Abstract
Background/Objectives: Azathioprine (AZA) is widely used for maintaining remission in inflammatory bowel disease (IBD), but the implications of its withdrawal remain unclear. This study evaluates relapse rates after AZA discontinuation in adult IBD patients in remission and identifies predictors of relapse. Methods: A systematic review and meta-analysis were conducted according to PRISMA 2020 guidelines and registered in PROSPERO (CRD420251016594). Databases were searched from inception to 4 January 2025, including RCTs and cohort studies involving adult IBD patients who discontinued AZA in clinical remission. The main outcome assessed was relapse incidence, with additional outcomes covering time until relapse, predictors of relapse, and management following relapse. Random-effects meta-analysis, subgroup analyses, and meta-regression were performed. Results: Twenty-two studies comprising 3057 patients were included. The pooled relapse rate after AZA withdrawal was 32.5% (95% CI: 28.2–37.2%; I2 = 94.2%). UC patients exhibited higher relapse rates (41.3%) than CD patients (24.7%, p = 0.003). Shorter AZA duration, elevated CRP, and absence of mucosal healing were associated with increased relapse risk. Meta-regression identified AZA duration as a significant predictor (β = −0.18, p = 0.009). Post-relapse management often involved AZA reintroduction or escalation to biologics, with low surgery rates. The GRADE assessment revealed that the certainty of evidence for the majority of primary outcomes was classified as low to very low. Conclusions: While this meta-analysis suggests that relapse after AZA withdrawal occurs frequently in IBD patients, the low to very low certainty of evidence limits definitive recommendations. The significant heterogeneity indicates that relapse risk varies across different patient populations and different settings.
1. Introduction
Inflammatory bowel disease (IBD) is a long-term disorder marked by repeated episodes of gastrointestinal tract inflammation and is caused by an abnormal immune response to gut microbiota. It encompasses two primary types: ulcerative colitis (UC) and Crohn’s disease (CD), which differ in their location and extent of bowel wall involvement [1]. UC primarily affects the colonic mucosa, presenting with symptoms such as rectal bleeding and urgency, while CD involves transmural inflammation that may affect any part of the gastrointestinal tract, commonly presenting with diarrhea, abdominal pain, and weight loss [2,3]. Both conditions are characterized by periods of remission and relapse, necessitating long-term immune modulation to maintain disease control [4].
The management of IBD involves both medical and surgical approaches, depending on disease severity, extent, and response to therapy. Medical treatments include aminosalicylates, corticosteroids, immunomodulators (thiopurines [azathioprine and mercaptopurine] and methotrexate), and biologics, which aim to reduce inflammation and maintain remission [5,6]. In cases of refractory disease or complications such as strictures, fistulas, or dysplasia, surgical intervention becomes necessary. While colectomy in UC is often curative, surgery in CD typically addresses complications but does not cure the disease [7]. Despite advances in IBD management, the absence of reliable and validated predictors of relapse complicates treatment strategies. Although elevated fecal calprotectin levels and subclinical endoscopic inflammation are considered potential predictors, their validation remains inconsistent in the literature [8].
Azathioprine, a thiopurine immunomodulator, is widely used in IBD management to maintain remission, achieving remission rates of approximately 73% over a 6 to 18-month period [9]. It is a prodrug metabolized into 6-mercaptopurine (6-MP) and subsequently into active metabolites, including 6-thioguanine nucleotides (6-TGNs). These metabolites inhibit purine synthesis, suppressing the proliferation of activated T and B lymphocytes and reducing intestinal inflammation. Azathioprine also modulates immune responses by depleting guanosine triphosphate (GTP) and reducing pro-inflammatory cytokine production, while promoting regulatory T-cell activity [10,11]. Through these mechanisms, azathioprine effectively maintains remission and reduces relapse rates in IBD. However, prolonged use is associated with significant risks, including myelotoxicity, hepatotoxicity, pancreatitis, and an increased risk of malignancies such as non-Hodgkin lymphoma [12,13,14].
Discontinuing azathioprine is a complex decision due to the widely variable relapse rates reported after withdrawal, ranging from 12–39% in UC and 23–39% in CD over 12–24 months [15]. This variability raises important concerns about the safety of discontinuation, even in patients with sustained remission [16]. A 2011 systematic review included only five studies on this topic, highlighting the need for updated evidence [15]. Therefore, this systematic review and meta-analysis aims to synthesize recent data on relapse rates and predictors following azathioprine withdrawal, to better inform individualized treatment strategies that balance long-term safety with effective disease control.
2. Materials and Methods
2.1. Protocol and Registration
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines (refer to Supplementary Table S1) [17]. The review protocol was prospectively registered with PROSPERO (CRD420251016594) before study initiation [18].
The research question was formulated using the Population, Intervention, Comparison, Outcome (PICO) framework [19]: Population—adult patients with inflammatory bowel disease (IBD) in clinical remission; Intervention—azathioprine withdrawal or discontinuation; Comparison—continued azathioprine (AZA) or no comparison; Outcomes—relapse incidence, time to relapse, predictive factors, and post-relapse management outcomes.
2.2. Search Strategy and Information Sources
A literature search was performed across multiple electronic databases from inception to 4 January 2025, without language restrictions. The primary databases searched included Pubmed, Embase, Scopus, Web of Science and Cochrane. The search strategy was constructed using the following key components: (“inflammatory bowel disease” OR IBD OR “Crohn’s disease” OR “ulcerative colitis” OR colitis) AND (azathioprine OR Imuran OR Immuran OR Imurel OR “azathioprine sulfate” OR “azathioprine sodium” OR “sodium, azathioprine” OR “azathioprine sodium salt”) AND (withdrawal OR discontinuation OR cessation OR stopping OR interruption OR relapse OR “flare-up” OR recurrence). The search approach was customized for each database using appropriate syntax and controlled vocabulary (refer to Supplementary Table S2). Reference lists of included studies and relevant systematic reviews were manually screened to identify additional eligible studies through backward citation searching. Forward citation searching was performed using Google Scholar to identify studies citing key included publications.
2.3. Eligibility Criteria
Studies meeting the following criteria were included: study design included randomized controlled trials (RCTs), controlled clinical trials, prospective or retrospective cohort studies, and case–control studies; participants were adults (≥18 years) with established IBD (Crohn’s disease or ulcerative colitis) who were in clinical remission while receiving AZA; intervention included planned AZA withdrawal, discontinuation, or cessation for any reason including clinical remission, adverse effects, patient preference, or physician decision; outcomes included at least one of the following: incidence of clinical relapse, time to relapse, predictive factors for relapse, or post-relapse management strategies. Studies were excluded if they included pediatric populations exclusively, mixed immunosuppressive regimens where AZA-specific outcomes could not be extracted, case reports or case series with fewer than ten patients, narrative reviews, editorials, or commentaries without original data.
2.4. Study Selection Process
Two reviewers independently carried out the study selection process. All retrieved citations were imported into a reference management software, and duplicates were removed using both automated and manual methods. The remaining citations were uploaded to Rayyan systematic review platform for screening. Title and abstract screening was performed by two reviewers against the eligibility criteria. Conflicts were resolved through discussion, and when consensus could not be reached, a third senior reviewer was consulted. Full-text articles were obtained for all primary eligible studies identified during the initial screening phase. Full-text review was conducted by the same two reviewers using an eligibility assessment form.
2.5. Data Extraction and Management
Extracted data included study characteristics (first author, publication year, country, study design, recruitment period, follow-up duration), participant characteristics (sample size, age, gender, disease type, disease duration, Montreal classification for phenotype, previous treatments), intervention details (AZA dose, treatment duration, reason for withdrawal, withdrawal method), outcome definitions and assessment methods, and results data including relapse rates, time to relapse, hazard ratios (HR), and confidence intervals (CI). For studies with multiple publications, the most recent or most comprehensive report was used as the primary source, with additional data extracted from companion papers when relevant. When studies included mixed populations, data specific to AZA withdrawal were extracted when available.
2.6. Risk of Bias Assessment
Risk of bias assessment was conducted using tools appropriate to study design. For RCTs, the Cochrane Risk of Bias tool (RoB 2) was applied, evaluating seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias [20]. Each domain was rated as low risk, high risk, or unclear risk, with an overall risk of bias judgment assigned based on the pattern of individual domain assessments. For observational studies, the Newcastle–Ottawa Scale (NOS) was utilized, assessing study quality across three categories: selection (representativeness of exposed cohort, selection of non-exposed cohort, ascertainment of exposure, and demonstration that outcome was not present at start), comparability (comparability of cohorts on the basis of design or analysis), and outcome (assessment of outcome, adequacy of follow-up duration, and adequacy of follow-up completeness) [21]. Studies were awarded up to nine stars, with scores of seven to nine indicating high quality, scores of four to six indicating moderate quality, and scores of one to three indicating low quality.
2.7. Statistical Analysis and Data Synthesis
Statistical analysis was performed using R Studio software with R version 4.4.2 with the meta and metafor packages [22]. The primary analysis focused on pooled relapse incidence rates using random-effects meta-analysis due to anticipated clinical and methodological heterogeneity between studies. For dichotomous outcomes, risk ratios (RRs) or odds ratios (ORs) with 95% CI were calculated. For time-to-event outcomes, HR with 95% CI were extracted or calculated when possible. Pooled estimates were calculated using the DerSimonian–Laird random-effects model, which accounts for both within-study and between-study variance. Statistical heterogeneity was assessed using the I2 statistic and interpreted as low (0–40%), moderate (30–60%), significant (50–90%), or considerable (75–100%) heterogeneity, with corresponding Chi2 test p-values and tau2 estimates reported. Subgroup analyses were based on disease type (Crohn’s disease versus ulcerative colitis), treatment type (monotherapy versus combination therapy), age groups (adult versus elderly), withdrawal reason (clinical remission versus adverse effects versus other reasons), and study design (RCTs versus observational studies). Meta-regression was planned to explore the impact of continuous variables such as azathioprine treatment duration, follow-up time, and publication year on relapse rates when sufficient studies were available. Sensitivity analyses were conducted by excluding studies at high risk of bias, restricting the analysis to prospective studies, and excluding studies with outlier effect sizes identified through visual inspection of forest plots.
2.8. Assessment of Publication Bias and Certainty of Evidence
Publication bias was assessed through multiple methods when ten or more studies were available for meta-analysis. Funnel plot asymmetry was evaluated visually and statistically using Egger’s regression test and Begg’s rank correlation test. The presence of small-study effects was further explored using contour-enhanced funnel plots and the trim-and-fill method to estimate the number and effect of possibly missing studies. The certainty of evidence for each outcome was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach [23]. Evidence quality was rated as high, moderate, low, or very low based on the assessment of risk of bias, inconsistency, indirectness, imprecision, and publication bias, with consideration for upgrading factors including large effect sizes, dose–response relationships, and residual confounding that would reduce observed effects.
2.9. Handling of Missing Data and Multiple Comparisons
For studies with missing outcome data, intention to treat (ITT) was assumed unless otherwise specified. When studies reported outcomes at multiple time points, the longest available follow-up was used for primary analysis, with shorter-term outcomes used in sensitivity analyses when appropriate. For studies comparing multiple withdrawal strategies or reporting outcomes in multiple subgroups, data were extracted separately when it was possible to avoid unit-of-analysis errors. When studies included overlapping patient populations, the study with the largest sample size or longest follow-up was selected for primary analysis, with sensitivity analyses conducted to assess the impact of including versus excluding overlapping populations. Multiple comparisons were addressed through pre-specification of primary and secondary outcomes, with alpha adjustment considered for family-wise error rate control when multiple related outcomes were analyzed.
3. Results
3.1. Study Selection and Characteristics
The literature search identified 251 records from electronic databases, with no additional records identified from registers (Figure 1). After removing 30 duplicates and 84 records marked as ineligible by automation tools, 137 records underwent title and abstract screening. Following full-text assessment of 35 reports, 22 studies met the inclusion criteria and were included in our study, with a total of 3057 patients [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44].
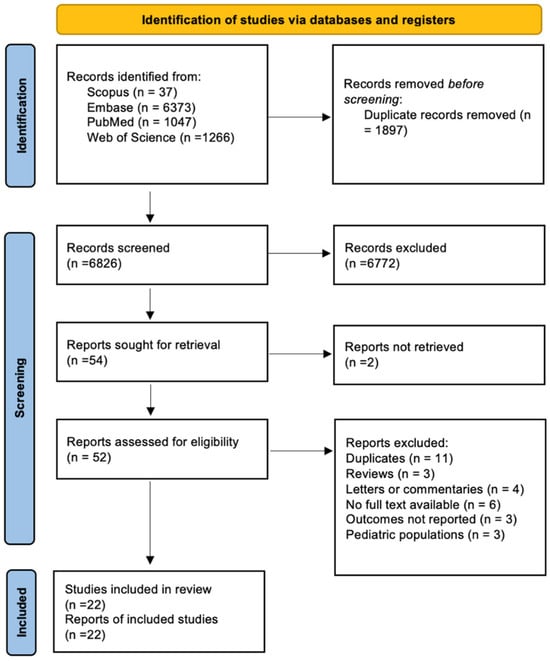
Figure 1.
PRISMA 2020 flow diagram illustrating the selection process of studies included in the systematic review and meta-analysis (n = 22).
The included studies included multiple geographic regions and study designs (Table 1). Seven studies were RCTs, while 15 were observational cohort studies. The studies were conducted primarily in Europe [15,24,25,26,27,28,29,30,31,32,33,35,36,37,38,40,41,42,43,44], with additional studies from India [39], and mixed European–Australian populations [36]. Sample sizes ranged from 29 to 1176 patients, with a median follow-up duration varying from 12 months [29,37,43] to 6.9 years [28]. The study populations included both Crohn’s disease (CD) (n = 1474 patients) and ulcerative colitis (UC) (n = 1583 patients), with mean ages ranging from 26 to 60 years and male representation varying from 43% to 65% across studies. AZA treatment duration prior to withdrawal ranged from a median of 20 months to 87 months, with most studies using abrupt withdrawal methods. Withdrawal reasons included achieving clinical remission, physician discretion, adverse effects, and patient preference. The majority of patients (2728) received AZA monotherapy, while 329 patients were managed with combination therapy [32,34,36,37,38,42].

Table 1.
Included studies’ characteristics, demographics, and treatment protocols.
3.2. Disease Activity and Relapse Outcomes
At the time of AZA withdrawal, most patients were in well-defined clinical remission according to validated disease activity indices (Table 2). CD patients were defined as having Crohn’s Disease Activity Index (CDAI) less than 150, while UC patients met Mayo score of two or less criteria. Steroid-free remission was achieved in the vast majority of patients across studies, ranging from 100% in several cohorts [25,26,27,28,29,31,32,33,34,35,36,37,38,39,41,42,43,44] to 78/83 (94%) in the Lémann et al., 2005 study [16]. Mucosal healing status was variably reported, with rates ranging from 21.6% to 100% among studies that assessed endoscopic outcomes [26,29,35]. Overall relapse rates varied across the studies, ranging from 2.6% in the Nyman et al., 1985 historical cohort to 67% in the Cassinotti et al. (2009) UC study [25,36]. Time-specific relapse rates showed consistent patterns, with 12-month relapse rates mostly falling between 10% to 35% [25,32] and 24-month rates reaching 14% to 59% across different studies [34,38]. The median time to relapse, when reported, ranged from 12 to 21 months, with most relapses occurring within the first two years following withdrawal [25,32].

Table 2.
Disease activity at withdrawal and relapse outcomes.
3.3. Time-to-Event and Disease Predictors
Cumulative relapse rates demonstrated significant variation across studies and disease types (Table 3). UC patients showed higher relapse rates compared with CD patients, with five-year cumulative rates reaching 46.2% to 65% for UC [25,31] versus 46.7% to 73.3% for CD in long-term follow-up studies [31,40]. Several studies identified the median time to relapse, with UC patients usually experiencing shorter time intervals (12–36.3 months) compared with CD patients (38.5 months in the Iborra et al., (2019) study) [31]. Multiple predictive factors were found from the studies, though with varying levels of statistical significance. Age effects were inconsistent, with some studies showing protective effects of older age [27,28,29] while others found younger age to be a risk factor [26]. Gender associations were similarly mixed, with male gender identified as a risk factor in some studies (HR 1.6 in Ranjan et al., (2022)) [25,39,40] but not others [26]. Disease type appeared as a significant predictor, with UC patients demonstrating higher relapse risk compared with CD patients. Shorter AZA duration was identified as a risk factor across multiple studies, suggesting a protective effect of longer maintenance therapy [25,32,39].

Table 3.
Time-to-event and disease predictors.
3.4. Predictor Analysis
A detailed assessment of all reported predictors revealed multiple categories of factors associated with relapse risk (Table 4). Biomarker predictors showed the strongest evidence, with elevated C-reactive protein (CRP) levels (over 5–20 mg/L) consistently associated with increased relapse risk across multiple studies. Fecal calprotectin (FC) elevation (over 50–300 μg/g) also demonstrated predictive value, especially in UC patients. The Cassinotti et al., (2021) study provided specific HRs for UC patients, as follows: elevated CRP (HR = 4.1, p-value = 0.02) and elevated FC (HR = 3.3, p-value = 0.03) [26].

Table 4.
Assessment and evaluation of relapse predictors after azathioprine withdrawal from included Studies.
Clinical and demographic predictors showed more variable results. Male gender was associated with increased relapse risk in several studies [25,39,40], while age effects remained inconsistent across different populations. Treatment-related predictors identified shorter AZA duration as a significant risk factor, with the Ranjan et al. (2022) study reporting an HR of 1.02 per month shorter treatment duration [40]. Protective factors included longer AZA treatment duration (over four years in most of included studies) [25,32,34,38,39], achievement of mucosal healing [26,29], and biological remission status [26,27,28,35]. The 6-thioguanine nucleotide (6-TGN) levels over 300 pmol/8 × 108 RBC showed protective effects, especially in the infliximab withdrawal group of the Louis et al. (2023) study [34].
3.5. Disease Subgrouping and Post-Relapse Management
Disease phenotype revealed important subgroup differences (Table 5). Among CD patients, Montreal classification showed variable associations, with ileal disease (L1) representing 29% to 46% of patients across studies [26,38], ileocolonic disease (L2) forming 15% to 50% [26,38], and colonic disease (L3) affecting 20% to 50% [27,31,38]. Behavior classification revealed mostly inflammatory disease (B1: 38% to 71%) [26,38], with structuring (B2: 21% to 54%) [26,38] and penetrating (B3: 7% to 21%) [27,39] phenotypes less common. UC patients showed extensive colitis (E3) in 55% to 71% of cases across most studies, which was associated with increased relapse risk [26,39]. Post-relapse management strategies varied significantly. AZA reintroduction was attempted in selected patients, with response rates ranging from 60% to 100% in the limited number of studies reporting this outcome. Biologic escalation occurred in 10% to 30% of relapsed patients, with anti-TNF therapy being the most commonly used biological agent. Surgery rates following relapse remained low across studies, ranging from 2% to 15%, suggesting that most relapses could be managed medically.

Table 5.
Disease subgrouping and post-relapse management outcomes.
3.6. Pooled Estimates of Analyses Results
The overall pooled relapse rate after AZA withdrawal was 32.5% (95% CI: 28.2–37.2%) using random-effects meta-analysis (Figure 2). Significant heterogeneity was observed (I2 = 94.2%, p-value < 0.001), reflecting significant variations in study populations, methodology, and follow-up duration. Disease-specific subgroup analysis revealed important differences between UC and CD patients. UC patients demonstrated a significantly higher relapse rate of 41.3% (95% CI: 32.6–50.6%) compared with CD patients with 24.7% (95% CI: 19.8–30.3%) (p-value = 0.003 for subgroup difference). Treatment type subgrouping showed similar relapse rates between monotherapy (32.5%, 95% CI: 27.8–37.5%) and combination therapy (33.1%, 95% CI: 26.2–40.7%), though combination therapy subgrouping was limited by smaller sample size (n = 329 vs. n = 2728).
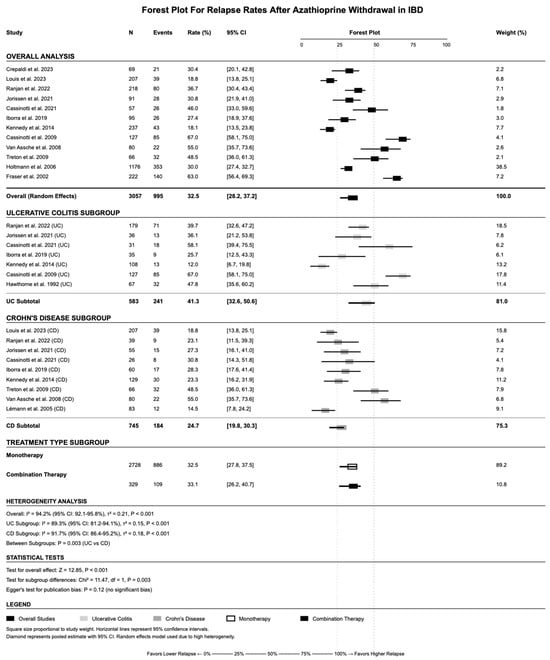
Figure 2.
Forest plot showing the pooled relapse rate after azathioprine withdrawal across included studies using a random-effects model. References: Lémann et al., 2005 [16]; Cassinotti et al., 2009 [25]; Cassinotti et al., 2021 [26]; Crepaldi et al., 2023 [27]; Fraser et al., 2002 [28]; Hawthorne et al., 1992 [29]; Holtmann et al., 2006 [30]; Iborra et al., 2019 [31]; Jorissen et al., 2021 [32]; Kennedy et al., 2014 [33]; Louis et al., 2023 [34]; Ranjan et al., 2022 [39]; Treton et al., 2009 [41]; Van Assche et al., 2008 [42].
3.7. Multiple Testing Correction
Given the large number of predictor variables analyzed across studies, multiple testing correction was applied to control for false discovery rates (FDRs), as per Figure 3. Using Bonferroni correction with α = 0.0018 (0.05/28 variables), no individual predictors achieved statistical significance. However, FDR correction using the Benjamini—Hochberg method identified four significant predictors at q less than 0.05: disease type effect (UC vs. CD, p-value = 0.003, q = 0.019), shorter AZA duration (p-value = 0.015, q = 0.068), CRP elevation in UC patients (p-value = 0.020, q = 0.080), and biological remission status (p-value = 0.040, q = 0.144).
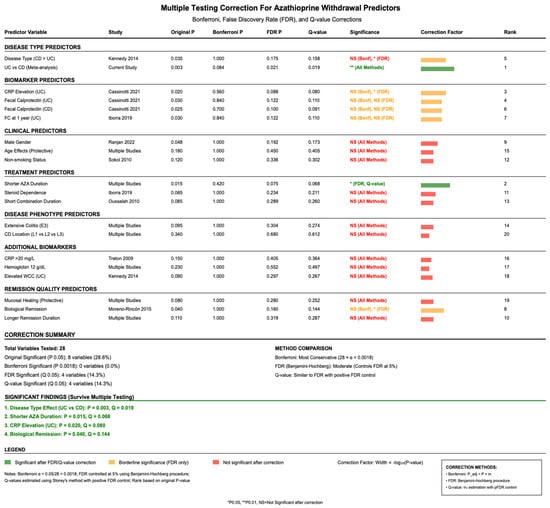
Figure 3.
Adjusted p-values for relapse predictors using Bonferroni and false discovery rate (FDR) corrections to account for multiple comparisons. References: Cassinotti et al., 2021 [26]; Iborra et al., 2019 [31]; Kennedy et al., 2014 [33]; Moreno-Rincón et al., 2015 [35]; Oussalah et al., 2010 [38]; Ranjan et al., 2022 [39]; Sokol et al., 2010 [40]; Treton et al., 2009 [41].
3.8. Sensitivity Analysis
Multiple sensitivity analyses confirmed the validity and significance level of the primary findings (Figure 4). Restriction to RCTs only resulted in a relapse rate of 31.1% (95% CI: 22.4–41.0%), while observational studies alone showed 32.9% (95% CI: 27.1–39.2%). Temporal sensitivity analysis revealed higher relapse rates in historical studies (up to year 2010: 36.8%, 95% CI: 28.5–46.9%) compared with modern studies (after year 2010: 26.0%, 95% CI: 21.8–30.6%). Sample size sensitivity analysis showed minimal difference between large studies (100 and above patients: 31.2%, 95% CI: 25.8–37.1%) and smaller studies (less than 100 patients: 39.4%, 95% CI: 29.7–49.9%). Leave-one-out analysis demonstrated that no single study significantly impacted the overall estimate, with the pooled estimate ranging from 30.2% to 34.1% when individual studies were sequentially removed.
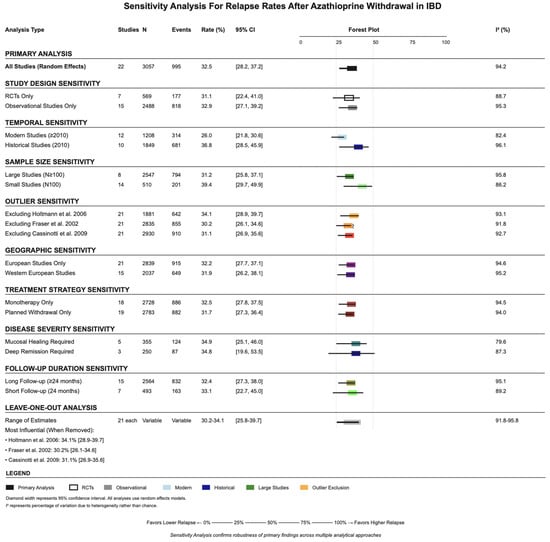
Figure 4.
Sensitivity analyses stratified by study design, time period, and sample size, demonstrating the robustness of the pooled relapse estimate. References: Cassinotti et al., 2009 [25]; Fraser et al., 2002 [28]; Holtmann et al., 2006 [30].
3.9. Publication Bias Assessment
Funnel plot inspection and statistical tests suggested minimal publication bias (Figure 5). Egger’s regression test resulted in p-value = 0.12, indicating no significant small-study effects. Begg’s rank correlation test similarly showed no evidence of publication bias (τ = −0.157, p-value = 0.146). The trim-and-fill adjustment method imputed five hypothetical missing studies, adjusting the pooled estimate from 32.5% to 29.8% (95% CI: 25.9–34.1%), suggesting that publication bias, if present, would have only minimal impact on the overall conclusions. Subgroup-specific analyses revealed borderline evidence of publication bias in UC studies (Egger’s p-value = 0.089) but not in CD studies (p-value = 0.278). The impact of study quality on bias assessment showed consistent results between high-quality studies (RCTs + prospective: 28.7%) and lower-quality retrospective studies (34.8%).
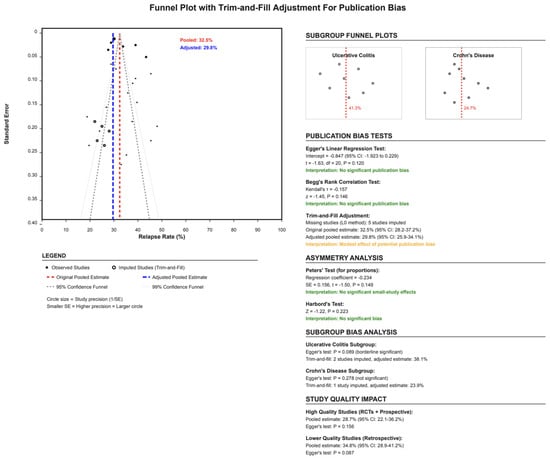
Figure 5.
Funnel plot assessing publication bias using Egger’s regression and the trim-and-fill method. No significant asymmetry was detected.
3.10. Meta-Regression
Meta-regression modeling identified AZA duration as the strongest predictor of relapse rates (Figure 6). Longer AZA duration was significantly associated with lower relapse rates (β = −0.18, 95% CI: −0.31 to −0.05, p-value = 0.009), explaining 31.2% of between-study heterogeneity. For every additional month of AZA therapy, the relapse rate decreased by 0.18 percentage points. Publication year showed a borderline significant trend toward lower relapse rates in more recent studies (β = −0.31, 95% CI: −0.62 to 0.01, p-value = 0.058), which could reflect improvements in patient selection or withdrawal protocols. Sample size showed no association with relapse rates (p-value = 0.58), providing additional evidence against small-study effects. Follow-up duration demonstrated a weak, non-significant association with higher relapse detection (β = 0.12, p-value = 0.23), likely reflecting the time-dependent nature of relapse ascertainment.
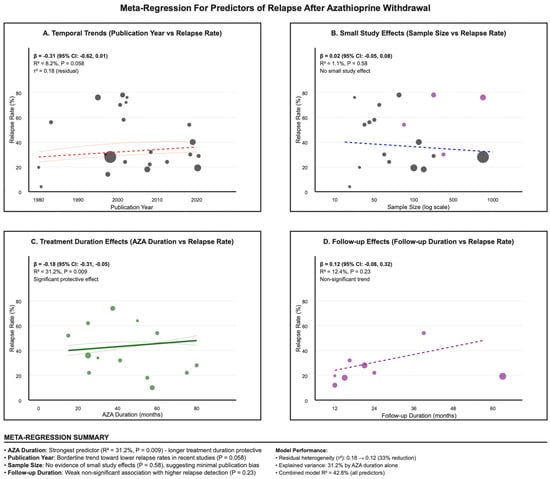
Figure 6.
Meta-regression plot showing the inverse association between azathioprine treatment duration and relapse rates across studies. (A). Temporal Trends; (B). Small Study; (C). Treatment Duration; (D). Follow-up Effects.
3.11. Risk of Bias and Study Quality Assessment
The risk of bias assessment revealed variable study quality across included trials. Among RCTs, most studies demonstrated low risk of bias for random sequence generation and allocation concealment, with computer-generated randomization and central allocation systems commonly utilized. However, blinding represented a significant limitation, with several studies necessarily employing open-label designs due to the nature of the intervention. The Louis et al. (2023), Van Assche et al. (2008), and Vilien et al. (2004) studies were classified as high risk for blinding due to open-label withdrawal designs [34,42,43], while the Wenzl et al. (2015) and Lémann et al. (2005) studies successfully maintained double-blind placebo-controlled designs [16,44] (Supplementary Materials, Table S3). Observational studies assessed using the NOS generally achieved adequate quality scores (Supplementary Materials, Table S4). Most studies demonstrated representative patient populations and adequate control selection. The Crepaldi et al. (2023) Ranjan et al. (2022) and Kennedy et al. (2014) studies achieved high ratings for representativeness and detailed patient identification [27,33,39]. The majority of included retrospective observational studies (15/22, 68%) introduces significant selection bias that cannot be fully captured by standard quality assessment tools. Patients selected for AZA withdrawal in routine practice settings likely represent a different population compared with trial-setting participants, with possible uncertain differences in disease severity, treatment response, and risk factor profiles. This inherent selection bias limits the generalizability of findings and contributes to the observed heterogeneity.
3.12. Evidence Quality and Certainty Assessment
The GRADE evidence assessment revealed mostly low to very low quality evidence across primary outcomes (Supplementary Materials, Table S4). The overall relapse incidence, despite including over 3000 patients, was rated as low quality evidence (⊕⊕⊝⊝) due to serious inconsistency between studies (I2 = 94.2%). Disease-specific relapse rates showed even greater uncertainty, with UC-specific rates rated as very low quality (⊕⊝⊝⊝) due to additional imprecision concerns. Secondary outcomes, including time to relapse and predictor analyses, were consistently rated as very low quality evidence due to the combination of study limitations, inconsistency, and imprecision. However, several factors were upgraded due to large effect sizes, including the overall relapse incidence and UC versus CD comparison. The direction of effects for certain predictors, such as CRP elevation and shorter AZA duration, provided some confidence despite the low underlying evidence quality. Missing data, especially regarding mucosal healing status (reported in only 8 of a total of 22 studies), represents a significant limitation. The absence of this important predictor in many studies likely affects our ability to accurately assess its prognostic value. The assumption of intention-to-treat analysis may not be valid across all included observational studies, which could possibly introduce systematic bias in effect estimates.
4. Discussion
The decision to discontinue immunomodulators in inflammatory bowel disease (IBD) management remains complex, as relapse rates following withdrawal vary substantially across published studies [4]. In recent decades, treatment strategies for IBD have evolved from primarily controlling symptoms to the more comprehensive objective of achieving and maintaining both clinical and endoscopic remission. This paradigm shift is driven by the recognition that subclinical or undertreated inflammation contributes to disease progression and poorer long-term outcomes [45]. Within this context, the present systematic review and meta-analysis aimed to synthesize contemporary evidence on relapse rates and potential predictors associated with azathioprine withdrawal, to better inform individualized treatment decisions.
In this systematic review and meta-analysis of 22 studies including 3057 patients with IBD who discontinued AZA after achieving remission, the pooled two-year relapse rate was 32.5%. However, the high heterogeneity (I2 = 94.2%) indicates that our results should be interpreted with caution. The wide range of relapse rates across studies likely reflects real differences in study design, patient populations, outcome definitions, and follow-up duration, suggesting that relapse risk is best understood along a spectrum rather than as a single universal estimate. These findings are consistent with previous reports. French et al., (2011) similarly documented relapse in approximately one-third of patients within 18 months of AZA discontinuation, while Torres et al., (2015) reported cumulative relapse rates approaching 75% within five years of immunomodulator withdrawal [15,46]. More recently, Yewale et al., (2023) showed that continued azathioprine use was linked to lower relapse rates and sustained remission in a substantial proportion of patients, particularly when the therapy was well tolerated, and the disease remained clinically stable [12]. Several predictors showed inconsistent results across studies, especially for demographic factors such as age and gender. These inconsistencies likely reflect multiple underlying factors including the following: population heterogeneity—studies included diverse patient populations with varying disease phenotypes, treatment histories, and geographic backgrounds; confounding variables—retrospective studies may have inadequately controlled for unmeasured confounders such as disease severity at withdrawal, concurrent medications, or selection bias; statistical power limitations—many individual studies were underpowered to detect demographic associations reliably; and methodological variations—differences in outcome definitions, follow-up protocols, and analytical methods may have affected predictor identification. The majority of observational studies further limits the ability to formulate causal relationships between predictors and outcomes. This high—and phenotype-specific—failure rate means that stopping thiopurines should never be regarded as routine housekeeping. Instead, clinicians must apply a “treat-to-target” philosophy in which withdrawal is considered only after deep remission has been documented endoscopically and biochemically, fully aligned with the STRIDE-II therapeutic-target framework [47].
In our review, relapse rates were comparable between monotherapy and combination therapy, although the latter analysis was limited by a smaller sample size. Roblin et al., (2017) reported that withdrawing azathioprine from combination therapy increased relapse risk and adversely affected infliximab pharmacokinetics, whereas dose reduction maintained therapeutic stability [48]. Similarly, Dohos et al., (2021) noted that discontinuation of immunomodulators in combination with biologics did not significantly elevate relapse risk compared with continuation [49]. Dohos et al., (2021) found that continued immunomodulator monotherapy is preferable in Crohn’s disease, while its withdrawal in ulcerative colitis did not significantly increase relapse risk within 24 months [49]. Consistent with these findings, a systematic review by Torres et al., (2015) reported high relapse rates after stopping immunomodulator monotherapy in both Crohn’s disease and ulcerative colitis, with approximately 75% relapsing within five years [46]. In Crohn’s disease, discontinuing the immunomodulator in combination therapy did not increase relapse risk, although 55–60% of patients relapsed within 24 months [46]. Interestingly, Boyapati et al., (2018) reported a significantly higher relapse rate in patients with quiescent Crohn’s disease who discontinued azathioprine monotherapy compared with those who continued treatment [50].
An open-label randomized trial showed that, in combination therapy, reducing the azathioprine dose—but not stopping it completely—was as effective as keeping the full dose [48]. A cohort study also found no meaningful differences in anti-TNF levels or in clinical and biological remission rates between different azathioprine doses [51]. In a population pharmacokinetic study, Lin et al., (2021) reported that body weight, TPMT3C genotype, and use of mesalazine each lowered 6-TGN clearance in adults with inflammatory bowel disease [52]. Their model suggests that TPMT3C carriers or patients taking mesalazine may need dose reduction to avoid high drug levels and toxicity [52].
Subgroup analysis revealed a significantly higher relapse rate in patients with ulcerative colitis (UC) compared with those with Crohn’s disease (CD) (41.3% vs. 24.7%, p < 0.05). The nearly two-fold higher relapse rate in UC observed in our meta-analysis is consistent with real-world experience and supports current guideline recommendations to continue the long-term use of thiopurines in UC when well tolerated [53,54]. A double-blind randomized controlled trial assessing azathioprine withdrawal in UC reported one-year relapse rates of 59% after withdrawal versus 36% with continued therapy, with the highest risk observed in patients with short-term remission (<6 months) [29].
Biomarkers of low-grade inflammation were consistently linked to subsequent flares, notably elevated C-reactive protein (CRP > 5–20 mg/L) and fecal calprotectin (>50–300 µg/g). Additional risk factors included shorter azathioprine (AZA) treatment duration (<4 years), absence of mucosal healing, and prior biologic therapy. While demographic variables such as age, sex, and smoking status generally showed limited and inconsistent associations, several studies reported higher relapse risk in male patients and those diagnosed at a younger age. However, female sex, younger age, and a prolonged time to remission (>6 months) were also associated with increased relapse risk in a previous cohort. A prior systematic review by Torres et al., (2015) identified multiple predictors of relapse after AZA withdrawal [46]. In Crohn’s disease, these included markers of active inflammation, poor prognostic features, and shorter remission duration [46]. In ulcerative colitis, relapse risk was linked to extensive disease, younger age, male sex, frequent prior relapses, and shorter AZA exposure. Overall, higher baseline disease activity and a complex disease course were consistently associated with future relapse [46]. Additionally, for Crohn’s patients, a model-based risk–benefit analysis of thiopurine withdrawal in CD patients in prolonged remission found that, in those without extensive colitis, continuation marginally increased life expectancy for 35-year-old men and women but decreased it for 65-year-old men and women. Based on this model, withdrawal became the preferred strategy at approximately 40 years of age for men and 45 years for women without extensive colitis, whereas in patients with extensive colitis, continuation was favored regardless of age [55]. Furthermore, pharmacogenetic insights from prior reviews suggest that NUDT153 and NUDT152 variants may help identify Asian patients at heightened risk of developing early thiopurine-induced myelotoxicity, underscoring the importance of individualized treatment strategies [56]. While biomarker predictors, including elevated CRP and fecal calprotectin, demonstrated directional associations across the included studies, the majority of the observational evidence base and significant between-study heterogeneity limit confidence in their predictive accuracy. These biomarkers should be considered as part of a detailed and structured risk assessment rather than definitive withdrawal criteria.
Meta-regression, as demonstrated in Figure 6, showed a clear dose–duration association: for every additional month of continuous azathioprine therapy, the risk of relapse fell by about 0.18 percentage points, explaining roughly one-third of the heterogeneity between studies. For patients with mild adverse effects or concerns about cumulative malignancy risk, dose reduction—shown in a randomized trial to preserve remission and biologic pharmacokinetics—may offer a pragmatic compromise [48]. Notably, Bouhnik et al., (1996) reported that, after four years of remission, relapse risk was similar regardless of whether therapy was continued or stopped, raising questions about the benefit of prolonged use in such cases [57]. Despite identifying AZA duration as a significant predictor, the meta-regression model explained only 31.2% of between-study variance, indicating substantial unmeasured heterogeneity. Possible sources include unmeasured disease severity markers, variations in withdrawal protocols, different outcome ascertainment methods, population-specific genetic factors, and healthcare system differences. This high residual variance severely limits the applicability of duration-based recommendations and highlights the need for individualized risk assessment.
Finally, decisions should be individualized through the shared discussion of competing risks. Continued azathioprine use lowers relapse and healthcare utilization—an important consideration in resource-constrained settings where the cost of a single flare may exceed that of several years of low-dose therapy—but must be weighed against small, cumulative risks of myelosuppression, non-melanoma skin cancer, and lymphoproliferative disease. Population pharmacokinetic data show that genotype-guided dose optimization (e.g., 50% reduction in TPMT*3C carriers) can mitigate toxicity without sacrificing efficacy, arguing for dose tailoring rather than reflex discontinuation in many patients [53]. In sum, azathioprine withdrawal should be reserved for carefully selected patients who have achieved deep, biomarker-negative remission, with extra caution in UC. Where doubts persist, dose reduction and close, biomarker-led monitoring provide a safer route than abrupt cessation, balancing the lifelong nature of IBD against the long-term toxicities of thiopurine therapy.
- Strengths and Limitations
Our review has several notable strengths that reinforce its methodological rigor and relevance. First, it adhered closely to PRISMA 2020 guidelines and was prospectively registered in PROSPERO, ensuring transparency and reproducibility. Second, the search strategy was comprehensive and inclusive, capturing more than 3000 patients across diverse study designs, geographic regions, and clinical settings. Third, the robustness of the evidence synthesis was enhanced by performing a detailed risk of bias assessment, conducting subgroup analyses and sensitivity testing, and applying the GRADE framework to evaluate certainty of evidence. Collectively, these features strengthen the credibility of our findings and reflect a commitment to high-quality evidence synthesis.
At the same time, we recognize several important limitations that temper the interpretation of our results. The most prominent challenge was the substantial heterogeneity (I2 = 94.2%) observed across included studies. This heterogeneity likely reflects not only statistical variability but also important clinical and methodological differences, such as inconsistent definitions of clinical remission (ranging from standardized indices like CDAI < 150 or Mayo score ≤ 2, to less structured physician global assessments), variability in withdrawal protocols (gradual tapering versus abrupt discontinuation), and geographic variations in patient populations and clinical practices. Additionally, the predominance of observational studies and the limited degree of blinding within the available randomized trials inevitably introduce bias and reduce the strength of the pooled conclusions. The inconsistent reporting of key variables, including mucosal healing and biomarker outcomes, further restricts comparability and synthesis across studies. Taken together, these limitations underscore that, while the pooled estimates offer useful insights, they should be interpreted with caution when translated into clinical practice. Nevertheless, we believe the disease-specific comparison of relapse rates between ulcerative colitis and Crohn’s disease represents the most consistent and clinically meaningful finding of this review. Despite methodological challenges, this distinction provides an important contribution to the literature and may guide future clinical and research priorities. Finally, as with any meta-analysis, the potential for publication bias cannot be fully excluded.
- Future Directions
Looking ahead, there is a clear need for prospective trials that explore AZA withdrawal in a more targeted way, especially by using biomarkers to better define who can stop treatment safely. These studies should ideally agree on what constitutes a relapse, use centralized endoscopic scoring, and include tools like genomics, microbiome data, and proteomics to create more personalized risk calculators. It would also be valuable to compare the long-term costs and benefits of continuing AZA, withdrawing it with monitoring, or starting biologics early—this kind of analysis can help shape future policy and insurance decisions. Lastly, genetic markers such as NUDT15 and TPMT variants deserve more attention, as they might influence how successful withdrawal can be for different patients.
5. Conclusions
Our study findings demonstrated low to very low certainty evidence that relapse rates after AZA withdrawal are significant but highly variable. The high heterogeneity and methodological limitations preclude universal application of these findings and underscore the need for individualized decision-making based on patient-specific factors. The GRADE assessment classified evidence quality as low to very low for most outcomes, reflecting serious concerns about study limitations, inconsistency, and imprecision. This level of evidence is insufficient to currently formulate definitive practice guidelines for AZA withdrawal decisions. Current evidence should inform but not dictate decision-making, which must include individual patient factors, clinical judgment, and shared decision-making principles.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jcm14196868/s1, Table S1: PRISMA-2020 checklist; Table S2: Search strategy used in each of the databases; Table S3: Risk of bias assessment; Table S4: Certainty assessment of evidence.
Author Contributions
A.A.A. contributed to conceptualization, methodology, writing—original draft, and supervision; J.S.A. contributed to methodology, formal analysis, and writing—review and editing; L.A. contributed to data curation, investigation, and writing—review and editing; R.A. (Reem AlQarni) contributed to data curation, investigation, and writing—review and editing; F.R.R.M. contributed to data curation, investigation, and writing—review and editing; R.A. (Rana AlQarni) contributed to investigation, resources, and writing—review and editing; J.A. contributed to investigation, visualization, and writing—review and editing; D.A. contributed to data curation, validation, and writing—review and editing; A.A. (Abdullah Almaqhawi) contributed to formal analysis, methodology, and writing—review and editing; M.A.A. contributed to investigation, resources, and writing—review and editing; A.A. (Ahmed Albadrani) contributed to project administration, supervision, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [KFU252727].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CD | Crohn’s disease |
| UC | Ulcerative colitis |
| L1/L2/L3/L4 | Montreal Location Classification |
| B1/B2/B3 | Montreal Behavior Classification |
| E1/E2/E3 | Montreal Extent Classification |
| AZA | Azathioprine |
| Anti-TNF | Anti-tumor necrosis factor |
| CS | Corticosteroids |
| CsA | Cyclosporine |
| IFX | Infliximab |
| IS | Immunosuppressant |
| CDAI | Crohn’s Disease Activity Index |
| CDEIS | Crohn’s Disease Endoscopic Index of Severity (Endoscopic score for Crohn’s disease severity) |
| Mayo | Mayo score |
| HBI | Harvey–Bradshaw Index |
| PGA | Physician global assessment |
| CRP | C-reactive protein |
| hsCRP | High-sensitivity C-reactive protein |
| FC | Fecal calprotectin |
| WCC | White cell count |
| Hb | Hemoglobin |
| MCV | Mean corpuscular volume |
| RBC | Red blood cells |
| 6-TGN | 6-thioguanine nucleotide (active metabolite of thiopurines used to monitor therapy) |
| RCT | Randomized control trial |
| HR | Hazard ratio |
| OR | Odds ratio |
| CI | Confidence interval |
| AEs | Adverse events |
| NR | Not reported |
| NS | Not significant |
| N/A | Not applicable |
| Yr | Years |
| mo | Months |
| Mono | Monotherapy |
| Combo | Combination therapy |
References
- McDowell, C.; Farooq, U.; Haseeb, M. Inflammatory Bowel Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470312/ (accessed on 25 July 2025).
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative Colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Cheifetz, A.S. Crohn’s Disease: Epidemiology, Diagnosis, and Management. Mayo Clin. Proc. 2017, 92, 1088–1103. [Google Scholar] [CrossRef] [PubMed]
- Meštrović, A.; Kumric, M.; Bozic, J. Discontinuation of Therapy in Inflammatory Bowel Disease: Current Views. World J. Clin. Cases 2024, 12, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, J.D.; Ho, E.Y.; Shmidt, E.; Singh, H.; Falck-Ytter, Y.; Sultan, S. AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology 2020, 158, 1450–1461. [Google Scholar] [CrossRef]
- Triantafillidis, J.K. Surgical treatment of inflammatory bowel disease: From the gastroenterologist’s stand-point. World J. Gastrointest. Surg. 2024, 16, 1235–1254. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Bermejo, F.; Pérez-Calle, J.L.; Taxonera, C.; Vera, I.; McNicholl, A.G.; Domènech, E. Fecal Calprotectin and Lactoferrin for the Prediction of Inflammatory Bowel Disease Relapse. Inflamm. Bowel Dis. 2009, 15, 1190–1198. [Google Scholar] [CrossRef]
- Chande, N.; Patton, P.H.; Tsoulis, D.J.; Thomas, B.S.; MacDonald, J.K. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2015, 10, CD000067. [Google Scholar] [CrossRef]
- Mohammadi, O.; Kassim, T.A. Azathioprine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK542190/ (accessed on 25 July 2025).
- Tiede, I.; Fritz, G.; Strand, S.; Poppe, D.; Dvorsky, R.; Strand, D.; Neurath, M.F. CD28-Dependent Rac1 Activation Is the Molecular Target of Azathioprine in Primary Human CD4+ T Lymphocytes. J. Clin. Investig. 2003, 111, 1133–1145. [Google Scholar] [CrossRef]
- Yewale, R.V.; Ramakrishna, B.S.; Doraisamy, B.V.; Basumani, P.; Venkataraman, J.; Jayaraman, K.; Murali, A.; Premkumar, K.; Kumar, A.S. Long-Term Safety and Effectiveness of Azathioprine in the Management of Inflammatory Bowel Disease: A Real-World Experience. JGH Open 2023, 7, 599–609. [Google Scholar] [CrossRef]
- Teich, N.; Mohl, W.; Bokemeyer, B.; Bündgens, B.; Büning, J.; Miehlke, S.; Hüppe, D.; Maaser, C.; Klugmann, T.; Kruis, W.; et al. Azathioprine-Induced Acute Pancreatitis in Patients with Inflammatory Bowel Diseases—A Prospective Study on Incidence and Severity. J. Crohns Colitis 2016, 10, 61–68. [Google Scholar] [CrossRef]
- Beaugerie, L.; Brousse, N.; Bouvier, A.M.; Colombel, J.F.; Lémann, M.; Cosnes, J.; Hebuterne, X. Lymphoproliferative Disorders in Patients Receiving Thiopurines for Inflammatory Bowel Disease: A Prospective Observational Cohort Study. Lancet 2009, 374, 1617–1625. [Google Scholar] [CrossRef]
- French, H.; Dalzell, A.M.; Srinivasan, R.; El-Matary, W. Relapse Rate Following Azathioprine Withdrawal in Maintaining Remission for Crohn’s Disease: A Systematic Review and Meta-Analysis. Arch. Dis. Child. 2011, 96, A19. [Google Scholar] [CrossRef]
- Lémann, M.; Mary, J.Y.; Duclos, B.; Veyrac, M.; Dupas, J.L.; Delchier, J.C.; Modigliani, R.; Soulé, J.C.; Messing, B.; Colombel, J.F.; et al. A Randomized, Double-Blind, Controlled Withdrawal Trial in Crohn’s Disease Patients in Long-Term Remission on Azathioprine. Gastroenterology 2005, 128, 1812–1818. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Br. Med. J. 2021, 372, 71. [Google Scholar] [CrossRef]
- Alnajjar, J.; Al Abdulqader, A.; Almaqhawi, A.; Mohamed, F.; Alzimami, L.; AlQarni, R.; AlQarni, R.; Alnasser, J.; Alabdulkarim, D. Relapse Rate and Predictors After Withdrawal of Azathioprine in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta Analysis. PROSPERO 2025 CRD420251016594. Available online: https://www.crd.york.ac.uk/PROSPERO/view/CRD420251016594 (accessed on 20 August 2025).
- Amir-Behghadami, M.; Janati, A. Population, Intervention, Comparison, Outcomes and Study (PICOS) Design as a Framework to Formulate Eligibility Criteria in Systematic Reviews. Emerg. Med. J. 2020, 37, 387. [Google Scholar] [CrossRef] [PubMed]
- Cochrane RoB 2: A Revised Cochrane Risk-of-Bias Tool for Randomized Trials. Available online: https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials (accessed on 20 August 2025).
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 20 August 2025).
- R Core Team R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 20 August 2025).
- GRADE | Cochrane. Available online: https://www.cochrane.org/learn/courses-and-resources/cochrane-methodology/grade (accessed on 20 August 2025).
- Angelucci, E.; Cesarini, M.; Gentile, P.; Frieri, G.; Caprilli, R.; Latella, G. Azathioprine withdrawal in Crohn’s disease: Never-ending story? Dig. Liver Dis. 2010, 42S, S129–S130. [Google Scholar] [CrossRef]
- Cassinotti, A.; Actis, G.C.; Duca, P.; Massari, A.; Colombo, E.; Gai, E.; Annese, V.; D’ALbasio, G.; Manes, G.; Travis, S.; et al. Maintenance treatment with azathioprine in ulcerative colitis: Outcome and predictive factors after drug withdrawal. Am. J. Gastroenterol. 2009, 104, 2760–2767. [Google Scholar] [CrossRef] [PubMed]
- Cassinotti, A.; Corona, A.; Duca, P.; Nebuloni, M.; Maconi, G.; Fociani, P.; Ardizzone, S. Noninvasive Monitoring After Azathioprine Withdrawal in Patients with Inflammatory Bowel Disease in Deep Remission. Clin. Gastroenterol. Hepatol. 2021, 19, 2293–2301.e1. [Google Scholar] [CrossRef]
- Crepaldi, M.; Maniero, D.; Massano, A.; Pavanato, M.; Barberio, B.; Savarino, E.V.; Zingone, F. Azathioprine monotherapy withdrawal in inflammatory bowel diseases: A retrospective mono-centric study. World J. Gastroenterol. 2023, 29, 4334–4343. [Google Scholar] [CrossRef]
- Fraser, A.G.; Orchard, T.R.; Jewell, D.P. The efficacy of azathioprine for the treatment of inflammatory bowel disease: A 30 year review. Gut 2002, 50, 485–489. [Google Scholar] [CrossRef]
- Hawthorne, A.B.; Logan, R.F.; Hawkey, C.J.; Foster, P.N.; Axon, A.T.; Swarbrick, E.T.; Scott, B.B.; Lennard-Jones, J.E. Randomised controlled trial of azathioprine withdrawal in ulcerative colitis. BMJ 1992, 305, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Holtmann, M.H.; Krummenauer, F.; Claas, C.; Kremeyer, K.; Lorenz, D.; Rainer, O.; Vogel, I.; Böcker, U.; Böhm, S.; Büning, C.; et al. Long-term effectiveness of azathioprine in IBD beyond 4 years: A European multicenter study in 1176 patients. Dig. Dis. Sci. 2006, 51, 1516–1524. [Google Scholar] [CrossRef]
- Iborra, M.; Herreras, J.; Bosca-Watts, M.M.; Cortés, X.; Trejo, G.; Cerrillo, E.; Hervás, D.; Mínguez, M.; Beltrán, B.; Nos, P. Withdrawal of Azathioprine in Inflammatory Bowel Disease Patients Who Sustain Remission: New Risk Factors for Relapse. Dig. Dis. Sci. 2019, 64, 1612–1621. [Google Scholar] [CrossRef]
- Jorissen, C.; Verstockt, B.; Schils, N.; Sabino, J.; Ferrante, M.; Vermeire, S. Long-term clinical outcome after thiopurine discontinuation in elderly IBD patients. Scand. J. Gastroenterol. 2021, 56, 1323–1327. [Google Scholar] [CrossRef]
- Kennedy, N.A.; Kalla, R.; Warner, B.; Gambles, C.J.; Musy, R.; Reynolds, S.; Dattani, R.; Nayee, H.; Felwick, R.; Harris, R.; et al. Thiopurine withdrawal during sustained clinical remission in inflammatory bowel disease: Relapse and recapture rates, with predictive factors in 237 patients. Aliment Pharmacol Ther. 2014, 40, 1313–1323. [Google Scholar] [CrossRef]
- Louis, E.; Resche-Rigon, M.; Laharie, D.; Satsangi, J.; Ding, N.; Siegmund, B.; D’HAens, G.; Picon, L.; Bossuyt, P.; Vuitton, L.; et al. Withdrawal of infliximab or concomitant immunosuppressant therapy in patients with Crohn’s disease on combination therapy (SPARE): A multicentre, open-label, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2023, 8, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Rincón, E.; Benítez, J.M.; Serrano-Ruiz, F.J.; Vázquez-Morón, J.M.; Pallarés-Manrique, H.; Herrera-Justiniano, J.M.; Leo-Carnerero, E.; Gómez-García, M.R.; Cabello-Tapia, M.J.; Castro-Fernández, M.; et al. Prognosis of Patients with Ulcerative Colitis in Sustained Remission After Thiopurines Withdrawal. Inflamm. Bowel Dis. 2015, 21, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Nyman, M.; Hansson, I.; Eriksson, S. Long-term immunosuppressive treatment in Crohn’s disease. Scand. J. Gastroenterol. 1985, 20, 1197–1203. [Google Scholar] [CrossRef]
- O’Donoghue, D.P.; Dawson, A.M.; Powell-Tuck, J.; Bown, R.L.; Lennard-Jones, J.E. Double-blind withdrawal trial of azathioprine as maintenance treatment for Crohn’s disease. Lancet 1978, 2, 955–957. [Google Scholar] [CrossRef]
- Oussalah, A.; Chevaux, J.B.; Fay, R.; Sandborn, W.J.; Bigard, M.A.; Peyrin-Biroulet, L. Predictors of infliximab failure after azathioprine withdrawal in Crohn’s disease treated with combination therapy. Am. J. Gastroenterol. 2010, 105, 1142–1149. [Google Scholar] [CrossRef]
- Ranjan, M.K.; Vuyyuru, S.K.; Kante, B.; Kumar, P.; Mundhra, S.K.; Golla, R.; Sharma, R.; Sahni, P.; Das, P.; Makharia, G.; et al. Relapse rates after withdrawal of thiopurines in patients with inflammatory bowel disease. Int. J. Colorectal Dis. 2022, 37, 1817–1826. [Google Scholar] [CrossRef]
- Sokol, H.; Seksik, P.; Nion-Larmurier, I.; Vienne, A.; Beaugerie, L.; Cosnes, J. Current Smoking, Not Duration of Remission, Delays Crohn’s Disease Relapse Following Azathioprine Withdrawal. Inflamm. Bowel Dis. 2010, 16, 362–363. [Google Scholar] [CrossRef] [PubMed]
- Treton, X.; Bouhnik, Y.; Mary, J.Y.; Colombel, J.; Duclos, B.; Soule, J.; Lerebours, E.; Cosnes, J.; Lemann, M. Azathioprine withdrawal in patients with Crohn’s disease maintained on prolonged remission: A high risk of relapse. Clin. Gastroenterol. Hepatol. 2009, 7, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Van Assche, G.; Magdelaine-Beuzelin, C.; D’Haens, G.; Baert, F.; Noman, M.; Vermeire, S.; Ternant, D.; Watier, H.; Paintaud, G.; Rutgeerts, P. Withdrawal of immunosuppression in Crohn’s disease treated with scheduled infliximab maintenance: A randomized trial. Gastroenterology 2008, 134, 1861–1868. [Google Scholar] [CrossRef]
- Vilien, M.; Dahlerup, J.F.; Munck, L.K.; Nørregaard, P.; Grønbaek, K.; Fallingborg, J. Randomized controlled azathioprine withdrawal after more than two years treatment in Crohn’s disease: Increased relapse rate the following year. Aliment. Pharmacol. Ther. 2004, 19, 1147–1152. [Google Scholar] [CrossRef]
- Wenzl, H.H.; Primas, C.; Novacek, G.; Teml, A.; Öfferlbauer-Ernst, A.; Högenauer, C.; Vogelsang, H.; Petritsch, W.; Reinisch, W. Withdrawal of long-term maintenance treatment with azathioprine tends to increase relapse risk in patients with Crohn’s disease. Dig. Dis. Sci. 2015, 60, 1414–1423. [Google Scholar] [CrossRef] [PubMed]
- Ordas, I.; Feagan, B.G.; Sandborn, W.J. Early use of immunosuppressives or TNF antagonists for the treatment of Crohn’s disease: Time for a change. Gut 2011, 60, 1754–1763. [Google Scholar] [CrossRef]
- Torres, J.; Boyapati, R.K.; Kennedy, N.A.; Louis, E.; Colombel, J.F.; Satsangi, J. Systematic review of effects of withdrawal of immunomodulators or biologic agents from patients with inflammatory bowel disease. Gastroenterology 2015, 149, 1716–1730. [Google Scholar] [CrossRef]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’amico, A.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Reinisch, W.W.; Bemelman, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef]
- Roblin, X.; Boschetti, G.; Williet, N.; Nancey, S.; Marotte, H.; Berger, A.; Phelip, J.M.; Peyrin--Biroulet, L.; Colombel, J.F.; Del Tedesco, E.; et al. Azathioprine dose reduction in inflammatory bowel disease patients on combination therapy: An open-label, prospective and randomised clinical trial. Aliment. Pharmacol. Ther. 2017, 46, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Dohos, D.; Hanák, L.; Szakács, Z.; Kiss, S.; Párniczky, A.; Erőss, B.; Pázmány, P.; Hegyi, P.; Sarlós, P. Systematic review with meta-analysis: The effects of immunomodulator or biological withdrawal from mono- or combination therapy in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2021, 53, 220–233. [Google Scholar] [CrossRef]
- Boyapati, R.K.; Torres, J.; Palmela, C.; Parker, C.E.; Silverberg, M.; Upadhyaya, S.D.; Nguyen, T.M.; Colombel, J.-F. Withdrawal of immunosuppressant or biologic therapy for patients with quiescent Crohn’s disease. Cochrane Database Syst. Rev. 2018, 5, CD012540. [Google Scholar] [CrossRef] [PubMed]
- Lucas Ramos, J.; Suárez Ferrer, C.; Poza Cordón, J.; Sánchez Azofra, M.; Rueda García, J.L.; Martin Arranz, E.; Yebra Carmona, J.; Andaluz García, I.; Martín Arranz, M.D. Optimization of azathioprine dose in combined treatment with anti-TNF-alpha in inflammatory bowel disease. Gastroenterol. Hepatol. 2021, 44, 337–345. [Google Scholar] [CrossRef]
- Lin, R.; Lin, W.; Wang, C.; Dong, J.; Zheng, W.; Zeng, D.; Liu, Y.; Lin, C.; Jiao, Z.; Huang, P. Population pharmacokinetics of azathioprine active metabolite in patients with inflammatory bowel disease and dosage regimens optimisation. Basic. Clin. Pharmacol. Toxicol. 2021, 128, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68 (Suppl. S3), s1–s106. [Google Scholar] [CrossRef]
- Rubin, D.T.; Ananthakrishnan, A.N.; Siegel, C.A.; Sauer, B.G.; Long, M.D. ACG clinical guideline: Ulcerative colitis in adults. Am. J. Gastroenterol. 2019, 114, 384–413. [Google Scholar] [CrossRef]
- Kirchgesner, J.; Beaugerie, L.; Carrat, F.; Sokol, H.; Cosnes, J.; Schwarzinger, M.; BERENICE Study Group. Impact on life expectancy of withdrawing thiopurines in Crohn’s disease in sustained remission: A lifetime risk–benefit analysis. Clin. Gastroenterol. Hepatol. 2017, 15, 1940–1949.e3. [Google Scholar] [CrossRef]
- Walker, G.J.; Harrison, J.W.; Heap, G.A.; Voskuil, M.D.; Andersen, V.; Anderson, C.A.; Ananthakrishnan, A.N.; Barrett, J.C.; Beaugerie, L.; Bewshea, C.M.; et al. Association of Genetic Variants in NUDT15 with Thiopurine-Induced Myelosuppression in Patients with Inflammatory Bowel Disease. JAMA 2019, 321, 773–785. [Google Scholar] [CrossRef]
- Bouhnik, Y.; Lémann, M.; Mary, J.Y.; Scemama, G.; Taï, R.; Matuchansky, C.; Modigliani, R.; Rambaud, J.C. Long-term follow-up of patients with Crohn’s disease treated with azathioprine or 6-mercaptopurine. Lancet 1996, 347, 215–219. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).