Hungry Bone Syndrome After Parathyroidectomy for Secondary Hyperparathyroidism: Pathogenesis and Contemporary Clinical Considerations
Abstract
1. Introduction
2. Parathyroid Hormone Physiology
3. Pathogenesis of Secondary Hyperparathyroidism in Chronic Kidney Disease
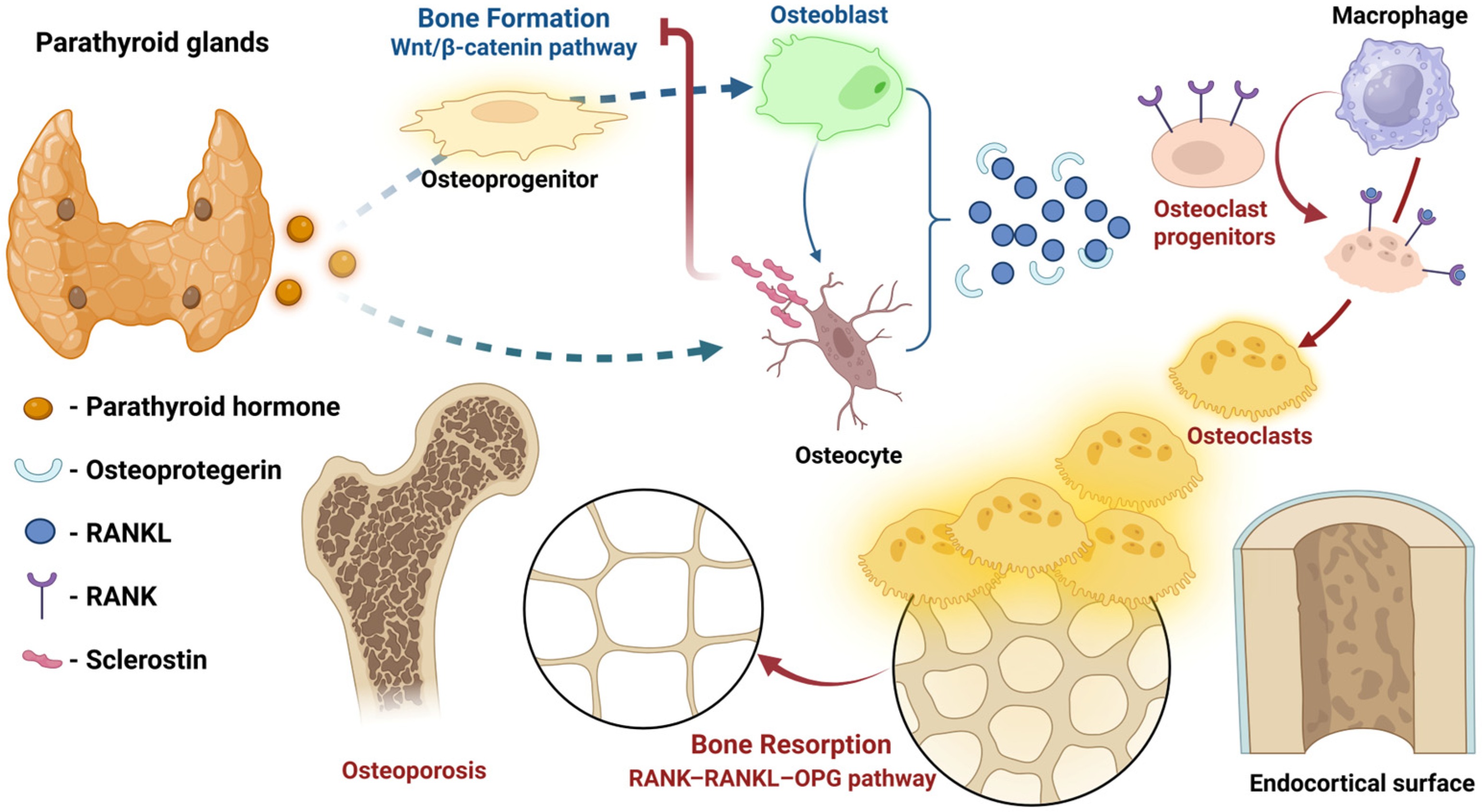
4. Molecular Targets and Signaling Pathways in Bone Tissue
4.1. The RANKL/RANK/OPG System
4.2. The Wnt/β-Catenin Signaling Pathway
4.3. Calcium-Sensing and Mineral Flux Dynamics
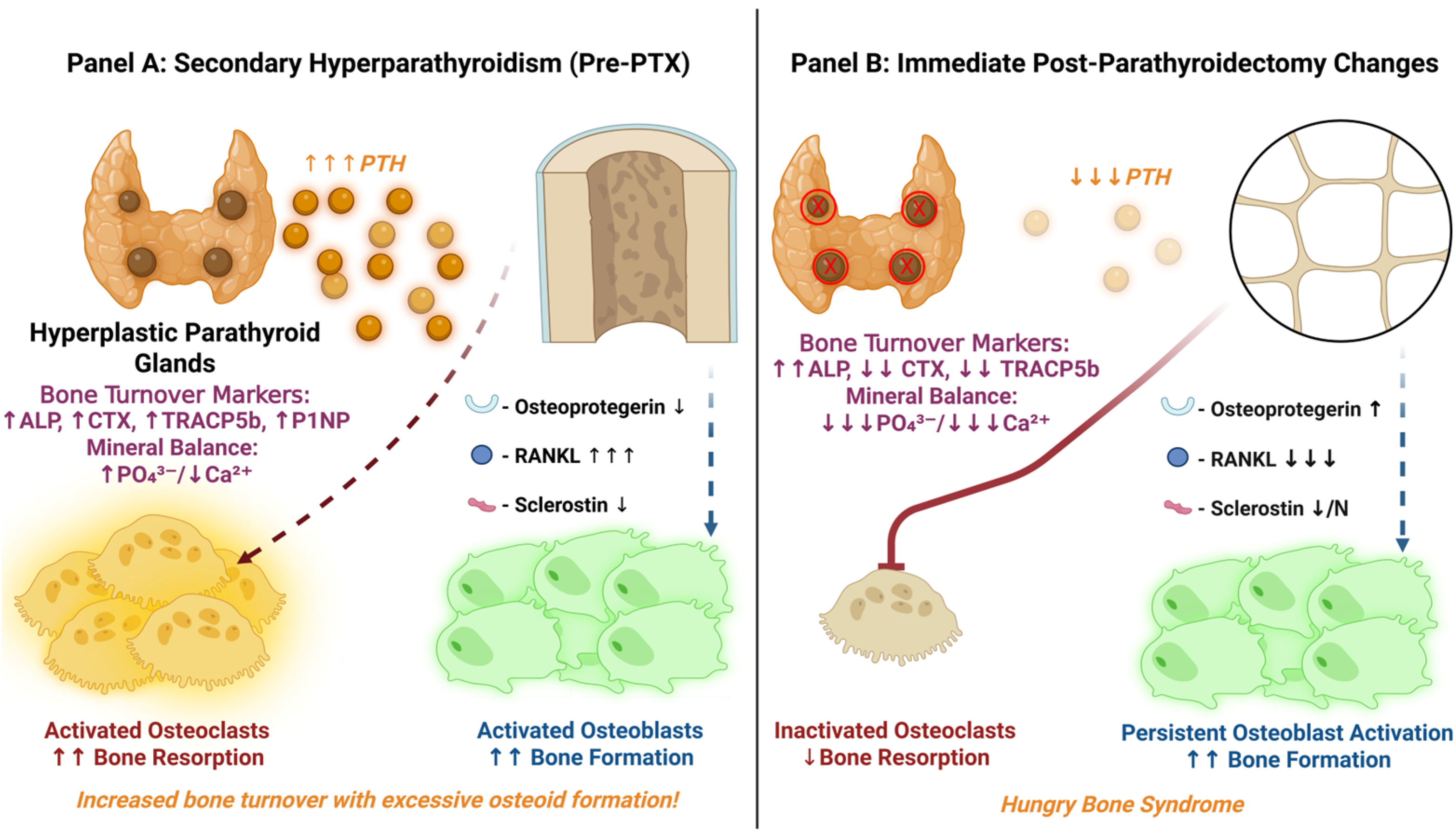
5. Bone Remodeling Dynamics Before and After Parathyroidectomy
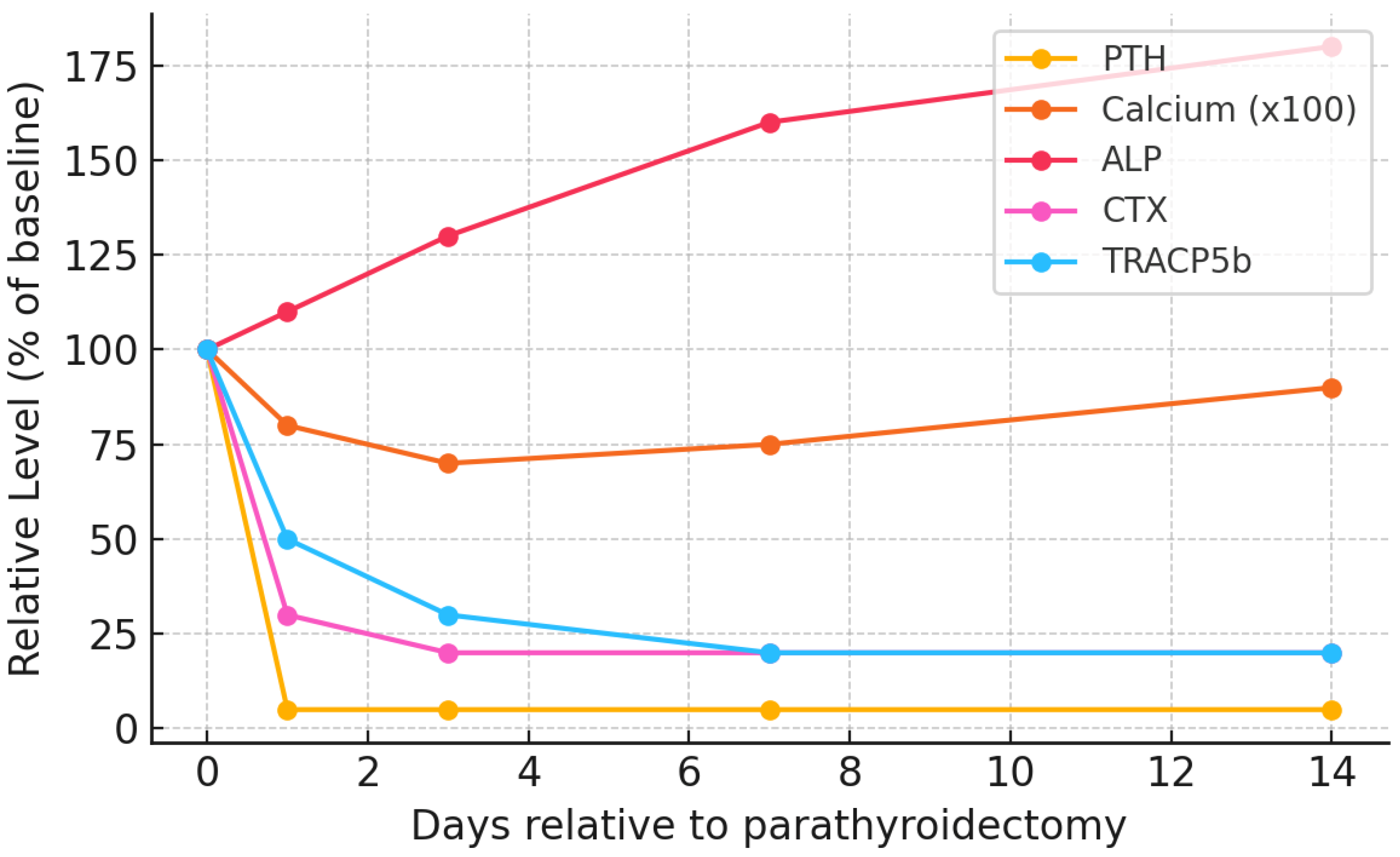
6. Timeline and Clinical Evolution
7. Risk Factors and Predictive Modeling for Hungry Bone Syndrome in Secondary Hyperparathyroidism
7.1. Key Biochemical and Clinical Predictors
- Preoperative PTH Level: Very high iPTH represents a surrogate marker for severe SHPT and high bone turnover. Many studies report significantly higher pre-PTX PTH in HBS patients versus non-HBS [11]. In general, the risk of HBS rises sharply at the extreme PTH levels seen in dialysis patients (e.g., an iPTH >1000 pg/mL was reported as an independent HBS predictor in a 130-patient cohort [18]). However, some cohorts (see Table 2) did not find PTH an independent risk factor when controlling for ALP [30], likely because PTH and ALP are collinear.
- Bone Turnover Markers: Elevated serum ALP (especially bone-specific ALP) is one of the strongest and most consistent predictors of HBS. In multiple studies, pre-op ALP was significantly higher in those who developed HBS [11,18,30,114,129]. ALP reflects osteoblastic activity and overall bone turnover; values >3–4× the ULN (previously proposed cutoff of ALP >420 U/L [18]) carry high associated risk. Other predictive bone formation markers (OC, P1NP) and resorption markers (CTX, TRAP-5b) are currently being investigated and, when available, may prove useful [11,114]. Essentially, their dynamics reflect a very high bone turnover state pre-PTX, which sets the stage for dramatic post-op remineralization.
- Preoperative Calcium and Vitamin D: Paradoxically, lower pre-op serum calcium level (within the context of ESRD) portends a greater drop post-PTX. Patients with autonomous hypercalcemia from tertiary HPT (or adynamic bone) actually have less bone uptake capacity and thus lower HBS risk. Conversely, a normal or low calcium in a severe SHPT patient indicates suppressed bone mineralization despite high turnover—the body maintains normocalcemia by inhibiting calcium incorporation into an expanded but under-mineralized osteoid matrix. After PTX, this “hungry” skeleton rapidly mineralizes, causing profound hypocalcemia as calcium floods into bone. Indeed, absence of pre-op hypercalcemia was a significant risk factor in multiple studies, with meta-analyses showing an odds ratio of 0.19 (95% CI: 0.11–0.31) for severe post-op hypocalcemia [129]. Severe 25-hydroxyvitamin D deficiency (common in CKD) could theoretically exacerbate HBS by limiting baseline calcium stores, but most patients are repleted before PTX; studies on vitamin D status and HBS risk have shown mixed results [11].
- Patient Factors (Age, Body Mass, Sex, Dialysis Vintage): Younger patients tend to mount more robust osteoblastic responses and have more metabolically active bone, which increases HBS susceptibility [11,18]. Indeed, age ≤45 was an independent predictor in multiple series [18,30]. Higher body weight (and by extension, greater skeletal mass) has also been linked to HBS risk [30]. The influence of sex is less clear. Some reports suggest males are at higher risk (potentially due to larger bone mass and lower estrogen levels predisposing to high-turnover lesions), but this has not been consistently observed. Nutritional status may play a role as well—one recent systematic review found that low pre-PTX albumin correlates with HBS [11], possibly reflecting frailty or chronic inflammation. Finally, the duration of pre-PTX dialysis may influence HBS risk, as longer dialysis vintage is associated with more severe CKD-mineral bone disorder. Multiple investigations have corroborated this notion [11,116].
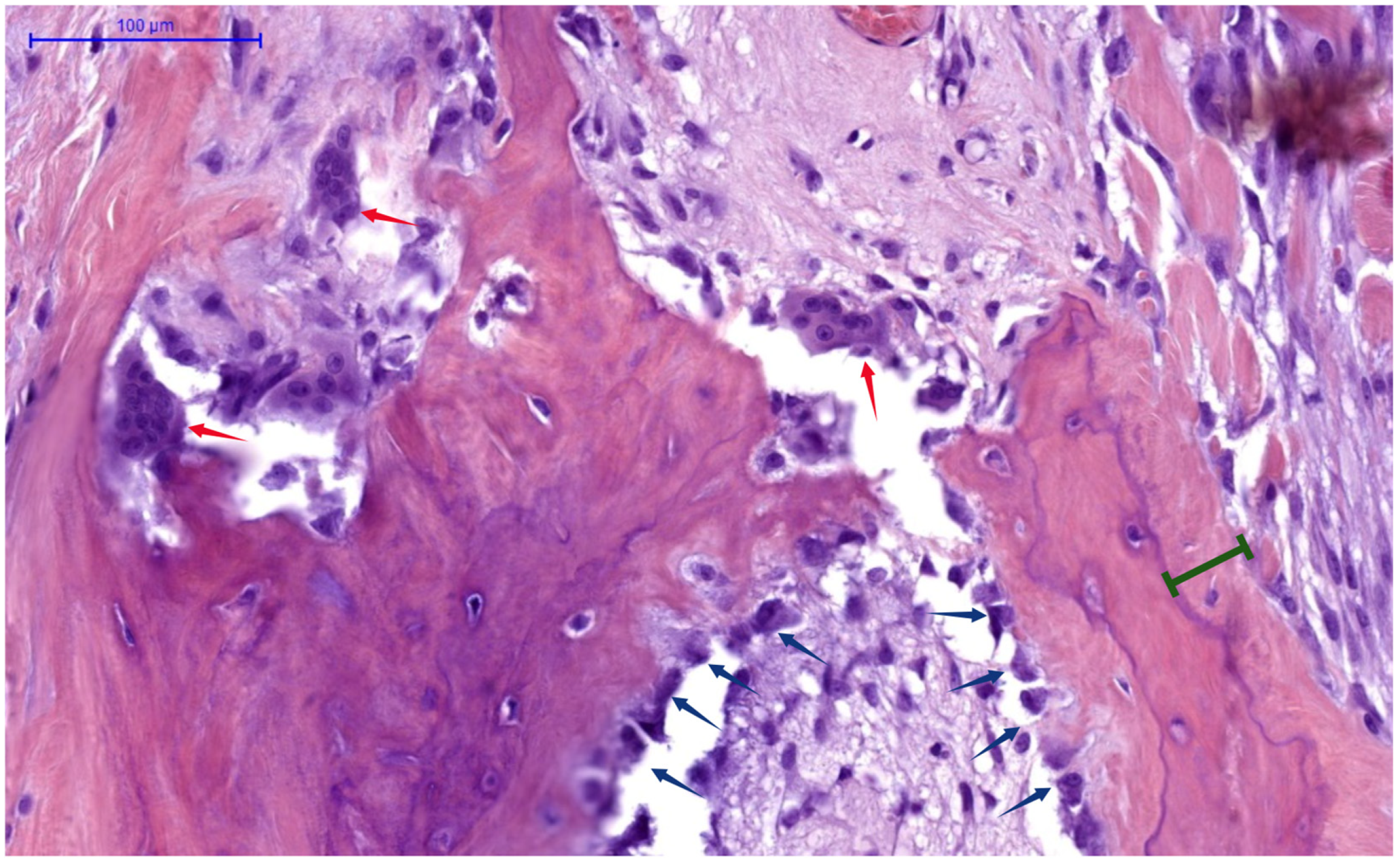
- Skeletal Burden of Disease: Patients with overt skeletal manifestations of SHPT (e.g., osteitis fibrosa cystica, subperiosteal bone resorption on X-ray, or brown tumors) inherently have very high bone turnover and large calcium deficits, predisposing them to severe HBS. In contrast, those with mixed uremic osteodystrophy or adynamic bone (often seen in longstanding diabetes or with calcimimetic overuse) have lower turnover and thus lower HBS risk. A bone biopsy (though rarely done pre-PTX) showing high turnover and abundant osteoid would strongly predict HBS. Similarly, very low pre-op bone mineral density could indicate high turnover bone loss.
- Surgical Factors: The extent and abruptness of PTH reduction at surgery significantly influence HBS development. Total PTX without autotransplantation causes the most complete and immediate PTH withdrawal, and thus confers the highest HBS risk [130]. Subtotal PTX or total PTX with a small autograft (e.g., forearm implant) theoretically leaves behind some PTH source to mitigate post-op hypocalcemia [131]. Even so, if the remnant tissue is insufficient or non-functioning, HBS can still occur. Thus, cohorts receiving autotransplant have shown inconsistent results: either marginally lower rates of severe HBS compared to those with solely total PTX [132], or no effect at all in any type of HPT patient [11]. The size and weight of resected parathyroid glands may also correlate with risk—larger glands (or higher total gland weight) theoretically indicate a greater burden of hyperactive tissue, which in turn implies more profound skeletal PTH effects pre-op [11]. Even so, results are scarce and inconsistent. Notably, in primary HPT, glands >1.7 cm had higher HBS occurrence risk [133], whereas in SHPT, removal of very large hyperplastic glands likewise portends HBS [117]. Concomitant thyroidectomy has been noted as a risk factor in primary HPT (likely due to longer associated operative time) [11], but in SHPT patients this is less common.
7.2. Emerging Predictive Models and Risk Scores
8. Prevention and Management Strategies
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| BMU | Basic multicellular unit (anatomic bone remodeling unit) |
| BTMs | Bone turnover markers |
| CaSR | Calcium-sensing receptor |
| CKD | Chronic kidney disease |
| cAMP | Cyclic adenosine monophosphate |
| CREB | cAMP-response element binding protein |
| C-PTHR | C-terminal parathyroid hormone receptor |
| CTX | C-terminal telopeptide of type I collagen (bone resorption marker) |
| CYP24A1 | 25-hydroxyvitamin D-24-hydroxylase |
| CYP27B1 | 25-hydroxyvitamin D-1α-hydroxylase |
| ESRD | End-stage renal disease |
| FGF-23 | Fibroblast growth factor-23 |
| GFR | Glomerular filtration rate |
| H&E | Hematoxylin and eosin (histological stain) |
| HBS | Hungry bone syndrome |
| HPT | Hyperparathyroidism |
| iPTH | Intact parathyroid hormone (full-length 1–84 PTH) |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MEF2 | Myocyte enhancer factor 2 |
| MLR | Multivariate logistic regression |
| NaPi-2a | Sodium-phosphate co-transporter type 2a |
| NaPi-2c | Sodium-phosphate co-transporter type 2c |
| NTX | N-terminal telopeptide of type I collagen (bone resorption marker) |
| OC | Osteocalcin (osteogenesis marker) |
| OPG | Osteoprotegerin |
| PKA | Protein kinase A |
| PKC | Protein kinase C |
| PTH | Parathyroid hormone |
| PTH1R | Type 1 parathyroid hormone receptor (classical PTH receptor) |
| PTX | Parathyroidectomy |
| P1NP | Procollagen type I N-terminal propeptide |
| RANK | Receptor activator of nuclear factor-κB (osteoclast precursor receptor) |
| RANKL | Receptor activator of nuclear factor-κB ligand |
| SHPT | Secondary hyperparathyroidism |
| TRACP5b | Tartrate-resistant acid phosphatase 5b (resorption marker) |
| TRPV5 | Transient receptor potential vanilloid 5 (renal calcium channel) |
| TRPV6 | Transient receptor potential vanilloid 6 (intestinal calcium channel) |
| ULN | Upper limit of normal (reference range) |
| VDR | Vitamin D receptor |
References
- Hedgeman, E.; Lipworth, L.; Lowe, K.; Saran, R.; Do, T.; Fryzek, J. International Burden of Chronic Kidney Disease and Secondary Hyperparathyroidism: A Systematic Review of the Literature and Available Data. Int. J. Nephrol. 2015, 2015, 184321. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Fang, Y.; Zhou, S.; Liu, X.; Li, Z. Estimating the Global Prevalence of Secondary Hyperparathyroidism in Patients with Chronic Kidney Disease. Front. Endocrinol. 2024, 15, 1400891. [Google Scholar] [CrossRef] [PubMed]
- Yajima, A.; Inaba, M.; Tominaga, Y.; Ito, A. Minimodeling Reduces the Rate of Cortical Bone Loss in Patients with Secondary Hyperparathyroidism. Am. J. Kidney Dis. 2007, 49, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [Google Scholar] [CrossRef] [PubMed]
- Shindo, M.; Lee, J.A.; Lubitz, C.C.; McCoy, K.L.; Orloff, L.A.; Tufano, R.P.; Pasieka, J.L. The Changing Landscape of Primary, Secondary, and Tertiary Hyperparathyroidism: Highlights from the American College of Surgeons Panel, “What’s New for the Surgeon Caring for Patients with Hyperparathyroidism”. J. Am. Coll. Surg. 2016, 222, 1240–1250. [Google Scholar] [CrossRef]
- Madorin, C.; Owen, R.P.; Fraser, W.D.; Pellitteri, P.K.; Radbill, B.; Rinaldo, A.; Seethala, R.R.; Shaha, A.R.; Silver, C.E.; Suh, M.Y.; et al. The Surgical Management of Renal Hyperparathyroidism. Eur. Arch. Otorhinolaryngol. 2012, 269, 1565–1576. [Google Scholar] [CrossRef]
- Schneider, R.; Slater, E.P.; Karakas, E.; Bartsch, D.K.; Schlosser, K. Initial Parathyroid Surgery in 606 Patients with Renal Hyperparathyroidism. World J. Surg. 2012, 36, 318–326. [Google Scholar] [CrossRef]
- Yeh, H.; Yeh, H.; Chiang, C.-C.; Yen, J.-C.; Wang, I.-K.; Liu, S.-H.; Lee, C.-C.; Weng, C.-H.; Huang, W.-H.; Hsu, C.-W.; et al. Hungry Bone Syndrome in Peritoneal Dialysis Patients after Parathyroid Surgery. Endocr. Connect. 2023, 12, e230107. [Google Scholar] [CrossRef]
- Bennett, G.A. Parathyroid Glands and Metabolic Bone Disease. Selected Studies. Am. J. Clin. Pathol. 1949, 19, 992. [Google Scholar] [CrossRef][Green Version]
- Muppidi, V.; Meegada, S.R.; Rehman, A. Secondary Hyperparathyroidism. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK557822/ (accessed on 1 October 2025).[Green Version]
- Mehta, R.; Rao, K.N.; Nagarkar, N.M.; Ghosh, A.; Sakale, H. Hungry Bone Syndrome Following Parathyroidectomy: A Comprehensive Systematic Review of Risk Factors. Indian J. Surg. 2024, 86, 27–39. [Google Scholar] [CrossRef]
- Carsote, M.; Nistor, C. Forestalling Hungry Bone Syndrome after Parathyroidectomy in Patients with Primary and Renal Hyperparathyroidism. Diagnostics 2023, 13, 1953. [Google Scholar] [CrossRef]
- Brasier, A.R.; Nussbaum, S.R. Hungry Bone Syndrome: Clinical and Biochemical Predictors of Its Occurrence after Parathyroid Surgery. Am. J. Med. 1988, 84, 654–660. [Google Scholar] [CrossRef]
- Witteveen, J.E.; van Thiel, S.; Romijn, J.A.; Hamdy, N.a.T. Hungry Bone Syndrome: Still a Challenge in the Post-Operative Management of Primary Hyperparathyroidism: A Systematic Review of the Literature. Eur. J. Endocrinol. 2013, 168, R45–R53. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Abraham, J.; Nakshabandi, A.; Lee, E. Treatment of Hypocalcemia in Hungry Bone Syndrome: A Case Report. Int. J. Surg. Case Rep. 2018, 51, 335–339. [Google Scholar] [CrossRef]
- Juárez-León, Ó.A.; Gómez-Sámano, M.Á.; Cuevas-Ramos, D.; Almeda-Valdés, P.; López-Flores, A.; La Torre, M.A.; Reza-Albarrán, A.A.; Gómez-Pérez, F.J. Atypical Parathyroid Adenoma Complicated with Protracted Hungry Bone Syndrome after Surgery: A Case Report and Literature Review. Case Rep. Endocrinol. 2015, 2015, 757951. [Google Scholar] [CrossRef]
- Ramesh, S.; Vekaria, S.; Fisher, J.C.; Wright, K.; Underwood, H.; Prescott, J.; Allendorf, J.; Patel, K.N.; Suh, I.; Sum, M. A Novel Risk Score to Predict Hungry Bone Syndrome After Parathyroidectomy for Renal Hyperparathyroidism. Endocr. Pract. 2023, 29, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Kritmetapak, K.; Kongpetch, S.; Chotmongkol, W.; Raruenrom, Y.; Sangkhamanon, S.; Pongchaiyakul, C. Incidence of and Risk Factors for Post-Parathyroidectomy Hungry Bone Syndrome in Patients with Secondary Hyperparathyroidism. Ren. Fail. 2020, 42, 1118–1126. Available online: https://pubmed.ncbi.nlm.nih.gov/33143476/ (accessed on 1 October 2025). [CrossRef]
- Misiorowski, W.; Czajka-Oraniec, I.; Kochman, M.; Zgliczyński, W.; Bilezikian, J.P. Osteitis Fibrosa Cystica-a Forgotten Radiological Feature of Primary Hyperparathyroidism. Endocrine 2017, 58, 380–385. [Google Scholar] [CrossRef]
- Huang, J.C.; Sakata, T.; Pfleger, L.L.; Bencsik, M.; Halloran, B.P.; Bikle, D.D.; Nissenson, R.A. PTH Differentially Regulates Expression of RANKL and OPG. J. Bone Miner. Res. 2004, 19, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.T.; Srinivasan, B.; Mödder, U.I.; Peterson, J.M.; McCready, L.K.; Riggs, B.L.; Dwyer, D.; Stolina, M.; Kostenuik, P.; Khosla, S. Effects of Parathyroid Hormone Treatment on Circulating Sclerostin Levels in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2010, 95, 5056–5062. [Google Scholar] [CrossRef] [PubMed]
- Yajima, A.; Inaba, M.; Tominaga, Y.; Nishizawa, Y.; Ikeda, K.; Ito, A. Increased Osteocyte Death and Mineralization inside Bone after Parathyroidectomy in Patients with Secondary Hyperparathyroidism. J. Bone Miner. Res. 2010, 25, 2374–2381. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.Y.; Thomas, W.E.; al-Dehaimi, A.W.; Assiri, A.M.; Eastell, R. Longitudinal Changes in Bone Mineral Density and Bone Turnover in Postmenopausal Women with Primary Hyperparathyroidism. J. Clin. Endocrinol. Metab. 1996, 81, 3487–3491. [Google Scholar] [CrossRef]
- Conigrave, A.D. The Calcium-Sensing Receptor and the Parathyroid: Past, Present, Future. Front. Physiol. 2016, 7, 563. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Komaba, H.; Takahashi, Y.; Sawada, K.; Tatsumi, R.; Kanai, G.; Suzuki, H.; Kakuta, T.; Fukagawa, M. Impact of Parathyroidectomy on Serum FGF23 and Soluble Klotho in Hemodialysis Patients With Severe Secondary Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2014, 99, E652–E658. [Google Scholar] [CrossRef] [PubMed]
- Uitterlinden, A.G.; Fang, Y.; Van Meurs, J.B.J.; Pols, H.A.P.; Van Leeuwen, J.P.T.M. Genetics and Biology of Vitamin D Receptor Polymorphisms. Gene 2004, 338, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, C.; Anastasopoulou, C. Hungry Bone Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK549880/ (accessed on 1 October 2025).
- Moe, S.M.; Drüeke, T.B. Management of Secondary Hyperparathyroidism: The Importance and the Challenge of Controlling Parathyroid Hormone Levels without Elevating Calcium, Phosphorus, and Calcium-Phosphorus Product. Am. J. Nephrol. 2003, 23, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Rauner, M.; Taipaleenmäki, H.; Tsourdi, E.; Winter, E.M. Osteoporosis Treatment with Anti-Sclerostin Antibodies—Mechanisms of Action and Clinical Application. J. Clin. Med. 2021, 10, 787. [Google Scholar] [CrossRef]
- Ho, L.-Y.; Wong, P.-N.; Sin, H.-K.; Wong, Y.-Y.; Lo, K.-C.; Chan, S.-F.; Lo, M.-W.; Lo, K.-Y.; Mak, S.-K.; Wong, A.K.-M. Risk Factors and Clinical Course of Hungry Bone Syndrome after Total Parathyroidectomy in Dialysis Patients with Secondary Hyperparathyroidism. BMC Nephrol. 2017, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Bergwitz, C.; Jüppner, H. Regulation of Phosphate Homeostasis by PTH, Vitamin D, and FGF23. Annu. Rev. Med. 2010, 61, 91–104. [Google Scholar] [CrossRef]
- Habener, J.F.; Potts, J.T.; Rich, A. Pre-Proparathyroid Hormone. Evidence for an Early Biosynthetic Precursor of Proparathyroid Hormone. J. Biol. Chem. 1976, 251, 3893–3899. [Google Scholar] [CrossRef]
- Centeno, P.P.; Herberger, A.; Mun, H.-C.; Tu, C.; Nemeth, E.F.; Chang, W.; Conigrave, A.D.; Ward, D.T. Phosphate Acts Directly on the Calcium-Sensing Receptor to Stimulate Parathyroid Hormone Secretion. Nat. Commun. 2019, 10, 4693. [Google Scholar] [CrossRef] [PubMed]
- Hruska, K.A.; Kopelman, R.; Rutherford, W.E.; Klahr, S.; Slatopolsky, E.; Greenwalt, A.; Bascom, T.; Markham, J. Metabolism in Immunoreactive Parathyroid Hormone in the Dog. The Role of the Kidney and the Effects of Chronic Renal Disease. J. Clin. Invest. 1975, 56, 39–48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leiker, A.J.; Yen, T.W.F.; Eastwood, D.C.; Doffek, K.M.; Szabo, A.; Evans, D.B.; Wang, T.S. Factors That Influence Parathyroid Hormone Half-Life: Are New Intraoperative Criteria Needed? JAMA Surg. 2013, 148, 602. [Google Scholar] [CrossRef] [PubMed]
- D’Amour, P. Circulating PTH Molecular Forms: What We Know and What We Don’t. Kidney Int. Suppl. 2006, 102, S29–S33. [Google Scholar] [CrossRef]
- Evenepoel, P.; Bover, J.; Ureña Torres, P. Parathyroid Hormone Metabolism and Signaling in Health and Chronic Kidney Disease. Kidney Int. 2016, 90, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Divieti, P.; Geller, A.I.; Suliman, G.; Jüppner, H.; Bringhurst, F.R. Receptors Specific for the Carboxyl-Terminal Region of Parathyroid Hormone on Bone-Derived Cells: Determinants of Ligand Binding and Bioactivity. Endocrinology 2005, 146, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Pioszak, A.A.; Xu, H.E. Molecular Recognition of Parathyroid Hormone by Its G Protein-Coupled Receptor. Proc. Natl. Acad. Sci. USA 2008, 105, 5034–5039. [Google Scholar] [CrossRef]
- Swarthout, J.T.; D’Alonzo, R.C.; Selvamurugan, N.; Partridge, N.C. Parathyroid Hormone-Dependent Signaling Pathways Regulating Genes in Bone Cells. Gene 2002, 282, 1–17. [Google Scholar] [CrossRef]
- Silva, B.C.; Bilezikian, J.P. Parathyroid Hormone: Anabolic and Catabolic Actions on the Skeleton. Curr. Opin. Pharmacol. 2015, 22, 41–50. [Google Scholar] [CrossRef]
- Hoenderop, J.G.J.; Nilius, B.; Bindels, R.J.M. Calcium Absorption across Epithelia. Physiol. Rev. 2005, 85, 373–422. [Google Scholar] [CrossRef]
- Forster, I.C.; Hernando, N.; Biber, J.; Murer, H. Proximal Tubular Handling of Phosphate: A Molecular Perspective. Kidney Int. 2006, 70, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- Young, K.; Beggs, M.R.; Grimbly, C.; Alexander, R.T. Regulation of 1 and 24 Hydroxylation of Vitamin D Metabolites in the Proximal Tubule. Exp. Biol. Med. 2022, 247, 1103–1111. [Google Scholar] [CrossRef]
- Fadda, G.Z.; Akmal, M.; Lipson, L.G.; Massry, S.G. Direct Effect of Parathyroid Hormone on Insulin Secretion from Pancreatic Islets. Am. J. Physiol. 1990, 258, E975–E984. [Google Scholar] [CrossRef] [PubMed]
- Calvi, L.M.; Adams, G.B.; Weibrecht, K.W.; Weber, J.M.; Olson, D.P.; Knight, M.C.; Martin, R.P.; Schipani, E.; Divieti, P.; Bringhurst, F.R.; et al. Osteoblastic Cells Regulate the Haematopoietic Stem Cell Niche. Nature 2003, 425, 841–846. [Google Scholar] [CrossRef]
- Jono, S.; Nishizawa, Y.; Shioi, A.; Morii, H. Parathyroid Hormone–Related Peptide as a Local Regulator of Vascular Calcification. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.L.; Wolf, M. Calcium and Phosphate Disorders: Core Curriculum 2024. Am. J. Kidney Dis. 2024, 83, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Komaba, H.; Fukagawa, M. Pathophysiology of Parathyroid Hyperplasia in Chronic Kidney Disease: Preclinical and Clinical Basis for Parathyroid Intervention. NDT Plus 2008, 1 (Suppl. S3), iii2–iii8. [Google Scholar] [CrossRef][Green Version]
- National Kidney Foundation. K/DOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease. Am. J. Kidney Dis. 2003, 42, S1–S201. [Google Scholar] [CrossRef]
- Van Pottelbergh, G.; Vaes, B.; Jadoul, M.; Matheï, C.; Wallemacq, P.; Degryse, J.-M. The Prevalence and Detection of Chronic Kidney Disease (CKD)-Related Metabolic Complications as a Function of Estimated Glomerular Filtration Rate in the Oldest Old. Arch. Gerontol. Geriatr. 2012, 54, e419–e425. [Google Scholar] [CrossRef] [PubMed]
- Iorga, C.; Iorga, C.R.; Andreiana, I.; Bengulescu, I.; Constantin, T.; Strambu, V. Advantages of Total Parathyroidectomy in Patients with Secondary Hyperparathyroidism Induced by End Stage Renal Disease. Front. Endocrinol. 2023, 14, 1191914. [Google Scholar] [CrossRef]
- De Francisco, A.L.M.; Cobo, M.A.; Setien, M.A.; Rodrigo, E.; Fresnedo, G.F.; Unzueta, M.T.; Amado, J.A.; Ruiz, J.C.; Arias, M.; Rodriguez, M. Effect of Serum Phosphate on Parathyroid Hormone Secretion during Hemodialysis. Kidney Int. 1998, 54, 2140–2145. [Google Scholar] [CrossRef]
- Shimada, T.; Hasegawa, H.; Yamazaki, Y.; Muto, T.; Hino, R.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Fukumoto, S.; Yamashita, T. FGF-23 Is a Potent Regulator of Vitamin D Metabolism and Phosphate Homeostasis. J. Bone Miner. Res. 2004, 19, 429–435. [Google Scholar] [CrossRef]
- Galitzer, H.; Ben-Dov, I.Z.; Silver, J.; Naveh-Many, T. Parathyroid Cell Resistance to Fibroblast Growth Factor 23 in Secondary Hyperparathyroidism of Chronic Kidney Disease. Kidney Int. 2010, 77, 211–218. [Google Scholar] [CrossRef]
- Hoyland, J.A.; Picton, M.L. Cellular Mechanisms of Renal Osteodystrophy. Kidney Int. 1999, 56, S8–S13. [Google Scholar] [CrossRef]
- Hocher, B.; Armbruster, F.P.; Stoeva, S.; Reichetzeder, C.; Grön, H.J.; Lieker, I.; Khadzhynov, D.; Slowinski, T.; Roth, H.J. Measuring Parathyroid Hormone (PTH) in Patients with Oxidative Stress--Do We Need a Fourth Generation Parathyroid Hormone Assay? PLoS ONE 2012, 7, e40242. [Google Scholar] [CrossRef]
- Zull, J.E.; Smith, S.K.; Wiltshire, R. Effect of Methionine Oxidation and Deletion of Amino-Terminal Residues on the Conformation of Parathyroid Hormone. Circular Dichroism Studies. J. Biol. Chem. 1990, 265, 5671–5676. [Google Scholar] [CrossRef] [PubMed]
- Disthabanchong, S.; Hassan, H.; McConkey, C.L.; Martin, K.J.; Gonzalez, E.A. Regulation of PTH1 Receptor Expression by Uremic Ultrafiltrate in UMR 106–01 Osteoblast-like Cells. Kidney Int. 2004, 65, 897–903. [Google Scholar] [CrossRef]
- Nii-Kono, T.; Iwasaki, Y.; Uchida, M.; Fujieda, A.; Hosokawa, A.; Motojima, M.; Yamato, H.; Kurokawa, K.; Fukagawa, M. Indoxyl Sulfate Induces Skeletal Resistance to Parathyroid Hormone in Cultured Osteoblastic Cells. Kidney Int. 2007, 71, 738–743. [Google Scholar] [CrossRef]
- Behets, G.J.; Viaene, L.; Meijers, B.; Blocki, F.; Brandenburg, V.M.; Verhulst, A.; D’Haese, P.C.; Evenepoel, P. Circulating Levels of Sclerostin but Not DKK1 Associate with Laboratory Parameters of CKD-MBD. PLoS ONE 2017, 12, e0176411. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast Differentiation and Activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Kitaura, H.; Marahleh, A.; Ohori, F.; Noguchi, T.; Shen, W.-R.; Qi, J.; Nara, Y.; Pramusita, A.; Kinjo, R.; Mizoguchi, I. Osteocyte-Related Cytokines Regulate Osteoclast Formation and Bone Resorption. Int. J. Mol. Sci. 2020, 21, 5169. [Google Scholar] [CrossRef]
- Novacescu, D. HBS Review. Created in BioRender. 2025. Available online: https://BioRender.com/lpuf2is (accessed on 1 October 2025).
- Hofbauer, L.C.; Khosla, S.; Dunstan, C.R.; Lacey, D.L.; Boyle, W.J.; Riggs, B.L. The Roles of Osteoprotegerin and Osteoprotegerin Ligand in the Paracrine Regulation of Bone Resorption. J. Bone Miner. Res. 2000, 15, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.M. Therapy for Patients with CKD and Low Bone Mineral Density. Nat. Rev. Nephrol. 2013, 9, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.A.; Martin, T.J. Coupling Signals between the Osteoclast and Osteoblast: How Are Messages Transmitted between These Temporary Visitors to the Bone Surface? Front. Endocrinol. 2015, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Latus, J.; Roesel, M.; Fritz, P.; Braun, N.; Ulmer, C.; Steurer, W.; Biegger, D.; Alscher, M.D.; Kimmel, M. Incidence of and Risk Factors for Hungry Bone Syndrome in 84 Patients with Secondary Hyperparathyroidism. Int. J. Nephrol. Renov. Dis. 2013, 6, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Stilgren, L.S.; Rettmer, E.; Eriksen, E.F.; Hegedüs, L.; Beck-Nielsen, H.; Abrahamsen, B. Skeletal Changes in Osteoprotegerin and Receptor Activator of Nuclear Factor-Kappab Ligand mRNA Levels in Primary Hyperparathyroidism: Effect of Parathyroidectomy and Association with Bone Metabolism. Bone 2004, 35, 256–265. [Google Scholar] [CrossRef]
- Okada, Y.; Montero, A.; Zhang, X.; Lorenzo, J.; Doetschman, T.; Coffin, J.D.; Hurley, M. Impaired Osteoclast Formation in Bone Marrow Cultures of Fgf2 Null Mice in Response to Parathyroid Hormone. J. Biol. Chem. 2003, 278, 21258–21266. [Google Scholar] [CrossRef] [PubMed]
- El-Masri, B.M.; Andreasen, C.M.; Laursen, K.S.; Kofod, V.B.; Dahl, X.G.; Nielsen, M.H.; Thomsen, J.S.; Brüel, A.; Sørensen, M.S.; Hansen, L.J.; et al. Mapping RANKL- and OPG-Expressing Cells in Bone Tissue: The Bone Surface Cells as Activators of Osteoclastogenesis and Promoters of the Denosumab Rebound Effect. Bone Res. 2024, 12, 62. [Google Scholar] [CrossRef]
- Liu, S.; Weiping, Z.; Li, S.; Cui, T.; Li, Z.; Zhang, B.; Li, Z.; Wu, J.; Liang, X.; Lin, Z.; et al. The Effect of Bovine Parathyroid Hormone Withdrawal on MC3T3-E1 Cell Proliferation and Phosphorus Metabolism. PLoS ONE 2015, 10, e0120402. [Google Scholar] [CrossRef][Green Version]
- Rico-Llanos, G.A.; Becerra, J.; Visser, R. Insulin-like Growth Factor-1 (IGF-1) Enhances the Osteogenic Activity of Bone Morphogenetic Protein-6 (BMP-6) in Vitro and in Vivo, and Together Have a Stronger Osteogenic Effect than When IGF-1 Is Combined with BMP-2. J. Biomed. Mater. Res. A 2017, 105, 1867–1875. [Google Scholar] [CrossRef]
- Bordukalo-Nikšić, T.; Kufner, V.; Vukičević, S. The Role Of BMPs in the Regulation of Osteoclasts Resorption and Bone Remodeling: From Experimental Models to Clinical Applications. Front. Immunol. 2022, 13, 869422. [Google Scholar] [CrossRef]
- Baron, R.; Kneissel, M. WNT Signaling in Bone Homeostasis and Disease: From Human Mutations to Treatments. Nat. Med. 2013, 19, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Bellido, T.; Ali, A.A.; Gubrij, I.; Plotkin, L.I.; Fu, Q.; O’Brien, C.A.; Manolagas, S.C.; Jilka, R.L. Chronic Elevation of Parathyroid Hormone in Mice Reduces Expression of Sclerostin by Osteocytes: A Novel Mechanism for Hormonal Control of Osteoblastogenesis. Endocrinology 2005, 146, 4577–4583. [Google Scholar] [CrossRef] [PubMed]
- Cejka, D.; Herberth, J.; Branscum, A.J.; Fardo, D.W.; Monier-Faugere, M.-C.; Diarra, D.; Haas, M.; Malluche, H.H. Sclerostin and Dickkopf-1 in Renal Osteodystrophy. Clin. J. Am. Soc. Nephrol. 2011, 6, 877. [Google Scholar] [CrossRef]
- Hu, L.; Chen, W.; Qian, A.; Li, Y.-P. Wnt/β-Catenin Signaling Components and Mechanisms in Bone Formation, Homeostasis, and Disease. Bone Res. 2024, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Leupin, O.; Kramer, I.; Collette, N.M.; Loots, G.G.; Natt, F.; Kneissel, M.; Keller, H. Control of the SOST Bone Enhancer by PTH Using MEF2 Transcription Factors. J. Bone Miner. Res. 2007, 22, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Huybrechts, Y.; Evenepoel, P.; Haarhaus, M.; Cavalier, E.; Dams, G.; Van Hul, W.; D’Haese, P.C.; Verhulst, A. Osteocytic Sclerostin Expression as an Indicator of Altered Bone Turnover. Nutrients 2023, 15, 598. [Google Scholar] [CrossRef]
- Asamiya, Y.; Tsuchiya, K.; Nitta, K. Role of Sclerostin in the Pathogenesis of Chronic Kidney Disease-Mineral Bone Disorder. Ren. Replace. Ther. 2016, 2, 8. [Google Scholar] [CrossRef][Green Version]
- Bonewald, L.F. The Amazing Osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef]
- Schaffler, M.B.; Cheung, W.-Y.; Majeska, R.; Kennedy, O. Osteocytes: Master Orchestrators of Bone. Calcif. Tissue Int. 2014, 94, 5–24. [Google Scholar] [CrossRef]
- Xiong, J.; Onal, M.; Jilka, R.L.; Weinstein, R.S.; Manolagas, S.C.; O’Brien, C.A. Matrix-Embedded Cells Control Osteoclast Formation. Nat. Med. 2011, 17, 1235–1241. [Google Scholar] [CrossRef]
- Goltzman, D.; Hendy, G.N. The Calcium-Sensing Receptor in Bone—Mechanistic and Therapeutic Insights. Nat. Rev. Endocrinol. 2015, 11, 298–307. [Google Scholar] [CrossRef]
- Maria, C.S.; Cheng, Z.; Li, A.; Wang, J.; Shoback, D.; Tu, C.-L.; Chang, W. Interplay between CaSR and PTH1R Signaling in Skeletal Development and Osteoanabolism. Semin. Cell Dev. Biol. 2016, 49, 11–23. [Google Scholar] [CrossRef]
- Chang, W.; Tu, C.; Chen, T.-H.; Bikle, D.; Shoback, D. The Extracellular Calcium-Sensing Receptor (CaSR) Is a Critical Modulator of Skeletal Development. Sci. Signal 2008, 1, ra1. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Sharma, S. Physiology, Calcium. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK482128/ (accessed on 1 October 2025).
- Jimenez, L.E.; Spinks, T.J.; Ranicar, A.S.; Joplin, G.F. Total Body Calcium Mass in Primary Hyperparathyroidism and Long-Term Changes. Calcif. Tissue Int. 1984, 36, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive Calcium Phosphate Materials and Applications in Bone Regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- Alexander, R.T.; Dimke, H. Effects of Parathyroid Hormone on Renal Tubular Calcium and Phosphate Handling. Acta Physiol. 2023, 238, e13959. [Google Scholar] [CrossRef] [PubMed]
- Raggatt, L.J.; Partridge, N.C. Cellular and Molecular Mechanisms of Bone Remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef] [PubMed]
- Hauge, E.M.; Qvesel, D.; Eriksen, E.F.; Mosekilde, L.; Melsen, F. Cancellous Bone Remodeling Occurs in Specialized Compartments Lined by Cells Expressing Osteoblastic Markers. J. Bone Miner. Res. 2001, 16, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, A.M.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R. Bone Histomorphometry: Standardization of Nomenclature, Symbols, and Units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 1987, 2, 595–610. [Google Scholar] [CrossRef]
- Malluche, H.H.; Mawad, H.W.; Monier-Faugere, M.-C. Renal Osteodystrophy in the First Decade of the New Millennium: Analysis of 630 Bone Biopsies in Black and White Patients. J. Bone Miner. Res. 2011, 26, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.D.; Wesseling, K.; Salusky, I.B. Role of Parathyroid Hormone and Therapy with Active Vitamin D Sterols in Renal Osteodystrophy. Semin. Dial. 2005, 18, 290–295. [Google Scholar] [CrossRef]
- Andersen, T.L.; Sondergaard, T.E.; Skorzynska, K.E.; Dagnaes-Hansen, F.; Plesner, T.L.; Hauge, E.M.; Plesner, T.; Delaisse, J.-M. A Physical Mechanism for Coupling Bone Resorption and Formation in Adult Human Bone. Am. J. Pathol. 2009, 174, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Ubara, Y.; Tagami, T.; Nakanishi, S.; Sawa, N.; Hoshino, J.; Suwabe, T.; Katori, H.; Takemoto, F.; Hara, S.; Takaichi, K. Significance of Minimodeling in Dialysis Patients with Adynamic Bone Disease. Kidney Int. 2005, 68, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Bazydlo, L.A.L.; Needham, M.; Harris, N.S. Calcium, Magnesium, and Phosphate. Lab. Med. 2014, 45, e44–e50. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, C.; Shen, C.; Zeng, M.; Yang, G.; Mao, H.; Zhang, B.; Yu, X.; Cui, Y.; Sun, B.; et al. Diagnostic Accuracy Study of Intraoperative and Perioperative Serum Intact PTH Level for Successful Parathyroidectomy in 501 Secondary Hyperparathyroidism Patients. Sci. Rep. 2016, 6, 26841. [Google Scholar] [CrossRef]
- Li, Y.; Feng, X.; Chen, N.; Song, S.; Yu, M.; Wang, Y.; Zhang, H.; Wang, L.; Chen, M.; Tian, N. Prognosis and Factors Related to Severe Secondary Hyperparathyroidism in Long-Term Peritoneal Dialysis Patients. Ren. Fail. 2024, 46, 2356022. [Google Scholar] [CrossRef]
- Magnusson, P.; Sharp, C.A.; Magnusson, M.; Risteli, J.; Davie, M.W.; Larsson, L. Effect of Chronic Renal Failure on Bone Turnover and Bone Alkaline Phosphatase Isoforms. Kidney Int. 2001, 60, 257–265. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, S.; Li, Z. Effects of Parathyroidectomy on Bone Metabolism in Haemodialysis Patients with Secondary Hyperparathyroidism. Scand. J. Clin. Lab. Invest. 2017, 77, 527–534. [Google Scholar] [CrossRef]
- Lu, K.-C.; Ma, W.-Y.; Yu, J.-C.; Wu, C.-C.; Chu, P. Bone Turnover Markers Predict Changes in Bone Mineral Density after Parathyroidectomy in Patients with Renal Hyperparathyroidism. Clin. Endocrinol. 2012, 76, 634–642. [Google Scholar] [CrossRef]
- Lu, K.-C.; Tseng, C.-F.; Wu, C.-C.; Yeung, L.-K.; Chen, J.-S.; Chao, T.-Y.; Janckila, A.J.; Yam, L.T.; Chu, P. Effects of Calcitriol on Type 5b Tartrate-Resistant Acid Phosphatase and Interleukin-6 in Secondary Hyperparathyroidism. Blood Purif. 2006, 24, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Zhao, L.; Wu, L.; Ji, Q. The Levels of Bone Turnover Markers and Parathyroid Hormone and Their Relationship in Chronic Kidney Disease. Clin. Chim. Acta 2023, 548, 117518. [Google Scholar] [CrossRef]
- Brown, J.E.; Thomson, C.S.; Ellis, S.P.; Gutcher, S.A.; Purohit, O.P.; Coleman, R.E. Bone Resorption Predicts for Skeletal Complications in Metastatic Bone Disease. Br. J. Cancer 2003, 89, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.-C.; Huang, C.-Y.; Liu, C.-C.; Wu, C.-J.; Chen, S.-Y.; Chu, P.; Wu, C.-C.; Lo, L.; Diang, L.-K.; Lu, K.-C. Serum Bone Resorption Markers after Parathyroidectomy for Renal Hyperparathyroidism: Correlation Analyses for the Cross-Linked N-Telopeptide of Collagen I and Tartrate-Resistant Acid Phosphatase. Sci. World J. 2012, 2012, 503945. [Google Scholar] [CrossRef][Green Version]
- Guo, C.Y.; Holland, P.A.; Jackson, B.F.; Hannon, R.A.; Rogers, A.; Harrison, B.J.; Eastell, R. Immediate Changes in Biochemical Markers of Bone Turnover and Circulating Interleukin-6 after Parathyroidectomy for Primary Hyperparathyroidism. Eur. J. Endocrinol. 2000, 142, 451–459. [Google Scholar] [CrossRef]
- Rajeev, P.; Movseysan, A.; Baharani, A. Changes in Bone Turnover Markers in Primary Hyperparathyroidism and Response to Surgery. Ann. R. Coll. Surg. Engl. 2017, 99, 559–562. [Google Scholar] [CrossRef]
- Xu, J.; Kong, N.; Bai, N.; Zhang, Z.; Cui, A.; Tan, S.; Xu, Q. Identification of Novel Risk Factors for Postoperative Severe Hypocalcemia in Patients with Primary Hyperparathyroidism Undergoing Parathyroidectomy: A Case Control Study. BMC Endocr. Disord. 2024, 24, 88. [Google Scholar] [CrossRef]
- Novodvorsky, P.; Lowry, A.F.; Lim, C.B.B.; Balasubramanian, S.P. Serum Magnesium Measurements After Parathyroidectomy for Primary Hyperparathyroidism: Should It Be Routine? World J. Surg. 2020, 44, 1898–1904. [Google Scholar] [CrossRef] [PubMed]
- Meriç, S.; Hacim, N.A. Preoperative Low Serum Magnesium Level Is a Significant Predictive Factor for Postoperative Hypomagnesemia in Patients Who Underwent Parathyroidectomy for Primary Hyperparathyroidism. Namık Kemal Med. J. 2022, 10, 53–58. [Google Scholar] [CrossRef]
- Ge, Y.; Yang, G.; Wang, N.; Zha, X.; Yu, X.; Mao, H.; Sun, B.; Zeng, M.; Zhang, B.; Xing, C. Bone Metabolism Markers and Hungry Bone Syndrome after Parathyroidectomy in Dialysis Patients with Secondary Hyperparathyroidism. Int. Urol. Nephrol. 2019, 51, 1443–1449. [Google Scholar] [CrossRef]
- Yang, G.; Zha, X.; Mao, H.; Yu, X.; Wang, N.; Xing, C. Hypocalcemia-Based Prediction of Hungry Bone Syndrome after Parathyroidectomy in Hemodialysis Patients with Refractory Secondary Hyperparathyroidism. J. Int. Med. Res. 2018, 46, 4985–4994. [Google Scholar] [CrossRef]
- Phimphilai, M.; Inya, S.; Manosroi, W. A Predictive Risk Score to Diagnose Hypocalcemia after Parathyroidectomy in Patients with Secondary Hyperparathyroidism: A 22-Year Retrospective Cohort Study. Sci. Rep. 2022, 12, 9548. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, B.; Zou, X.; Wei, T.; Gong, R.; Zhu, J.; Li, Z. A Nomogram to Predict Hungry Bone Syndrome After Parathyroidectomy in Patients With Secondary Hyperparathyroidism. J. Surg. Res. 2020, 255, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Carling, T.; Rastad, J.; Åkerström, G.; Westin, G. Vitamin D Receptor (VDR) and Parathyroid Hormone Messenger Ribonucleic Acid Levels Correspond to Polymorphic VDR Alleles in Human Parathyroid Tumors1. J. Clin. Endocrinol. Metab. 1998, 83, 2255–2259. [Google Scholar] [CrossRef] [PubMed]
- Lhaneche, L.; Hald, J.D.; Domingues, A.; Hannouche, D.; Delepine, M.; Zelenika, D.; Boland, A.; Ostertag, A.; Cohen-Solal, M.; Langdahl, B.L.; et al. Variations of SOST mRNA Expression in Human Bone Are Associated with DNA Polymorphism and DNA Methylation in the SOST Gene. Bone 2016, 92, 107–115. [Google Scholar] [CrossRef]
- Cole, D.E.; Peltekova, V.D.; Rubin, L.A.; Hawker, G.A.; Vieth, R.; Liew, C.C.; Hwang, D.M.; Evrovski, J.; Hendy, G.N. A986S Polymorphism of the Calcium-Sensing Receptor and Circulating Calcium Concentrations. Lancet 1999, 353, 112–115. [Google Scholar] [CrossRef]
- Chávez, K.V.; Márquez-González, H.; Chavez-Tostado, M. The Usefulness of Intraoperative PTH as a Predictor for Successful Parathyroidectomy in Secondary Hyperparathyroidism. Front. Surg. 2021, 8, 696469. [Google Scholar] [CrossRef]
- Chandran, M.; Bilezikian, J.P.; Salleh, N.M.; Ying, H.; Lau, J.; Lee, J.; deJong, M.C.; Chan Maung, A.; Parameswaran, R. Hungry Bone Syndrome Following Parathyroidectomy for Primary Hyperparathyroidism in a Developed Country in the Asia Pacific. A Cohort Study. Osteoporos. Sarcopenia 2022, 8, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.-C.; Liu, C.-L.; Lee, J.-J.; Liu, T.-P.; Wu, C.-J.; Cheng, S.-P. Osteocalcin Is an Independent Predictor for Hungry Bone Syndrome After Parathyroidectomy. World J. Surg. 2020, 44, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Dau, L.N.; Nguyen, N.D.B.; Do, M.D.; Lam, H.V. Extremely High Preoperative Parathyroid Hormone Associated With Severe Postoperative Hungry Bone Syndrome-Induced Delirium in a Case of Parathyroid Carcinoma in Vietnam: A Case Report. Cureus 2025, 17, e78213. [Google Scholar] [CrossRef]
- Sung, J.K.; Kim, J.-Y.; Ryu, D.-W.; Lee, J.-W.; Youn, Y.-J.; Yoo, B.-S.; Choe, K.-H. A Case of Hypocalcemia-Induced Dilated Cardiomyopathy. J. Cardiovasc. Ultrasound 2010, 18, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Radu, C.P.; Daniealopol, V.; Santini, A.; Darie, R.; Sala, D.T. Fatal Hypocalcaemia Due to Hungry Bone Syndrome with Secondary Refractory HyperParathyroidism After Parathyroidectomy: A Case Report. J. Crit. Care. Med. 2019, 5, 140–144. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, X.; Ni, H.; Deng, H. Predictive Value of Ionized Calcium in Critically Ill Patients: An Analysis of a Large Clinical Database MIMIC II. PLoS ONE 2014, 9, e95204. [Google Scholar] [CrossRef]
- Cheng, S.-P.; Liu, C.-L.; Chen, H.-H.; Lee, J.-J.; Liu, T.-P.; Yang, T.-L. Prolonged Hospital Stay after Parathyroidectomy for Secondary Hyperparathyroidism. World J. Surg. 2009, 33, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Lou, Y.; Cui, Y.; Liu, S.; Cui, W.; Sun, G. Risk Factors for Hypocalcemia in Dialysis Patients with Refractory Secondary Hyperparathyroidism after Parathyroidectomy: A Meta-Analysis. Ren. Fail. 2022, 44, 503–512. [Google Scholar] [CrossRef]
- Mustafa, R.; Begum, H.A.; Mannath, S.N.; Fernandez James, C. Severe Prolonged Hypocalcemia Following Four-Gland Parathyroidectomy in a Patient With Established Renal Failure. Cureus 2024, 16, e67964. [Google Scholar] [CrossRef] [PubMed]
- Coman, A.; Tarta, C.; Aiordachioae, G.A.; Goldis, D.; Utu, D.; Marian, M.; Dobrescu, A.; Buleu, F.; Olariu, S. Predictors of Hungry Bone Syndrome and Reintervention After Subtotal Versus Total Parathyroidectomy for Secondary Hyperparathyroidism in Dialysis Patients: A Single-Center Cohort. J. Clin. Med. 2025, 14, 4944. [Google Scholar] [CrossRef]
- Hiramitsu, T.; Hasegawa, Y.; Futamura, K.; Okada, M.; Goto, N.; Narumi, S.; Watarai, Y.; Tominaga, Y.; Ichimori, T. Treatment for Secondary Hyperparathyroidism Focusing on Parathyroidectomy. Front. Endocrinol. 2023, 14, 1169793. [Google Scholar] [CrossRef] [PubMed]
- Guillén Martínez, A.J.; Smilg Nicolás, C.; Moraleda Deleito, J.; Guillén Martínez, S.; García-Purriños García, F. Risk Factors and Evolution of Calcium and Parathyroid Hormone Levels in Hungry Bone Syndrome after Parthyroidectomy for Primary Hyperparathyroidism. Endocrinol. Diabetes Nutr. (Engl. Ed.) 2020, 67, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Amjad, W.; Ginzberg, S.P.; Passman, J.E.; Heintz, J.; Kelz, R.R.; Wachtel, H. Predictive Risk Score for Postparathyroidectomy Hungry Bone Syndrome in Patients With Secondary Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2024, 109, 603–610. [Google Scholar] [CrossRef]
- Gao, D.; Liu, Y.; Cui, W.; Lu, X.; Lou, Y. A Nomogram Prediction Model for Hungry Bone Syndrome in Dialysis Patients with Secondary Hyperparathyroidism after Total Parathyroidectomy. Eur. J. Med. Res. 2024, 29, 208. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Guo, Y.; Mo, Q.; Ma, J. Prediction Model of Postoperative Severe Hypocalcemia in Patients with Secondary Hyperparathyroidism Based on Logistic Regression and XGBoost Algorithm. Comput. Math. Methods Med. 2022, 2022, 8752826. [Google Scholar] [CrossRef]
- Landsberg, A.; Brockman, N.K.; Sevinc, E.; McClurg, C.; Elliott, M.J.; Girard, L.-P.; James, M.T.; Leung, A.A.; Pannu, N.I.; Pasternak, M.; et al. Interventions to Reduce the Risk of Hypocalcemia After Parathyroidectomy for People With Advanced Chronic Kidney Disease: A Systematic Review. Can. J. Kidney Health Dis. 2025, 12, 20543581251358144. [Google Scholar] [CrossRef] [PubMed]
- Grube, M.; Weber, F.; Kahl, A.L.; Kribben, A.; Mülling, N.; Reinhardt, W. Effect of High Dose Active Vitamin D Therapy on the Development of Hypocalcemia After Subtotal Parathyroidectomy in Patients on Chronic Dialysis. Int. J. Nephrol. Renov. Dis. 2021, 14, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Lidor, C.; Edelstein, S. Calcitriol Increases Ca2+-ATPase Activity. Biochem. Biophys. Res. Commun. 1987, 144, 713–717. [Google Scholar] [CrossRef]
- Mayilvaganan, S.; Vijaya Sarathi, H.A.; Shivaprasad, C. Preoperative Zoledronic Acid Therapy Prevent Hungry Bone Syndrome in Patients with Primary Hyperparathyroidism. Indian. J. Endocrinol. Metab. 2017, 21, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-T.; Sheu, W.H.-H.; Tu, S.-T.; Kuo, S.-W.; Pei, D. Bisphosphonate Pretreatment Attenuates Hungry Bone Syndrome Postoperatively in Subjects with Primary Hyperparathyroidism. J. Bone Miner. Metab. 2006, 24, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A.; Stearns, M.P. Administration of Pamidronate Helps Prevent Immediate Postparathyroidectomy Hungry Bone Syndrome. Nephrology 2007, 12, 386–390. [Google Scholar] [CrossRef]
- Aschenbrenner, D.S. New Boxed Warning for Osteoporosis Drug. AJN Am. J. Nurs. 2024, 124, 18. [Google Scholar] [CrossRef]
- Gong, L.; Tang, W.; Lu, J.; Xu, W. Thermal Ablation versus Parathyroidectomy for Secondary Hyperparathyroidism: A Meta-Analysis. Int. J. Surg. 2019, 70, 13–18. [Google Scholar] [CrossRef]
- Alsafran, S.; Sherman, S.K.; Dahdaleh, F.S.; Ruhle, B.; Mercier, F.; Kaplan, E.L.; Angelos, P.; Grogan, R.H. Preoperative Calcitriol Reduces Postoperative Intravenous Calcium Requirements and Length of Stay in Parathyroidectomy for Renal-Origin Hyperparathyroidism. Surgery 2019, 165, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Niramitmahapanya, S.; Sunthornthepvarakul, T.; Deerochanawong, C.; Sarinnapakorn, V.; Athipan, P. Role of Loading Calcitriol to Control Hypocalcemia after Parathyroidectomy in Chronic Kidney Disease. J. Med. Assoc. Thai 2011, 94, 295–302. [Google Scholar]
- Bollerslev, J.; Rejnmark, L.; Zahn, A.; Heck, A.; Appelman-Dijkstra, N.M.; Cardoso, L.; Hannan, F.M.; Cetani, F.; Sikjaer, T.; Formenti, A.M.; et al. European Expert Consensus on Practical Management of Specific Aspects of Parathyroid Disorders in Adults and in Pregnancy: Recommendations of the ESE Educational Program of Parathyroid Disorders (PARAT 2021). Eur. J. Endocrinol. 2021, 186, R33–R63. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.H.; Tan, H.C.L.; Loke, S.C.; Arulanantham, S.A. Novel Calcium Infusion Regimen after Parathyroidectomy for Renal Hyperparathyroidism. Nephrology 2017, 22, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.; Vilayur, E.; Gao, M.; Sankoorikal, C.; Bendinelli, C. Calcitriol Loading before Total Parathyroidectomy with Autotransplant in Patients with End-Stage Kidney Disease: Does It Prevent Postoperative Hypocalcaemia? Intern. Med. J. 2019, 49, 886–893. [Google Scholar] [CrossRef]
- Silarat, P.; Saeseow, S.; Pathumarak, A.; Srisuwarn, P.; Suvikapakornkul, R.; Disthabanchong, S. Improved Clinical Outcomes Associated With Hungry Bone Syndrome Following Parathyroidectomy in Dialysis Patients. Endocr. Pract. 2024, 30, 1079–1088. [Google Scholar] [CrossRef]
- Kaya, B.; Altun, E.; Paydas, S.; Balal, M. The Efficacy of Dipyridamole in the Treatment of Hypophosphatemia- Hypocalcemia for Hungry Bone Syndrome in a Hemodialysis Patient. Saudi J. Kidney Dis. Transplant. 2015, 26, 363. [Google Scholar] [CrossRef]
- Goyal, A.; Anastasopoulou, C.; Ngu, M.; Singh, S. Hypocalcemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK430912/ (accessed on 1 October 2025).
- Li, B.; Lv, X.; Li, X.; Hou, X.; Xu, F. Postoperative Hungry Bone Syndrome in Primary Hyperparathyroidism: A Case Report. Medicine 2024, 103, e39717. [Google Scholar] [CrossRef] [PubMed]
| Phase (Post-PTX) | Time Frame | Key Biochemical Changes | Clinical Features & Management Considerations |
|---|---|---|---|
| Immediate | 0–6 h | PTH drops >90% within minutes; Ca2+ initially stable (due to buffers). | Typically, asymptomatic [121,122]. Initiate calcium prophylaxis (e.g., IV calcium), especially if pre-op PTH was high [12]. Monitor Ca2+ q6h [27]. |
| Early Acute | 12–72 h | Ca2+ starts to fall (notably by 18–24 h); PO43− rapidly falls by 24–48 h; PTH at nadir. | Subtle symptoms (perioral tingling, distal paresthesias, muscle cramps) may ensue. Positive Chvostek/Trousseau may be elicited [27]. Intensive Ca2+ monitoring (q6–8h) required; adjust IV/oral Ca2+ upwards as needed [27]. Ensure serum Mg2+ is normal [14]. Calcitriol analogues should be initiated [27]. |
| Subacute | Day 3–14 | Ca2+ reaches nadir (often day 5–7); ALP peaks (2–3× pre-op); PO43− often <2 mg/dL; Mg2+ low as well. BTMs (CTX, TRAP5b) at nadir. | Severe hypocalcemia symptoms may appear: tetany, seizures, bronchospasm, laryngospasm, arrhythmias. Cardiac monitoring mandatory [15,16]. Highest IV Ca2+ requirements (oftentimes >10 g calcium gluconate/24 h) [12]. PO43− supplementation if <1.5 mg/dL [12]; administer IV Mg2+ as needed [14]. Calcitriol doses should be maximized [12]. |
| Recovery | 2 weeks—12 months | Ca2+ gradually normalizes (weeks); ALP declines toward normal (months); PTH remains low (if total PTX) or low-normal (if subtotal/PTX+autograft). | Symptoms resolve. Taper IV Ca2+, then oral Ca2+ over weeks-months. High calcitriol dose continued until ALP normalizes and there is no hypocalcemia on minimal Ca2+ supplementation [15]. In ~10–15% of patients, oral Ca2+/vitamin D needed >1 year (“protracted HBS”) [16]. Endocrine follow-up for permanent hypoparathyroidism (if persistent) [27]. |
| Study (Year) | Patient Population (n, Details) | HBS Incidence (Definition) | Significant Preoperative Risk Factors for HBS |
|---|---|---|---|
| Ho et al., 2017 [30] | 62 dialysis patients; tPTX(-)AT; 10-year single-center cohort. | 27.4% (corrected Ca2+ ≤ 2.1 mmol/L, lasting for ≥4 days, within 1st month post-PTX) | Younger age, higher body weight, higher ALP and lower Ca2+ predicted HBS in MLR analysis. iPTH was elevated in HBS vs. non-HBS, but not an independent predictor (when adjusted for ALP). |
| Ge et al., 2019 [114] | 115 dialysis patients; tPTX(+)AT; 2.5-year single-center cohort. | 87.8% (total Ca2+ ≤ 2.1 mmol/L and/or hypocalcemia for ≥4 days post-PTX) | Higher ALP and lower Ca2+ predicted HBS in MLR analysis. Younger age, higher ALP and higher iPTH positively correlated with HBS severity. Specific bone metabolism dynamics were revealed (post-PTX: iPTH, CT, CTX and TRACP5b rapidly decreased, while OC and ALP increased more slowly). |
| Kritmetapak et al., 2020 [18] | 130 dialysis patients; PTX technique at surgeons discretion; 6-year single-center cohort. | 82.3% (Ca2+ nadir <8.4 mg/dL within the first 3 days post-PTX and/or requiring IV Ca2+ for symptoms) | PTH >1000 pg/mL, ALP >420 U/L, age ≤45 years and absence of hypercalcemia (corrected Ca2+ <10.2 mg/dL) were significantly associated with HBS in MLR analysis. |
| Phimphilai et al., 2022 [116] | 179 dialysis patients; mostly tPTX(+)AT (79.3%); 22- year single-center cohort. | 82.1% (corrected Ca2+ <8.5 mg/dL for >3 days post-PTX) | Longer dialysis vintage (≥5 years), higher PO43− (≥5 mg/dL); higher ALP (≥387 U/L) and mean difference between iPTH pre- and post-PTX (>97%) were independent risk factors for hypocalcemia in MLR analysis. |
| Gao et al., 2022 [129] | 2990 ESRD patients with rSHPT (13 studies, from 2013–2021); variable PTX techniques (including ablation). | Not applicable (Meta-analysis of risk factors for post-PTX hypocalcemia) | Lower Ca2+, higher ALP and higher iPTH were associated with post-PTX hypocalcemia (i.e., Ca2+ <8.4 mg/dL within first 3 days post-PTX). Age was not significant. |
| Mehta et al., 2024 [11] | 2598 patients undergoing PTX for primary, secondary and tertiary HPT (18 studies, from 2006–2021); variable PTX techniques. | 43.6% for SHPT cohort (Systematic review of risk HBS factors) | Younger age, larger glands, previous dialysis, longer dialysis vintage, Ca2+ close to normal, higher iPTH (i.e., >90% drop in PTH post-PTX), higher ALP, higher OC, lower albumin, and higher TRACP5b were HBS predictors in the SHPT cohort |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coman, A.; Tarta, C.; Marian, M.; Popa, D.I.; Olariu, S.; Rosu, M.; Utu, D.; Buleu, F.; Macovei-Oprescu, A.-M.; Novacescu, D.; et al. Hungry Bone Syndrome After Parathyroidectomy for Secondary Hyperparathyroidism: Pathogenesis and Contemporary Clinical Considerations. J. Clin. Med. 2025, 14, 7104. https://doi.org/10.3390/jcm14197104
Coman A, Tarta C, Marian M, Popa DI, Olariu S, Rosu M, Utu D, Buleu F, Macovei-Oprescu A-M, Novacescu D, et al. Hungry Bone Syndrome After Parathyroidectomy for Secondary Hyperparathyroidism: Pathogenesis and Contemporary Clinical Considerations. Journal of Clinical Medicine. 2025; 14(19):7104. https://doi.org/10.3390/jcm14197104
Chicago/Turabian StyleComan, Adina, Cristi Tarta, Marco Marian, Daian Ionel Popa, Sorin Olariu, Mihai Rosu, Diana Utu, Florina Buleu, Anca-Monica Macovei-Oprescu, Dorin Novacescu, and et al. 2025. "Hungry Bone Syndrome After Parathyroidectomy for Secondary Hyperparathyroidism: Pathogenesis and Contemporary Clinical Considerations" Journal of Clinical Medicine 14, no. 19: 7104. https://doi.org/10.3390/jcm14197104
APA StyleComan, A., Tarta, C., Marian, M., Popa, D. I., Olariu, S., Rosu, M., Utu, D., Buleu, F., Macovei-Oprescu, A.-M., Novacescu, D., Zara, F., & Murariu, M. (2025). Hungry Bone Syndrome After Parathyroidectomy for Secondary Hyperparathyroidism: Pathogenesis and Contemporary Clinical Considerations. Journal of Clinical Medicine, 14(19), 7104. https://doi.org/10.3390/jcm14197104







