Abstract
Background: Left ventricular thrombus (LVT) remains a well-recognized complication following myocardial infarction (MI). Whilst vitamin K antagonists (VKAs) have traditionally been the cornerstone of management, direct oral anticoagulants (DOACs) have been increasingly utilized despite limited data to support this. We sought to perform an up-to-date meta-analysis of all randomized controlled trials (RCTs) comparing DOACs to VKAs for LVT resolution. Methods: A systematic search of major scientific databases was performed to identify RCTs published until May 2025. The primary efficacy endpoint was complete LVT resolution at 3 months. The risk ratio (RR) and 95% confidence intervals (CIs) of the individual RCTs were pooled via the inverse-variance method and random-effects model. Results: Seven RCTs involving 554 patients with a mean age of 54 years were included in the meta-analysis. At 3 months, there was no difference in the rate of LVT resolution between those in the DOAC arm and the warfarin arm (86% vs. 81%, RR 1.01 [95%CI 0.93–1.10], p = 0.76). There was low heterogeneity at I2 = 15%. There was no difference in major or clinically significant bleeding or in the composite of stroke or thromboembolic complications, although the 95%CIs were wide. Conclusions: DOACs appear to be comparable to warfarin in achieving LVT resolution at 3 months. These findings support the consideration of DOACs as alternatives to VKAs in selected patients for LVT resolution. Further adequately powered trials and head-to-head comparisons between DOACs are required to confirm their safety.
1. Introduction
Left ventricular thrombus (LVT) is a well-recognized complication of myocardial infarction (MI) [1]. Patients with reduced left ventricular ejection fraction (LVEF), anterior myocardial infarction, elevated troponin levels and inflammatory markers have been identified as risk factors for developing LVT [2]. Despite advancements in reperfusion techniques, LVT is still observed post-MI in nearly 20% of patients with anterior ST-segment elevation myocardial infarction (STEMI) and impaired LV function by cardiac magnetic resonance (CMR) [3].
In addition to acute MI, dilated cardiomyopathy (DCM) is another important substrate for LVT formation. Historically, echocardiographic series reported prevalence rates between 10 and 30% [4,5]. However, more recent cohort studies suggest a lower prevalence rate of 2.3% among over 3000 patients with DCM at a single tertiary center in Beijing [6] and 7.1% in a multicenter analysis of 1267 patients with non-ischemic cardiomyopathy [7]. Randomized data specific to DCM-associated LVT are still lacking, as most RCTs involving anticoagulation for LVT focus on post-MI populations, with few studies reporting outcomes specifically for patients with DCM. The burden of LVT formation is associated with increased risk of stroke, distal embolization and other major cardiovascular events [8].
Traditionally, vitamin K antagonists (VKAs), such as warfarin, have been the cornerstone of LVT management, guided by evidence extrapolated from pre-reperfusion era studies and real-world experience. However, limitations including narrow therapeutic window, food and drug interactions, and variable time in therapeutic range pose challenges to optimal management. The availability of direct oral anticoagulants (DOACs) has transformed anticoagulation in other clinical settings, offering predictable pharmacokinetics, fewer interactions, and no requirement for routine monitoring. To date, in the absence of large, randomized controlled trials (RCTs), the comparative effectiveness of DOACs remains uncertain [1].
Despite this, DOACs are increasingly prescribed off-label for LVT, supported by small RCTs and meta-analyses of predominantly observational studies suggesting comparable efficacy and safety to VKAs [9,10,11]. A recent meta-analysis of RCTs only showed no difference in LVT resolution between DOAC and warfarin, although the sample size was small [10]. Recently, RIVAWAR trial result was presented at the American College of Cardiology (ACC) 2025 annual congress. This single-center, open-label, randomized study including 261 patients, showed that by 12 weeks, there were no significant differences in LVT resolution between rivaroxaban and warfarin [12].
Therefore, we aimed to perform an up-to-date meta-analysis of all RCTs comparing DOAC against warfarin for the treatment of LVT resolution.
2. Materials and Methods
This meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [13].
2.1. Eligibility Criteria
We included all RCTs enrolling patients with a confirmed diagnosis of LVT that compared direct oral anticoagulants (DOACs) versus vitamin K antagonists (VKAs) and reported LVT resolution during follow-up, whether published as full-text papers or abstracts.
2.2. Search Strategy
A systematic search was performed using PubMed/MEDLINE, Ovid/Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL) to identify RCTs published in English up to May 2025. The search strategy combined terms including “direct oral anticoagulants,” “vitamin K antagonists,” “randomized controlled trials,” and “left ventricular thrombus”.
2.3. Study Selection
Two authors (MH, SA) independently screened titles and abstracts for eligibility and performed full-text review and data extraction. Discrepancies were resolved by consensus with a third reviewer (HB). As this study utilized previously published data, institutional review board approval and informed consent were not required. Eligible RCTs included in this meta-analysis are summarized in Table 1.
2.4. Data Extraction and Quality Assessment
Extracted variables included study design, patient population, anticoagulant regimen (DOAC vs. VKA), inclusion and exclusion criteria, clinical outcomes, and duration of follow-up. Risk of bias for individual studies was assessed in accordance with the Cochrane risk-of-bias assessment tool (Figure 1) [14].
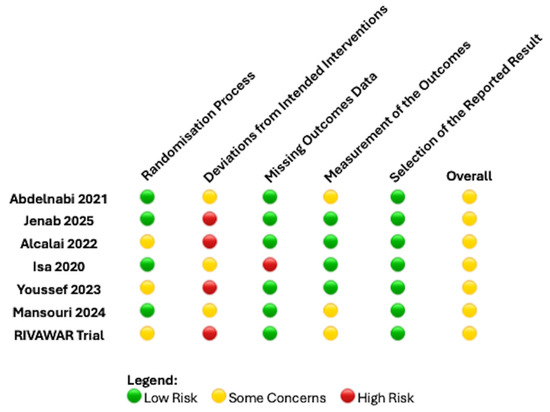
Figure 1.
Risk of bias summary by Cochrane risk assessment tool for the RCTs included [11,12,15,16,17,18,19].

Table 1.
Characteristics of studies included.
Table 1.
Characteristics of studies included.
| Study | Total Number of Patients and Country | RCT Type and Intervention | Inclusion and Exclusion Criteria | Follow-Up Duration | Endpoints |
|---|---|---|---|---|---|
| Isa et al. 2020 [17] | n = 27 Malaysia | Pilot, prospective, single-center, randomized, single-blinded outcome study Apixaban 5 mg BD or 2.5 mg BD for 12 weeks, based on recommendation Warfarin with initial heparin infusion, aiming for a target INR of 2–3 | Inclusion criteria:
Exclusion criteria:
| 15 weeks (Echocardiography 12 weeks) | Primary outcome: 12 weeks percentage of LVT mean size reduction or total resolution Secondary outcomes: All-cause mortality Ischemic stroke Worsening heart failure |
| Abdelnabi et al. 2021 [19] | n = 79 Egypt Bulgaria | Prospective, open-label, multi- center, RCT Rivaroxaban 20 mg daily Warfarin with initial enoxaparin until reaching target INR 2–3 | Inclusion criteria
Exclusion criteria
| 1, 3, 6 months | Primary outcomes: Presence or absence of LVT as assessed by 2D transthoracic echocardiography Secondary outcomes: Stroke or systemic embolism Major bleeding |
| Alcalai et al. 2022 [18] | n = 35 Israel | Multicentre, national, randomized open-label non-inferiority clinical trial Apixaban 5 mg BD or 2.5 mg BD for 3 months dose based on recommendations Warfarin targeting INR 2–3 | Inclusion criteria:
Exclusion criteria:
| 3 months | Primary outcome: Presence and dimensions of LVT as assessed by 2D echocardiography Secondary outcomes: Stroke or systemic embolism Major bleeding All-cause mortality |
| Youssef et al. 2023 [15] | n = 50 Saudi Arabia | Open-label RCT Apixaban 5 mg BD Warfarin targeting INR 2–3 | Inclusion criteria:
Exclusion criteria:
| 1, 3, 6 months | Primary outcome: Resolution of LVT in 3 months Secondary outcomes: Resolution of LVT in 6 months Safety outcome: MACE or any relevant bleeding according to the BARC classification |
| Mansouri et al. 2024 [16] | n = 52 Iran | open- label non-inferiority RCT Rivaroxaban 20 mg daily Warfarin 5 mg loading dose aiming INR 2–3 | Inclusion criteria:
Exclusion criteria:
| 3 months | Primary outcome: Resolution of LVT on TTE in 3 months Secondary outcomes: Bleeding Systemic embolic events Rehospitalization MACE Echocardiographic measures focusing on changes in thrombus size, mobility, and morphology |
| Jenab et al. 2025 [11] | n = 50 Iran | Pilot, open-label, parallel-group RCT with a 1:1 allocation ratio, concealed allocation sequences, and blinded outcome assessments Rivaroxaban (15 mg daily) plus clopidogrel (75 mg daily) plus aspirin (80 mg daily, only during the first 7 days) Warfarin overlapping with enoxaparin, until reaching an INR goal of 2.0–2.5, plus clopidogrel (75 mg daily plus aspirin (80 mg daily, only during the first 7 days) | Inclusion criteria:
Exclusion criteria:
| 3 months | Primary outcome: 3-month non-contrast 2D TTE-based complete LVT resolution Other outcomes: SSE at 3 months MACE) at 3 months All-cause death at 3 months Main safety outcomes: Major bleeding events based on ISTH definition at 3 months |
| RIVAWAR trial [12] | n = 261 Pakistan | Investigator initiated, single-center trial, open-label, RCT with a 2:1 allocation ratio Rivaroxaban 20 mg once daily Warfarin targeting INR 2–3 | Inclusion criteria:
Exclusion criteria:
| 3 months | Primary outcome: presence of LVT on TTE at 12 weeks post-randomization Secondary outcome: All-cause mortality Ischemic stroke Major bleeding |
CrCl: creatinine clearance; LVT: left ventricular thrombus; TTE: transthoracic echocardiography; MACE: major adverse cardiovascular events; BARC: bleeding Academic Research Consortium; MPHV: mechanical prosthetic heart valve; RCT: randomized controlled trial; SSE: stroke and systemic emboli.
2.5. Endpoints
The primary efficacy endpoint was complete resolution of LVT at 3 months. Secondary endpoint of interest was the incidence of major or clinically significant bleeding events as reported by the individual RCTs and the occurrence of stroke or thromboembolic complications.
2.6. Statistical Analysis
Statistical analyses were conducted using RevMan version 5.4 (Cochrane Collaboration, Nordic Cochrane Centre). Pooled risk ratios (RRs) with 95% confidence intervals (CIs) were calculated using the inverse-variance method and a random-effects model to account for variability across trials. Heterogeneity across trials was assessed using the I2 statistic, with thresholds of 25%, 50%, and 75% denoting low, moderate, and high heterogeneity, respectively. To estimate the comparative efficacy and safety of apixaban versus rivaroxaban in the absence of direct head-to-head RCTs, we performed an adjusted indirect comparison using the Bucher method. Specifically, we compared apixaban and rivaroxaban indirectly through their respective comparisons with the shared control group (warfarin). A two-sided p-value < 0.05 was considered statistically significant.
3. Results
The PRISMA diagram is shown in Figure 2. A total of 564 records were identified through database searches. No additional records were retrieved from trial registries or other sources. Prior to the screening phase, 32 duplicate entries were removed. An additional 110 records were excluded for reasons unrelated to duplication—such as non-English language, conference abstracts without full texts, or studies unrelated to the research question—leaving 422 records for title and abstract screening.
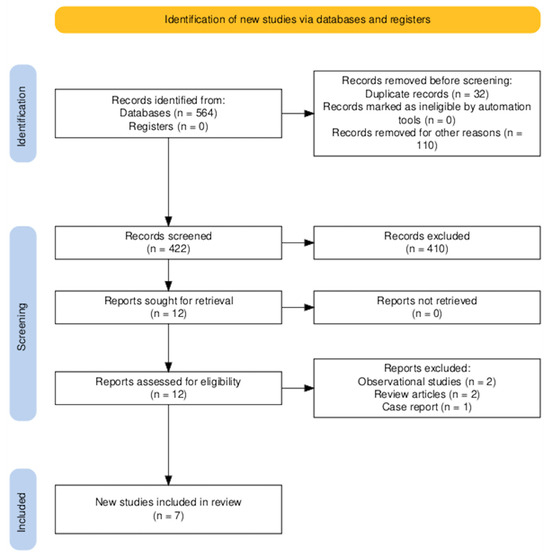
Figure 2.
PRISMA flow diagram of the study selection process.
Of these, 410 were excluded based on title and abstract review, predominantly due to irrelevance to the predefined inclusion criteria. Twelve full-text articles were retrieved for detailed assessment. All full-texts were successfully obtained and evaluated.
Following full-text review, five studies were excluded as shown in Figure 2. Consequently, seven RCTs [11,12,15,16,17,18,19] involving 554 patients were included in this meta-analysis. Six RCTs [11,16,18,19,20,21] were published as articles and one was available as abstract form only. This RCT was recently reported at the ACC congress 2025 and was recently published as an editorial comment article [17]. All RCTs have some concern regarding the risk of bias as shown in Figure 1 and this was predominantly due to the open-label nature of all these RCTs included. The average age of the patients was 54 years old and only 23% were female (gender not reported by Youssef et al. [15]). 44% had diabetes mellitus, 56% had a history of hypertension and 35% were current smokers. 20% of patients had LVT due to an underlying diagnosis of dilated cardiomyopathy. The follow-up period was 3 months in 5 RCTs [11,13,16,17,18] and 6 months in 2 RCTs [15,19].
3.1. LVT Resolution at 3 Months
LVT resolution data were not available in the trial paper by Isa et al. [19] but these data were subsequently acquired (LVT resolution at 3 months) by contacting the corresponding author [10]. At 3 months, there was no difference in the rate of LVT resolution between those in the DOAC arm and the warfarin arm (86% vs. 81%, RR 1.01 [95% confidence interval (CI) 0.93–1.10], p = 0.76), as seen in Figure 3. There was low heterogeneity at I2 = 15%.
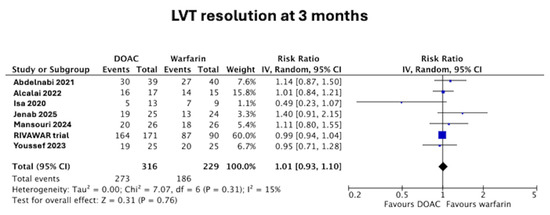
Figure 3.
Forest plot of LVT resolution at 3 months in DOAC vs. warfarin treatment groups [11,12,15,16,17,18,19].
3.2. Major or Clinically Significant Bleeding
Only Youssef et al. [15] explicitly used the Bleeding Academic Research Consortium (BARC) definition. The other trials either did not report a standardized bleeding definition or did not provide detailed safety endpoint methodology. There was no difference in the major or clinically significant bleeding between those in the DOAC arm and the warfarin arm (2.8% vs. 4.4%, RR 0.75 [95%CI 0.26–2.19], p = 0.60), as seen in Figure 4. There was low heterogeneity at I2 = 8%.
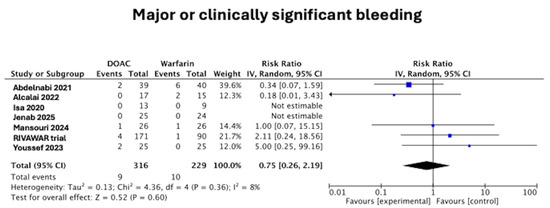
Figure 4.
Forest plot of major or clinically significant bleeding in DOAC vs. warfarin treatment groups [11,12,15,16,17,18,19].
3.3. Stroke or Thromboembolic Complications
There was no difference in the composite of stroke or thromboembolic complications between those in the DOAC arm and the warfarin arm (2.2% vs. 3.4%, RR 0.75 [95%CI 0.12–4.25], p = 0.72). Heterogeneity was moderate at I2 = 40%.
3.4. Apixaban Versus Rivaroxaban for LVT Resolution at 3 Months
Three RCTs each compared apixaban against warfarin [15,17,18] and four RCTs compared rivaroxaban against warfarin [11,13,16,19]. Indirect comparison of apixaban versus rivaroxaban showed numerically lower LVT resolution rate with apixaban (40/55, 73%) when compared to rivaroxaban (233/261, 89%) but this was not statistically significant (RR 0.90, 95%CI 0.70–1.15).
4. Discussion
In this meta-analysis, we included seven RCTs involving 554 patients with LVT. We found no significant difference in LVT resolution at 3 months between patients treated with DOACs and those treated with warfarin. These findings are aligned with emerging real-world data [19] and add to the growing body of evidence suggesting that DOACs may be a safe and effective alternative to vitamin K antagonists for LVT.
The comparable efficacy observed in LVT resolution supports the biological plausibility that factor Xa inhibition, central to both rivaroxaban and apixaban, is sufficient to prevent thrombus propagation and facilitate resolution [21]. Notably, thrombus resolution was numerically higher in the rivaroxaban-treated patients compared to those treated with apixaban in indirect comparisons, though this difference did not reach statistical significance. This discrepancy could reflect pharmacokinetic differences, patient selection, or trial-level heterogeneity, but should be interpreted cautiously due to the absence of direct head-to-head trials.
Safety outcomes were also similar between groups. The pooled rate of major or clinically relevant bleeding was low and not significantly different between DOACs and warfarin, with low heterogeneity. Only one trial employed a standardized definition for bleeding outcomes, highlighting the need for consistent safety endpoint reporting in future trials. Furthermore, the CIs for both bleeding and embolic outcomes were quite wide, indicating that these RCTs, even after pooling in a meta-analysis, were not adequately powered. Larger RCTs powered for these safety outcomes are warranted.
It is worth noting that a substantial proportion of included patients had LVT secondary to dilated cardiomyopathy, a group for whom anticoagulation strategy remains even less well-defined. None of the RCTs reported disaggregated outcomes for this group. This lack of etiologic subgroup reporting represents a key limitation of the evidence base and of our meta-analysis. The consistency of treatment effects across this heterogeneous population suggests that DOACs may be broadly applicable; however, it is possible that LVT in the setting of DCM may carry different risks of thrombus persistence or embolization. Subgroup-specific data, dedicated trials or patient-level pooled analyses are warranted to clarify optimal anticoagulation strategy in this population.
Notably, recent analyses, including the meta-analysis included in the RCT by Jenab et al. [11], have highlighted the substantial heterogeneity in imaging modalities, timing of follow-up, and reporting of thrombus characteristics across RCTs, factors which may have influenced observed resolution rates. Standardized imaging protocols, consistent definitions of thrombus morphology, and extended follow-up are needed in future trials to clarify these important clinical outcomes. The ongoing RCTs such as the EARLYmyo-LVT trial (N = 280) (NCT03764241), WaRMIN trial (N = 196) (NCT05794399) are using CMR for the assessment of LVT resolution. Rivaroxaban in Left Ventricular Thrombus trial (N = 320) (NCT04970576) is using transthoracic echocardiography (TTE) and the ACTonLVT trial (N = 320) (NCT05892042) is using either TTE or CMR for the assessment of LVT resolution.
Strengths and Limitations
This is the most comprehensive synthesis to date of randomized data comparing DOACs and warfarin for LVT. A key strength of this analysis is the exclusive inclusion of RCTs, which reduces confounding and selection bias inherent in prior meta-analyses relying heavily on observational data. We also addressed data gaps by contacting authors to obtain unpublished outcomes, improving the completeness and robustness of our pooled analysis.
Nonetheless, several limitations should be acknowledged. First, the total number of patients remains modest, and event rates were low, particularly for safety endpoints, which limits the power to detect small differences. Second, there was heterogeneity in DOAC dosing, background antiplatelet therapy, and imaging modalities used for LVT assessment across trials. Third, the indirect comparison between apixaban and rivaroxaban should be considered hypothesis-generating only and should not guide clinical decision-making in the absence of head-to-head evidence. Fourth, the reliance on one abstract-only trial, open-label design of all the RCTs included and may have introduced bias. Fifth, only one RCT used a standardized definition for bleeding and future trials need to take this into account during the trial design stage, to minimize heterogeneity when pooling future RCTs in this field. Sixth, we performed a study-level rather than a patient-level meta-analysis. Lastly, none of the ongoing RCTs are powered for hard clinical outcomes. Therefore, there is a pressing need for the community to conduct RCTs adequately powered for hard clinical outcomes in this field to provide robust efficacy and safety data.
5. Conclusions
In conclusion, this meta-analysis of RCTs demonstrates that DOACs are comparable to warfarin in achieving LVT resolution at 3 months. The meta-analysis was underpowered for safety outcomes but there was no signal of harm. These findings support the consideration of DOACs as a reasonable alternative to VKAs in selected patients with LVT, particularly in settings where warfarin use is logistically challenging. Future adequately powered trials for hard clinical outcomes are needed to inform clinical practice and guideline recommendations.
Author Contributions
Conceptualization, H.B.; methodology, H.B. and J.M.; software, H.B.; validation, N.H. and M.H. and S.A.; formal analysis, H.B.; investigation, H.B.; resources, H.B.; data curation, M.H., N.H. and S.A.; writing—original draft preparation, J.M., S.A. and M.H.; writing—review and editing, H.B. and S.B.W.; visualization, H.B. and S.B.W.; supervision, H.B. and S.B.W.; project administration, H.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Levine, G.N.; McEvoy, J.W.; Fang, J.C.; Ibeh, C.; McCarthy, C.P.; Misra, A.; Shah, Z.I.; Shenoy, C.; Spinler, S.A.; Vallurupalli, S.; et al. Management of Patients at Risk for and With Left Ventricular Thrombus: A Scientific Statement From the American Heart Association. Circulation 2022, 146, e205–e223. [Google Scholar] [CrossRef]
- Holzknecht, M.; Reindl, M.; Tiller, C.; Lechner, I.; Perez Cabrera, R.; Mayr, A.; Brenner, C.; Klug, G.; Bauer, A.; Metzler, B.; et al. Clinical Risk Score to Predict Early Left Ventricular Thrombus After ST-Segment Elevation Myocardial Infarction. Cardiovasc. Imaging 2021, 14, 308–310. [Google Scholar] [CrossRef]
- Bulluck, H.; Chan, M.H.H.; Paradies, V.; Yellon, R.L.; Ho, H.H.; Chan, M.Y.; Chin, C.W.L.; Tan, J.W.; Hausenloy, D.J. Incidence and predictors of left ventricular thrombus by cardiovascular magnetic resonance in acute ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention: A meta-analysis. J. Cardiovasc. Magn. Reson. 2018, 20, 72. [Google Scholar] [CrossRef]
- Gottdiener, J.S.; Gay, J.A.; VanVoorhees, L.; DiBianco, R.; Fletcher, R.D. Frequency and embolic potential of left ventricular thrombus in dilated cardiomyopathy: Assessment by 2-dimensional echocardiography. Am. J. Cardiol. 1983, 52, 1281–1285. [Google Scholar] [CrossRef]
- Ciaccheri, M.; Castelli, G.; Cecchi, F.; Nannini, M.; Santoro, G.; Troiani, V.; Zuppiroli, A.; Dolara, A. Lack of correlation between intracavitary thrombosis detected by cross sectional echocardiography and systemic emboli in patients with dilated cardiomyopathy. Br. Heart J. 1989, 62, 26–29. [Google Scholar] [CrossRef]
- Wu, H.S.; Dong, J.Z.; Du, X.; Hu, R.; Jia, C.Q.; Li, X.; Wu, J.H.; Ruan, Y.F.; Yu, R.H.; Long, D.Y.; et al. Risk Factors for Left Ventricular Thrombus Formation in Patients with Dilated Cardiomyopathy. Semin. Thromb Hemost 2023, 49, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Adi, D.; Wu, Y.; Aizezi, A.; Li, Y.P.; Kerem, M.; Wei, X.; Liu, F.; Ma, X.; Ma, Y.T. A nomogram to predict ventricular thrombus in dilated cardiomyopathy patients. J. Thromb. Thrombolysis 2024, 57, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Velangi, P.S.; Choo, C.; Chen, K.A.; Kazmirczak, F.; Nijjar, P.S.; Farzaneh-Far, A.; Okasha, O.; Akcakaya, M.; Weinsaft, J.W.; Shenoy, C. Long-Term Embolic Outcomes After Detection of Left Ventricular Thrombus by Late Gadolinium Enhancement Cardiovascular Magnetic Resonance Imaging: A Matched Cohort Study. Circulation. Cardiovasc. Imaging 2019, 12, e009723. [Google Scholar] [CrossRef] [PubMed]
- Gogos, C.; Anastasiou, V.; Papazoglou, A.S.; Daios, S.; Didagelos, M.; Kamperidis, N.; Moschovidis, V.; Papadopoulos, S.F.; Iatridi, F.; Sarafidis, P.; et al. Direct Oral Anticoagulants Versus Vitamin K Antagonists for the Management of Left Ventricular Thrombus After Myocardial Infarction: A Meta-Analysis. Am. J. Cardiol. 2024, 232, 18–25. [Google Scholar] [CrossRef]
- Khalid, S.; Joseph, T.; Isa, W.Y.H.W.; Bulluck, H. Letter to the Editor Regarding “A Meta-Analysis of RCTs Comparing DOACs Against Warfarin for the Treatment of Left Ventricular Thrombus”. Heart Lung Circ. 2024, 33, e53–e54. [Google Scholar] [CrossRef] [PubMed]
- Jenab, Y.; Sadeghipour, P.; Mohseni-Badalabadi, R.; Kaviani, R.; Hosseini, K.; Pasebani, Y.; Khederlou, H.; Rafati, A.; Mohammadi, Z.; Jamalkhani, S.; et al. Direct oral anticoagulants or warfarin in patients with left ventricular thrombus after ST-elevation myocardial infarction: A pilot trial and a prespecified meta-analysis of randomised trials. EuroIntervention 2025, 21, 82–92. [Google Scholar] [CrossRef]
- Vranckx, P.; Halvorsen, S. Cracking the Clot: The RIVAWAR Trial Challenges Warfarin’s Reign in Left Ventricular Thrombus Post-Acute Coronary Syndrome. Eur. Heart J. Acute Cardiovasc. Care 2025, 14, 243–244. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj 2011, 343, d5928. [Google Scholar] [CrossRef]
- Youssef, A.A.; Alrefae, M.A.; Khalil, H.H.; Abdullah, H.I.; Khalifa, Z.S.; Al Shaban, A.A.; Wali, H.A.; AlRajab, M.R.; Saleh, O.M.; Nashy, B.N. Apixaban in Patients With Post-Myocardial Infarction Left Ventricular Thrombus: A Randomized Clinical Trial. CJC Open 2023, 5, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, P.; Jazi, Z.A.; Mansouri, M.H.; Dehghan, H.; Zavar, R.; Hashemi, S.M.; Sattar, F.; Sadeghi, M.; Amirpour, A.; Abdar, M. Evaluation of the efficacy and safety of rivaroxaban compared to warfarin in patients with left ventricular apical thrombus: A randomized clinical trial. Thromb J. 2024, 22, 66. [Google Scholar] [CrossRef] [PubMed]
- Isa, W.Y.H.W.; Hwong, N.; Mohamed Yusof, A.K.; Yusof, Z.; Loong, N.S.; Wan-Arfah, N.; Naing, N.N. Apixaban versus Warfarin in Patients with Left Ventricular Thrombus: A Pilot Prospective Randomized Outcome Blinded Study Investigating Size Reduction or Resolution of Left Ventricular Thrombus. J. Clin. Prev. Cardiol. 2020, 9, 150–154. [Google Scholar] [CrossRef]
- Alcalai, R.; Butnaru, A.; Moravsky, G.; Yagel, O.; Rashad, R.; Ibrahimli, M.; Planer, D.; Amir, O.; Elbaz-Greener, G.; Leibowitz, D. Apixaban vs. warfarin in patients with left ventricular thrombus: A prospective multicentre randomized clinical trialdouble dagger. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Abdelnabi, M.; Saleh, Y.; Fareed, A.; Nossikof, A.; Wang, L.; Morsi, M.; Eshak, N.; Abdelkarim, O.; Badran, H.; Almaghraby, A. Comparative Study of Oral Anticoagulation in Left Ventricular Thrombi (No-LVT Trial). J. Am. Coll. Cardiol. 2021, 77, 1590–1592. [Google Scholar] [CrossRef]
- Yao, Z.; Gue, Y.; Lip, G.Y.H. Comparison of Direct Oral Anticoagulants and Vitamin K Antagonists for Left Ventricular Thrombus: A Global Retrospective Study. Am. J. Med. 2025, 138, 468–476. [Google Scholar] [CrossRef]
- Romualdi, E.; Ageno, W. Investigational factor Xa inhibitors for thrombosis and acute coronary syndromes. Expert. Opin. Investig. Drugs 2011, 20, 495–505. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).