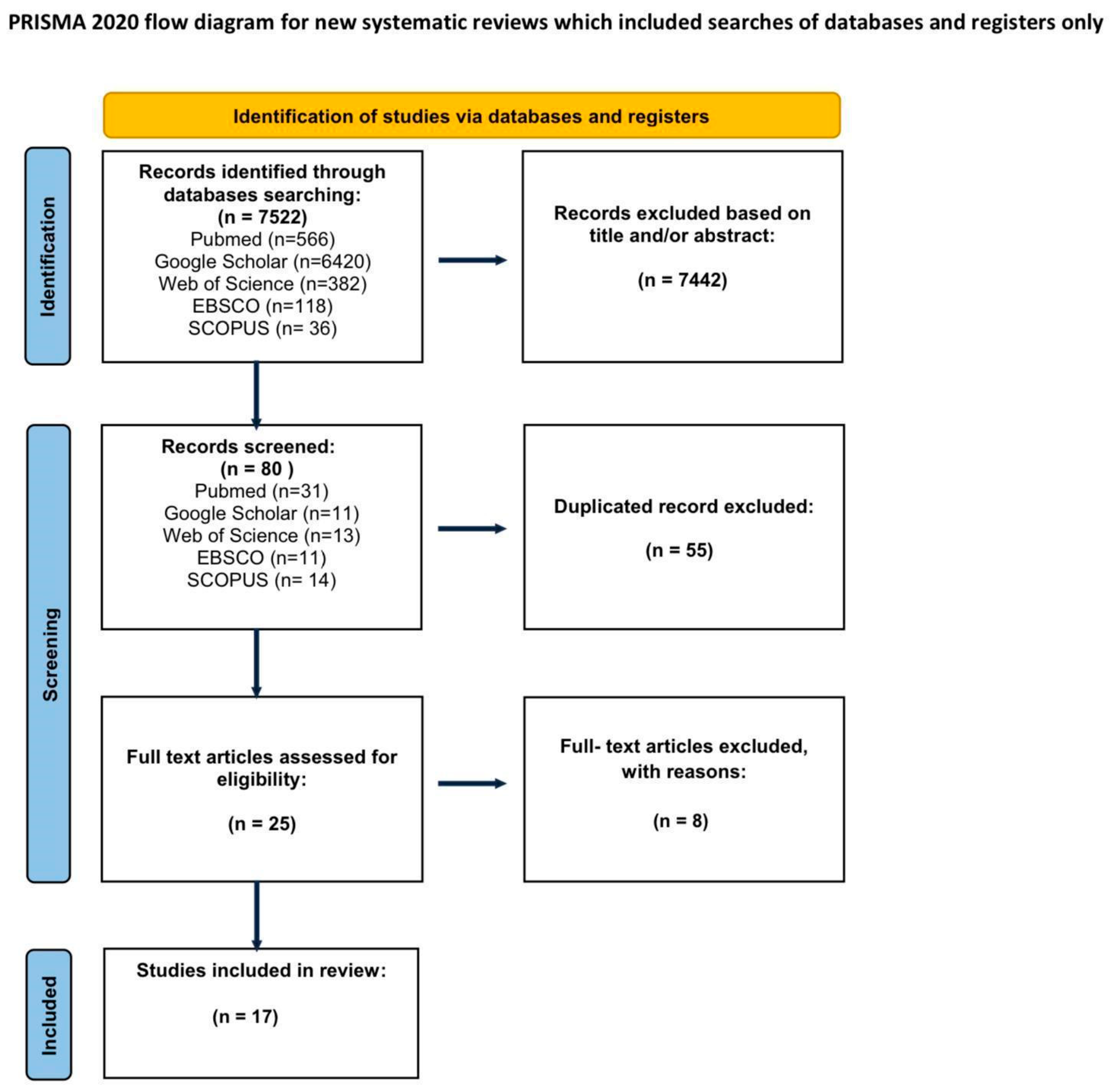
In Vivo Confocal Microscopy in the Surgical Treatment of Keratinocyte Carcinomas: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Type of Cancer
2.2. Type of Surgery
2.3. The Use of CM
2.4. Conclusions Reported by Original Studies
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madan, V.; Lear, J.T.; Szeimies, R.M. Non-melanoma skin cancer. Lancet 2010, 375, 673–685. [Google Scholar] [CrossRef]
- Cakir, B.Ö.; Adamson, P. Epidemiology and economic burden of nonmelanoma skin cancer. Facial Plast. Surg. Clin. N. Am. 2012, 20, 419–422. [Google Scholar] [CrossRef]
- Attal, Z.G.; Shalata, W.; Soklakova, A.; Tourkey, L.; Shalata, S.; Abu Saleh, O.; Abu Salamah, F.; Alatawneh, I.; Yakobson, A. Advanced and metastatic non-melanoma skin cancer: Epidemiology, risk factors, clinical features, and treatment options. Biomedicines 2024, 12, 1448. [Google Scholar] [CrossRef]
- Rutkowski, P.; Owczarek, W.; Nejc, D.; Jeziorski, A.; Wysocki, W.M.; Słowińska, M.; Dudzisz-Śledź, M.; Wiśniewski, P.; Tchórzewska-Korba, H.; Szumera-Ciećkiewicz, A.; et al. Expert recommendation on diagnostic-therapeutic management in skin carcinomas. Oncol. Clin. Pract. 2022, 18, 69–91. [Google Scholar] [CrossRef]
- Rutkowski, P.; Owczarek, W.; Nejc, D.; Jeziorski, A.; Wysocki, W.M.; Słowińska, M.; Dudzisz-Śledź, M.; Wiśniewski, P.; Koseła-Paterczyk, H.; Kiprian, D.; et al. Skin carcinomas. Oncol. Clin. Pract. 2020, 16, 143–162. [Google Scholar] [CrossRef]
- Cribier, B.; Scrivener, Y.; Grosshans, E. Tumors arising in nevus sebaceus: A study of 596 cases. Dermatopathology 2000, 7, 103–112. [Google Scholar] [CrossRef]
- Gogineni, E.; Cai, H.; Carillo, D.; Rana, Z.; Bloom, B.; Potters, L.; Gaballa, H.; Ghaly, M. Computed tomography-based flap brachytherapy for non-melanoma skin cancers of the. J. Contemp. Brachytherapy 2021, 13, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Sol, S.; Boncimino, F.; Todorova, K.; Waszyn, S.E. Therapeutic approaches for non-melanoma skin cancer: Standard of care and emerging modalities. Int. J. Mol. Sci. 2024, 25, 7056. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Strange, R.C.; Lear, J.T. Clinical review: Basal cell carcinoma. BMJ 2003, 327, 794–798. [Google Scholar] [CrossRef]
- Navarrete-Dechent, C.; Cordova, M.; Aleissa, S.; Liopyris, K.; Dusza, S.W.; Phillips, W.; Rossi, A.M.; Lee, E.H.; Marghoob, A.A.; Nehal, K.S. Reflectance confocal microscopy confirms residual basal cell carcinoma on clinically negative biopsy sites before Mohs micrographic surgery: A prospective study. J. Am. Acad. Dermatol. 2019, 81, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Aleissa, S.; Navarrete-Dechent, C.; Cordova, M.; Sahu, A.; Dusza, S.W.; Phillips, W.; Rossi, A.; Lee, E.; Nehal, K.S. Presurgical evaluation of basal cell carcinoma using combined reflectance confocal microscopy-optical coherence tomography: A prospective study. J. Am. Acad. Dermatol. 2020, 82, 962–968. [Google Scholar] [CrossRef]
- Longo, C.; Ragazzi, M.; Rajadhyaksha, M.; Nehal, K.; Bennassar, A.; Pellacani, G.; Malvehy Guilera, J. In vivo and ex vivo confocal microscopy for dermatologic and Mohs surgeons. Dermatol. Clin. 2016, 34, 497–504. [Google Scholar] [CrossRef]
- Atak, M.F.; Farabi, B.; Navarrete-Dechent, C.; Rubinstein, G.; Rajadhyaksha, M.; Jain, M. Confocal microscopy for diagnosis and management of cutaneous malignancies: Clinical impacts and innovation. Diagnostics 2023, 13, 854. [Google Scholar] [CrossRef]
- Shahriari, N.; Grant-Kels, J.M.; Rabinovitz, H.; Oliviero, M.; Scope, A. Reflectance confocal microscopy: Principles, basic terminology, clinical indications, limitations, and practical considerations. J. Am. Acad. Dermatol. 2021, 84, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Braghiroli, N.F.; Sugerik, S.; Freitas LARde Oliviero, M.; Rabinovitz, H. The skin through reflectance confocal microscopy—Historical background, technical principles, and its correlation with histopathology. An. Bras. Dermatol. 2022, 97, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Venturi, F.; Pellacani, G.; Farnetani, F.; Maibach, H.; Tassone, D.; Dika, E. Noninvasive diagnostic techniques in the preoperative setting of Mohs micrographic surgery: A review of the literature. Dermatol. Ther. 2022, 35, e15638. [Google Scholar] [CrossRef] [PubMed]
- Venturini, M.; Gualdi, G.; Zanca, A.; Lorenzi, L.; Pellacani, G.; Calzavara-Pinton, P.G. A new approach for presurgical margin assessment by reflectance confocal microscopy of basal cell carcinoma. Br. J. Dermatol. 2016, 174, 380–385. [Google Scholar] [CrossRef]
- Longo, C.; Pampena, R.; Bombonato, C.; Gardini, S.; Piana, S.; Mirra, M.; Raucci, M.; Kyrgidis, A.; Pellacani, G.; Ragazzi, M. Diagnostic accuracy of ex vivo fluorescence confocal microscopy in Mohs surgery of basal cell carcinomas: A prospective study on 753 margins. Br. J. Dermatol. 2019, 180, 1473–1480. [Google Scholar] [CrossRef]
- Xiong, Y.Q.; Ma, S.J.; Mo, Y.; Huo, S.T.; Wen, Y.Q.; Chen, Q. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: A meta-analysis. J. Cancer Res. Clin. Oncol. 2017, 143, 1627–1635. [Google Scholar] [CrossRef]
- Edwards, S.J.; Osei-Assibey, G.; Patalay, R.; Wakefield, V.; Karner, C. Diagnostic accuracy of reflectance confocal microscopy using VivaScope for detecting and monitoring skin lesions: A systematic review. Clin. Exp. Dermatol. 2017, 42, 266–275. [Google Scholar] [CrossRef]
- Pan, Z.Y.; Lin, J.R.; Cheng, T.T.; Wu, J.Q.; Wu, W.Y. In vivo reflectance confocal microscopy of basal cell carcinoma: Feasibility of preoperative mapping of cancer margins. Dermatol. Surg. 2012, 38, 1945–1950. [Google Scholar] [CrossRef]
- Lupu, M.; Voiculescu, V.M.; Caruntu, A.; Tebeica, T.; Caruntu, C. Preoperative evaluation through dermoscopy and reflectance confocal microscopy of the lateral excision margins for primary basal cell carcinoma. Diagnostics 2021, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Richarz, N.A.; Boada, A.; Jaka, A.; Bassas, J.; Ferrándiz, C.; Carrascosa, J.M.; Yélamos, O. Challenges for new adopters in pre-surgical margin assessment by handheld reflectance confocal microscope of basal cell carcinoma: A prospective single-center study. Dermatol. Pract. Concept. 2022, 12, e2022210. [Google Scholar] [CrossRef]
- Tannous, Z.; Torres, A.; González, S. In vivo real-time confocal reflectance microscopy: A noninvasive guide for Mohs micrographic surgery facilitated by aluminum chloride, an excellent contrast enhancer. Dermatol. Surg. 2003, 29, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Gualdi, G.; Venturini, M.; Zanca, A.; Calzavara-Pinton, P.G.; Pellacani, G. Pre-surgical basal cell carcinoma margin definition: The SMART approach. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 474–476. [Google Scholar] [CrossRef]
- Teixeira, D.A.; Rezze, G.G.; Pinhal, M.A.S.; Paschoal, F.M. Reflectance confocal microscopy as a tool for screening surgical margins of basal cell carcinoma. An. Bras. Dermatol. 2018, 93, 601–604. [Google Scholar] [CrossRef]
- Flores, E.; Yélamos, O.; Cordova, M.; Kose, K.; Phillips, W.; Lee, E.H.; Rossi, A.; Nehal, K.; Rajadhyaksha, M. Peri-operative delineation of non-melanoma skin cancer margins in vivo with handheld reflectance confocal microscopy and video-mosaicking. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Shavlokhova, V.; Vollmer, M.; Vollmer, A.; Gholam, P.; Saravi, B.; Hoffmann, J.; Engel, M.; Elsner, J.; Neumeier, F.; Freudlsperger, C. In vivo reflectance confocal microscopy of wounds: Feasibility of intraoperative basal cell carcinoma margin assessment. Ann. Transl. Med. 2021, 9, 1716. [Google Scholar] [CrossRef]
- Ahlgrimm-Siess, V.; Laimer, M.; Rabinovitz, H.S.; Oliviero, M.; Hofmann-Wellenhof, R.; Marghoob, A.A.; Scope, A. Confocal microscopy in skin cancer. Curr. Dermatol. Rep. 2018, 7, 105–118. [Google Scholar] [CrossRef]
- Stefanski, M.; Le Guern, A.; Visseaux, L.; Ehret, M.; Colomb, M.; Jeudy, G.; Le Duff, F.; Vourc’h, M.; Baroudjian, B.; Perea-Villacorta, R.; et al. Real-life practice of reflectance confocal microscopy in France: A prospective multicenter study. J. Am. Acad. Dermatol. 2024, 91, 51–56. [Google Scholar] [CrossRef]
- Schüle, D.; Breuninger, H.; Schippert, W.; Dietz, K.; Moehrle, M. Confocal laser scanning microscopy in micrographic surgery (three-dimensional histology) of basal cell carcinomas. Br. J. Dermatol. 2009, 161, 698–700. [Google Scholar] [CrossRef] [PubMed]
- Scope, A.; Mahmood, U.; Gareau, D.S.; Kenkre, M.; Lieb, J.A.; Nehal, K.S.; Rajadhyaksha, M. In vivo reflectance confocal microscopy of shave biopsy wounds: Feasibility of intraoperative mapping of cancer margins. Br. J. Dermatol. 2010, 163, 1218. [Google Scholar] [CrossRef]
- Flores, E.S.; Cordova, M.; Kose, K.; Phillips, W.; Rossi, A.; Nehal, K.; Rajadhyaksha, M. Intraoperative imaging during Mohs surgery with reflectance confocal microscopy: Initial clinical experience. J. Biomed. Opt. 2015, 20, 061103. [Google Scholar] [CrossRef]
- Shavlokhova, V.; Vollmer, M.; Gholam, P.; Saravi, B.; Vollmer, A.; Hoffmann, J.; Engel, M.; Freudlsperger, C. Deep learning on basal cell carcinoma in vivo reflectance confocal microscopy data. J. Pers. Med. 2022, 12, 1471. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, B.; Salgarelli, A.C.; Mandel, V.D.; Bellini, P.; Reggiani, C.; Farnetani, F.; Pellacani, G.; Magnoni, C. Non-melanoma skin cancer of the head and neck: The aid of reflectance confocal microscopy for the accurate diagnosis and management. G. Ital. Dermatol. Venereol. 2017, 152, 169–177. [Google Scholar] [CrossRef] [PubMed]
| Authors | Type of Surgery | Number of Patients | Type of Use RCM | Type of Cancer | The Effect of Using RCM | Timing of RCM Use | Confocal Microscope Type | Depth Limit (μm) |
|---|---|---|---|---|---|---|---|---|
| Pan et al. 2012 [21] | CLASSIC REMOVAL | 9 | EXCISION MARGIN | BCC | Objective: The accuracy of RCM in determining the lateral margins of BCC in vivo compared to histology was 92.3%. | PREOPERATIVELY | Vivascope 1500 | 200 |
| Venturini et al. 2016 [17] | MOHS | 3 | EXCISION MARGIN | BCC | Subjective: Preoperative results corresponded to the results of intraoperative histopathological examination. | PREOPERATIVELY | Vivascope 1500 | 200 |
| Lupu et al. 2021 [22] | CLASSIC REMOVAL | 7 | EXCISION MARGIN | BCC | Objective: The accuracy of RCM in determining the lateral boundaries of BCC in vivo compared to histology was 93.1% (95% CI 0.77–0.99), with a sensitivity of 66.67% and a specificity of 100%. | PREOPERATIVELY | VivaScope | 200–250 |
| Richarz et al. 2022 [23] | MOHS | 17 | EXCISION MARGIN | BCC | Objective: No significant differences between the RCM-predicted and the actual surface area of the surgical defect, 2.95 cm2 vs 2.52 cm2. | PREOPERATIVELY | Vivascope 3000 | 200–250 |
| Tannous et al. 2003 [24] | MOHS | 5 | EXCISION MARGIN | BCC | Subjective: Confocal microscopy in vivo can be a useful tool in supporting Mohs surgery, and aluminum chloride effectively enhances tumor contrast in this imaging technique. | INTRAOPERATIVELY | Vivascope 1000 | No information |
| Gualdi et al. 2016 [25] | CLASSIC REMOVAL | 1 | EXCISION MARGIN | BCC | Objective: RCM detected that islands of BCC cancer extended 0.1 mm beyond the initial incision. Subjective: Allowing the surgeon to redraw the surgical margin (implying a positive impact). | INTRAOPERATIVELY | No information | No information |
| Teixeira et al. 2018 [26] | MOHS | 8 | EXCISION MARGIN | BCC | Objective: In six cases (50%), the margins were free of cancer in the first stage of MMS. Subjective: RCM enabled full determination of the lateral boundaries of the tumor in all cases, leading to the preservation of healthy skin. | INTRAOPERATIVELY | Vivascope 1500 | 300 |
| Flores et al. 2019 [27] | MOHS | 17 | EXCISION MARGIN | BCC, SCC | Objective: RCM videos and video-mosaics showed imaging quality in 91% of NMSC lesions pre-operatively and 83% intra-operatively. Sensitivity/specificity were 71%/86% and 86%/81% for two RCM video-mosaic evaluators, and overall agreement was 80% and 83% with histopathology. Subjective: The imaging quality was acceptable (resolution and contrast) | PREOPERATIVELY, INTRAOPERATIVELY | Vivascope 3000 | 120–150 |
| Shavlokhova et al. 2021 [28] | CLASSIC REMOVAL | 50 | EXCISION MARGIN | BCC | Objective: RCM in vivo identified BCC at wound margins with a sensitivity of 88.5% and a specificity of 91.7% compared to imaging of intact skin, and with a sensitivity of 97.8% and a specificity of 90.7% compared to histopathology. Subjective: This method allows for the identification of BCC at wound margins. | INTRAOPERATIVELY | Vivascope 3000 | 350 |
| Ahlgrimm-Siess et al. 2018 [29] | CLASSIC REMOVAL | 224 | DIAGNOSTIC METHOD | BCC | Objective: Sensitivity of 97% and specificity of 93% were demonstrated in the diagnosis of BCC | PREOPERATIVELY | Vivascope 1500 | 250 |
| Navarrete-Dechent et al. 2019 [10] | MOHS | 47 | DIAGNOSTIC METHOD | BCC | Objective: Sensitivity, specificity, positive predictive value, and negative predictive value of RCM were 92.8%, 68.4%, 86.6%, and 81.2%, respectively. Subjective: Due to the strong correlation between RCM imaging and histopathological outcomes, RCM can be a valuable tool for assessing residual BCC at the biopsy site, which may help reduce the number of unnecessary surgeries. | PREOPERATIVELY | Vivascope 3000 | 200 |
| Stefanski et al. 2024 [30] | CLASSIC REMOVAL | 410 | DIAGNOSTIC METHOD | BCC, SCC | Objective: 50.6% of patients avoided biopsy, and the correlation between RCM and histopathology was 82.76%. Subjective: Thanks to RCM, patients avoided biopsy (implying a positive outcome). | PREOPERATIVELY | Vivascope 1500 | 200 |
| Schüle et al. 2009 [31] | MOHS | 66 | DIAGNOSTIC METHOD | BCC | Objective: The concordance coefficients between RCM and histopathology were low. Subjective: The effect was poor. | INTRAOPERATIVELY | Vivascope 2500 | No information |
| Scope et al. 2010 [32] | MOHS | 20 | DIAGNOSTIC METHOD | BCC, SCC | Objective: Wounds were fully visualized using RCM in 33% of cases (out of n = 39), and in 4 cases, RCM visualized BCC, later confirmed histologically. Subjective: RCM imaging of wounds is feasible but limited in visualizing all cases. | INTRAOPERATIVELY | Vivascope 2000 | 300 |
| Flores et al. 2015 [33] | MOHS | 25 | DIAGNOSTIC METHOD | BCC, SCC | Subjective: Due to the good correlation of RCM imaging with histopathology, RCM shows potential in intraoperative detection of cancer presence at wound edges, which could shorten surgery time and improve its effectiveness. | INTRAOPERATIVELY | Vivascope 3000 | 150 |
| Shavlokhova et al. 2022 [34] | CLASSIC REMOVAL | 62 | DIAGNOSTIC METHOD | BCC | Objective: In seven of 10 (70%) cases, cancer margins were identified; in three of 10 (30%) cases, margins could not be detected. All frozen biopsies of surgical margins were negative, and in 12 of 13 (92.3%) cases, RCM margins were negative. Subjective: The effect was partial, and with a larger database, greater effectiveness in automatic detection of BCC is possible. | INTRAOPERATIVELY | Vivascope 3000 | No information |
| Ferrari et al. 2017 [35] | CLASSIC REMOVAL | 141 | DIAGNOSTIC METHOD | BCC, SCC | Subjective: RCM is a valuable diagnostic tool that allows for in vivo imaging of tissues, contributing to more accurate diagnostics of NSC of the head and neck, saving patient time and costs for the public healthcare system. | PREOPERATIVELY | No information | No information |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojarska, M.; Kokot, K.; Bernecka, P.; Domańska, N.; Libik, A.; Bunevich, D.; Nowakowska, D.; Dzido, M.; Borzyszkowska, W.; Kazimierczak, W.; et al. In Vivo Confocal Microscopy in the Surgical Treatment of Keratinocyte Carcinomas: A Systematic Review. J. Clin. Med. 2025, 14, 5779. https://doi.org/10.3390/jcm14165779
Wojarska M, Kokot K, Bernecka P, Domańska N, Libik A, Bunevich D, Nowakowska D, Dzido M, Borzyszkowska W, Kazimierczak W, et al. In Vivo Confocal Microscopy in the Surgical Treatment of Keratinocyte Carcinomas: A Systematic Review. Journal of Clinical Medicine. 2025; 14(16):5779. https://doi.org/10.3390/jcm14165779
Chicago/Turabian StyleWojarska, Monika, Klaudia Kokot, Paulina Bernecka, Natalia Domańska, Agata Libik, Dana Bunevich, Dominika Nowakowska, Magdalena Dzido, Wiktoria Borzyszkowska, Wojciech Kazimierczak, and et al. 2025. "In Vivo Confocal Microscopy in the Surgical Treatment of Keratinocyte Carcinomas: A Systematic Review" Journal of Clinical Medicine 14, no. 16: 5779. https://doi.org/10.3390/jcm14165779
APA StyleWojarska, M., Kokot, K., Bernecka, P., Domańska, N., Libik, A., Bunevich, D., Nowakowska, D., Dzido, M., Borzyszkowska, W., Kazimierczak, W., & Jankau, J. (2025). In Vivo Confocal Microscopy in the Surgical Treatment of Keratinocyte Carcinomas: A Systematic Review. Journal of Clinical Medicine, 14(16), 5779. https://doi.org/10.3390/jcm14165779








