Abstract
Study Design: Retrospective Case–Control. Objectives: Sleep disturbances negatively impact quality of life and increase illness susceptibility. Chronic pain is a risk factor for sleep disruption, particularly in patients with degenerative spinal conditions. Existing studies suggest that degenerative cervical myelopathy (DCM) patients often experience sleep disturbances, possibly due to spinal cord compression and pain. However, most research is limited to small, single-center studies, creating a need for broader analyses. Methods: We utilized the Merative Explorys Dataset, focusing on electronic health record data of patients diagnosed with DCM and sleep disorders identified via ICD codes. Comorbidities analyzed included depression/bipolar disorder, chronic pulmonary disease, migraine, osteoarthritis, hypertension, malignancy, diabetes, and cerebrovascular disease. Patient demographic information (age, race, sex, and body mass index (BMI)) was included as covariates. Logistic regression analyses were performed to evaluate the association between each comorbidity and the risk of sleep disturbance. Results: Among 40,551 DCM patients, significant predictors of sleep disturbance included higher BMI (OR: 1.05, 95% CI: 1.05–1.06), depression/bipolar disorder (OR: 1.65, 95% CI: 1.56–1.74), chronic pulmonary disease (OR: 1.26, 95% CI: 1.20–1.33), migraine (OR: 1.32, 95% CI: 1.22–1.43), and hypertension (OR: 1.16, 95% CI: 1.10–1.23). Conclusions: This large-scale analysis demonstrates the multifactorial nature of sleep disturbances in DCM, highlighting strong associations with BMI and respiratory conditions, suggesting a contributory role of sleep-disordered breathing. The identification of migraines as a risk factor highlights the need for multidisciplinary management. Addressing modifiable risk factors such as BMI and mental health may improve sleep quality in DCM patients.
1. Introduction
Sleep is an essential function of the human body and plays a major role in maintaining cognitive function and physiologic processes such as glucose metabolism and overall metabolic regulation [,]. Given its role in multiple physiological domains, sleep disturbance and deficits are associated with decreased quality of life, increased illness susceptibility, and increased mortality [,,]. Sleep deprivation is a global issue affecting nearly 20% of people across nations []. In the United States, only 65.2% of individuals report healthy sleep, and more than one-third of respondents report sleeping less than seven hours per night [,].
Among the numerous established risk factors for sleep disturbance, chronic pain is of particular interest. Approximately 50% of patients with chronic low back pain experience significant sleep disturbance, while 42% of individuals with chronic neck pain and chronic back pain report similar problems. Similarly, studies have demonstrated that sleep disturbances are prevalent in patients with degenerative spinal disease, including degenerative cervical myelopathy (DCM), lumbar spinal stenosis, and adult spinal deformity [,,]. These studies propose that the mechanisms driving sleep disturbances in DCM patients may be related to pain, paresthesias, sleep breathing disorders and sleep movement disorders, or the possible disruption of melatonin pathways in the cervical spinal cord. Another hypothesis is that cervical stenosis may alter cerebrospinal fluid dynamics, impairing waste clearance and increasing posterior fossa pressure, which could in turn exacerbate sleep apnea and other sleep-related disturbances []. Along these lines, surgically treated patients who receive decompression of the spinal cord show greater improvements in sleep disturbance symptoms than those managed conservatively [].
Sleep disturbances have long been overlooked in spine surgery literature, where most of the studies focus on surgical outcomes [,,,,,,,,]. Available data remain inadequate, especially regarding DCM. The majority of studies on the cervical spine examine cervical spinal cord injury [,,]. When focusing specifically on DCM, what sparse literature exists is restricted by single-centered studies with small patients samples [], which may introduce bias in epidemiological analyses and risk factor identification. Accordingly, augment this literature, our study sought to leverage a large population-based patient dataset to identify risk factors of sleep disturbance in patients with DCM.
2. Materials and Methods
2.1. Study Design and Population
This retrospective case–control study leveraged the Merative Explorys Therapeutic Dataset, a large de-identified, population-based repository aggregating electronic health records (EHRs) from multiple U.S. healthcare systems. Adult patients with a diagnosis of DCM were identified using International Classification of Diseases, Tenth Revision (ICD-10) codes. In order to reduce the likelihood of including sleep disorders that may be unrelated to underlying DCM, relevant cases were defined as individuals who received a subsequent diagnosis of a sleep disorder (also identified via ICD-10 codes) at least six months after the index date of DCM diagnosis. Controls were selected from the same DCM cohort and included patients who did not receive any sleep disorder diagnosis after being diagnosed with DCM. Demographic and comorbidities were extracted from EHR. Supplementary Table S1 provides the ICD-10 codes used.
2.2. Statistical Analysis
All statistical analyses were performed using reproducible scripts in R Studio (v4.2 2024.02.29) First, descriptive analyses were performed to characterize the demographic and clinical profiles of the study population. Continuous variables were summarized using means and standard deviations (SD) or medians and interquartile ranges (IQR) as appropriate, while categorical variables were described using frequencies and percentages. Univariable analyses were performed to explore the association between each potential risk factor and the likelihood of developing sleep disorders following a diagnosis of DCM.
For multivariable analyses, a logistic regression model was constructed to estimate the adjusted associations between candidate predictors and the risk of sleep disorders among DCM patients. The independent variables used in the model were those considered clinically relevant or consistently reported in the literature. To ensure model stability and interpretability, two variable selection methods were employed. First, a stepwise selection approach was used to iteratively refine the model based on the Akaike Information Criterion (AIC). Collinearity diagnostics, including variance inflation factors (VIFs), were examined to ensure that no excessive correlation existed among the predictors. Final estimates were reported as adjusted odds ratios (OR) with 95% confidence intervals (95% CI). Statistical significance was defined as p < 0.05.
2.3. Missing Data Handling
To address missing values, a multiple imputation strategy was employed. Ten imputed datasets were created using a chained equations approach, wherein each incomplete variable was regressed on other variables iteratively. Each dataset underwent 50 iterations to refine the imputed values, thereby capturing uncertainty and improving accuracy. After imputation, the 10 completed datasets were combined using Rubin’s rules to produce final estimates.
3. Results
3.1. Descriptive Characteristics of the Study Population
A total of 40,551 patients with DCM were included in this study. Table 1 and Figure 1 summarize the demographic and clinical characteristics of the study population stratified by the presence or absence of sleep disorders following DCM diagnosis. Of the total cohort, 74.8% (n = 30,322) did not develop sleep disorders, while 25.2% (n = 10,229) did. The median (IQR) age was 61 (53–70) years in the group with sleep disorders was 61 (52–71) years in the group without. Body mass index (BMI) was significantly higher among patients who developed sleep disorders, with a median (IQR) of 30.0 (25.8–35.1), compared to those without sleep disorders, whose median BMI was 27.8 (24.1–32.0) (p < 0.001).

Table 1.
Descriptive Statistics of Potential Risk Factors for Sleep Disturbance in Degenerative Cervical Myelopathy patients. Demographic details, clinical characteristics, and comorbidity prevalence stratified by the presence or absence of subsequent sleep disorders.
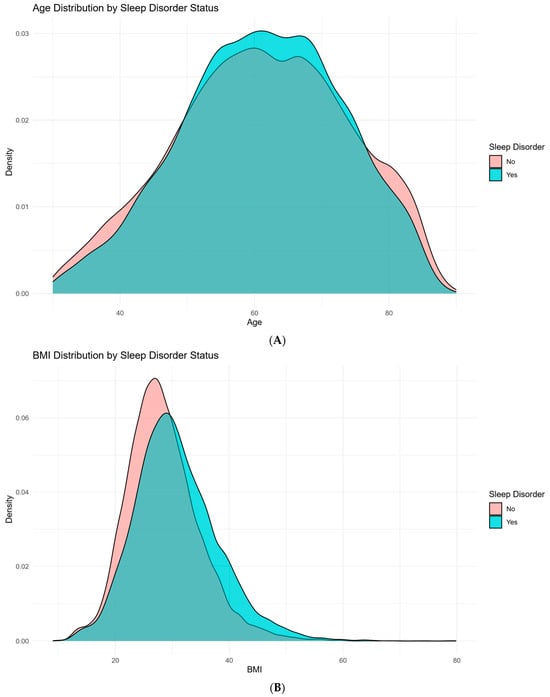
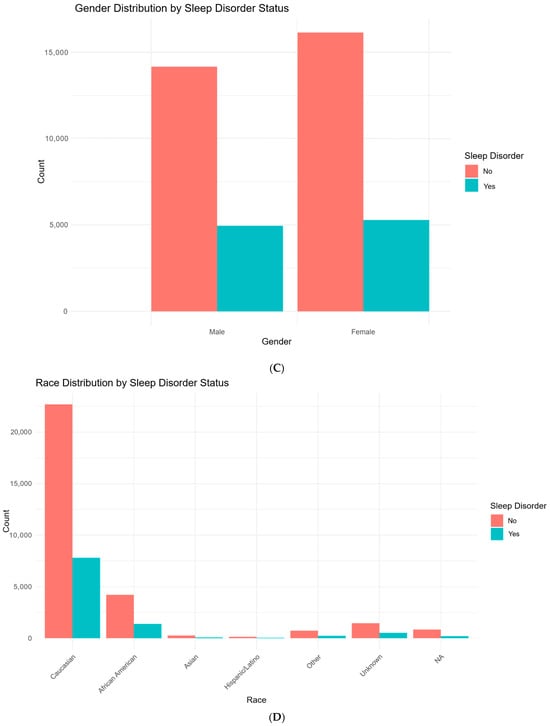
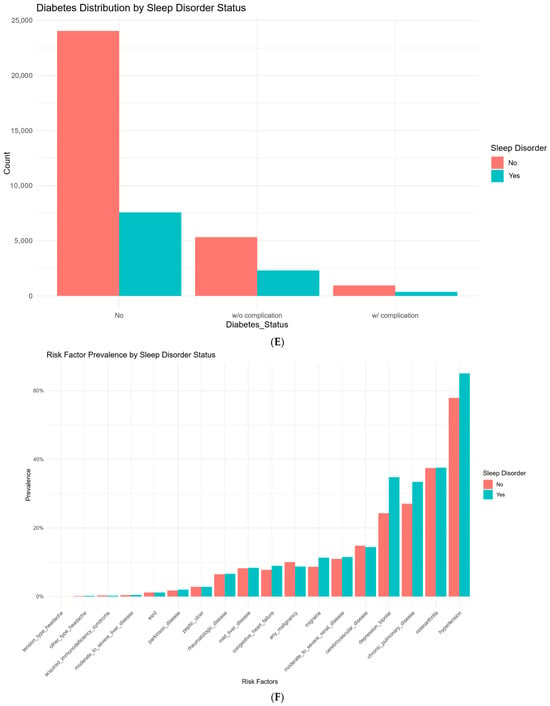
Figure 1.
Demographic and clinical characteristics stratified by sleep disorder status in patients with degenerative cervical myelopathy (DCM). (A): Distribution of age in DCM patients, comparing those with and without sleep disorders. (B): Distribution of body mass index (BMI) in DCM patients, comparing those with and without sleep disorders. (C): Distribution of gender in DCM patients, comparing those with and without sleep disorders. (D): Distribution of race in DCM patients, comparing those with and without sleep disorders. (E): Prevalence of Diabetes Status (no diabetes, diabetes without complications, and diabetes with complications) in DCM patients with and without sleep disorders. (F): Distribution of risk factor prevalence in DCM patients, comparing those without and without sleep disorders.
Several comorbidities were more prevalent in the sleep disorder group. Hypertension was present in 65.1% of those with sleep disorders, compared to 57.9% in the non-sleep disorder group (p < 0.001). Likewise, conditions such as congestive heart failure (p < 0.001), chronic pulmonary disease (p < 0.001), and migraine (p < 0.001) were more common in individuals who developed sleep disorders. Notably, depression or bipolar disorder was reported in 34.8% of patients with sleep disorders compared to 24.3% of those without (p < 0.001).
3.2. Univariable Analysis
Table 2 and Figure 2 present the OR and 95% CI for each predictor evaluated in the univariable logistic regression analysis. Depression or bipolar disorder (OR: 1.66, 95% CI: 1.58–1.75; p < 0.001) and diabetes (OR: 1.38, 95% CI: 1.30–1.46; p < 0.001) were strongly associated with sleep disorders. Chronic pulmonary disease (OR: 1.35, 95% CI: 1.29–1.42; p < 0.001) and hypertension (OR: 1.35, 95% CI: 1.29–1.42; p < 0.001) were each associated with elevated odds of sleep disorders, as was migraine (OR: 1.35, 95% CI 1.25–1.45; p < 0.001). Body mass index (BMI) demonstrated a positive association, with each unit increase linked to a higher risk of sleep disorders (OR 1.05, 95% CI: 1.05–1.05; p < 0.001). Congestive heart failure also showed a modest but statistically significant association (OR 1.16, 95% CI: 1.07–1.26; p < 0.001).

Table 2.
Univariable Logistic Regression Results of Predictors of Sleep Disturbance in Degenerative Cervical Myelopathy patients: Provides coefficients, odds ratios (ORs), 95% confidence intervals (CIs), and p-values for each variable tested for association with sleep disorder.
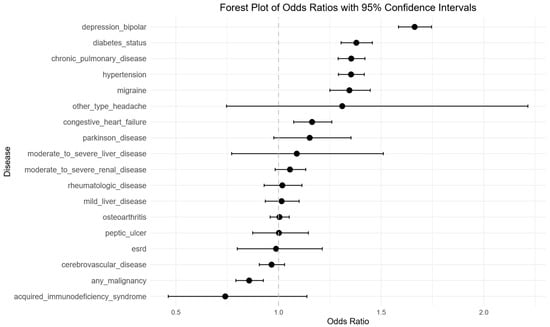
Figure 2.
Univariable logistic regression analysis of risk factors for sleep disorder in degenerative cervical myelopathy patients. Forest plot illustrating odds ratios (OR’s) and 95% confidence intervals (CIs) for each evaluated predictor, demonstrating statistically significant associations with depression/bipolar, diabetes status, chronic pulmonary disease, hypertension, migraine. Negative associations were seen with any malignancy.
3.3. Multivariable Analysis
A multivariable logistic regression model was constructed using predictors selected through a stepwise procedure. This yielded a final model that identified several significant sleep disorder predictors (Figure 3). Among these, chronic pulmonary disease (OR: 1.27, 95% CI: 1.20–1.33, p < 0.001), depression or bipolar disorder (OR: 1.65, 95% CI: 1.57–1.74, p < 0.001), migraine (OR: 1.32, 95% CI: 1.22–1.43, p < 0.001), hypertension (OR: 1.16, 95% CI: 1.10–1.22, p < 0.001), and diabetes without complications (OR: 1.14, 95% CI: 1.07–1.21, p < 0.001) were associated with an increased likelihood of developing sleep disorders. Higher BMI and older age were also positively related to risk (OR of approximately 1.05 per unit increase in BMI and 1.01 per year increase in age) (p < 0.001 for both).
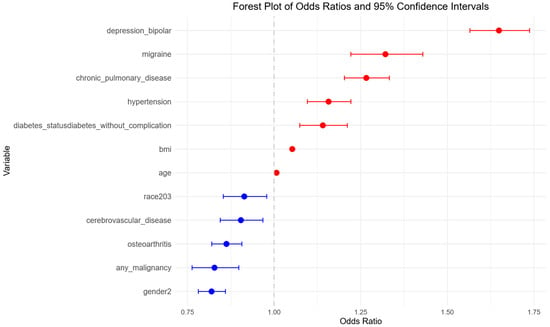
Figure 3.
Multivariable logistic regression analysis of risk factors for sleep disorders in degenerative cervical myelopathy patients. Forest plot showing adjusted odds ratios (ORs) and 95% confidence intervals (CIs) from the final logistic regression model, highlighting independent predictors of sleep disorders. Blue points represent predictors with ORs < 1.00. Red points represent predictors with ORs ≥ 1.00.
In contrast, osteoarthritis (OR: 0.86, 95% CI: 0.82–0.91, p < 0.001), any malignancy (OR: 0.83, 95% CI: 0.76–0.90, p < 0.001), cerebrovascular disease (OR: 0.90, 95% CI: 0.84–0.97, p = 0.004), and being African American compared to Caucasian (OR: 0.91, 95% CI: 0.85–0.98, p = 0.01), and female gender (OR: 0.82, 95% CI: 0.78–0.86, p < 0.001) were associated with reduced risk of sleep disorder.
4. Discussion
To our knowledge, this study provides the largest analysis examining risk factors associated with sleep disturbance in patients with DCM. Using a robust dataset of 40,551 DCM patients, we identified several significant predictors of sleep disturbance, including higher BMI, depression or bipolar disorder, chronic pulmonary disease, migraines, and hypertension. These findings expand our understanding of the multifactorial nature of sleep disorders in DCM patients, underscoring the role that both physiologic and psychological comorbidities play in sleep outcomes.
Our logistic regression analysis revealed that higher BMI (OR: 1.05, 95% CI: 1.05–1.06), depression or bipolar scores (OR: 1.65, 95% CI: 1.56–1.74), chronic pulmonary disease (OR 1.26 95% CI: 1.20–1.33), migraine (OR 1.32, 95% CI 1.22–1.43), and hypertension (OR 1.16, 95% CI 1.10–1.23) were independently associated with increased odds of sleep disturbance. These risk factors align with findings from prior research on degenerative spinal disease, but our study further highlights their relevance to patients suffering specifically from DCM [,]. Additionally, we found that age, although statistically significant, had only a modest effect size (OR: 1.01, 95% CI 1.00-1.01) suggesting that its clinical impact may be less profound than reported in other studies [].
The mechanisms underlying sleep disturbance in DCM are likely complex and multifactorial, including both neurological and systemic factors. Emerging evidence highlights the role of microglial regulation and neuroinflammation in sleep disturbance across neurological conditions. Microglia influence sleep–wake regulation and become activated in response to stress and sleep fragmentation or deprivation. This activation can lead to release of pro-inflammatory cytokines and further disrupt sleep [,,]. In the context of DCM, chronic spinal cord compression may similarly engage these pathways, suggesting that neuroinflammatory cascades could represent an additional biological mechanism linking DCM to sleep disturbance.
We complement this mechanistic discussion by identifying key risk factors for sleep disturbance in this population. Our results are consistent with the findings of Kim et al., who identified depression, chronic pain, and neuropsychiatric conditions to be significant predictors of sleep disturbance in patients with spinal disease []. Our study builds on these results by incorporating other risk factors such as chronic pulmonary disease and hypertension, which have not been emphasized in previous studies on sleep disturbances in DCM patients. The significant associations between sleep disturbance and chronic pulmonary disease and BMI suggest that respiratory conditions, potentially through mechanisms such as sleep-disordered breathing, hypersomnias, or obesity hypoventilation syndrome, play a notable role in exacerbating sleep issues in this population [,].
Furthermore, our findings regarding migraines are novel in this population and align with broader research which have found migraines to have a major impact on sleep [,]. Altered sleep architecture, pain associated with migraines [], and disruptions in calcitonin gene-related peptide (CGRP) likely contribute to poor sleep quality [,]. Prior studies focusing on spinal pathology have not substantially published on this link, making our study an additional notable iteration upon prior work.
Given the growing recognition of mental health as a critical determinant of outcomes in spine surgery populations [,,,], our findings emphasize a significant association between depression or bipolar disorder and sleep disturbances in DCM. Depression is well-documented to disrupt sleep architecture, contributing to difficulties with both sleep initiation and maintenance. In the context of DCM, this psychological burden may be further amplified by chronic pain and neurological symptoms, compounding the risk for sleep disruption. These results underscore the need for routine mental health screening and management in this population [].
The identification of these risk factors has clinical implications for the management of DCM patients. Addressing modifiable risk factors such as BMI should be a priority in the pre- and perioperative period. Managing patient’s weight could potentially reduce the risk of sleep related breathing disorders and improve sleep quality. As in spinal cord injury, DCM may result in neural pathway disruption which may impair circadian regulation through altered melatonin production [,]. Pain and paresthesias, which are hallmarks of DCM, contribute to sleep disturbances by increasing nocturnal arousals and reducing sleep quality.
Given the associations between these multiple different conditions (some of which tend to occur together in a comorbid fashion) and sleep disturbance in DCM, a multidisciplinary approach is necessary to address this problem. Collaborations across multiple specialties could address the broad spectrum of comorbidities that contribute to sleep disturbance in this patient population. Screening these patients beforehand and connecting them with the right provider to preemptively treat these underlying comorbidities could significantly enhance patient quality of life. In practice, the identified predictors may serve as a framework for risk stratification: for example, patients with elevated BMI or chronic pulmonary disease could be prioritized for evaluation of sleep-disordered breathing, while those with depression or migraines may benefit from early referral to psychiatry or neurology. This could improve early recognition, allow proactive intervention, and ensure that high-risk patients are stratified appropriately for perioperative management.
Interestingly, certain conditions such as osteoarthritis (OR: 0.86, 95% CI: 0.82–0.91), any malignancy (OR: 0.83, 95% CI: 0.76–0.90), cerebrovascular disease (OR: 0.90, 95% CI: 0.84–0.97), and Black race compared to White (OR: 0.91, 95% CI: 0.85–0.97) were associated with lower odds of sleep disturbance. While these findings may initially appear counterintuitive, it is possible that patients with these conditions are more likely to be prescribed medications such as opioids, muscle relaxants, or sedating agents, which may incidentally improve sleep symptoms. Alternatively, these associations may reflect unmeasured confounders or biases in reporting. Future studies should consider integrating medication profiles and longitudinal follow-up to better understand these inverse relationships.
Limitations
Our study has several limitations. The retrospective nature of the data collection introduces potential bias, particularly in the accuracy of recorded diagnoses. Additionally, we were unable to include objective measures of sleep quality such as polysomnography, which would have provided a more direct assessment of sleep disturbances in this population. Further research with prospective data and objective sleep assessments is needed to confirm the results of our study. Moreover, we were unable to account for medication use in this cohort, including antidepressants, hypnotics, and analgesics, which may be important confounding factors influencing sleep. Another important limitation is that our study did not examine the severity of DCM with sleep disturbance. This is relevant, as one might expect patients with more severe disease to have worse sleep outcomes. However, even with patients being categorized as having severe DCM, assessment can be challenging []. Future studies incorporating validated severity scales alongside objective sleep measures should be conducted. Another limitation is our reliance on ICD coding to identify comorbidities such as depression or migraine. This may introduce bias, with the potential for both under- and overestimation of these conditions; however, it is an acceptable and widely used method in large population studies [,,,]. Lastly, interventions aimed at modulating the identified risk factors, particularly in patients undergoing surgical intervention for DCM, should be evaluated for their impact on sleep outcomes.
5. Future Directions and Applications
Future work should move behind large sized retrospective HER analyses and expand to prospective cohort studies that integrate both clinical and biological markers of sleep disturbance in DCM. This could include objective sleep assessments, serum or CSF biomarkers of neuroinflammation, and validated scales of DCM severity (Modified Japanese Orthopedic Association Score) to better capture the link between disease burden and sleep outcomes. In parallel, translational studies examining the role of microglial activation, circadian disruption and cerebrospinal fluid dynamics may provide mechanistic insight into the pathways underlying sleep disturbance in this population.
Clinically, our findings highlight opportunities for earlier intervention. Risk factors such as BMI, pulmonary disease and depression could be incorporated into preoperative screening tools to identify patients at greatest risk for poor sleep quality. These predictors may also be used to stratify patients and tailor perioperative management; for example, through targeted weight optimization, pulmonary evaluation, and depression or migraine management via specialty care. Such strategies may ultimately improve not only sleep quality but also overall recovery trajectories and quality of life for patients with DCM.
6. Conclusions
Our study utilizes a large dataset of patients with DCM to provide robust evidence that BMI, depression/bipolar status, chronic pulmonary disease, migraines, and hypertension are significant predictors of sleep disturbance for these patients. Our findings highlight the multifactorial nature of sleep disorders in this population and underscore the need for multidisciplinary approaches to the pre- and perioperative care of DCM patients as well as further research regarding DCM and sleep.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm14197110/s1, Table S1: ICD-10 Codes of Comorbid Conditions: ICD-10 codes of comorbid conditions. Comprehensive list of ICD-10 codes used to identify each comorbid conditions included in this study.
Author Contributions
Conceptualization, S.Y., K.J., J.Z., J.K.G.; Writing—Original Draft, S.Y., K.J.; Data Curation, J.Z.; Formal Analysis, J.Z.; Investigation, J.Z.; Writing—Review and Editing, S.Y., K.J., M.A.R.-C., A.P., A.D., F.A., W.M., G.B., D.P.M.G., B.P., A.T.Y., D.H., W.Z.R., C.A.M., J.K.G., Project Administration, J.K.G., Supervision, J.K.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by U.S. Department of Defense (DoD) Grant, Foundation for Barnes-Jewish Hospital, Washington University/BJC Healthcare Big Ideas Competition, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Grant Number: 1K23AR082986-01A1.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Washington University in St. Louis (protocol code 202306053 and 28 December 2023).
Informed Consent Statement
Patient consent was waived because this study utilized de-identified, population-level data from The Merative Explorys database. As such, no identifiable patient information was utilized and no specific consents were necessary.
Data Availability Statement
The data used in this study were obtained from the Merative™ MarketScan® Research Databases and are available to licensed users through commercial agreement with Merative.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Cirelli, C.; Tononi, G. Is sleep essential? PLoS Biol. 2008, 6, e216. [Google Scholar] [CrossRef]
- Knutson, K.L.; Spiegel, K.; Penev, P.; Van Cauter, E. The Metabolic Consequences of Sleep Deprivation. Sleep Med. Rev. 2007, 11, 163–178. [Google Scholar] [CrossRef]
- Yakdan, S.; Rahhal, N.; Al Chaar, S.; Alhaddad, J.; Al Akoum, M.; Chahine, Y.; Najem, R.; Chahine, M.N. Prevalence of Obstructive Sleep Apnea Among Lebanese Patients With Chronic Kidney Disease: Its Repercussion on Disease Trajectory and Its Effect on Patients’ Quality of Life. Int. J. Nephrol. 2025, 2025, 1427467. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.A.; Huecker, M.R. Sleep Deprivation. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK547676/ (accessed on 4 May 2025).
- Liew, S.C.; Aung, T. Sleep deprivation and its association with diseases—A review. Sleep. Med. 2021, 77, 192–204. [Google Scholar] [CrossRef]
- Stranges, S.; Tigbe, W.; Gómez-Olivé, F.X.; Thorogood, M.; Kandala, N.-B. Sleep problems: An emerging global epidemic? Findings from the INDEPTH WHO-SAGE study among more than 40,000 older adults from 8 countries across Africa and Asia. Sleep 2012, 35, 1173–1181. [Google Scholar] [CrossRef]
- Liu, Y.; Wheaton, A.G.; Chapman, D.P.; Cunningham, T.J.; Lu, H.; Croft, J.B. Prevalence of Healthy Sleep Duration among Adults--United States, 2014. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Oh, J.-K.; Kim, S.W.; Yee, J.S.; Kim, T.-H. Risk factors for sleep disturbance in patients with cervical myelopathy and its clinical significance: A cross-sectional study. Spine J. 2021, 21, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, J.; Kim, S.W.; Oh, J.-K.; Park, M.S.; Kim, Y.-W.; Kim, T.-H. Prevalence of sleep disturbance in patients with lumbar spinal stenosis and analysis of the risk factors. Spine J. 2020, 20, 1239–1247. [Google Scholar] [CrossRef]
- Kim, H.-J.; Hong, S.-J.; Park, J.-H.; Ki, H. Sleep Disturbance and Its Clinical Implication in Patients with Adult Spinal Deformity: Comparison with Lumbar Spinal Stenosis. Pain. Res. Manag. 2020, 2020, 6294151. [Google Scholar] [CrossRef]
- Khachatryan, T.; Robinson, J.S. The possible impact of cervical stenosis on cephalad neuronal dysfunction. Med. Hypotheses 2018, 118, 13–18. [Google Scholar] [CrossRef]
- Kim, J.; Kim, G.; Kim, S.W.; Oh, J.-K.; Park, M.S.; Kim, Y.-W.; Kim, T.-H. Changes in sleep disturbance in patients with cervical myelopathy: Comparison between surgical treatment and conservative treatment. Spine J. 2021, 21, 586–597. [Google Scholar] [CrossRef]
- Zhang, J.K.; Javeed, S.; Greenberg, J.K.; Yakdan, S.; Kaleem, M.I.; Botterbush, K.S.; Benedict, B.; Dibble, C.F.; Sun, P.; Sherrod, B.; et al. Diffusion MRI Metrics Characterize Postoperative Clinical Outcomes After Surgery for Cervical Spondylotic Myelopathy. Neurosurgery 2025, 96, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Yakdan, S.; Zhang, J.; Benedict, B.; Xu, Z.; Javeed, S.; Zhang, J.K.; Steel, B.A.; Gupta, V.P.; Botterbush, K.; Piccirillo, J.F.; et al. Multidomain postoperative recovery trajectories after lumbar and thoracolumbar spine surgery. Spine J. 2025; in press. [Google Scholar] [CrossRef]
- Yakdan, S.M.; Herrera, M.; Wehbe, N.; Al Akoum, M.; Kaleem, M.I.; Ruiz-Cardozo, M.A.; Joseph, K.; Assaf, N.; Dimassi, H. Outcome of spine surgery in the context of spinal metastatic disease: The National Surgical Quality Improvement Program. J. Craniovertebral Junction Spine 2024, 15, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.K.; Frumkin, M.; Xu, Z.; Zhang, J.; Javeed, S.; Zhang, J.K.; Benedict, B.; Botterbush, K.; Yakdan, S.; Molina, C.A.; et al. Preoperative Mobile Health Data Improve Predictions of Recovery From Lumbar Spine Surgery. Neurosurgery 2024, 95, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Yakdan, S.; Benedict, B.; Botterbush, K.; Lagisetty, A.; Kaleem, M.I.; Alessio, R.; Hardi, A.; Javeed, S.; Ruiz-Cardozo, M.A.; Yahanda, A.T.; et al. Randomized controlled trials comparing cervical disc arthroplasty and anterior cervical discectomy and fusion outcomes in degenerative spine disease: A systematic review and meta-analysis. J. Neurosurg. Spine 2025. epub ahead of printing. [Google Scholar] [CrossRef]
- Zhang, J.K.; Yakdan, S.; Kaleem, M.I.; Javeed, S.; Greenberg, J.K.; Botterbush, K.S.; Benedict, B.; Reis, M.; Hongsermeier-Graves, N.; Twitchell, S.; et al. Spinal cord metrics derived from diffusion MRI: Improvement in prognostication in cervical spondylotic myelopathy compared with conventional MRI: Presented at the 2024 AANS/CNS Joint Section on Disorders of the Spine and Peripheral Nerves. J. Neurosurg. Spine 2024, 41, 639–647. [Google Scholar] [CrossRef]
- Bhan, R.; Yakdan, S.; Diaz, C.; Zhang, J.; Xu, Z.; Joseph, K.; Plog, B.; Yahanda, A.T.; Zhang, J.K.; Javeed, S.; et al. Association between preoperative activity levels and postoperative complications in adult spinal deformity surgery. Spine Deform. 2025; epub ahead of printing. [Google Scholar] [CrossRef]
- Zhang, J.K.; Yakdan, S.; Zhang, J.; Javeed, S.; Benedict, B.; Frumkin, M.; Ogunlade, J.; Gupta, M.; Piccirillo, J.F.; Bess, S.; et al. Establishing objective markers of physical activity to identify early improvement after lumbar spine surgery: Presented at the 2025 AANS/CNS Joint Section on Disorders of the Spine and Peripheral Nerves. J. Neurosurg. Spine, 2025; epub ahead of printing. [Google Scholar] [CrossRef]
- Joseph, K.B.; Bui, T.T.B.; Yahanda, A.T.; Gupta, V.P.; Vogl, S.B.; Yakdan, S.M.; Galla, J.T.B.; Ruiz-Cardozo, M.A.; Barot, K.; Chakladar, S.B.; et al. Mechanical Failures as Predicted by Achieving Local vs Global T4-L1 Hip Axis Goals: A Single Center Experience. Spine, 2025; epub ahead of printing. [Google Scholar] [CrossRef]
- Sankari, A.; Minic, Z.; Farshi, P.; Shanidze, M.; Mansour, W.; Liu, F.; Mao, G.; Goshgarian, H.G. Sleep disordered breathing induced by cervical spinal cord injury and effect of adenosine A1 receptors modulation in rats. J. Appl. Physiol. 2019, 127, 1668–1676. [Google Scholar] [CrossRef]
- Yang, T.-H.; Xirasagar, S.; Cheng, Y.-F.; Wu, C.-S.; Kao, Y.-W.; Shia, B.-C.; Lin, H.-C. Association of cervical spondylosis with obstructive sleep apnea. Sleep Med. 2020, 71, 54–58. [Google Scholar] [CrossRef]
- Berlowitz, D.J.; Brown, D.J.; Campbell, D.A.; Pierce, R.J. A longitudinal evaluation of sleep and breathing in the first year after cervical spinal cord injury. Arch. Phys. Med. Rehabil. 2005, 86, 1193–1199. [Google Scholar] [CrossRef]
- Yakdan, S.; Zhang, J.; Botterbush, K.; Kaleem, M.I.; Benedict, B.; Xu, Z.; Frumkin, M.; Javeed, S.; Zhang, J.K.; Ogunlade, J.; et al. Sleep Physiology in Spine Disease: A Comparative Study of Cervical and Lumbar Patients. Clin. Spine Surg. 2025. epub ahead of printing. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.H.; Kim, T.-H. Changes in Sleep Problems in Patients Who Underwent Surgical Treatment for Degenerative Spinal Disease with a Concurrent Sleep Disorder: A Nationwide Cohort Study in 3183 Patients during a Two-Year Perioperative Period. J. Clin. Med. 2022, 11, 7402. [Google Scholar] [CrossRef]
- Kim, J.; Kang, M.S.; Kim, T.-H. Prevalence of Sleep Disturbance and Its Risk Factors in Patients Who Undergo Surgical Treatment for Degenerative Spinal Disease: A Nationwide Study of 106,837 Patients. J. Clin. Med. 2022, 11, 5932. [Google Scholar] [CrossRef]
- Garofalo, S.; Picard, K.; Limatola, C.; Nadjar, A.; Pascual, O.; Tremblay, M. Role of Glia in the Regulation of Sleep in Health and Disease. In Comprehensive Physiology, 1st ed.; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2020; pp. 687–712. [Google Scholar] [CrossRef]
- Mishra, I.; Pullum, K.B.; Eads, K.N.; Strunjas, A.R.; Ashley, N.T. Peripheral Sympathectomy Alters Neuroinflammatory and Microglial Responses to Sleep Fragmentation in Female Mice. Neuroscience 2022, 505, 111–124. [Google Scholar] [CrossRef]
- Deurveilher, S.; Golovin, T.; Hall, S.; Semba, K. Microglia dynamics in sleep/wake states and in response to sleep loss. Neurochem. Int. 2021, 143, 104944. [Google Scholar] [CrossRef]
- Du, D.; Zhang, G.; Xu, D.; Liu, L.; Hu, X.; Chen, L.; Li, X.; Shen, Y.; Wen, F. Prevalence and clinical characteristics of sleep disorders in chronic obstructive pulmonary disease: A systematic review and meta-analysis. Sleep Med. 2023, 112, 282–290. [Google Scholar] [CrossRef] [PubMed]
- McNicholas, W.T.; Hansson, D.; Schiza, S.; Grote, L. Sleep in chronic respiratory disease: COPD and hypoventilation disorders. Eur. Respir. Rev. 2019, 28, 190064. [Google Scholar] [CrossRef]
- Tiseo, C.; Vacca, A.; Felbush, A.; Filimonova, T.; Gai, A.; Glazyrina, T.; Hubalek, I.A.; Marchenko, Y.; Overeem, L.H.; Piroso, S.; et al. Migraine and sleep disorders: A systematic review. J. Headache Pain. 2020, 21, 126. [Google Scholar] [CrossRef] [PubMed]
- Cevoli, S.; Giannini, G.; Favoni, V.; Pierangeli, G.; Cortelli, P. Migraine and sleep disorders. Neurol Sci. 2012, 33 (Suppl. 1), 43–46. [Google Scholar] [CrossRef]
- Stanyer, E.C.; Creeney, H.; Nesbitt, A.D.; Holland, P.R.; Hoffmann, J. Subjective Sleep Quality and Sleep Architecture in Patients With Migraine: A Meta-analysis. Neurology 2021, 97, e1620–e1631. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, S.; Ossipov, M.H.; Johnson, K.W. The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain 2017, 158, 543–559. [Google Scholar] [CrossRef]
- Yakdan, S.; Benedict, B.; Javeed, S.; Zhang, J.K.; Ruiz-Cardozo, M.A.; Kelly, M.P.; Neuman, B.; Goodin, B.R.; Ray, W.Z.; Rodebaugh, T.L.; et al. Utility of the psychache scale in patients undergoing surgery for degenerative lumbar disease: A prospective single-center study. Eur. Spine J. 2025. epub ahead of printing. [Google Scholar] [CrossRef]
- Javeed, S.; Benedict, B.; Yakdan, S.; Saleem, S.; Zhang, J.K.; Botterbush, K.; Frumkin, M.R.; Hardi, A.; Neuman, B.; Kelly, M.P.; et al. Implications of Preoperative Depression for Lumbar Spine Surgery Outcomes: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2024, 7, e2348565. [Google Scholar] [CrossRef]
- Javeed, S.; Yakdan, S.; Benedict, B.; Saleem, S.; Kaleem, M.; Zhang, J.K.; Frumkin, M.R.; Hardi, A.; Neuman, B.; Kelly, M.P.; et al. Influence of Preoperative Depression on Cervical Spine Surgery Outcomes: A Systematic Review and Meta-Analysis. Global Spine J. 2025. epub ahead of printing. [Google Scholar] [CrossRef]
- Benedict, B.; Frumkin, M.; Botterbush, K.; Javeed, S.; Zhang, J.K.; Yakdan, S.; Neuman, B.J.; Steinmetz, M.P.; Ghogawala, Z.; Kelly, M.P.; et al. Using Multimodal Assessments to Reevaluate Depression Designations for Spine Surgery Candidates. J. Bone Joint Surg. Am. 2024, 106, 1704–1712. [Google Scholar] [CrossRef]
- Vorvolakos, T.; Leontidou, E.; Tsiptsios, D.; Mueller, C.; Serdari, A.; Terzoudi, A.; Nena, E.; Tsamakis, K.; Constantinidis, T.C.; Tripsianis, G. The association between sleep pathology and depression: A cross-sectional study among adults in Greece. Psychiatry Res. 2020, 294, 113502. [Google Scholar] [CrossRef]
- Whelan, A.; Halpine, M.; Christie, S.D.; McVeigh, S.A. Systematic review of melatonin levels in individuals with complete cervical spinal cord injury. J. Spinal Cord. Med. 2020, 43, 565–578. [Google Scholar] [CrossRef]
- Hartley, S.; Daville, R.; Jonathan, L.; Raverot, V.; Di Maria, J.; Bossard, I.; Bensmail, D.; Quera-Salva, M.A.; Leotard, A. Melatonin secretion and sleep disorders in patients with spinal cord injuries. Spinal Cord 2024, 62, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.R.; Jentzsch, T.; Wilson, J.R.; Moghaddamjou, A.; Jiang, F.; Rienmueller, A.; Badhiwala, J.H.; Akbar, M.A.; Nater, A.; Oitment, C.; et al. Inter-rater Reliability of the Modified Japanese Orthopedic Association Score in Degenerative Cervical Myelopathy: A Cross-sectional Study. Spine 2021, 46, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Menai, M.; van Hees, V.T.; Elbaz, A.; Kivimaki, M.; Singh-Manoux, A.; Sabia, S. Accelerometer assessed moderate-to-vigorous physical activity and successful ageing: Results from the Whitehall II study. Sci. Rep. 2017, 7, srep45772. [Google Scholar] [CrossRef]
- Khurshid, S.; Weng, L.-C.; Al-Alusi, M.A.; Halford, J.L.; Haimovich, J.S.; Benjamin, E.J.; Trinquart, L.; Ellinor, P.T.; McManus, D.D.; Lubitz, S.A. Accelerometer-derived physical activity and risk of atrial fibrillation. Eur. Heart J. 2021, 42, 2472–2483. [Google Scholar] [CrossRef]
- Master, H.; Annis, J.; Huang, S.; Beckman, J.A.; Ratsimbazafy, F.; Marginean, K.; Carroll, R.; Natarajan, K.; Harrell, F.E.; Roden, D.M.; et al. Association of step counts over time with the risk of chronic disease in the All of Us Research Program. Nat. Med. 2022, 28, 2301–2308. [Google Scholar] [CrossRef] [PubMed]
- Yakdan, S.; Benedict, B.; Singh, P.; Frumkin, M.R.; Goodin, B.R.; Neuman, B.; Cheng, A.L.; Wang, J.; Kelly, M.P.; Ray, W.Z.; et al. Association of activity with the risk of developing musculoskeletal pain in the All of Us research program. J. Pain 2025, 35, 105516. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).