13,14]. Despite the safety and effectiveness of these strategies, they are still a matter of debate. A reliable bedside tool of risk prediction could help stratify cardiac surgery patients at low to high risk of POAF and tailor the use of preventive pharmacological strategies (Figure 2).
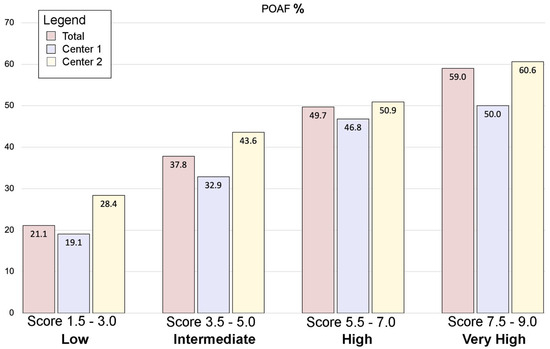
Figure 2.
The POLARIS score. A total of 5739 patients were included in the present study: 3467 from Center 1 and 2272 from Center 2. POAF occurred in 32.7% of patients (1874/5739). After model optimization, seven variables were identified to significantly influence POAF and were used to construct the POLARIS score. The four clusters of increasing risk showed a progressive increase in POAF incidence. POAF = postoperative atrial fibrillation.
Previously reported risk models for POAF in cardiac surgery patients had a small cohort and restricted their analysis only to CABG patients, as shown by Amar et al. [15], thus excluding a large population of valve surgery patients generally recognized as patients at high risk of POAF [5,16,17,18]. In other studies with larger cohorts, Mathew et al. [19] and Magee et al. [18] still limited their investigations to CABG patients, but they also included postoperative variables, thus hampering the possibility of reliably predicting POAF preoperatively and possibly using prophylactic treatments, especially in the case of urgent or emergent operations. Similar to our analysis, Mariscalco et al. [1] used a large cohort of patients undergoing multiple different cardiac surgery operations to build a POAF score that showed moderate discrimination in a validation cohort. Their predictive score was derived from 17,262 patients, had an AUC of 0.64, and had also been externally validated by splitting the overall population into thirds (2/3 for derivation and 1/3 for validation) [20,21]. Other studies have used smaller cohorts of patients and validated their models with bootstrap samples. Tran et al. used a cohort of 999 patients to build a POAF score that was validated in 5000 bootstrap samples with a c-statistic of 0.69 [22]. Mahoney et al. used 624 patients to build a model with a c-statistic of 0.65 that was validated with 1500 bootstrap samples [16].
In the present study, we retrospectively investigated a large population of more than 5700 all-comer patients who underwent cardiac surgery in two different institutions (Center 1, Spedali Civili di Brescia, University of Brescia, Italy—3467 pts; Center 2, Centro Cardiologico Monzino, University of Milan, Italy—2272 pts). All types of interventions, isolated or combined multiple procedures, as well as urgent and emergent cases, were included. Patients with a history of preoperative AF were excluded regardless of the presence of sinus rhythm at the time of hospitalization in order to avoid overestimating by counting and misinterpreting recurrences of a preexisting arrhythmia as a new onset POAF. We intentionally extended our investigation to all comers without preoperative AF who underwent cardiac surgery to reflect a real-world analysis. Moreover, it should be noted that patients with a history of AF undergoing cardiac surgery would most benefit from concomitant ablation procedures [23,24].
In the present analysis, POAF occurred in one-third of the overall population (32.7%), consistent with previous studies where POAF was reported variably, ranging from 20% to 40% in cardiac surgery patients [3].
Multivariable analysis and stepwise logistic regression performed in the derivation cohort (Center 1) highlighted seven preoperative factors influencing the incidence of POAF (Table 5): Mitral valve surgery (either repair or replacement) was the strongest-ranked predictor (OR: 1.90), followed by aortic valve surgery (OR: 1.44), pulmonary hypertension (OR: 1.39), chronic renal failure (OR: 1.31), obesity (OR: 1.20), and age (OR: 1.03), while a minimally invasive approach (either for aortic valve, mitral valve or ascending aorta) was found to be a protective predictor (OR: 0.65). Although age, chronic kidney disease, obesity, and pulmonary hypertension were previously reported as determinants of postoperative AF in cardiac surgery patients, valve surgery, in particular mitral valve surgery, provided per se the highest risk of POAF in our derivation cohort, as similarly reported in a recent review [4]. A diffused atrium injury due to the large atriotomy required during mitral valve surgery has been largely investigated as a sufficient cause of diffuse inflammation able to induce changes in atrial electrophysiology, thus promoting POAF in animal models [3].
Following our derivation analysis, we interestingly found that the use of minimally invasive approaches reduced the incidence of POAF, although this finding remains controversial [25]. While POAF reduction has been reported in some studies [26,27], no difference between minimally invasive and full sternotomy approaches was found by others [28,29]. Both systemic and local inflammation play a role in increasing the incidence of POAF. While the use of the CPB in cardiac surgery was associated with systemic hyper-inflammation and oxidative stress, thus increasing the incidence of POAF, the limited pericardial opening during minimally invasive approaches may locally reduce the release of pro-inflammatory molecules. Although not fully elucidated, the contact between inflammatory cells and cardiac tissue is of paramount importance in the development of POAF in cardiac surgery patients [30,31]. Moreover, in our cohort, a minimally invasive approach was associated with peripheral cannulation for the use of the CPB: avoiding purse-string at the level of the right atrium may further reduce the atrial injury and the subsequent local apoptosis and inflammation induced by the surgical trauma [32,33]. However, a definitive role of peripheral cannulation in the incidence of POAF should require further investigations.
After the identification of the above-mentioned predictors, the following multivariable logistic regression was performed in the derivation cohort (Center 1), and the POLARIS score was then built proportionally with the relative ORs values (Table S1). We therefore tested the POLARIS in both the derivation and the validation cohorts, obtaining a reliable calibration and discrimination power (Hosmer–Lemeshow p-value = 0.878 and 0.554, respectively). A calibration plot visually assesses the agreement between predicted probabilities and observed outcomes by comparing observed event rates to predicted probabilities across risk groups. Ideally, points align closely with the diagonal line, indicating perfect calibration. The Hosmer–Lemeshow test provides a statistical measure of calibration by dividing predictions into groups (generally 10) and comparing observed and expected event rates within each group. A high p-value suggests good calibration, while a low p-value indicates discrepancies. However, the test’s sensitivity to group size and sample size may limit its reliability, making calibration plots a valuable complementary tool for evaluating model performance.
After stratifying the POLARIS score in 4 clusters of risk (low = 1.5–3; intermediate = 3.5–5; high = 5.5–7; very high = 7.5–9), we demonstrated an increase in the incidence of POAF according to the progression of the categories towards higher risks in the validation cohort. Furthermore, this result was confirmed by means of Spearman’s analysis, identifying a linear positive correlation between the POLARIS score and the rate of POAF in the validation cohort (Spearman’s rho: 0.636). These results might be even more remarkable considering the big differences in terms of preoperative characteristics between the derivation (Center 1) and the validation (Center 2) cohorts, thus suggesting the possibility of widely applying this score to larger, random cardiac surgery populations.
Among other POAF predicting scores, the CHA2DS2-VASc score was also found to predict POAF [34], even though it was originally designed and currently used to assess the one-year risk of thromboembolic events in non-anticoagulated individuals with atrial fibrillation [35]. Patients at higher risk (CHA2DS2-VASc > 3) are suggested to receive a prophylactic treatment [20,21,34]. As a result, a comparison between the POLARIS and the CHA2DS2-VASc score was performed. The regression analysis revealed a significant correlation between the two scores, thus empowering the validity of the POLARIS.
Finally, we investigated the impact of the type of cardioplegia on POAF rates. We found that cold cardioplegic solutions have a higher incidence of POAF compared to off-pump or warm cardioplegia, which is in line with a previous study [36]. Therefore, according to these results, warm cardioplegia might be preferred in patients at high risk of POAF. However, this factor is difficult to mitigate, being the type of myocardial protection a “surgeon preference” and difficult to modify.
4.1. Future Perspectives
Further research will be started in our institution by combining AAD prophylaxis in high-risk patients in order to investigate whether or not this therapy may impact the incidence of POAF in this subset of patients. Indeed, current literature recommends the use of AAD to reduce the incidence of POAF in patients considered at high risk [4,37]; however, this recommendation is infrequently applied in practice. The POLARIS score aims to help identify higher-risk patients, potentially encouraging physicians to implement such prophylactic measures.
In high-risk patients, the lack of AF symptomatology should not preclude the use of specific precautions. Indeed, it is fundamental to emphasize that POAF holds significant clinical relevance even in the absence of AF-related symptoms, as asymptomatic patients face the same adverse outcomes as those who are symptomatic [38].
Lastly, considering the positive results obtained in the current study, we would encourage further investigation and confirmation of whether the use of a minimally invasive approach in high-risk patients could be beneficial.
4.2. Limitations
The POLARIS score model showed good accuracy when tested on the validation cohort. Moreover, this model was mainly based on and developed on preoperative clinical factors, but the multifactorial etiology of POAF may require implementation with preoperative AF-specific echocardiographic (such as atrial dimensions or diastolic dysfunction), biomarkers (like BNP, neutrophil–lymphocyte ratio, or genetic predisposition to excessive fibrosis), and hemodynamic data that, given the retrospective nature of the study, were not available. Regarding echocardiographic data, it is important to note that ejection fraction was tested in the univariable regression analysis but was found to be non-significant. Valve insufficiencies were not included in the model because they were closely related to the type of procedure, and including them would have introduced multicollinearity. Future prospective studies may help to further improve the predictivity of the score. Additionally, the model needs to be tested prospectively in order to enhance its clinical and fine-tuning usefulness for follow-up AF monitoring.
5. Conclusions
The POLARIS score is a simple-to-use bedside tool and may represent a user-friendly predictive score for POAF. It showed a good ability to stratify the risk of POAF in all-comer patients who underwent cardiac surgery. Precise identification of patients at high and very high risk of developing AF postoperatively may implement clinical practice with the introduction of preoperative antiarrhythmic prophylaxis, further reducing the incidence of POAF and, potentially, its clinical sequelae. This would be more relevant, particularly in patients with diastolic function impairment in whom atrial contribution during diastole is of paramount importance to maintain an adequate cardiac output during the postoperative period.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm14020650/s1, Figure S1: Histogram of POAF occurrence by risk score; Figure S2: Predictiveness curve of the derivation cohort; Figure S3: Derivation cohort ROC curve; Figure S4: Validation cohort ROC curve; Figure S5: Predictiveness curve of the validation cohort; Table S1: Creation of the POLARIS score from the ORs; Table S2: Cardioplegia use by POAF outcome; Table S3: Cardioplegia regression analysis on POAF outcome; TRIPOD Checklist.
Author Contributions
Conceptualization, F.R., M.B., G.P., L.D.B. and C.M.; methodology, F.R., M.B., C.T., G.M., G.P. and L.D.B.; software, M.B. and C.T.; validation, F.R., M.B., S.P., G.M. and L.D.B.; formal analysis, M.B. and C.T.; investigation, F.R., M.B., G.S., S.P., G.P., G.M., G.B. and L.D.B.; resources, S.B. and C.M.; data curation, M.B., C.T., G.S., S.P., G.M. and L.D.B.; writing—original draft preparation, F.R., M.B. and L.D.B.; writing—review and editing, all authors; visualization, M.B., G.S., S.P. and G.M.; supervision, F.R., G.B., S.B., L.D.B. and C.M.; project administration, F.R., M.B. and L.D.B.; funding acquisition, not applicable. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, approved by the Ethics Committee at Spedali Civili di Brescia, Brescia, Italy, with number NP4980 (15/10/2021) and waived for patient informed consent.
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Claudio Muneretto: Consulting Fee for Estech, LivaNova and Allergan. Gianluigi Bisleri: Atricure (speakers’ bureau, advisory board), Medtronic (speakers’ bureau, advisory board). The remaining authors have nothing to disclose. Given the aim of the study to develop a predictive model for an objective outcome independent of any device-related factors, the reported disclosures did not impact the study results in any way.
Abbreviations
The following abbreviations are used in this manuscript:
| AAD | Antiarrhythmic drugs |
| AF | Atrial fibrillation |
| CVA | Cerebrovascular accident |
| POAF | Postoperative atrial fibrillation |
| POLARIS | POstoperative atriaL fibrillAtion in caRdIac Surgery |
Appendix A
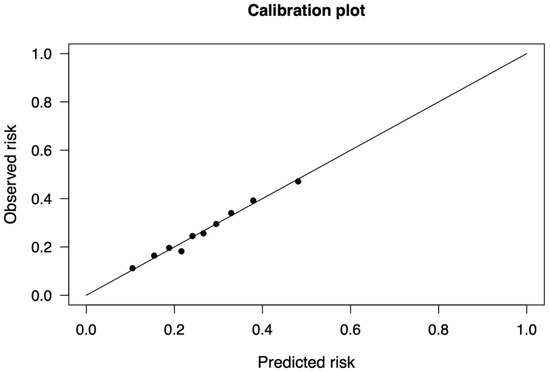
Figure A1.
Calibration curve of the derivation cohort. The model was calibrated using the Hosmer–Lemeshow goodness-of-fit test. Here the derivation cohort is evaluated.
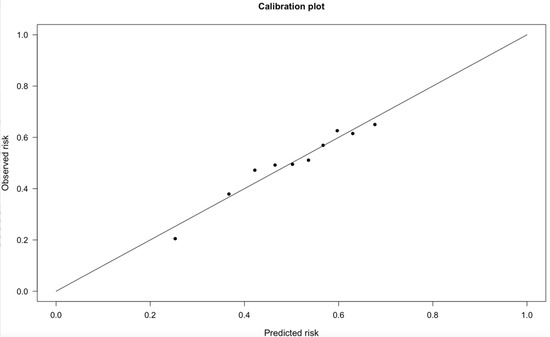
Figure A2.
Calibration curve of the validation cohort. The model was calibrated using the Hosmer–Lemeshow goodness-of-fit test. Here the validation cohort is evaluated.
References
- Mariscalco, G.; Biancari, F.; Zanobini, M.; Cottini, M.; Piffaretti, G.; Saccocci, M.; Banach, M.; Beghi, C.; Angelini, G.D. Bedside Tool for Predicting the Risk of Postoperative Atrial Fibrillation after Cardiac Surgery: The POAF Score. J. Am. Heart Assoc. 2014, 3, e000752. [Google Scholar] [CrossRef]
- Greenberg, J.W.; Lancaster, T.S.; Schuessler, R.B.; Melby, S.J. Postoperative Atrial Fibrillation Following Cardiac Surgery: A Persistent Complication. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2017, 52, 665–672. [Google Scholar] [CrossRef]
- Dobrev, D.; Aguilar, M.; Heijman, J.; Guichard, J.-B.; Nattel, S. Postoperative Atrial Fibrillation: Mechanisms, Manifestations and Management. Nat. Rev. Cardiol. 2019, 16, 417–436. [Google Scholar] [CrossRef]
- Gaudino, M.; Di Franco, A.; Rong, L.Q.; Piccini, J.; Mack, M. Postoperative Atrial Fibrillation: From Mechanisms to Treatment. Eur. Heart J. 2023, 44, ehad019. [Google Scholar] [CrossRef]
- Thorén, E.; Hellgren, L.; Jidéus, L.; Ståhle, E. Prediction of Postoperative Atrial Fibrillation in a Large Coronary Artery Bypass Grafting Cohort. Interact. Cardiovasc. Thorac. Surg. 2012, 14, 588–593. [Google Scholar] [CrossRef]
- Parise, O.; Parise, G.; Vaidyanathan, A.; Occhipinti, M.; Gharaviri, A.; Tetta, C.; Bidar, E.; Maesen, B.; Maessen, J.G.; La Meir, M.; et al. Machine Learning to Identify Patients at Risk of Developing New-Onset Atrial Fibrillation after Coronary Artery Bypass. J. Cardiovasc. Dev. Dis. 2023, 10, 82. [Google Scholar] [CrossRef]
- Yang, H.; Yuan, C.; Yang, J.; Xiang, H.; Lan, W.; Tang, Y. A Novel Predictive Model for New-Onset Atrial Fibrillation in Patients after Isolated Cardiac Valve Surgery. Front. Cardiovasc. Med. 2022, 9, 949259. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD Statement. BMJ 2015, 350, g7594. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J.G.M. Refining Clinical Risk Stratification for Predicting Stroke and Thromboembolism in Atrial Fibrillation Using a Novel Risk Factor-Based Approach: The Euro Heart Survey on Atrial Fibrillation. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef]
- Demography—Elderly Population—OECD Data. Available online: https://www.oecd.org/en/data/indicators/elderly-population.html (accessed on 1 March 2023).
- Goh, S.L.; Yap, K.H.; Chua, K.C.; Chao, V.T.T. Does Preoperative Statin Therapy Prevent Postoperative Atrial Fibrillation in Patients Undergoing Cardiac Surgery? Interact. Cardiovasc. Thorac. Surg. 2015, 20, 422–428. [Google Scholar] [CrossRef][Green Version]
- Klinger, R.Y.; Thunberg, C.A.; White, W.D.; Fontes, M.; Waldron, N.H.; Piccini, J.P.; Hughes, G.C.; Podgoreanu, M.V.; Stafford-Smith, M.; Newman, M.F.; et al. Intraoperative Magnesium Administration Does Not Reduce Postoperative Atrial Fibrillation After Cardiac Surgery. Anesth. Analg. 2015, 121, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Lagier, D.; Nee, L.; Guieu, R.; Kerbaul, F.; Fenouillet, E.; Roux, N.; Giorgi, R.; Theron, A.; Grisoli, D.; Gariboldi, V.; et al. Peri-Operative Oral Caffeine Does Not Prevent Postoperative Atrial Fibrillation after Heart Valve Surgery with Cardiopulmonary Bypass: A Randomised Controlled Clinical Trial. Eur. J. Anaesthesiol. 2018, 35, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Thein, P.M.; White, K.; Banker, K.; Lunny, C.; Mirzaee, S.; Nasis, A. Preoperative Use of Oral Beta-Adrenergic Blocking Agents and the Incidence of New-Onset Atrial Fibrillation After Cardiac Surgery. A Systematic Review and Meta-Analysis. Heart Lung Circ. 2018, 27, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Amar, D.; Shi, W.; Hogue, C.W.; Zhang, H.; Passman, R.S.; Thomas, B.; Bach, P.B.; Damiano, R.; Thaler, H.T. Clinical Prediction Rule for Atrial Fibrillation After Coronary Artery Bypass Grafting. J. Am. Coll. Cardiol. 2004, 44, 1248–1253. [Google Scholar] [CrossRef]
- Mahoney, E.M.; Thompson, T.D.; Veledar, E.; Williams, J.; Weintraub, W.S. Cost-Effectiveness of Targeting Patients Undergoing Cardiac Surgery for Therapy with Intravenous Amiodarone to Prevent Atrial Fibrillation. J. Am. Coll. Cardiol. 2002, 40, 737–745. [Google Scholar] [CrossRef]
- Zaman, A.G.; Archbold, R.A.; Helft, G.; Paul, E.A.; Curzen, N.P.; Mills, P.G. Atrial Fibrillation after Coronary Artery Bypass Surgery: A Model for Preoperative Risk Stratification. Circulation 2000, 101, 1403–1408. [Google Scholar] [CrossRef]
- Magee, M.J.; Herbert, M.A.; Dewey, T.M.; Edgerton, J.R.; Ryan, W.H.; Prince, S.; Mack, M.J. Atrial Fibrillation after Coronary Artery Bypass Grafting Surgery: Development of a Predictive Risk Algorithm. Ann. Thorac. Surg. 2007, 83, 1707–1712, discussion 1712. [Google Scholar] [CrossRef]
- Mathew, J.P.; Fontes, M.L.; Tudor, I.C.; Ramsay, J.; Duke, P.; Mazer, C.D.; Barash, P.G.; Hsu, P.H.; Mangano, D.T.; Investigators of the Ischemia Research and Education Foundation; et al. A Multicenter Risk Index for Atrial Fibrillation after Cardiac Surgery. JAMA 2004, 291, 1720–1729. [Google Scholar] [CrossRef]
- Burgos, L.M.; Seoane, L.; Parodi, J.B.; Espinoza, J.; Galizia Brito, V.; Benzadón, M.; Navia, D. Postoperative Atrial Fibrillation Is Associated with Higher Scores on Predictive Indices. J. Thorac. Cardiovasc. Surg. 2019, 157, 2279–2286. [Google Scholar] [CrossRef]
- Cameron, M.J.; Tran, D.T.T.; Abboud, J.; Newton, E.K.; Rashidian, H.; Dupuis, J.-Y. Prospective External Validation of Three Preoperative Risk Scores for Prediction of New Onset Atrial Fibrillation After Cardiac Surgery. Anesth. Analg. 2018, 126, 33–38. [Google Scholar] [CrossRef]
- Tran, D.T.T.; Perry, J.J.; Dupuis, J.-Y.; Elmestekawy, E.; Wells, G.A. Predicting New-Onset Postoperative Atrial Fibrillation in Cardiac Surgery Patients. J. Cardiothorac. Vasc. Anesth. 2015, 29, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Dominici, C.; Chello, M. Concomitant Surgical Ablation for Treatment of Atrial Fibrillation in Patients Undergoing Cardiac Surgery. Rev. Cardiovasc. Med. 2022, 23, 101. [Google Scholar] [CrossRef] [PubMed]
- Baudo, M.; Rosati, F.; Lapenna, E.; Di Bacco, L.; Benussi, S. Surgical Options for Atrial Fibrillation Treatment during Concomitant Cardiac Procedures. Ann. Cardiothorac. Surg. 2024, 13, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Maimari, M.; Baikoussis, N.G.; Gaitanakis, S.; Dalipi-Triantafillou, A.; Katsaros, A.; Kantsos, C.; Lozos, V.; Triantafillou, K. Does Minimal Invasive Cardiac Surgery Reduce the Incidence of Post-Operative Atrial Fibrillation? Ann. Card. Anaesth. 2020, 23, 7–13. [Google Scholar] [CrossRef]
- Mihos, C.G.; Santana, O.; Lamas, G.A.; Lamelas, J. Incidence of Postoperative Atrial Fibrillation in Patients Undergoing Minimally Invasive versus Median Sternotomy Valve Surgery. J. Thorac. Cardiovasc. Surg. 2013, 146, 1436–1441. [Google Scholar] [CrossRef]
- Glower, D.D.; Desai, B.S.; Hughes, G.C.; Milano, C.A.; Gaca, J.G. Aortic Valve Replacement via Right Minithoracotomy versus Median Sternotomy: A Propensity Score Analysis. Innovations 2014, 9, 75–81, discussion 81. [Google Scholar] [CrossRef]
- Brinkman, W.T.; Hoffman, W.; Dewey, T.M.; Culica, D.; Prince, S.L.; Herbert, M.A.; Mack, M.J.; Ryan, W.H. Aortic Valve Replacement Surgery: Comparison of Outcomes in Matched Sternotomy and PORT ACCESS Groups. Ann. Thorac. Surg. 2010, 90, 131–135. [Google Scholar] [CrossRef]
- Murtuza, B.; Pepper, J.R.; Stanbridge, R.D.; Darzi, A.; Athanasiou, T. Does Minimal-Access Aortic Valve Replacement Reduce the Incidence of Postoperative Atrial Fibrillation? Tex. Heart Inst. J. 2008, 35, 428–438. [Google Scholar]
- Scott, L.; Li, N.; Dobrev, D. Role of Inflammatory Signaling in Atrial Fibrillation. Int. J. Cardiol. 2019, 287, 195–200. [Google Scholar] [CrossRef]
- Yao, C.; Veleva, T.; Scott, L.; Cao, S.; Li, L.; Chen, G.; Jeyabal, P.; Pan, X.; Alsina, K.M.; Abu-Taha, I.; et al. Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation. Circulation 2018, 138, 2227–2242. [Google Scholar] [CrossRef]
- Schuessler, R.B.; Ishii, Y.; Khagi, Y.; Diabagate, K.; Boineau, J.P.; Damiano, R.J. The Effects of Inflammation on Heart Rate and Rhythm in a Canine Model of Cardiac Surgery. Heart Rhythm. 2012, 9, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, K.; Nakashima, H.; Saku, K. The HMG-CoA Reductase Inhibitor Atorvastatin Prevents Atrial Fibrillation by Inhibiting Inflammation in a Canine Sterile Pericarditis Model. Cardiovasc. Res. 2004, 62, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Zeng, M.; Liu, Y.; Xu, Y.; Bai, Y.; Cao, L.; Ling, Z.; Fan, J.; Yin, Y. CHA2DS2-VASc Score for Identifying Patients at High Risk of Postoperative Atrial Fibrillation After Cardiac Surgery: A Meta-Analysis. Ann. Thorac. Surg. 2020, 109, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Y.; Zhang, A.-D.; Lu, H.-Y.; Guo, J.; Wang, F.-F.; Li, Z.-C. CHADS2 versus CHA2DS2-VASc Score in Assessing the Stroke and Thromboembolism Risk Stratification in Patients with Atrial Fibrillation: A Systematic Review and Meta-Analysis. J. Geriatr. Cardiol. JGC 2013, 10, 258–266. [Google Scholar] [CrossRef]
- Mauro, M.D.; Calafiore, A.M.; Di Franco, A.; Nicolini, F.; Formica, F.; Scrofani, R.; Antona, C.; Messina, A.; Troise, G.; Mariscalco, G.; et al. Association Between Cardioplegia and Postoperative Atrial Fibrillation in Coronary Surgery. Int. J. Cardiol. 2021, 324, 38–43. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Developed with the Special Contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Karakasis, P.; Pamporis, K.; Siontis, K.C.; Theofilis, P.; Samaras, A.; Patoulias, D.; Stachteas, P.; Karagiannidis, E.; Stavropoulos, G.; Tzikas, A.; et al. Major Clinical Outcomes in Symptomatic vs. Asymptomatic Atrial Fibrillation: A Meta-Analysis. Eur. Heart J. 2024, ehae694. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).