Abstract
Objective: The purpose of this study was to report the clinical characteristics and prognosis of spontaneous isolated abdominal aortic dissection (SIAAD) based on the dissection length. Methods: Between March 2012 and September 2023, 159 of 7572 patients with aortic dissection were diagnosed with SIAAD and enrolled in the retrospective study. We proposed a new morphologic classification: extensive SIAAD (e-SIAAD) and focal SIAAD (f-SIAAD), based on whether the dissection length exceeds 50 mm or not. The clinical baseline, computed tomography angiography (CTA) findings, and long-term follow-up of the two types were compared. Results: SIAAD prevalence was 2.1%. Patients with f-SIAAD were significantly older (63.74 ± 10.97 vs. 50.70 ± 10.10 years, p < 0.001), had more atherosclerosis risk factors, arteriosclerosis, and penetrating aortic ulcers compared to e-SIAAD patients. Conversely, e-SIAAD presented more acutely (72.97% vs. 34.12%, p = 0.001), exhibited more frequent symptoms (85.14% vs. 61.18%, p = 0.0037), larger dissection diameters (31.89 ± 10.99 vs. 24.41 ± 11.28 mm, p = 0.001), and greater involvement of the renal and iliac arteries. Treatment involved medical management (30%), endovascular repair (65%), or surgery (2.5%), without significant differences between groups. In-hospital mortality was higher in f-SIAAD (six deaths vs. one in e-SIAAD). During median follow-up of 48 months (range, 6–148 months), mortality was higher in f-SIAAD (70% vs. 90% estimated 10-year survival). Conclusions: SIAAD classification by dissection length revealed significant differences in clinical presentation, CTA characteristics, and prognosis. Focal dissections correlated with advanced age, severe arteriosclerosis, and poorer long-term outcomes, emphasizing the need for tailored management approaches.
1. Introduction
Spontaneous isolated abdominal aorta dissection (SIAAD) is an infrequent and potentially fatal condition, accounting for 1% to 4% of all aortic dissections [1,2,3]. In SIAAD, the dissection flap is confined to the abdominal aorta, with no extension into the thoracic aorta. This distinguishes it from the classic DeBakey/Stanford classifications of aortic dissection, which primarily describe thoracic involvement. The first SIAAD was reported nearly two centuries ago, in 1822 [4]. Yet, even after 185 years since that report, the epidemiology, pathophysiology, and optimal treatment strategy remain unclear. Risk factors include hypertension, atherosclerosis, and connective tissue disorders.
Most published experience consists of individual case reports and small case series, which limit our understanding of this rare entity [5,6,7]. In those studies, the classification of SIAAD was primarily based on the anatomical relationship between the location of the primary entry tear and the orifices of abdominal vessels (such as suprarenal and infrarenal) to guide endovascular repair and surgery [8]. However, little research has focused on the classification of the morphological characteristics of SIAAD itself. Some SIAADs, like thoracic aortic dissection, cause a relatively long dissection; yet other SIAADs cause a quite short dissection. This may suggest different pathological features between the extensive (long) and focal (short) SIAADs.
Accordingly, the aim of this retrospective study was to explore and compare the risk factors, clinical characteristics, treatment modalities, and long-term follow-up results between patients with the extensive and focal SIAAD.
2. Methods
This retrospective study was conducted at Tongji Hospital, a single high-volume tertiary care center in Wuhan, China, over a 12-year period from March 2012 to December 2023. Of the 7572 patients diagnosed with aortic dissection (AD) during this time, 159 cases met the criteria for SIAAD. SIAAD was defined as any AD exclusively involving the aorta below the diaphragm on spiral computed tomography angiography (CTA) and confirmed by at least two experienced interventional cardiologists. Patients with intramural hematoma (IMH), thoracic AD, and iatrogenic and traumatic SIAAD were excluded. Residual abdominal AD after endovascular repair for thoracoabdominal AD was also excluded.
The Ethics Committee of Tongji Hospital approved this retrospective observational study, Huazhong University of Science and Technology (TJ-IRB20211102). All aspects of the study comply with the principles outlined in the Declaration of Helsinki.
All patients were diagnosed by computed tomography angiography (CTA) using a 64-detector row scanner (LightSpeed VCT; GE Healthcare, Milwaukee, WI, USA). A non-ionic contrast medium containing 300 mg iodine/mL iopromide (Ultravist 370®; Bayer Healthcare, Berlin, Germany) was administered intravenously in a total volume of 120 mL.
Data Collection and Classifications
Demographics, cardiovascular risk factors, clinical presentations, aortic CTA features, treatment modality, and in-hospital and long-term outcomes were collected through medical records and via telephone follow-up or mailed questionnaire when necessary.
To objectively establish the threshold distinguishing extensive (e-SIAAD) from focal SIAAD (f-SIAAD), we analyzed the distribution of dissection lengths (Figure 1). Notably, a clear bimodal distribution was observed, with a natural separation occurring near 50 mm, where a distinct reduction in frequency was evident. For the sake of convenience, we defined the dissection length >50 mm as e-SIAAD (n = 74) and that ≤50 mm as f-SIAAD (n = 85).
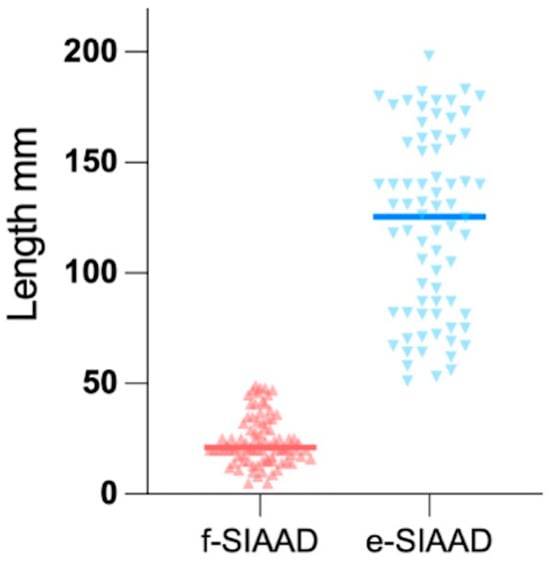
Figure 1.
A clear bimodal distribution is evident, with a distinct reduction in frequency near 50 mm, supporting the classification into focal (f-SIAAD, ≤50 mm) and extensive (e-SIAAD, >50 mm) subtypes. The mean dissection length of extensive-SIAAD (118.93 ± 43.65 mm) is significantly greater than that of focal-SIAAD (24.48 ± 10.92 mm; p < 0.001). SIAAD, spontaneous isolated abdominal aortic dissection.
According to the time interval from symptom onset to hospital admission, SIAAD was divided into two groups: acute (≤2 weeks) and chronic (>2 weeks) SIAAD [9]. According to the location of the primary entry site, SIAAD was categorized into three groups: supraceliac (above the celiac artery), paravisceral (between the celiac artery and the lowest renal artery), and infrarenal (below the lowest renal artery) [10].
Other imaging data on aortic CTA (like SIAAD maximum diameter, false lumen thrombosis, aortic arteriosclerosis, abdominal aortic aneurysm [AAA], penetrating aortic ulcer [PAU], and branch artery involvement) were also collected and evaluated. PAU was defined as the presence of a filling defect in the aorta, without the intimal flap and true/false lumens.
3. Statistical Analysis
Categorical data were presented as frequency and percentage. Continuous variables were presented as mean ± standard deviation for normally distributed data, or as median with interquartile range (IQR) for non-normally distributed data.
A comparison between the extensive and focal SIAAD was performed using the Fisher exact test for categorical data and the independent Student t-test for continuous data. Kaplan–Meier survival analysis was performed for time-to-event data, and differences between survival curves were assessed using the log-rank test.
Statistical analyses were performed with the SPSS software (version 21.0) and GraphPad Prism version 9.5.0 (GraphPad Software, San Diego, CA, USA). A two-sided value of p < 0.05 was considered statistically significant.
4. Results
4.1. Patient Characteristics and Clinical Presentation
The prevalence of SIAAD in this study was 2.1% (159 per 7572 AD patients). The baseline characteristics of SIAAD patients are described in Table 1. The mean age was 57.7 ± 12.4 years (range, 31–86 years), and 119 (74.8%) patients were men. SIAAD patients often had a history of hypertension (112, 70.44%), with a relatively high prevalence of coronary artery disease (56, 35.22%). There were 115 (72.33%) patients with SIAAD-related symptoms on admission. Abdominal pain was the most common symptom (62, 38.99%). A total of 83 (52.2%) of patients were admitted to the hospital in the acute phase of SIAAD. Of 67 (47.8%) chronic SIAAD patients, 24 (36%) had SIAAD-related symptoms; 17 (25%) were asymptomatic; and 26 (39%) patients were admitted to the hospital for other conditions: among these, acute coronary syndrome was slightly the most frequently observed. Notably, less than half of the patients (77, 48.43%) were initially diagnosed with dissection upon admission.

Table 1.
Baseline characteristics of SIAAD according to the dissection length.
The number ratio of f-SIAAD (n = 85) and e-SIAAD (n = 74) was close to 1:1. Compared with patients with e-SIAAD, those with f-SIAAD were much older (66 ± 14 vs. 49 ± 13 years, p < 0.001) and more likely to have a history of hypertension, diabetes mellitus, coronary artery disease, and chronic renal insufficiency. Moreover, patients with f-SIAAD were more frequently admitted to hospital in chronic phase of SIAAD (56 [66.88%] vs. 20 [27.03%], p = 0.001) and had less SIAAD-related symptoms (including chest, abdominal, back, and lumbar pain) (52 [61.18%] vs. 63 [85.14%], p = 0.0037). Furthermore, AD as the initial diagnosis on admission was less frequently reported in f-SIAAD than in e-SIAAD (21 [42.71%] vs. 51 [68.92%], p = 0.001). There was no statistically significant difference in terms of sex, smoking, and alcohol between the patients with f-SIAAD and e-SIAAD.
4.2. CTA Imaging Findings
The representative CTA images of e-SIAAD and f-SIAAD are presented in Figure 2, and the aortic CTA characteristics are reported in Table 2. Mean dissection length was 68.4 ± 56.3 mm (5–198 mm). Most primary entries (103, 64.78%) were located under the lowest renal artery (infrarenal-SIAAD). The length of dissection was significantly longer in e-SIAAD than that in f-SIAAD (118.93 ± 43.65 vs. 24.48 ± 10.92 mm, p < 0.001) (Figure 1). Compared to e-SIAAD, f-SIAA had a higher frequency of infrarenal primary entry, aortic arteriosclerosis, and penetrating aortic ulcer. In contrast, e-SIAAD had a bigger maximum dissection diameter and was more often associated with false lumen thrombosis and more involvement of the renal artery and common iliac artery.
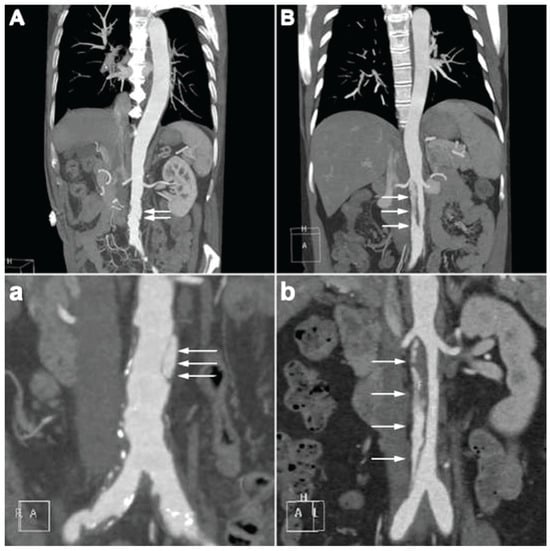
Figure 2.
(A,a) the characteristic of focal-SIAAD on aortic CTA is the presence of a short intimal flap in the abdominal aorta, often with aortic arteriosclerosis. (B,b) the characteristic of extensive-SIAAD on aortic CTA is the presence of a long intimal flap in the abdominal aorta, normally without apparent aortic arteriosclerosis. Two partial enlarged images of (A,B) are shown in (a,b), respectively. White arrows denote the intimal flap and false lumen. CTA, computed tomography angiography; SIAAD, spontaneous isolated abdominal aortic dissection.

Table 2.
Aortic CTA findings of SIAAD according to the dissection length.
4.3. Management and In-Hospital Outcomes
As shown in Table 3, management of SIAAD patients was medical in 48 (30.19%), endovascular in 104 (65.41%), and surgical in 4 (2.52%) patients. Initial medical therapy was administered with a β-blocker in 101 (63%) patients, an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker in 55 (34%) patients, and a calcium channel blocker in 126 (79%) patients. Anti-platelet agents (aspirin and clopidogrel) and statins were more commonly used in f-SIAAD than in e-SIAAD.

Table 3.
In-hospital management and outcome of SIAAD according to the dissection length.
There were 40 (25.16%) patients (12 f-SIAAD and 28 e-SIAAD) who had AAA. The abdominal aorta was replaced in four patients, two e-SIAAD, both of whom had aortic rupture, and two f-SIAAD had giant AAA (diameter > 70 mm). Notably, one f-SIAAD patient (man, 70 years) experienced severe aortic rupture immediately before surgery. Despite emergency replacement of the abdominal aorta under cardiopulmonary bypass, the patient remained hemodynamically unstable postoperatively and subsequently passed away. Overall, the in-hospital mortality rate, irrespective of e-SIAAD or f-SIAAD, was relatively low (7, 4%), with six f-SIAAD and one e-SIAAD cases. The mean hospital stay was 11.4 days, with 9 days for patients treated medically and 13 days for those undergoing surgery or endovascular repair. There were seven death cases in the hospital, among which six were treated medically and one with open surgery. The patient with open surgery underwent emergency abdominal aorta replacement for severe aortic rupture before surgery but remained unstable postoperatively and subsequently died. Of the six patients managed conservatively, two died from acute aortic rupture, for which their families refused surgery; one patient’s dissection involved the superior mesenteric artery (SMA), resulting in intestinal necrosis. This complication progressed to acute diffuse peritonitis and ultimately resulted in multiorgan failure and death. One patient was admitted for acute myocardial infarction (AMI) and died of heart failure during hospitalization. One patient admitted with acute liver failure during hospitalization developed severe pneumonia and respiratory failure, ultimately resulting in death. One patient admitted with advanced lung cancer also died during the hospital stay.
4.4. Long-Term Outcomes
After discharge, 11 patients died during follow-up and their detailed information is shown in Table 4. Mortality was reported in one patient treated surgically, one patient who underwent endovascular repair, and nine patients who received medical treatment. The median follow-up was 48 months (range, 6–148 months). The Kaplan–Meier estimated survival rate at 1 year was ~98%, at 5 years ~95%, and at 10 years ~80% for the entire cohort (Figure 3A). When stratified by dissection type, there was a notable divergence in survival curves (Figure 3B). Patients with f-SIAAD had lower survival over time than those with e-SIAAD. The estimated 5-year survival for f-SIAAD was approximately 78%, compared to ~95% for e-SIAAD. By 10 years, about 70% of f-SIAD patients were alive vs. ~90% of e-SIAAD (though few e-SIAAD had 10-year follow-up given many were younger at baseline).

Table 4.
Information of death cases during hospitalization and follow-up.
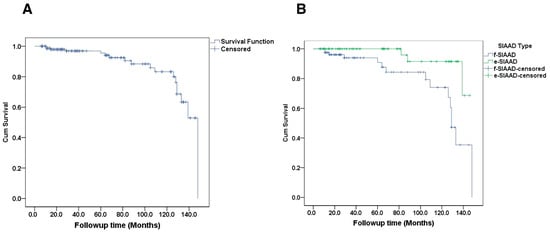
Figure 3.
(A) Survival curve in patients with SIAAD. (B) the cumulative survival rate in focal-SIAAD is significantly lower than that in extensive-SIAAD (p = 0.010). SIAAD, spontaneous isolated abdominal aortic dissection.
Repeated aortic CTA was obtained in 56 (35%) of 159 patients during follow-up. Of 108 patients initially treated with surgery or endovascular repair, 42 had CTA follow-up, and all images showed a reduction in aortic diameter in the dissection segment. Of 48 patients initially treated with a conservative strategy, 14 (10 f-SIAAD and 4 e-SIAAD) had CTA follow-up. In the 10 f-SIAAD patients, the dissection image showed progressive enlargement in three (one patient had a giant AAA and refused surgery), improvement in one, and unchanged in six patients. In the four e-SIAAD patients, the dissection image showed progressive enlargement in one (late endovascular repair was scheduled for this patient) and improvement in three patients.
5. Discussion
The main findings of this study are as follows: (1) Patients with f-SIAAD were much older and had more cardiovascular risk factors (such as hypertension and diabetes mellitus), coronary artery disease, and chronic renal insufficiency than those with e-SIAAD; (2) Patients with e-SIAAD were more often admitted to the hospital in the acute phase and more frequently had SIAAD-related symptoms than those with f-SIAAD; (3) Aortic CTA showed that f-SIAAD was more often located at the infrarenal abdominal aorta and had arteriosclerosis and PAU.
In contrast, e-SIAAD more frequently had false lumen thrombosis and involved the renal and common iliac artery. Moreover, e-SIAAD also had wider and longer dissections; (4) The long-term prognosis in patients with f-SIAAD had a worse long-term survival than those with e-SIAAD, despite the focal dissections being anatomically smaller.
This retrospective study proposed a new morphological classification of SIAAD based on dissection length. As shown in Figure 1, a clear bimodal distribution emerges with natural separation near 50 mm, which we used as the cut-point for distinguishing f-SIAAD and e-SIAAD. In a previous study with detailed data on each patient, an apparent gap was observed in the ascending order of SIAAD length, with a mean dissection length of 54 mm [11]. From a procedural perspective, a segment of 5 cm is frequently referenced as the minimum length required for secure stent graft anchoring during endovascular repair, underscoring the practical significance of this threshold [12]. Importantly, when patients were grouped by this criterion, clear distinctions in both clinical presentation and anatomical features emerged, reinforcing the validity of this approach. Therefore, a cut-point of 50 mm may be reasonable.
The prevalence of SIAAD in our study aligns with previous studies, which range from 1% to 4% [1,13]. However, the true prevalence of SIAAD was likely underestimated, as a significant portion of patients in the study either presented without symptoms or had their dissection discovered incidentally. According to the International Registry of Acute Aortic Dissection (IRAD) study, up to 95% of patients experience dissection-related symptoms [2]. This could partly explain why less than half of the patients, 77 (48.43%), in this study were initially diagnosed with AD on admission. Moreover, compared to e-SIAAD, f-SIAAD patients were more likely to be admitted to the hospital in the chronic phase of AD and less likely to be initially diagnosed with AD on admission, mainly because more patients in f-SIAAD were asymptomatic than in e-SIAAD, suggesting that f-SIAAD is more likely to be missed than e-SIAAD.
SIAAD is often linked to hypertension, atherosclerosis, and preexisting aneurysmal changes [14,15]. In our updated data, AAA was present in 25% of cases. In addition, our data further expanded previous reports. Clinically, we observed that, compared with patients with e-SIAAD, those with f-SIAAD were older and more likely to have hypertension, diabetes mellitus, and coronary artery disease. Furthermore, aortic CTA image data showed that f-SIAAD had more aortic arteriosclerosis and PAU than e-SIAAD. Therefore, we believe that there might be some underlying pathology to explain these significant differences between f-SIAAD and e-SIAAD. A possible explanation might be that f-SIAAD is strongly associated with arteriosclerosis, and the presence of scarring and medial atrophy caused by arteriosclerosis limits the propagation of f-SIAAD. In addition to the length difference, we also found that, compared to e-SIAAD, the primary entry site of f-SIAAD was more commonly located in the infrarenal district. In contrast, e-SIAAD had a higher propensity for proximal entry: 37.8% of e-SIAAD had the primary entry tear above the renal arteries (suprarenal, involving the segment between the celiac axis and the lowest renal artery) compared to only 12.9% in f-SIAAD.
Management for SIAAD includes medical treatment, endovascular repair, and surgery. In the IRAD study, medical therapy was performed in two-thirds of patients [2], whereas endovascular repair or surgery was adopted in most patients in other published studies. Treatments varied in different studies, and there is no available therapeutic guideline for SIAAD. Contrary to the IRAD findings, in this study, EVAR was the primary treatment in 65% of patients, making it the predominant strategy. This aligns with recent publications that report increasing use of EVAR for isolated abdominal dissections [16,17]. The rationale for EVAR in SIAAD is to exclude the entry tear and reinforce the dissected segment, thus preventing false lumen pressurization and potential aneurysm formation or rupture (Figure 4). Our results show that EVAR achieved excellent aortic remodeling in all treated patients, with no aorta-related deaths in that group. This finding echoes other reports, which have shown high success and low mortality rates for EVAR in SIAAD [16,18]. In fact, a recent systematic review found endovascular treatment of SIAAD to be associated with good long-term aortic remodeling and low complication rates [19].
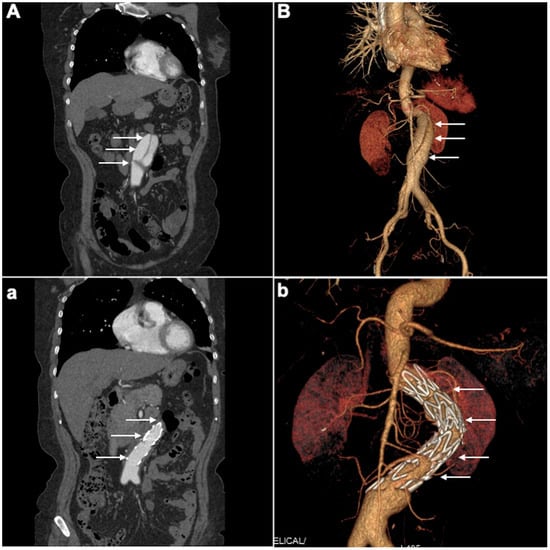
Figure 4.
(A) pre-EVAR CTA intimal flap separates true and false lumens within the abdominal aorta. (a) post-EVAR CTA successful stent-graft coverage of entry tear with absence of false lumens opacification. (B) Pre-EVAR 3D-CTA visualizes the SIAAD extending through the infrarenal aorta with evidence of a dissecting aneurysm and a prominent false lumen. (b) Post-EVAR 3D-CTA complete exclusion of the false lumen and successful aneurysm repair, with the endovascular stent graft visible. White arrows denote the intimal flap, false lumen, and stent-graft deployment. 3D-CTA, Three-dimensional Computed Tomography Angiogram; SIAAD, spontaneous isolated abdominal aortic dissection.
Overall, the short- and long-term clinical outcomes of SIAAD were good. In our study, medical therapy was adopted in 30% of patients, endovascular therapy was performed in 65%, and surgical treatment was performed in 2.5%. Given the larger sample size of 159 patients and the extended follow-up period compared to previous smaller studies, clearer insights into therapeutic outcomes were possible. However, significant differences between treatment modalities remained limited. This reflects findings in the context of uncomplicated type B (thoracic) dissections, where randomized trials (e.g., ADSORB) showed improved aortic remodeling with TEVAR but no early survival difference [20]. The fact that about one-third of our patients were managed medically and did well indicates that not every SIAAD requires stenting. For the f-SIAAD patients, 15 patients died in the hospital and the follow-up period, while only 4 were aorta-related deaths. Additionally, patients with f-SIAAD were frequently associated with old age, more co-morbidities, and fewer SIAAD-related symptoms. Hence, strict medical management focusing on the control of risk factors, heart rate, and blood pressure is advisable as a suitable strategy for f-SIAAD patients. For the e-SIAAD patients, only three patients died in hospital and during the follow-up period. Of these, one was attributed to an aorta-related cause, while the causes of the remaining two deaths were unknown. Additionally, the rate of early outcome for false lumen thrombosis was relatively high (41%), with some cases even being completely thrombotic (Figure 5(B1,B2)). Therefore, it is suggested that, in uncomplicated e-SIAAD patients, conservative treatment can occasionally be appropriate. Still, given the lack of reliable predictors of which will enlarge, ongoing surveillance to identify signs of dissection, AAA progression, and rupture is prudent for both f-SIAAD and e-SIAAD patients.
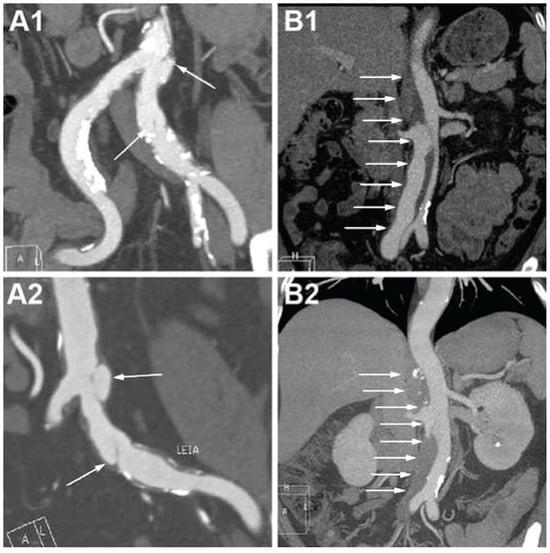
Figure 5.
CTA images on admission and at follow-up in focal and extensive SIAAD. (A1) F-SIAAD on admission; (A2) F-SIAAD remained unchanged at 24 months after discharge; (B1) E-SIAAD on admission; (B2) the false lumen of E-SIAAD was almost completely thrombotic at 24 months after discharge. White arrows denote the intimal flap and false lumen. CTA, computed tomography angiography; SIAAD, spontaneous isolated abdominal aortic dissection.
Interestingly, although the dissection length was much shorter in f-SIAAD than e-SIAAD, the long-term clinical follow-up tended to be worse in f-SIAAD. There are several reasons behind this “paradoxical” phenomenon. First, patients with f-SIAAD were much older and had more cardiovascular risk factors (like hypertension and diabetes) than those with e-SIAAD. Second, f-SIAAD was more often associated with arteriosclerosis and coronary artery disease. Third, f-SIAAD patients more frequently had chronic renal insufficiency than e-SIAAD patients. Hence, the life expectancy was speculated to be “paradoxically” shorter in f-SIAAD than e-SIAAD.
In conclusion, this study introduces a novel morphological classification of SIAAD into two subtypes: focal (f-SIAAD) and extensive (e-SIAAD), demonstrating significant prognostic and therapeutic implications. While our findings support the clinical utility of this classification, further prospective multicenter research is required to validate its predictive value and refine management strategies.
6. Conclusions
SIAAD may be further divided into focal and extensive SIAAD according to the dissection length. The significant distinctions in age, risk factors, clinical presentations, and CTA features between incident arteriosclerosis and vascular conditions in e-SIAAD and f-SIAAD indicate possible different mechanisms underlying them. Despite these differences, prompt diagnosis and appropriate management are key to good outcomes in both groups.
Endovascular aortic repair has become the first-line treatment for many patients, reflecting a shift in practice and offering excellent aortic remodeling. Conservative management remains a valid approach in select cases; however, careful monitoring is necessary to detect any late aortic enlargement. For selected patients with uncomplicated e-SIAAD, conservative management with close surveillance to identify signs of disease progression is justifiable. However, in cases of complicated e-SIAAD or high-risk anatomical features, endovascular intervention is now widely favored due to its safety, efficacy, and favorable impact on aortic remodeling, making it the preferred treatment modality in current practice. Moreover, the overall response to medical therapy in SIAAD was positive regardless of the trend that f-SIAAD may result in a worse long-term clinical prognosis than e-SIAAD. Ongoing research and collaboration will further refine these strategies, leading to the development of formal guidelines to assist in the care of patients with this uncommon form of AD.
7. Limitations
There are several limitations in our study. First, this study was observational and retrospective, conducted at a single high-volume center, which may introduce selection bias in patient inclusion and management decisions. Second, we cannot compare outcomes based on treatment approach in the two groups because of the same retrospective design reason. Ideally, patients with SIAAD would be randomized in a trial to analyze the results of the different treatment modalities. Third, due to the study’s nature, aortic CTA was not performed routinely during follow-up, and repeated CTA images were obtained in only a percentage of patients. Therefore, there is a bias in evaluating aortic remodeling in patients with SIAAD due to the limited data available. Fourth, while our sample size is substantial for this rare condition, the low frequency of critical outcome events (e.g., deaths, aortic ruptures, re-interventions) may reduce the statistical power of subgroup analyses, increasing the risk that observed differences may reflect random variation rather than true associations. Fifth, dissection length was not indexed to patient size, which may introduce anatomical bias, as the clinical implications of a 50 mm dissection may vary according to individual patient anatomy. Future studies should normalize measurements to improve classification accuracy. Finally, as f-SIAAD is strongly associated with aortic arteriosclerosis and PAU, it is often difficult to clearly distinguish between f-SIAAD and “intra-plaque dissection” caused by plaque rupture or PAU expansion based on imaging alone; as long as the “intra-plaque dissection” satisfies the definition of f-SIAAD (intimal flap length ≤ 50 mm and true/false lumens), we classified it as f-SIAAD. Finally, our results and proposed classification threshold require validation in larger, prospective, multicenter studies before they can be widely adopted in clinical practice.
Author Contributions
Conceptualization, H.Z., S.S. and X.H.; Methodology, H.Z., S.S. and X.H.; Formal analysis, S.S. and H.M.; Investigation, S.S., X.H. and Y.H.; Resources, H.Z.; Writing—original draft, S.S. and X.H.; Data curation, S.S., X.H., F.A., Y.H. and A.H.; Writing—original draft, S.S. and X.H.; Writing—review and editing, H.Z.; Visualization, S.S., H.M., F.A. and A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Chinese Society of Cardiology Foundation (CSCF2020B03) and the National Natural Science Foundation of China (8187021109, 8207021929, 82100510 and 81800411).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Tongji Hospital approved this retrospective observational study, Huazhong University of Science and Technology (TJ-IRB20211102) on 1 November 2021.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tsubouchi, H.; Onishi, H.; Maeno, K.; Nakagaichi, M.; Tsukushi, I.; Kitano, Y.; Makino, Y.; Hayashi, H.; Terasawa, H.; Kabuto, H. Study of Risk Factors and Image Findings of Isolated Abdominal Incidentally Detected Aortic Dissection. J. Clin. Ultras. 2025, 53, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, S.; Tsai, T.; Eagle, K.A.; Isselbacher, E.M.; Froehlich, J.; Cooper, J.V.; Rampoldi, V.; Upchurch, G.R., Jr.; International Registry of Acute Aortic Dissection (IRAD) investigators. Acute abdominal aortic dissection: Insight from the International Registry of Acute Aortic Dissection (IRAD). J. Vasc. Surg. 2007, 46, 913–919.e911. [Google Scholar] [CrossRef] [PubMed]
- Handa, N.; Nishina, T.; Nishio, I.; Asano, M.; Noda, K.; Ueno, Y. Endovascular stent-graft repair for spontaneous dissection of infra-renal abdominal aorta. Ann. Vasc. Surg. 2010, 24, 955.e1–955.e4. [Google Scholar] [CrossRef] [PubMed]
- Böckler, D.; Massoni, C.B.; Geisbüsch, P.; Hakimi, M.; von Tengg-Kobligk, H.; Hyhlik-Dürr, A. Single-center experience in the management of spontaneous isolated abdominal aortic dissection. Langenbecks Arch. Surg. 2016, 401, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, L.P.; Bernardes, M.V.; dos Santos Souza, J.E.; Pereira, R.M. Isolated abdominal aortic dissection in a young female patient. J. Vasc. Surg. 2016, 63, 243. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-P.; Liu, F.-E.; Duan, Q.; Ye, R.; Xiao, J.-Q. Spontaneous isolated dissection at renal upper abdominal aortic: A rare case report. Int. J. Clin. Exp. Med. 2015, 8, 8190. [Google Scholar] [PubMed]
- Farber, A.; Wagner, W.H.; Cossman, D.V.; Cohen, J.L.; Walsh, D.B.; Fillinger, M.F.; Cronenwett, J.L.; Lauterbach, S.R.; Levin, P.M. Isolated dissection of the abdominal aorta: Clinical presentation and therapeutic options. J. Vasc. Surg. 2002, 36, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Mózes, G.; Gloviczki, P.; Park, W.M.; Schultz, H.L.; Andrews, J.C. Spontaneous dissection of the infrarenal abdominal aorta. Semin. Vasc. Surg. 2002, 15, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, F.; Cannavale, A.; O’Sullivan, G.J.; Gazzetti, M.; Cirelli, C.; Lucatelli, P.; Santoni, M.; Catalano, C. Endovascular repair of acute and chronic aortic type B dissections: Main factors affecting aortic remodeling and clinical outcome. JACC Cardiovasc. Interv. 2016, 9, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.-q.; Li, D.-l.; Lai, M.-c.; Chen, X.-d.; Jin, W.; Zhang, H.-k.; Li, M. Endovascular treatment of isolated abdominal aortic dissection and postoperative aortic remodeling. J. Vasc. Surg. 2015, 61, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Kouvelos, G.N.; Vourliotakis, G.; Arnaoutoglou, E.; Papa, N.; Avgos, S.; Peroulis, M.; Papadopoulos, G.; Matsagkas, M.I. Endovascular treatment for isolated acute abdominal aortic dissection. J. Vasc. Surg. 2013, 58, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Chaikof, E.L.; Dalman, R.L.; Eskandari, M.K.; Jackson, B.M.; Lee, W.A.; Mansour, M.A.; Mastracci, T.M.; Mell, M.; Murad, M.H.; Nguyen, L.L. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 2018, 67, 2–77.e2. [Google Scholar] [CrossRef] [PubMed]
- Jonker, F.H.; Schlösser, F.J.; Moll, F.L.; Muhs, B.E. Dissection of the abdominal aorta. Current evidence and implications for treatment strategies: A review and meta-analysis of 92 patients. J. Endovasc. Ther. 2009, 16, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Maguire, E.M.; Pearce, S.W.; Xiao, R.; Oo, A.Y.; Xiao, Q. Matrix metalloproteinase in abdominal aortic aneurysm and aortic dissection. Pharmaceuticals 2019, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Jawadi, N.; Bisdas, T.; Torsello, G.; Stavroulakis, K.; Donas, K.P. Endovascular treatment of isolated abdominal aortic dissections: Long-term results. J. Endovasc. Ther. 2014, 21, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiao, Y.; Li, W.; He, C.; Zhang, H.; Zhang, T. Management of isolated abdominal aortic dissection: Indications and strategies for treatment. Ann. Vasc. Surg. 2024, 99, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Vandy, F.; Upchurch, G.R., Jr. Endovascular aneurysm repair: Current status. Circ. Cardiovasc. Interv. 2012, 5, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ma, T.; Guo, D.; Xu, X.; Chen, B.; Jiang, J.; Yang, J.; Shi, Z.; Zhu, T.; Shi, Y. Endovascular treatment of acute and chronic isolated abdominal aortic dissection. Vascular 2018, 26, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zafar, M.; Qiu, J.; Huang, Y.; Chen, Y.; Yu, C.; Elefteriades, J.A. A systematic review and meta-analysis of isolated abdominal aortic dissection. J. Vasc. Surg. 2019, 70, 2046–2053.e6. [Google Scholar] [CrossRef]
- Yang, J.; Shi, Y.; Jiang, J. Thoracic endovascular aortic repair or best medical therapy for uncomplicated type B aortic dissection? A meta-analysis. J. Cardiovasc. Surg. 2015, 63, 288–298. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).