Abstract
Objectives: To analyze the impact of implantoplasty on the mechanical resistance of dental implants, considering different implantoplasty designs and implant types. Methods: A systematic review was conducted in accordance with PRISMA guidelines. A search was performed in four databases: PubMed, Scopus, Web of Science, and Embase, along with a manual search for additional relevant studies. In vitro studies assessing the mechanical resistance of dental implants subjected to implantoplasty were included. A total of 136 studies were identified; after duplicate removal using Rayyan, and screening by title and abstract, 17 studies were ultimately selected after full-text assessment. Results: In vitro studies on external hexagon implants showed that fracture resistance in control groups ranged from 773.1 N to 1660 N for implants with a 4 mm diameter, and from 478.1 N to 1650 N after implantoplasty. For 3.5 mm diameter implants, values ranged from 548.8 N to 1276.1 N in control groups, and from 465.9 N to 1211.7 N after implantoplasty. In internal hexagon connections, fracture resistance after implantoplasty ranged between 321.7 N and 739 N. Conical connections exhibited a broader range of resistance values after implantoplasty, from 315.9 N to 2395.3 N. Conclusions: Implantoplasty reduces the mechanical strength of dental implants. Increased implantoplasty length correlates with decreased resistance, particularly affecting narrow implants. The prosthetic connection most affected by this procedure is the external hexagon, followed by the internal hexagon, with the conical connection being the most resistant.
1. Introduction
Dental implants have become a reliable and predictable solution for the rehabilitation of edentulous patients or those with partial tooth loss. However, biological complications such as peri-implantitis, a progressive and irreversible condition [1,2], pose a significant challenge to the long-term success of implant treatments. Peri-implantitis is characterized by inflammation, bleeding, and suppuration of peri-implant tissues, along with progressive bone loss around the implant [2,3,4], which can compromise its stability and functionality.
Implantoplasty, an adjunctive surgical therapy, is recommended for supracrestal bone defects, horizontal bone loss with exposed threads in non-aesthetic areas, depending on patient needs and satisfaction [5]. This procedure has been proposed as an effective procedure to reduce bacterial load on the implant surface and improve peri-implant tissue integration. It involves mechanical removal of the implant threads and rough surface [6], thereby smoothing the surface and reducing bacterial adhesion [7]. Various techniques and instruments can be used, which favor reduced bacterial colonization, promote fibroblast growth, and enhance healing [8,9,10,11,12]. Nevertheless, concerns remain regarding the effect of implantoplasty on implant structural integrity and the surrounding tissues.
Implantoplasty thins the implant walls and adversely affects their mechanical resistance, which depends on several factors, including implant diameter, platform design, and exposure to functional loads [13]. The removal of material during implantoplasty may compromise the structural integrity of the implant, reduce its load-bearing capacity, and increase the risk of fractures and long-term mechanical failure [14]. Additionally, it may cause overheating [10,15] and lead to harmful titanium particle deposition in surrounding tissues [15], which is further exacerbated by implant corrosion [16].
The objective of this study is to evaluate the effect of implantoplasty on the mechanical integrity of dental implants. Although several systematic reviews have addressed this topic, none have comprehensively assessed all the parameters considered in the present work. Accordingly, this review focuses on the available literature that includes experimental analyses of fracture and fatigue resistance before and after implantoplasty. This systematic review aims to provide a more thorough and integrated perspective, with the goal of determining whether implantoplasty has a significant impact on the structural and biomechanical integrity of dental implants.
2. Materials and Methods
2.1. Protocol and Registration
A systematic literature review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020 checklist [17]. The review protocol was registered in the Open Science Framework under the registration: https://doi.org/10.17605/OSF.IO/TU98A, accesed on 28 May 2025.
2.2. Research Question
The research question was formulated using the PICO framework (Patient, Intervention, Comparison, Outcome):
What is the fracture resistance of dental implants with peri-implantitis subjected to implantoplasty?
- Population: Dental implants with peri-implantitis
- Intervention: Implantoplasty
- Comparison: Untreated dental implant
- Outcome: Fracture resistance
2.3. Outcome Measures
The outcome measures were the fracture resistance and cyclic fatigue resistance of dental implants subjected to implantoplasty, a mechanical process of removing the threads and surface roughness to prevent bacterial plaque accumulation. The measurements were performed using a universal testing machine, according to ISO 14801:2007 and 2017 standards [18].
2.4. Eligibility Criteria
- Inclusion criteria: In vitro studies involving dental implants subjected to implantoplasty, and studies performing fatigue tests without limitation of diameter and length of dental implants.
- Exclusion criteria: In vivo studies, Randomized clinical trials, in silico studies, orthodontic implants, case reports.
2.5. Information Sources and Search Strategy
The databases searched up to March 2025 included PubMed, Embase, Scopus, and Web of Science. The search strategy used Boolean operators (AND, OR) combining MeSH and non-MeSH terms. No filters were applied for date or language.
Two independent reviewers (M.A.L.V and F.S.R) performed the search, study selection, and data extraction. Any disagreements were resolved by a third author. A manual search of references from selected articles was also conducted.
PubMed: (((fatigue[Title/Abstract]) OR (fracture resistance[Title/Abstract])) OR (strength[Title/Abstract])) AND (implantoplasty)[Title/Abstract]
Embase: (‘fatigue’/exp OR fatigue OR (cyclic AND (‘loading’/exp OR loading)) OR ((‘fracture’/exp OR fracture) AND (‘resistance’/exp OR resistance)) OR ‘strength’/exp OR strength) AND (‘implantoplasty’/exp OR implantoplasty)
Scopus: (‘fatigue’/exp OR fatigue OR (cyclic AND (‘loading’/exp OR loading)) OR ((‘fracture’/exp OR fracture) AND (‘resistance’/exp OR resistance)) OR ‘strength’/exp OR strength) AND (‘implantoplasty’/exp OR implantoplasty) (((fatigue) OR (fracture resistance[Title/Abstract])) OR (strength[Title/Abstract])) AND (implantoplasty)[Title/Abstract]
Web of Science: ((((TS = (fatigue)) OR TS = (cyclic loading)) OR TS = (fracture resistance)) OR TS = (strength)) AND TS = (implantoplasty)
2.6. Study Selection
After removing duplicates with Rayyan (https://rayyan.ai/cite, accessed on 21 August 2025), two independent reviewers selected the studies based on title and abstract. Full-text articles were then assessed, and studies not meeting the inclusion criteria, such as those not evaluating implant resistance or without implantoplasty, were excluded.
2.7. Data Extraction
Extracted variables included author, year, sample size, implant brand and material, implant diameter and length, prosthetic connection type, abutment details, torque, ISO standards, fixation materials, use of protective hemispherical cap, implantoplasty length, technique, burs and equipment used, polishing instruments, magnification, testing machine, chewing simulator, compression test, load cell, cyclic speed, measurement software, test temperature, SEM microscope used, fracture force and fatigue results. Microsoft Excel was used for data management and collection.
2.8. Risk of Bias
Risk of bias assessment followed the QUIN tool (risk of bias tool for assessing in vitro studies). Of the 17 in vitro studies evaluated, the result is of medium risk of bias, according to the QUIN evaluation tool, and its rating scale. Risk of Bias is shown in Table 1.

Table 1.
Risk of Bias—QUIN tool (quin assessment tool for in vitro studies), Scores for studies are awarded according to the following. Adequately specified = 2; inadequately specified = 1; not specified = 0; not applicable indicates that this category would not be counted.
3. Results
3.1. Development of the Study Selection
The search was conducted in March 2025 and identified 136 articles: 19 in PubMed, 16 in Embase, 35 in Web of Science, and 66 in Scopus. After removing duplicates, 90 articles remained. After screening titles and abstracts, 66 studies were excluded. A total of 21 articles were selected for full-text review, and 4 were excluded for not meeting inclusion criteria, such as not evaluating implant resistance or lacking implantoplasty procedures (Figure 1).
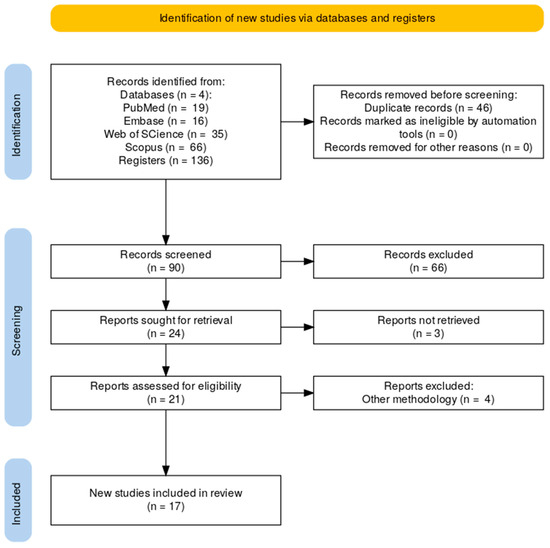
Figure 1.
PRISMA Flow Diagram.
The Meta-analysis was not performed due to the heterogeneity of the studies and the differences in dental implant designs across the studies.
3.2. Quality Assesment
Seventeen studies have medium quality based on the QUIN evaluation criterion used for in vitro studies, the scores thus obtained were used to grade the in vitro study as high, medium, or low risk (>70% = low risk of bias, 50% to 70% = medium risk of bias, and <50% = high risk of bias), due to the type of studies there is no randomization of the sample or a clear explanation of the sample calculation, as well as blinding the research advisor (see Table 1).
3.3. Study Characteristics
Sample sizes in the selected studies ranged from 18 to 315 implants. Brands included Straumann [18,19], TRI Vent implants [20,25], Biomimetic Ocean, Avinent [21,22,25,26,28], Implacil-Bortoli [23], Neodent [8], Klockner Implant System [27,33], Astra Tech Dentsply Sirona [30], and Conelog [31]. The prosthetic platforms used were external hexagon [8,22,23,26,27], internal hexagon [6,9,19,23,24,25,28,29,31,32], and conical connection [21,23,30]. All studies adhered to ISO 14801 standards [18] (see Table 2).

Table 2.
Implants Used in the Studies: Manufacturer, Dimensions, Connection Type, and Prosthetic Platform.
Implantoplasty lengths ranged from 1.5 mm [30], 3 mm [19,24,26,30,31], 4 mm [9], 4.5 mm [31] to 5 mm [6,20,23,25,26,29,32], 6 mm [8,27], and up to 7 mm [26] (Table 3). Implantoplasty was performed with a lathe in studies [6,9,19,23,25], or manually by a clinical expert in studies [8,9,20,22,24,26,27,28,29,30,32].

Table 3.
Implantoplasty: Length, Technique, Operator, and Instruments Used.
3.4. Fracture Resistance Results
External hexagon implants (Table 4): 4 mm diameter implants showed control group fracture resistance between 773.1 N and 1660 N, and between 478.1 N and 1650 N after implantoplasty. 3.5 mm implants ranged from 548.82 N to 1276.1 N in control groups and 465.95 N to 1211.7 N after implantoplasty. Some studies compared different implantoplasty techniques or evaluated crown-to-implant ratios [22].

Table 4.
Fracture Test Results of External Hexagon Implants.
Internal hexagon implants (Table 5): used diameters varied, with lengths of 10–11 mm. Fracture resistance ranged from 812 N [9] to 3325 N [20] in control groups, and from 321.7 N [20] to 739 N [9] post-implantoplasty.

Table 5.
Fracture Test Results of Internal Hexagon Implants.
Conical connection implants (Table 6): fracture resistance ranged from 348 N [31] to 2296.68 N [30] in controls, and 315.9 N [31] to 2395.3 N [30] after implantoplasty.

Table 6.
Fracture Test Results of Conical Connection Implants.
3.5. Cyclic Loading Results
Some studies [6,19,25,32] assessed implantoplasty after cyclic chewing simulations with predominant values of 2,000,000 cycles (Table 7).

Table 7.
Fracture Results of Implants after Cyclic Loading.
One study [28] showed results after cyclic loading using 5 × 106 cycles at 15 Hz, with decreasing loads in 5% increments (Table 8).

Table 8.
Implantoplasty Results under Cyclic Loading.
4. Discussion
Implantoplasty, when used in conjunction with surgical procedures, can be considered a viable alternative for the treatment of peri-implantitis [6]. Clinical decision-making should take into account the implant diameter and type of prosthetic connection when selecting implantoplasty as an adjunctive therapy, as it can help maintain the health of peri-implant tissues [1,2]. When properly performed with suitable materials, the procedure reduces bacterial plaque accumulation in the cervical region of the implant. However, it may also compromise the screw and abutment, especially considering the crown-to-implant ratio or lever arm effect.
Various implant designs and prosthetic connections—external hexagon, internal hexagon, and conical—of different diameters and lengths were used in the in vitro studies, allowing a broader understanding of implant behavior after undergoing implantoplasty.
Multiple methodologies and instruments have been employed for performing implantoplasty. The study by Costa-Berenguer et al. [8] is one of the most frequently cited, along with those by Tsampli, De Souza Júnior, Sahrmann, and Ramel [9,10,11,12]. These methods range from conventional rotary instrumentation (using carbide burs and high-speed handpieces) to more recent techniques involving ultrasonic tips with abrasive stones. Each method generates different levels of surface roughness and may induce issues such as thermal damage to peri-implant tissues—especially in structurally weakened implants [10,15] as well as inflammatory responses from titanium particle deposition in soft tissues [33], often linked to implant corrosion [16].
All included studies adhered to ISO 14801:2016 standards [18] for dynamic loading tests in dental implants. However, this standard does not account for peri-implantitis conditions, which introduces variability in measurement distances and load distribution, potentially influencing final results.
Material removal during implantoplasty, while beneficial for decontamination, inevitably weakens the implant structure. Narrow implants are more susceptible to mechanical failure post-procedure [19,20,31], while standard-diameter implants tend to retain greater structural resistance. Longer implantoplasty depths are associated with further decreases in mechanical resistance.
With respect to prosthetic connections, resistance increases as the connection becomes more internal. Thus, implants with external hex connections showed the lowest resistance [8,21,22,23,26,27], followed by internal hex [9,20,21,23], with conical connections being the most resistant [21,23,30,31].
Despite the growing body of evidence, no study has quantitatively assessed the amount of titanium lost during implantoplasty or its correlation with mechanical resistance at various preparation depths. Similarly, few studies have conducted cyclic fatigue tests with clear, standardized reporting.
In this study, we identified several limitations. Since the instruments and techniques used to perform implantoplasty are diverse and the time and calibration of the equipment used are parameters that could influence the outcome of implantoplasty or the degree of wear [9,10,11], it would be beneficial to measure the degree of corrosion [33], the amount of titanium released and the temperature rise [15] caused by the wear that could be released. Furthermore, by using in vitro studies, we inherently assume the limitations of each individual study, highlighting the lack of articles that use implants with a similar length, diameter and prosthetic connection, which makes direct comparisons difficult, and variability in their evaluation method. Therefore, it would be valuable for future research to consider conducting a review of clinical studies with several follow-up periods to obtain more reliable and clinically relevant results.
5. Conclusions
Implantoplasty decreases the mechanical resistance of dental implants, particularly in narrow-diameter implants. Increased implantoplasty length correlates with reduced implant strength.
Among prosthetic connections, the external hexagon is the most affected by the procedure, followed by the internal hexagon. The conical connection exhibits the highest mechanical resistance.
Author Contributions
All the authors contributed to the writing, reviewing, and editing of the study. Primary author and development of systematic review: M.L.V., collaboration un systematic review and verification of results: M.F.S.-R., A.R.-C. and R.A.-P., development of the manuscript: M.L.V., J.A.-L. and M.F.S.-R., metal-analysis and statistic analysis: C.L.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external fundings.
Institutional Review Board Statement
Ethics review and approval for this study was waived because it was an in vitro study.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Smeets, R.; Henningsen, A.; Jung, O.; Heiland, M.; Hammächer, C.; Stein, J.M. Definition, etiology, prevention and treatment of peri-implantitis—A review. Head Face Med. 2014, 10, 34. [Google Scholar] [CrossRef]
- Berglundh, T.; Jepsen, S.; Stadlinger, B.; Terheyden, H. Peri-implantitis and its prevention. Clin. Oral Implants Res. 2019, 30, 150–155. [Google Scholar] [CrossRef]
- Dreyer, H.; Grischke, J.; Tiede, C.; Eberhard, J.; Schweitzer, A.; Toikkanen, S.E.; Glöckner, S.; Krause, G.; Stiesch, M. Epidemiology and risk factors of peri-implantitis: A systematic review. J. Periodontal Res. 2018, 53, 657–681. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.; Gonzalo, E.; Gil Villagra, L.J.; Miegimolle, B.; Suarez, M.J. What is the prevalence of peri-implantitis? A systematic review and meta-analysis. BMC Oral Health 2022, 22, 449. [Google Scholar] [CrossRef] [PubMed]
- Khoury, F.; Keeve, P.L.; Ramanauskaite, A.; Schwarz, F.; Koo, K.-T.; Sculean, A.; Romanos, G. Surgical treatment of peri-implantitis—Consensus report of working group 4. Int. Dent. J. 2019, 69, 18–22. [Google Scholar] [CrossRef]
- Stavropoulos, A.; Bertl, K.; Isidor, F.; von Steyern, P.V. Implantoplasty and the risk of fracture of narrow implants with advanced bone loss: A laboratory study. Clin. Oral Implants Res. 2023, 34, 1038–1046. [Google Scholar] [CrossRef]
- Bianchini, M.; Galarraga-Vinueza, M.; Bedoya, K.; Correa, B.; Magini, R.d.S.; Schwarz, F. Implantoplasty Enhancing Peri-implant Bone Stability Over a 3-Year Follow-up: A Case Series. Int. J. Periodontics Restor. Dent. 2020, 40, e1–e8. [Google Scholar] [CrossRef]
- Costa-Berenguer, X.; García-García, M.; Sánchez-Torres, A.; Sanz-Alonso, M.; Figueiredo, R.; Valmaseda-Castellon, E. Effect of implantoplasty on fracture resistance and surface roughness of standard diameter dental implants. Clin. Oral Implants Res. 2018, 29, 46–54. [Google Scholar] [CrossRef]
- Tsampli, A.; Rues, S.; Kappel, H.; Rammelsberg, P.; Kappel, S. In vitro pilot study comparing a novel implantoplasty sonic instrumentation protocol with a conventional protocol using burs. Clin. Oral Implants Res. 2024, 35, 340–349. [Google Scholar] [CrossRef]
- De Souza Júnior, J.M.; De Souza, J.G.O.; Neto, A.L.P.; Iaculli, F.; Piattelli, A.; Bianchini, M.A. Analysis of effectiveness of different rotational instruments in implantoplasty: An in vitro study. Implant Dent. 2016, 25, 341–347. [Google Scholar] [CrossRef]
- Sahrmann, P.; Luso, S.; Mueller, C.; Ender, A.; Attin, T.; Stawarczyk, B.; Schmidlin, P. Titanium Implant Characteristics After Implantoplasty: An In Vitro Study on Two Different Kinds of Instrumentation. Int. J. Oral Maxillofac. Implants 2019, 34, 1299–1305. [Google Scholar] [CrossRef]
- Ramel, C.F.; Lüssi, A.; Özcan, M.; Jung, R.E.; Hämmerle, C.H.F.; Thoma, D.S. Surface roughness of dental implants and treatment time using six different implantoplasty procedures. Clin. Oral Implants Res. 2016, 27, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, D.; Banerjee, S.; Parasrampuria, N.; Pal, D. Efficacy of implantoplasty in management of peri-implantitis: A systematic review. J. Indian Prosthodont. Soc. 2023, 23, 210–217. [Google Scholar] [CrossRef]
- Keeve, P.L.; Koo, K.T.; Ramanauskaite, A.; Romanos, G.; Schwarz, F.; Sculean, A.; Khoury, F. Surgical Treatment of Periimplantitis with Non-Augmentative Techniques. Implant Dent. 2019, 28, 177–186. [Google Scholar] [CrossRef]
- Sharon, E.; Shapira, L.; Wilensky, A.; Abu-Hatoum, R.; Smidt, A. Efficiency and Thermal Changes during Implantoplasty in Relation to Bur Type. Clin. Implant Dent. Relat. Res. 2013, 15, 292–296. [Google Scholar] [CrossRef]
- Lozano, P.; Peña, M.; Herrero-Climent, M.; Rios-Santos, J.V.; Rios-Carrasco, B.; Brizuela, A.; Gil, J. Corrosion Behavior of Titanium Dental Implants with Implantoplasty. Materials 2022, 15, 1563. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- UNI EN ISO 14801:2016; Dentistry—Implants—Dynamic Loading Test for Endosseous Dental Implants. Asociación Española de Normalización: Génova, Italy; Madrid, Spain, 2017.
- Bertl, K.; Isidor, F.; von Steyern, P.V.; Stavropoulos, A. Does implantoplasty affect the failure strength of narrow and regular diameter implants? A laboratory study. Clin. Oral Investig. 2021, 25, 2203–2211. [Google Scholar] [CrossRef]
- Chan, H.-L.; Oh, W.-S.; Ong, H.S.; Fu, J.-H.; Steigmann, M.; Sierraalta, M.; Wang, H.-L. Impact of Implantoplasty on Strength of the Implant-Abutment Complex. Int. J. Oral Maxillofac. Implants 2013, 28, 1530–1535. [Google Scholar] [CrossRef]
- Camps-Font, O.; González-Barnadas, A.; Mir-Mari, J.; Figueiredo, R.; Gay-Escoda, C.; Valmaseda-Castellón, E. Fracture resistance after implantoplasty in three implant-abutment connection designs. Med. Oral Patol. Oral Y Cirugia Bucal 2020, 25, E691–E699. [Google Scholar] [CrossRef]
- Leitão-Almeida, B.; Camps-Font, O.; Correia, A.; Mir-Mari, J.; Figueiredo, R.; Valmaseda-Castellón, E. Effect of crown to implant ratio and implantoplasty on the fracture resistance of narrow dental implants with marginal bone loss: An in vitro study. BMC Oral Health 2020, 20, 329. [Google Scholar] [CrossRef]
- Gehrke, S.; Junior, J.; Dedavid, B.; Shibli, J. Analysis of Implant Strength After Implantoplasty in Three Implant-Abutment Connection Designs: An In Vitro Study. Int. J. Oral Maxillofac. Implants 2016, 31, e65–e70. [Google Scholar] [CrossRef] [PubMed]
- Diéguez-Pereira, M.; Chávarri-Prado, D.; Viteri-Agustín, I.; Montalban-Vadillo, O.; Pérez-Pevida, E.; Brizuela-Velasco, A. Effect of implantoplasty on the elastic limit of dental implants of different diameters. Int. J. Implant. Dent. 2021, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Jorio, I.C.; Stawarczyk, B.; Attin, T.; Schmidlin, P.R.; Sahrmann, P. Reduced fracture load of dental implants after implantoplasty with different instrumentation sequences. An in vitro study. Clin. Oral Implants Res. 2021, 32, 881–892. [Google Scholar] [CrossRef]
- Leitão-Almeida, B.; Camps-Font, O.; Correia, A.; Mir-Mari, J.; Figueiredo, R.; Valmaseda-Castellón, E. Effect of bone loss on the fracture resistance of narrow dental implants after implantoplasty. An in vitro study. Med. Oral Patol. Oral Cir. Bucal 2021, 26, e611–e618. [Google Scholar] [CrossRef]
- Sivolella, S.; Brunello, G.; Michelon, F.; Concheri, G.; Graiff, L.; Meneghello, R. Implantoplasty: Carbide burs vs diamond sonic tips. An in vitro study. Clin. Oral Implants Res. 2021, 32, 324–336. [Google Scholar] [CrossRef]
- Camps-Font, O.; Toledano-Serrabona, J.; Juiz-Camps, A.; Gil, J.; Sánchez-Garcés, M.A.; Figueiredo, R.; Gay-Escoda, C.; Valmaseda-Castellón, E. Effect of Implantoplasty on Roughness, Fatigue and Corrosion Behavior of Narrow Diameter Dental Implants. J. Funct. Biomater. 2023, 14, 61. [Google Scholar] [CrossRef]
- Fonseca, D.; de Tapia, B.; Pons, R.; Aparicio, C.; Guerra, F.; Messias, A.; Gil, J. The Effect of Implantoplasty on the Fatigue Behavior and Corrosion Resistance in Titanium Dental Implants. Materials 2024, 17, 2944. [Google Scholar] [CrossRef]
- Goh, R.; Li, K.C.; Atieh, M.A.; Ma, S.; Oliver, A.; Giraldo, D.; Tawse-Smith, A. The Effect of Implantoplasty on Fracture Resistance and Implant Surface Changes: An In Vitro and Finite Element Analysis Study. Clin. Implant Dent. Relat. Res. 2024, 27, e13409. [Google Scholar] [CrossRef]
- Graf, T.; Güth, J.-F.; Schweiger, J.; Erdelt, K.-J.; Edelhoff, D.; Stimmelmayr, M. Biomechanical behavior of implants with different diameters in relation to simulated bone loss—An in vitro study. Clin. Oral Investig. 2023, 27, 5887–5894. [Google Scholar] [CrossRef]
- Shah, S.D.; Zheng, F.; Seghi, R.R.; Lee, D.J. Strength of titanium-zirconium alloy implants with a conical connection after implantoplasty. J. Prosthet. Dent. 2022, 132, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Toledano-Serrabona, J.; Bosch, B.M.; Díez-Tercero, L.; Gil, F.J.; Camps-Font, O.; Valmaseda-Castellón, E.; Gay-Escoda, C.; Sánchez-Garcés, M.Á. Evaluation of the inflammatory and osteogenic response induced by titanium particles released during implantoplasty of dental implants. Sci. Rep. 2022, 12, 15790. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).