Abstract
Background/Objectives: Prior studies using administrative data have found that antenatal anemia is a risk factor for severe maternal morbidity (SMM). However, administrative definitions, including the commonly used definition from the Centers for Disease Control and Prevention (CDC), have a poor positive predictive value for some SMM components. We tested the relationship between hemoglobin level at delivery admission and SMM, as defined by gold-standard chart review. Methods: This was a retrospective case–control study of deliveries at a high-acuity hospital in Los Angeles, California, from 2016 to 2019. Administrative data were screened to identify patients with CDC SMM. Control-patients were selected at random from screen-negative individuals. Medical records for all individuals were reviewed for gold-standard SMM criteria, and clinical data were abstracted. Confirmed-positive and confirmed-negative patients were compared using bivariate analyses. Multiple logistic regression models were developed to test the relationship between admission hemoglobin level and gold-standard SMM. Results: Of 4202 eligible individuals, 275 (6.5%) screened positive for SMM. Of these, 107 (38.5%) met gold-standard SMM criteria; 285 confirmed-negative controls were retained for analysis. Case-patients were more likely than control-patients to have anemia on delivery admission (43.9% vs. 24.2%, p < 0.01) and had lower admission hemoglobin levels (11.2 ± 1.7 g/dL vs. 11.9 ± 1.3 g/dL, p < 0.01). After controlling for covariates, admission hemoglobin was independently and inversely associated with gold-standard SMM (aOR = 0.76, 95% CI 0.60–0.96, p = 0.02). Conclusions: Lower hemoglobin level at delivery admission was associated with an increased risk of developing gold-standard SMM.
1. Introduction
Severe maternal morbidity (SMM) is a composite measure of adverse outcomes that represent a “near-miss” for maternal mortality. Anemia has been identified as one potential risk factor for SMM [,,,,]. The majority of prior studies examining this relationship have relied on administrative coding (such as the International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10], and Current Procedural Terminology [CPT] coding systems) used in medical billing and hospital discharge records to identify cases of SMM [,,,]. However, administrative definitions have a poor positive predictive value for some SMM components and risk overestimating the association between anemia and SMM [,]. This is particularly true for maternal morbidity related to hemorrhage and associated blood transfusion because administrative data do not document the number of units given. A commonly used SMM definition from the Centers for Disease Control and Prevention (CDC) is based on administrative data; using this definition, transfusion of as little as one unit of red blood cells (RBCs) meets SMM criteria []. In comparison, in-depth medical record review is considered the gold-standard for identifying cases of SMM [,].
Anemia is both highly prevalent in the obstetric population—affecting an estimated 11.5% of pregnancies in the United States []—and potentially modifiable. A better understanding of its relationship with maternal morbidity could inform strategies to improve maternal health outcomes. We performed a case–control study of deliveries at a high-acuity hospital in Los Angeles, California, to test the relationship between maternal hemoglobin at delivery admission and gold-standard SMM. We hypothesized that a lower admission hemoglobin level would be associated with increased risk of SMM.
2. Materials and Methods
This was a study of all childbirth admissions to Los Angeles County + University of Southern California Hospital from 2016 to 2019. Data for this cohort were provided by the California Maternal Quality Care Collaborative (CMQCC) Maternal Data Center [] and consisted of linked patient-level hospital discharge and birth certificate data. Deliveries < 20 gestational weeks, pregnancy terminations, and deliveries with missing or erroneous information were excluded from the study cohort.
Eligible patients from the CMQCC cohort were screened for SMM using the CDC definition [] (Figure 1). This definition includes 21 adverse outcomes, each defined by ICD-10 codes. Coding for CDC SMM was obtained courtesy of Fridman et al. []. All screen-positive patients were included as potential cases. A similar number of potential control-patients were randomly selected from screen-negative deliveries in the study period.
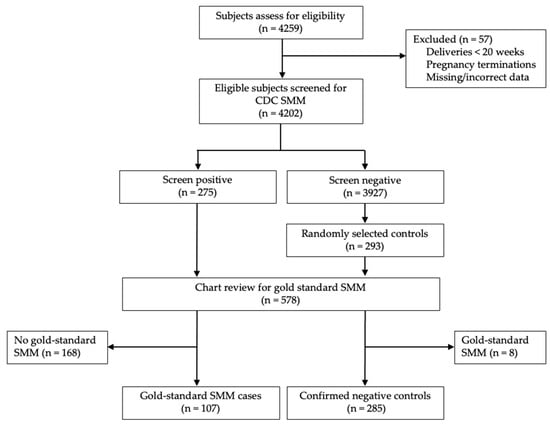
Figure 1.
Flow diagram of eligible patients.
Medical records for screen-positive and screen-negative individuals were reviewed for the presence of clinical gold-standard criteria as defined by Main et al. []. This gold-standard definition is composed of 21 adverse outcomes previously validated by a combination of hospital quality review and expert consensus. A comparison of CDC SMM and gold-standard SMM definitions is provided in Appendix A. For example, using the CDC SMM definition, an individual meets criteria for SMM if they have an ICD-10 code for blood product transfusion during their delivery admission. In comparison, the gold-standard definition used in this study requires transfusion of 4 or more units of blood product, or any transfusion plus associated procedures/interventions (e.g., hysterectomy or uterine artery embolization) [].
Data regarding antepartum hemoglobin levels were obtained from medical record review. Admission hemoglobin was defined as the hemoglobin level at the time of admission to the labor and delivery unit for childbirth. Presence of anemia was determined based on admission hemoglobin and defined as first- and third-trimester hemoglobin ≤ 11 g/dL, and second-trimester hemoglobin ≤ 10.5 g/dL []. Anemia was further subcategorized as mild (hemoglobin 9.0–10.5 g/dL in the second trimester and 9.0–11.0 g/dL in the third trimester), moderate (hemoglobin 7.0–8.9 g/dL), or severe (hemoglobin < 7.0 g/dL) []. SMM risk factors were previously described in a scoping review [] and were identified for our patient population using administrative data. The remaining baseline patient characteristics were also obtained using administrative data, including patient age, race, ethnicity, body mass index (BMI), insurance type, and pregnancy history and characteristics.
Case-patients were screen-positive individuals with gold-standard SMM confirmed on chart review. Control-patients included only screen-negative individuals with gold-standard SMM excluded on chart review. Bivariate analyses were used to compare baseline characteristics and hemoglobin levels between cases and controls and to compare the frequency of individual gold-standard SMM components between cases with and without anemia.
Multiple logistic regression models were developed to evaluate the relationship between delivery admission hemoglobin level and gold-standard SMM. Backward, forward, and stepwise selection methods were used, with gold-standard SMM case status as the outcome and antepartum risk factors for SMM as covariates. Risk factors were included as covariates in the models based on the following criteria from bivariate analyses: p < 0.15, a maximum of 5 missing patients, and a minimum of 5 patients per predictor group. Only covariates that contributed to the model (p < 0.05) were retained. Black race was forced in the models as black race is known to increase risk for both SMM and anemia [,]. After evaluating the association with hemoglobin level, the models were re-run to test the associations between the outcome and (1) anemia (yes/no) and (2) anemia severity (none vs. mild vs. moderate/severe).
Seven investigators participated in the chart review. All underwent training to ensure abstraction consistency. Interrater reliability for 12 test records was found to be in very good agreement: kappa = 0.92 (95% CI 0.89 to 0.94). All calculations were performed using SAS (v 9.4, SAS Institute Inc., Cary, NC, USA). Categorical data were presented as number (percentage), and continuous data were presented as mean ± standard deviation (SD) or median (range). Odds ratios (ORs) and 95% confidence intervals (CIs) were reported. A p-value < 0.05 was considered statistically significant. This study was exempt by the Institutional Review Board at the University of Southern California Health Sciences Campus (Protocol #APP-19-06341).
3. Results
Of 4259 individuals who delivered in the study period, 4202 met the inclusion criteria. Screening with the CDC SMM definition identified 275 individuals (6.5%). Among these, 107 cases of gold-standard SMM (38.9%) were confirmed on chart review. Among 293 randomly selected controls, 8 (2.7%) were excluded for meeting gold-standard SMM criteria on chart review. The final analytic sample consisted of 107 cases and 285 controls.
Baseline characteristics were compared between cases and controls (Table 1). Case-patients were less likely to have a government payer (91.6% vs. 97.3%, p < 0.01). Groups were otherwise similar with regard to demographic characteristics. Regarding obstetric characteristics and comorbidities, case-patients were more likely to deliver preterm or by cesarean section. Case-patients were also more likely to have a multifetal gestation, fetal growth restriction, placenta previa or accreta spectrum disorder, pre-eclampsia, and medical comorbidities including cardiac, pulmonary, renal, and thyroid disease.

Table 1.
Baseline and hemoglobin characteristics of individuals with and without gold-standard severe maternal morbidity.
With regard to anemia characteristics, case-patients had lower mean admission hemoglobin levels (11.2 ± 1.7 g/dL vs. 11.9 ± 1.3 g/dL, p < 0.01) and were significantly more likely to have anemia on delivery admission (43.9% vs. 24.2%, p < 0.01) (Table 1). Case-patients were also more likely to have moderate or severe anemia compared to control-patients (moderate: 8.4% vs. 2.5%; severe: 0.9% vs. 0.0%; p < 0.01).
The frequency of SMM components was compared between case-patients with and without anemia (Table 2). The most common indicators for SMM among those with anemia included blood transfusion and pre-eclampsia. Those with anemia were more likely to receive a gold-standard blood transfusion (38.3% vs. 18.3%, p = 0.03) and were less likely to have gold-standard pre-eclampsia, though this finding did not achieve statistical significance (38.3 vs. 58.3%, p = 0.05). There was no other statistically significant difference between groups with regard to the frequency of SMM components. It is important to note that case numbers for individual SMM components were small, and the study was not powered to detect a difference in the individual components.

Table 2.
Gold-standard severe maternal morbidity in individuals with and without anemia.
A multiple logistic regression model was developed to evaluate the independent effect of admission hemoglobin on case status. Admission hemoglobin was independently and inversely associated with SMM in the adjusted model (OR 0.76, 95% CI 0.60–0.96, p = 0.02) (Table 3). Other patient characteristics associated with SMM included preterm delivery, abnormal placentation, maternal cardiac disease, maternal renal disease, and gestational hypertension/pre-eclampsia. The c-statistic was 0.91. The Hosmer–Lemeshow test had a p-value of 0.74, indicating that the model was well-calibrated. Neither anemia as a categorical variable nor anemia severity contributed significantly to the model.

Table 3.
Multiple logistic regression model for severe maternal morbidity by admission hemoglobin.
4. Discussion
In this case–control study, we demonstrated an inverse and independent relationship between delivery admission hemoglobin and gold-standard SMM. After controlling for relevant covariates, we found that for every one-point increase in hemoglobin level, a subject was 24% less likely to develop SMM.
Our findings are consistent with one prior study evaluating the relationship between anemia and gold-standard SMM: Guignard et al. identified SMM cases by medical record review using a definition derived from Delphi consensus. They found that anemia was associated with an increased odds of severe maternal morbidity among deliveries in France (aOR 1.8, 95% CI 1.5–2.1) [].
The majority of other studies examining the relationship between anemia and SMM have used the CDC’s administrative definition of SMM [,,,], in which one unit of RBCs would qualify as SMM. However, transfusion of one unit of RBCs may not represent true maternal morbidity []. Indeed, a proactive approach to transfusion may represent efforts to reduce maternal morbidity. An alternative analytic approach is to test the relationship between administrative SMM and anemia but exclude the codes for blood transfusion []. Yet an assessment of the relationship between anemia and SMM would be incomplete without considering transfusion: blood transfusion represents a surrogate for hemorrhage, a leading cause of maternal morbidity and mortality []. We previously found that excluding transfusion codes from the CDC definition would miss 27.3% of gold-standard SMM cases []. Our use of a gold-standard definition of transfusion (large volume transfusion and/or need for procedural interventions such as hysterectomy) permitted identification of true clinical maternal morbidity related to hemorrhage.
Prior studies have found a greater risk of SMM with increasing anemia severity [,]. In our bivariate analysis, individuals with moderate or severe anemia were more likely to develop SMM. However, this association was no longer statistically significant after adjustment for confounders. It is important to note that our study was not powered to detect differences in SMM risk by anemia severity.
Comparing rates of SMM components between case-patients with and without anemia, we found that pre-eclampsia accounted for a greater proportion of SMM cases among those without anemia, though this did not achieve statistical significance (p = 0.05). We suspect our results are attributable to the timing of hemoglobin level ascertainment in our population. We evaluated hemoglobin level on delivery admission, a time when patients are likely to be presenting in the acute stages of pre-eclampsia. Acute pre-eclampsia can lead to intravascular depletion and hemoconcentration, with a subsequent spurious increase in hemoglobin []. Besides pre-eclampsia and transfusion, there was no difference in the rate of individual SMM components between cases with and without anemia, though our study was not powered to detect a difference for the individual components.
The relationship between anemia and SMM is likely multifactorial and extends beyond an increased risk for blood transfusion. Lower red blood cell volume compromises hemostatic mechanisms and the ability to compensate for bleeding during delivery, increasing the risk of postpartum hemorrhage, coagulopathy, and hysterectomy [,,,]. Anemia has also been identified as an independent risk factor for infection (including sepsis) [,,], cesarean delivery [], and hypertensive disorders of pregnancy [,,,]. Finally, anemia may be a surrogate for other factors that predispose to SMM, including inflammation, infectious disease, malnutrition, and socioeconomic deprivation/barriers to care [,,].
Predicting individuals at risk for SMM poses an ongoing challenge to providers []. Based on our findings, anemia at delivery admission may serve as an early warning sign for increased risk of rare but serious maternal complications. Recognizing this association can help providers prioritize prompt clinical assessment of anemic patients who exhibit a clinical status change and facilitate the early mobilization of the multidisciplinary obstetric team if needed.
Targeted interventions to prevent SMM in individuals with anemia should focus on the specific SMM components most relevant to this population. We found that gold-standard blood transfusion was among the most common indicators for SMM for individuals with anemia. Several interventions to address the risk of life-threatening hemorrhage in this group can be considered: First, hospital systems can adopt hemorrhage risk assessment tools that incorporate anemia into the risk calculation and ensure these tools are standardized across delivery admissions. Second, providers can ensure adequate preparation for hemorrhage, including ready access to uterotonics, tranexamic acid, and intrauterine compression devices. Finally, providers can ensure immediate blood product availability in the event that a life-threatening hemorrhage does occur. The CMQCC Obstetric Hemorrhage Safety Bundle is one potential tool that uses hemoglobin level at childbirth admission to guide hemorrhage risk stratification, prevention, and management []. This tool has been shown to reduce rates of SMM overall and mitigate racial disparities in SMM [].
Prenatal interventions to identify and treat anemia are another important consideration when discussing tools to prevent SMM. Society guidelines recommend anemia testing and treatment beginning at the first prenatal visit based on the physiological demands of pregnancy and the observed association between anemia and individual adverse pregnancy outcomes []. However, there remains insufficient direct evidence to determine the impact of anemia treatment on composite SMM [], and more research is needed to guide recommendations in this area.
Our study has a number of strengths. To our knowledge, ours is the first study to test the relationship between anemia and gold-standard SMM within a US cohort. Use of a gold-standard definition of SMM excluded false-positive SMM cases, thus preventing the risk of overestimating the association between SMM and anemia that can be seen with administrative definitions. Additionally, the study site was a county hospital primarily serving an underinsured and socioeconomically disadvantaged population, which may mitigate socioeconomic disadvantage as a confounder for the relationship between anemia and SMM.
Our study has several limitations. Using administrative data to screen for gold-standard SMM missed some SMM cases. Additionally, even though we limited the outcome and independent variable to data abstracted from the medical record, the remaining SMM risk factors were obtained from administrative data, and thus, the limits of using these data apply to our analyses []. Finally, admission hemoglobin was not categorized to distinguish between those patients with antenatal anemia vs. those with an acute episode of bleeding prior to admission; the latter would also be more likely to experience SMM and could falsely elevate the estimated OR between admission hemoglobin and SMM.
5. Conclusions
Our study demonstrated that hemoglobin level on delivery admission was inversely related to the odds of developing gold-standard SMM. Anemia should be considered a red flag warning for patients at risk for developing adverse peripartum outcomes, including SMM. Providers should consider implementing standardized hemorrhage risk assessment and prevention tools to help reduce the risk of hemorrhage-related SMM in this population. Research is needed to investigate whether antenatal anemia treatment can reduce rates of composite SMM.
Author Contributions
Conceptualization, S.P.F., K.N.O., A.R.R., L.M.K. and N.M.C.; data curation, S.P.F.; formal analysis, L.M.K.; investigation, S.P.F., K.N.O., A.R.R., J.V.P., A.G., J.D.W. and G.B.; methodology, S.P.F., I.S., L.M.K. and N.M.C.; project administration, L.M.K. and N.M.C.; resources, L.M.K. and N.M.C.; software, L.M.K.; supervision, S.P.F. and N.M.C.; validation, I.S. and L.M.K.; writing—original draft preparation, S.P.F., A.R.R. and J.D.W.; writing—review and editing, S.P.F., I.S., L.M.K. and N.M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was classified as exempt by the Institutional Review Board at the University of Southern California Health Sciences Campus (Protocol #APP-19-06341 approved on 19 June 2020).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the data.
Data Availability Statement
Data is available upon request to the corresponding author.
Acknowledgments
We thank the Maternal Quality Indicators Working Group (MQI) for providing the SAS code for SMM and its risk factors. MQI is based at the University of California at Los Angeles and Cedars-Sinai Medical Center and includes Moshe Fridman, Kimberly D. Gregory, Lisa A. Nicholas, Naomi Greene, and Samia Saeb.
Conflicts of Interest
L. Korst assists with research studies as an independent contractor. The remaining authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CDC | Centers for Disease Control and Prevention |
| CI | Confidence interval |
| CMQCC | California Maternal Quality Care Collaborative |
| ICD10 | International Classification of Diseases, Tenth Revision, Clinical Modification |
| OR | Odds ratio |
| RBC | Red blood cell |
| SMM | Severe maternal morbidity |
Appendix A

Table A1.
Indicators for severe maternal morbidity (SMM).
Table A1.
Indicators for severe maternal morbidity (SMM).
| CDC SMM Indicators [] | Gold-Standard SMM Indicators from Main et al. [] |
|---|---|
| Acute myocardial infarction | Acute myocardial infarction |
| Aneurysm | Aneurysm |
| Acute renal failure | Acute renal failure
|
| Adult respiratory distress syndrome | Adult respiratory distress syndrome
|
| Amniotic fluid embolism | Amniotic fluid embolism |
| Cardiac arrest/ventricular fibrillation | Cardiac arrest/ventricular fibrillation |
| Conversion of cardiac rhythm | Conversion of cardiac rhythm
|
| Disseminated intravascular coagulation | Disseminated intravascular coagulation |
| Eclampsia | Eclampsia and severe pre-eclampsia
|
| Heart failure/arrest during surgery or procedure | Heart failure/arrest during surgery or procedure
|
| Puerperal cerebrovascular disorders | Puerperal cerebrovascular disorders |
| Pulmonary edema/acute heart failure | Pulmonary edema/acute heart failure |
| Severe anesthesia complications | Severe anesthesia complications
|
| Sepsis | Sepsis
|
| Shock | Shock |
| Sickle cell disease with crisis | Sickle cell disease with crisis |
| Air and thrombotic embolism | Air and thrombotic embolism |
| Blood product transfusion | Blood product transfusion
|
| Hysterectomy | Hysterectomy and surgical complications
|
| Temporary tracheostomy | Temporary tracheostomy |
| Ventilation | Ventilation (other than ventilation for a surgical procedure) |
ARDS, acute respiratory distress syndrome; IV, intravenous; ICU, intensive care unit; HELLP, hemolysis, elevated liver enzymes, and low platelets.
References
- Harrison, R.K.; Lauhon, S.R.; Colvin, Z.A.; McIntosh, J.J. Maternal anemia and severe maternal morbidity in a US cohort. Am. J. Obstet. Gynecol. MFM 2021, 3, 100395. [Google Scholar] [CrossRef]
- Ray, J.G.; Davidson, A.; Berger, H.; Dayan, N.; Park, A.L. Haemoglobin levels in early pregnancy and severe maternal morbidity: Population-based cohort study. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 1154–1164. [Google Scholar] [CrossRef]
- Nyarko, S.H.; Greenberg, L.T.; Saade, G.R.; Phibbs, C.S.; Buzas, J.S.; Lorch, S.A.; Rogowski, J.; Passarella, M.; Boghossian, N.S. Association between iron deficiency anemia and severe maternal morbidity: A retrospective cohort study. Ann. Epidemiol. 2024, 100, 10–15. [Google Scholar] [CrossRef]
- Beckert, R.H.; Baer, R.J.; Anderson, J.G.; Jelliffe-Pawlowski, L.L.; Rogers, E.E. Maternal anemia and pregnancy outcomes: A population-based study. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2019, 39, 911–919. [Google Scholar] [CrossRef]
- Guignard, J.; Deneux-Tharaux, C.; Seco, A.; Beucher, G.; Kayem, G.; Bonnet, M.P.; EPIMOMS Group. Gestational anaemia and severe acute maternal morbidity: A population-based study. Anaesthesia 2021, 76, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Main, E.K.; Abreo, A.; McNulty, J.; Gilbert, W.; McNally, C.; Poeltler, D.; Lanner-Cusin, K.; Fenton, D.; Gipps, T.; Melsop, K.; et al. Measuring severe maternal morbidity: Validation of potential measures. Am. J. Obstet. Gynecol. 2016, 214, 643.e1–643.e10. [Google Scholar] [CrossRef] [PubMed]
- Fabricant, S.P.; Opara, K.N.; Paul, J.V.; Blissett, G.; Rau, A.R.; White, J.D.; Girma, A.; Sriprasert, I.; Korst, L.M.; Mitchell, E.N. The Positive Predictive Value of Hospital Discharge Data for Identifying Severe Maternal Morbidity With and Without Blood Transfusion. Jt. Comm. J. Qual. Patient Saf. 2023, 49, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. How Does CDC Identify Severe Maternal Morbidity? 26 December 2019. Available online: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/smm/severe-morbidity-ICD.htm (accessed on 7 September 2021).
- Snowden, J.M.; Lyndon, A.; Kan, P.; El Ayadi, A.; Main, E.; Carmichael, S.L. Severe Maternal Morbidity: A Comparison of Definitions and Data Sources. Am. J. Epidemiol. 2021, 190, 1890–1897. [Google Scholar] [CrossRef]
- World Health Organization. Prevalence of Anaemia in Pregnant Women (aged 15–49) (%). 2021. Available online: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/4552 (accessed on 7 September 2021).
- California Maternal Quality Care Collaborative. Maternal Data Center. 2021. Available online: https://www.cmqcc.org/maternal-data-center (accessed on 5 December 2021).
- Fridman, M.; Korst, L.M.; Reynen, D.J.; Nicholas, L.A.; Greene, N.; Saeb, S.; Troyan, J.L.; Gregory, K.D. Severe Maternal Morbidity in California Hospitals: Performance Based on a Validated Multivariable Prediction Model. Jt. Comm. J. Qual. Patient Saf. 2021, 47, 686–695. [Google Scholar] [CrossRef]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. 2011. Available online: https://www.who.int/publications/i/item/WHO-NMH-NHD-MNM-11.1 (accessed on 7 September 2021).
- Galloway, R. Anemia Prevention and Control: What Works. USAID, World Bank, PAHO/WHO, Micronutrient Initiative, FAO, and UNICEF. 2003. Available online: https://documents1.worldbank.org/curated/en/234691468160187223/pdf/561740WP0v20Bo1PUBLIC10anemia1Part2.pdf (accessed on 7 September 2021).
- Korst, L.M.; Gregory, K.D.; Nicholas, L.A.; Saeb, S.; Reynen, D.J.; Troyan, J.L.; Greene, N.; Fridman, M. A scoping review of severe maternal morbidity: Describing risk factors and methodological approaches to inform population-based surveillance. Matern. Health Neonatol. Perinatol. 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Howell, E.A. Reducing disparities in severe maternal morbidity and mortality. Clin. Obstet. Gynecol. 2018, 61, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Anemia in Pregnancy: Practice Bulletin, Number 233. Obstet. Gynecol. 2021, 138, e55–e64. [CrossRef] [PubMed]
- Cresswell, J.A.; Alexander, M.; Chong, M.Y.C.; Link, H.M.; Pejchinovska, M.; Gazeley, U.; Ahmed, S.M.A.; Chou, D.; Moller, A.B.; Simpson, D.; et al. Global and regional causes of maternal deaths 2009-20: A WHO systematic analysis. Lancet. Glob. Health 2025, 13, e626–e634. [Google Scholar] [CrossRef]
- Silver, H.M.; Seebeck, M.; Carlson, R. Comparison of total blood volume in normal, preeclamptic, and nonproteinuric gestational hypertensive pregnancy by simultaneous measurement of red blood cell and plasma volumes. Am. J. Obstet. Gynecol. 1998, 179, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Teng, F.; Branch, E.; Chu, S.; Joseph, K.S. Maternal and Perinatal Morbidity and Mortality Associated With Anemia in Pregnancy. Obstet. Gynecol. 2019, 134, 1234–1244. [Google Scholar] [CrossRef]
- Surbek, D.; Vial, Y.; Girard, T.; Breymann, C.; Bencaiova, G.A.; Baud, D.; Hornung, R.; Taleghani, B.M.; Hösli, I. Patient blood management (PBM) in pregnancy and childbirth: Literature review and expert opinion. Arch. Gynecol. Obstet. 2020, 301, 627–641. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Q.; Song, Y.; Fang, L.; Huang, L.; Sun, Y. Nutritional factors for anemia in pregnancy: A systematic review with meta-analysis. Front. Public Health 2022, 10, 1041136. [Google Scholar] [CrossRef]
- Jung, J.; Rahman, M.M.; Rahman, M.S.; Swe, K.T.; Islam, M.R.; Rahman, M.O.; Akter, S. Effects of hemoglobin levels during pregnancy on adverse maternal and infant outcomes: A systematic review and meta-analysis. Ann. N. Y. Acad. Sci. 2019, 1450, 69–82. [Google Scholar] [CrossRef]
- Ge, S.; Ali, S.; Haldane, V.; Bekdache, C.; Tang, G.H.; Sholzberg, M. An approach to Hemequity: Identifying the barriers and facilitators of iron deficiency reduction strategies in low- to middle-income countries. Br. J. Haematol. 2025, 206, 428–442. [Google Scholar] [CrossRef]
- Society for Maternal-Fetal Medicine (SMFM); Lappen, J.R.; Pettker, C.M.; Louis, J.M. Society for Maternal-Fetal Medicine Consult Series #54: Assessing the risk of maternal morbidity and mortality. Am. J. Obstet. Gynecol. 2021, 224, B2–B15. [Google Scholar] [CrossRef]
- California Maternal Quality Care Collaborative. Obstetric Hemorrhage Toolkit. 24 March 2015. Available online: https://www.cmqcc.org/node/2036 (accessed on 24 September 2021).
- Main, E.K.; Chang, S.C.; Dhurjati, R.; Cape, V.; Profit, J.; Gould, J.B. Reduction in racial disparities in severe maternal morbidity from hemorrhage in a large-scale quality improvement collaborative. Am. J. Obstet. Gynecol. 2020, 223, 123.e1–123.e14. [Google Scholar] [CrossRef] [PubMed]
- Cantor, A.G.; Holmes, R.; Bougatsos, C.; Atchison, C.; DeLoughery, T.; Chou, R. Screening and Supplementation for Iron Deficiency and Iron Deficiency Anemia During Pregnancy: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2024, 332, 914–928. [Google Scholar] [CrossRef] [PubMed]
- Lain, S.J.; Hadfield, R.M.; Raynes-Greenow, C.H.; Ford, J.B.; Mealing, N.M.; Algert, C.S.; Roberts, C.L. Quality of data in perinatal population health databases: A systematic review. Med. Care 2012, 50, e7–e20. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).