Abstract
Background: Keratinocyte carcinomas (KCs), including basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs), are the most prevalent malignancies globally, particularly affecting sun-exposed facial areas. Achieving clear surgical margins in these regions is essential to ensure oncologic control while preserving cosmetic outcomes. Reflectance confocal microscopy (RCM) is a noninvasive imaging technique that enables real-time, high-resolution visualization of skin structures and may aid in margin assessment during KC surgery. This systematic review aims to evaluate the role of in vivo RCM in the surgical treatment of KCs. Methods: This review followed PRISMA guidelines. A comprehensive search of PubMed, Scopus, Web of Science, Medline, and EBSCO databases was conducted for studies published between January 1992 and December 2024. Inclusion criteria focused on clinical studies utilizing in vivo RCM for diagnostic or surgical applications in KC management. Results: Eighteen studies involving 1112 patients were included. RCM was used preoperatively in 5 studies and intraoperatively in another 5. Nine studies assessed margin delineation, while eight focused on diagnostic accuracy. RCM improved diagnostic confidence and allowed for more precise margin assessment, potentially reducing the extent of surgical excision in cosmetically sensitive areas. However, its broader clinical adoption is limited by operator dependency, procedural complexity, and lack of standardization. Conclusions: RCM shows promise as a supportive tool in KC surgery, particularly for preoperative planning. While its diagnostic utility is well established, its intraoperative role requires further validation. Larger, standardized, and cost-effective studies are needed to confirm its impact on surgical outcomes and patient quality of life.
1. Introduction
Keratinocyte carcinomas (KCs) are the most diagnosed human cancers globally [1,2]. The term “keratinocyte carcinomas” mainly refers to basal cell carcinomas (BCC) and squamous cell carcinomas (SCC), but may also include cutaneous lymphomas, adnexal tumors, Merkel cell carcinomas, and other rare skin neoplasms [1]. BCC is the most common (75–80%), followed by SCC (15–20%) [3], with BCC being significantly more prevalent [3,4,5]. KCs arise from epidermal cells, and UV radiation exposure promotes malignant cell proliferation [6]. Thus, they typically appear on sun-exposed areas such as the neck or limbs. Lesions in the head and neck, especially in the H zone, are considered high-risk due to the likelihood of deeper invasion and recurrence [7]. This zone includes the nose, eyelids, periorbital area, ears, and central, marked by proximity to critical structures and varied skin thickness [7].
Surgical excision with a margin of healthy tissue remains the standard treatment for high-risk KCs [8]. However, unpredictable tumor growth patterns and partial spontaneous healing make it difficult to define lesion borders, increasing the risk of incomplete excision or unnecessary tissue removal—impacting oncological safety and aesthetics. While not highly lethal, KCs cause significant morbidity [9]. Based on retrospectively collected data, including a cohort of 500 patients from our center, the average excised tumor size was 14.77 mm, ranging from 2 mm to 80 mm. Even with a 2–4 mm excision margin in a histopathology exam, removing a 14.77 mm tumor can lead to irreversible damage to the eyelids, mouth, or nose, requiring complex reconstruction to restore function and quality of life.
Mohs micrographic surgery is the gold standard [4], involving layer-by-layer excision and intraoperative microscopic analysis. Only affected margins are extended, ensuring complete tumor removal with minimal healthy tissue loss [4]. Despite its precision, Mohs is costly, time-consuming, and resource-intensive, limiting patient access and increasing wait times. Early intervention is crucial, as larger lesions are harder to remove with satisfactory functional and cosmetic results.
Confocal microscopy (CM) offers a promising alternative for preoperative tumor margin assessment [10,11,12]. This laser-based imaging tool provides black-and-white images resembling histology. CM includes reflectance CM (RCM) and ex vivo CM (EVCM). RCM enables in vivo imaging with histology-like sensitivity, using only reflectance contrast, without dyes [13]. CM bridges dermoscopy and histopathology, expanding diagnostic capabilities [14,15,16,17] and enabling noninvasive assessment comparable to biopsy [18,19]. It shortens diagnostic and treatment pathways [14,15], facilitating diagnosis without tissue disruption [20]. Given these advantages, CM is also used in the surgical management of skin cancers, potentially improving cosmetic outcomes and reducing resource use. It may minimize surgical trauma, enhance recovery, and improve patients’ quality of life.
The aim of our work is to conduct a systematic review of the literature on the use of confocal microscopy (CM) in treating keratinocyte carcinomas (KCs). Despite its promise, CM’s clinical application in KC management remains inconsistent. Although several studies have evaluated its diagnostic and perioperative value, unresolved questions include standardization, reproducibility, operator dependency, efficacy across subtypes and anatomical sites, and cost-effectiveness relative to Mohs surgery. Furthermore, high-quality, large-scale studies on recurrence, surgical margins, and cosmetic and functional outcomes are lacking. Therefore, this review aims to evaluate current evidence, identify knowledge gaps, and suggest future clinical research priorities.
2. Materials and Methods
This study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRIMSA) guidelines. A systematic search of the PubMed, Web of Science, Scopus, Medline, and EBSCO databases for articles published between January 1992 and December 2024 was performed using search terms designed to identify studies on the use of CM as an adjunct method in the surgical treatment of KCs. The following search query was used: “(confocal microscopy OR confocal imaging OR RCM) AND (nonmelanoma OR bcc OR skin cancer OR scc OR keratinocyte carcinomas)” AND (surgical removal OR surgery OR Mohs OR presurgical OR intraoperative).
Inclusion criteria were that the articles provided the use of CM as an adjunct method in the surgical treatment of keratinocyte carcinomas. The exclusion criteria involved studies that included the application of ex vivo CM, the exclusive use of CM as a diagnostic method, the use CM without marking the tumor margins, the use of CM as an adjunct method in the surgical treatment of melanoma, the application of combined techniques, e.g., optical coherence tomography (OCT), and marking the margins for removal by means other than surgery, e.g., radiotherapy or laser ablation. Unrelated articles that were discovered by keyword matching were also excluded. Abstracts, case reports, conference papers, letters, editorials, and articles written in languages other than English were excluded during the initial screening of titles and citations. Studies duplicated in databases were removed using Mendeley Software (Reference Manager Version 2.130.2). After the initial search was performed by one researcher, duplicate records were removed. Two independent reviewers screened the remaining titles and abstracts according to predefined inclusion and exclusion criteria. Based on this screening, potentially relevant full-text articles were identified and retrieved. Full-text articles were then independently assessed by the same two reviewers to determine the in vivo application of CM in the surgical treatment of skin cancer on the face. We specifically evaluated whether the study involved tumor resection, the use of RCM (in vivo and/or ex vivo), and whether clinical outcomes and conclusions relevant to CM application were reported. Disagreements at any stage were resolved by consensus in consultation with a third and fourth reviewer. Final decisions were made under the supervision of the first author. A flow diagram of the study selection process is presented in Figure 1.
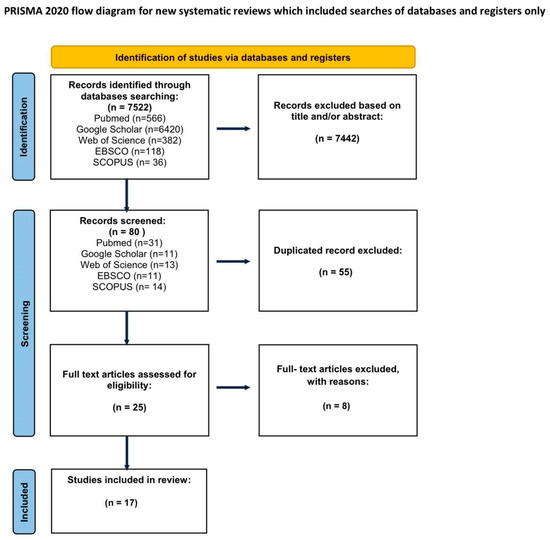
Figure 1.
PRISMA flowchart.
The initial search identified 7522 articles. The initial search identified 7522 articles. After excluding articles that do not match the criteria, 90 studies were included for abstract review. Finally, 25 were selected for full text appraisal, of which 17 met all the inclusion criteria and were included in this review. This systematic review included 17 articles, including over 1112 patients (Table 1). Due to significant variations in the technique and purpose of RCM application, the publications are too diverse to adhere to a meta-analysis. The articles included in the review are presented in Table 1.

Table 1.
Articles included in the systematic review.
2.1. Type of Cancer
In 12 studies, the treatment of basal cell carcinoma of the skin was examined, while in 5 studies, both basal cell and squamous cell carcinoma treatments were examined.
2.2. Type of Surgery
In 10 studies describing 208 patients, CM was used as an adjunct method for Mohs surgery. In 7 articles, describing 904 patients, the use of RCM as an adjunct method during conventional surgical removal of skin cancer was evaluated.
2.3. The Use of CM
In 5 studies, excision margins were evaluated preoperatively, while in 5 studies, the margins were determined intraoperatively.
In 8 studies, the possibility of using CM as a method of confirming cancer diagnosis was evaluated, including in 4 studies concerning the use of RCM in conventional surgery on 817 patients and in 4 articles concerning its use in Mohs surgery on 158.
In 8 articles, the use of RCM to assess margins was evaluated, including in 4 studies concerning conventional surgery on 47 patients and 6 concerning Mohs surgery on 70 patients.
2.4. Conclusions Reported by Original Studies
Across the reviewed literature, most studies concluded that CM, and particularly reflectance confocal microscopy (RCM), holds promise as an adjunct tool in both diagnosis and surgical planning for skin cancer. Its ability to provide high-resolution, real-time imaging of the superficial dermis and epidermis was frequently cited as a significant advantage. Studies noted that CM contributed to improved diagnostic confidence and more accurate delineation of tumor margins, which in turn supported tissue-sparing surgical strategies. This was especially relevant in the management of recurrent tumors or lesions with poorly defined clinical borders.
However, several limitations were also consistently reported. These included the steep learning curve associated with image acquisition and interpretation, the time-intensive nature of the procedure, and limited imaging depth, which restricts the visualization of deeper or infiltrative tumor components. Such challenges have likely contributed to the variability in clinical adoption observed across studies.
In summary, while the extracted data confirm that CM demonstrates valuable diagnostic and intraoperative applications, its routine integration into surgical workflows remains inconsistent. This variability is largely attributable to technical constraints and the need for further standardization. The implications of these findings are discussed in greater detail in the following section.
3. Discussion
This systematic review provides a structured overview of currently available evidence on the use of confocal microscopy (CM), particularly reflectance confocal microscopy (RCM), in the surgical treatment of KC. While the number of included studies is limited, and data are heterogeneous, our findings highlight patterns of clinical application and point toward areas requiring further investigation [16,18,22]. Our analysis aimed to evaluate the clinical utility of CM as a complementary technique in determining surgical margins and confirming diagnosis and to assess whether it could improve the accuracy and outcomes of skin cancer surgery [29]. Given the growing interest in CM and its potential role in dermatologic surgery, this review contributes to consolidating the current understanding and identifying key knowledge gaps [13,15,17]. Although the topic remains under-researched, our findings provide an updated overview of the clinical contexts in which CM has been implemented and evaluated [12,16].
There are increasing reports on the use of CM in conventional excision and Mohs micrographic surgery [18,21,24,28,32,33]. Most studies conclude that it can improve intraoperative decision-making, especially in poorly defined or recurrent tumors [13,31]. The studies reviewed consistently indicate that CM can assist the surgeon in complete tumor resection and reduce the risk of residual disease [17,18,22,27]. Its ability to image the epidermis and superficial dermis in high resolution in vivo allows visualization of tumor architecture in real time without the need for tissue processing [12,14,15]. However, the total number of cases described in the literature remains limited—only 137 patients have been evaluated for CM-based margin assessment—highlighting that the current evidence base is narrow and comes from small, heterogeneous patient cohorts [16,23,30].
Several limitations probably contribute to the fact that CM is rarely used in the assessment of surgical margins. First, the method is very time-consuming and requires expert interpretation and manual scanning of lesion margins [13,23,34]. Second, the variability of image acquisition protocols and the lack of standardized criteria for defining the positivity of margins limit reproducibility and clinical acceptability [20,26,29]. In addition, CM is generally limited in its ability to visualize deeper dermal structures, which may be a disadvantage in infiltrating or deeply invasive subtypes of BCC [18,22,31]. These factors may partly explain the muted clinical enthusiasm for CM in the assessment of margins, despite its theoretical advantages [13,15].
In contrast, the use of CM for diagnostic purposes is supported by more robust data. In 995 patients included in the studies examined, CM showed high sensitivity and specificity in identifying lesions, with performance metrics comparable to those of histopathology [19,20,29,30]. These results suggest that CM could play a more important role in non-invasive diagnosis or as a triage tool prior to surgical planning [13,14]. However, some authors reported that CM was less effective when used intraoperatively in the context of Mohs surgery, possibly due to technical limitations such as difficulties in correlating confocal images with frozen section histology or delays in acquisition and interpretation [27,28,33].
Despite these limitations, CM is particularly promising in selected patient groups and clinical scenarios. In young patients or when treating lesions in cosmetic or functional risk areas such as the central face, eyelids, or lips, precise margin control is crucial to avoid overtreatment and reduce the need for extensive reconstruction [12,17,25]. In such cases, CM can support a customized surgical approach that limits excision to the affected areas while preserving healthy tissue [28]. In addition, the non-invasive nature of CM makes it particularly attractive for situations where biopsy is contraindicated or undesirable [14,29,35].
Our review also demonstrates that conclusions reported by the included studies were generally aligned in recognizing CM’s potential clinical value. Across the articles, CM was frequently associated with improved visualization of tumor architecture, more informed intraoperative decisions, and enhanced diagnostic accuracy [13,16,22]. At the same time, multiple studies noted the technical and procedural barriers to its wider adoption, including operator dependency, cost, and limited availability [20,23,29].
The current literature is limited not only by small sample sizes but also by methodological inconsistencies and insufficient data on long-term outcomes. A key limitation of the current evidence is the lack of stratification by tumor subtype and histological differentiation. Most studies did not distinguish between low-risk and high-risk BCCs, nor did they report SCC differentiation grades consistently [3,5,19,29]. This is clinically relevant, as more aggressive tumors may extend beyond the imaging capabilities of confocal microscopy [16,31,34]. Without subgroup analysis, it is difficult to assess CM’s effectiveness in complex cases where precise margin control is essential. Future studies should address this gap by explicitly classifying tumors to better evaluate CM’s diagnostic and intraoperative value, as well as aim to validate the performance of CM in larger, multicenter cohorts using standardized imaging protocols and objective endpoints [13,16,23,30]. In addition, cost-effectiveness analyses are needed to determine whether the integration of CM into routine clinical practice offers measurable benefits in terms of lower recurrence rates, lower costs to the healthcare system, or improved patient-reported outcomes [29].
In summary, confocal microscopy appears to be a promising adjunct in the surgical treatment of KCs, especially when used in well-selected cases [13,16,18,22]. The findings from this review support the role of CM primarily in diagnostic settings and, to a more limited extent, in intraoperative margin evaluation [19,20,27]. Broader implementation will require technological improvements, procedural standardization, and stronger clinical evidence [14,23,30]. Standardization of CM protocols and further exploration of its clinical value are needed to fully define its role in dermatologic surgery [16]. Given the increasing incidence of skin cancers worldwide, any tool that can improve surgical precision and minimize the risk of recurrence deserves close attention and further investigation [1,2,8].
Author Contributions
Conceptualization, M.W.; methodology, A.L. and W.B.; software, D.B.; validation, J.J.; formal analysis, K.K.; investigation, P.B.; resources, N.D., D.N., and M.D.; data curation, K.K. and W.K.; writing—original draft preparation, M.W.; writing—review and editing, K.K.; visualization, M.W.; supervision, J.J.; project administration, M.W.; funding acquisition, M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study did not require Institutional Review Board approval, as it did not involve research on human subjects.
Informed Consent Statement
Due to the nature of this study, informed consent from participants was not required.
Data Availability Statement
Available on request.
Acknowledgments
Manuscript preparation was supported during Harvard Medical School’s Polish Clinical Scholars Research Training Program, organized by Agencja Badań Medycznych (ABM, English: Medical Research Agency, Warsaw, Poland).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Madan, V.; Lear, J.T.; Szeimies, R.M. Non-melanoma skin cancer. Lancet 2010, 375, 673–685. [Google Scholar] [CrossRef]
- Cakir, B.Ö.; Adamson, P. Epidemiology and economic burden of nonmelanoma skin cancer. Facial Plast. Surg. Clin. N. Am. 2012, 20, 419–422. [Google Scholar] [CrossRef]
- Attal, Z.G.; Shalata, W.; Soklakova, A.; Tourkey, L.; Shalata, S.; Abu Saleh, O.; Abu Salamah, F.; Alatawneh, I.; Yakobson, A. Advanced and metastatic non-melanoma skin cancer: Epidemiology, risk factors, clinical features, and treatment options. Biomedicines 2024, 12, 1448. [Google Scholar] [CrossRef]
- Rutkowski, P.; Owczarek, W.; Nejc, D.; Jeziorski, A.; Wysocki, W.M.; Słowińska, M.; Dudzisz-Śledź, M.; Wiśniewski, P.; Tchórzewska-Korba, H.; Szumera-Ciećkiewicz, A.; et al. Expert recommendation on diagnostic-therapeutic management in skin carcinomas. Oncol. Clin. Pract. 2022, 18, 69–91. [Google Scholar] [CrossRef]
- Rutkowski, P.; Owczarek, W.; Nejc, D.; Jeziorski, A.; Wysocki, W.M.; Słowińska, M.; Dudzisz-Śledź, M.; Wiśniewski, P.; Koseła-Paterczyk, H.; Kiprian, D.; et al. Skin carcinomas. Oncol. Clin. Pract. 2020, 16, 143–162. [Google Scholar] [CrossRef]
- Cribier, B.; Scrivener, Y.; Grosshans, E. Tumors arising in nevus sebaceus: A study of 596 cases. Dermatopathology 2000, 7, 103–112. [Google Scholar] [CrossRef]
- Gogineni, E.; Cai, H.; Carillo, D.; Rana, Z.; Bloom, B.; Potters, L.; Gaballa, H.; Ghaly, M. Computed tomography-based flap brachytherapy for non-melanoma skin cancers of the. J. Contemp. Brachytherapy 2021, 13, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Sol, S.; Boncimino, F.; Todorova, K.; Waszyn, S.E. Therapeutic approaches for non-melanoma skin cancer: Standard of care and emerging modalities. Int. J. Mol. Sci. 2024, 25, 7056. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Strange, R.C.; Lear, J.T. Clinical review: Basal cell carcinoma. BMJ 2003, 327, 794–798. [Google Scholar] [CrossRef]
- Navarrete-Dechent, C.; Cordova, M.; Aleissa, S.; Liopyris, K.; Dusza, S.W.; Phillips, W.; Rossi, A.M.; Lee, E.H.; Marghoob, A.A.; Nehal, K.S. Reflectance confocal microscopy confirms residual basal cell carcinoma on clinically negative biopsy sites before Mohs micrographic surgery: A prospective study. J. Am. Acad. Dermatol. 2019, 81, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Aleissa, S.; Navarrete-Dechent, C.; Cordova, M.; Sahu, A.; Dusza, S.W.; Phillips, W.; Rossi, A.; Lee, E.; Nehal, K.S. Presurgical evaluation of basal cell carcinoma using combined reflectance confocal microscopy-optical coherence tomography: A prospective study. J. Am. Acad. Dermatol. 2020, 82, 962–968. [Google Scholar] [CrossRef]
- Longo, C.; Ragazzi, M.; Rajadhyaksha, M.; Nehal, K.; Bennassar, A.; Pellacani, G.; Malvehy Guilera, J. In vivo and ex vivo confocal microscopy for dermatologic and Mohs surgeons. Dermatol. Clin. 2016, 34, 497–504. [Google Scholar] [CrossRef]
- Atak, M.F.; Farabi, B.; Navarrete-Dechent, C.; Rubinstein, G.; Rajadhyaksha, M.; Jain, M. Confocal microscopy for diagnosis and management of cutaneous malignancies: Clinical impacts and innovation. Diagnostics 2023, 13, 854. [Google Scholar] [CrossRef]
- Shahriari, N.; Grant-Kels, J.M.; Rabinovitz, H.; Oliviero, M.; Scope, A. Reflectance confocal microscopy: Principles, basic terminology, clinical indications, limitations, and practical considerations. J. Am. Acad. Dermatol. 2021, 84, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Braghiroli, N.F.; Sugerik, S.; Freitas LARde Oliviero, M.; Rabinovitz, H. The skin through reflectance confocal microscopy—Historical background, technical principles, and its correlation with histopathology. An. Bras. Dermatol. 2022, 97, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Venturi, F.; Pellacani, G.; Farnetani, F.; Maibach, H.; Tassone, D.; Dika, E. Noninvasive diagnostic techniques in the preoperative setting of Mohs micrographic surgery: A review of the literature. Dermatol. Ther. 2022, 35, e15638. [Google Scholar] [CrossRef] [PubMed]
- Venturini, M.; Gualdi, G.; Zanca, A.; Lorenzi, L.; Pellacani, G.; Calzavara-Pinton, P.G. A new approach for presurgical margin assessment by reflectance confocal microscopy of basal cell carcinoma. Br. J. Dermatol. 2016, 174, 380–385. [Google Scholar] [CrossRef]
- Longo, C.; Pampena, R.; Bombonato, C.; Gardini, S.; Piana, S.; Mirra, M.; Raucci, M.; Kyrgidis, A.; Pellacani, G.; Ragazzi, M. Diagnostic accuracy of ex vivo fluorescence confocal microscopy in Mohs surgery of basal cell carcinomas: A prospective study on 753 margins. Br. J. Dermatol. 2019, 180, 1473–1480. [Google Scholar] [CrossRef]
- Xiong, Y.Q.; Ma, S.J.; Mo, Y.; Huo, S.T.; Wen, Y.Q.; Chen, Q. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: A meta-analysis. J. Cancer Res. Clin. Oncol. 2017, 143, 1627–1635. [Google Scholar] [CrossRef]
- Edwards, S.J.; Osei-Assibey, G.; Patalay, R.; Wakefield, V.; Karner, C. Diagnostic accuracy of reflectance confocal microscopy using VivaScope for detecting and monitoring skin lesions: A systematic review. Clin. Exp. Dermatol. 2017, 42, 266–275. [Google Scholar] [CrossRef]
- Pan, Z.Y.; Lin, J.R.; Cheng, T.T.; Wu, J.Q.; Wu, W.Y. In vivo reflectance confocal microscopy of basal cell carcinoma: Feasibility of preoperative mapping of cancer margins. Dermatol. Surg. 2012, 38, 1945–1950. [Google Scholar] [CrossRef]
- Lupu, M.; Voiculescu, V.M.; Caruntu, A.; Tebeica, T.; Caruntu, C. Preoperative evaluation through dermoscopy and reflectance confocal microscopy of the lateral excision margins for primary basal cell carcinoma. Diagnostics 2021, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Richarz, N.A.; Boada, A.; Jaka, A.; Bassas, J.; Ferrándiz, C.; Carrascosa, J.M.; Yélamos, O. Challenges for new adopters in pre-surgical margin assessment by handheld reflectance confocal microscope of basal cell carcinoma: A prospective single-center study. Dermatol. Pract. Concept. 2022, 12, e2022210. [Google Scholar] [CrossRef]
- Tannous, Z.; Torres, A.; González, S. In vivo real-time confocal reflectance microscopy: A noninvasive guide for Mohs micrographic surgery facilitated by aluminum chloride, an excellent contrast enhancer. Dermatol. Surg. 2003, 29, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Gualdi, G.; Venturini, M.; Zanca, A.; Calzavara-Pinton, P.G.; Pellacani, G. Pre-surgical basal cell carcinoma margin definition: The SMART approach. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 474–476. [Google Scholar] [CrossRef]
- Teixeira, D.A.; Rezze, G.G.; Pinhal, M.A.S.; Paschoal, F.M. Reflectance confocal microscopy as a tool for screening surgical margins of basal cell carcinoma. An. Bras. Dermatol. 2018, 93, 601–604. [Google Scholar] [CrossRef]
- Flores, E.; Yélamos, O.; Cordova, M.; Kose, K.; Phillips, W.; Lee, E.H.; Rossi, A.; Nehal, K.; Rajadhyaksha, M. Peri-operative delineation of non-melanoma skin cancer margins in vivo with handheld reflectance confocal microscopy and video-mosaicking. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Shavlokhova, V.; Vollmer, M.; Vollmer, A.; Gholam, P.; Saravi, B.; Hoffmann, J.; Engel, M.; Elsner, J.; Neumeier, F.; Freudlsperger, C. In vivo reflectance confocal microscopy of wounds: Feasibility of intraoperative basal cell carcinoma margin assessment. Ann. Transl. Med. 2021, 9, 1716. [Google Scholar] [CrossRef]
- Ahlgrimm-Siess, V.; Laimer, M.; Rabinovitz, H.S.; Oliviero, M.; Hofmann-Wellenhof, R.; Marghoob, A.A.; Scope, A. Confocal microscopy in skin cancer. Curr. Dermatol. Rep. 2018, 7, 105–118. [Google Scholar] [CrossRef]
- Stefanski, M.; Le Guern, A.; Visseaux, L.; Ehret, M.; Colomb, M.; Jeudy, G.; Le Duff, F.; Vourc’h, M.; Baroudjian, B.; Perea-Villacorta, R.; et al. Real-life practice of reflectance confocal microscopy in France: A prospective multicenter study. J. Am. Acad. Dermatol. 2024, 91, 51–56. [Google Scholar] [CrossRef]
- Schüle, D.; Breuninger, H.; Schippert, W.; Dietz, K.; Moehrle, M. Confocal laser scanning microscopy in micrographic surgery (three-dimensional histology) of basal cell carcinomas. Br. J. Dermatol. 2009, 161, 698–700. [Google Scholar] [CrossRef] [PubMed]
- Scope, A.; Mahmood, U.; Gareau, D.S.; Kenkre, M.; Lieb, J.A.; Nehal, K.S.; Rajadhyaksha, M. In vivo reflectance confocal microscopy of shave biopsy wounds: Feasibility of intraoperative mapping of cancer margins. Br. J. Dermatol. 2010, 163, 1218. [Google Scholar] [CrossRef]
- Flores, E.S.; Cordova, M.; Kose, K.; Phillips, W.; Rossi, A.; Nehal, K.; Rajadhyaksha, M. Intraoperative imaging during Mohs surgery with reflectance confocal microscopy: Initial clinical experience. J. Biomed. Opt. 2015, 20, 061103. [Google Scholar] [CrossRef]
- Shavlokhova, V.; Vollmer, M.; Gholam, P.; Saravi, B.; Vollmer, A.; Hoffmann, J.; Engel, M.; Freudlsperger, C. Deep learning on basal cell carcinoma in vivo reflectance confocal microscopy data. J. Pers. Med. 2022, 12, 1471. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, B.; Salgarelli, A.C.; Mandel, V.D.; Bellini, P.; Reggiani, C.; Farnetani, F.; Pellacani, G.; Magnoni, C. Non-melanoma skin cancer of the head and neck: The aid of reflectance confocal microscopy for the accurate diagnosis and management. G. Ital. Dermatol. Venereol. 2017, 152, 169–177. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).