Abstract
Background/Objectives: As the incidence of diabetes mellitus (DM) is increasing rapidly worldwide, it is anticipated that an increasing number of women will enter pregnancy with pregestational diabetes mellitus (PGDM) in the future. Compelling evidence suggests that hyperglycemia in pregnancy is related to multiple adverse perinatal outcomes. This systematic review and meta-analysis aims to assess and quantify the association of PGDM with a range of adverse perinatal outcomes, providing a comprehensive understanding of its impact on pregnancy. Methods: The data sources of this systematic review and meta-analysis were Medline/PubMed, Scopus and Cochrane Library (January 1999 to August 2023), complemented by hand-searching for additional references. Observational studies reporting perinatal outcomes of pregnancies with PGDM diagnosed before pregnancy versus control pregnancies were eligible for inclusion. A systematic review and meta-analysis were conducted as per the PRISMA guidelines. Pooled estimate odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to determine the risk of adverse pregnancy outcomes between PGDM and control pregnancies. Results: The systematic search of the literature yielded 81 observational studies meeting inclusion criteria and in total, 137,237,640 pregnancies were included in the analysis. A total of 19 adverse perinatal outcomes were assessed, revealing a significant association with PGDM. In pregnancies with PGDM there was an increased risk of adverse perinatal outcomes, including gestational hypertension (OR 3.16, 95% CI 2.65–3.77), preeclampsia (OR 4.46, 95% CI 3.94–5.05), preterm delivery (OR 3.46, 95% CI 3.06–3.91), cesarean delivery (OR 3.12, 95% CI 2.81–3.47), induction of labor (OR 2.92, 95% CI 2.35–3.63), macrosomia (OR 2.23, 95% CI 1.76–2.83), LGA neonates (OR 3.95, 95% CI 3.47–4.49), low 5-min Apgar score (OR 2.49, 95% CI 2.07–2.99), shoulder dystocia (OR 3.05, 95% CI 2.07–4.50), birth trauma (OR 1.40, 95% CI 1.22–1.62), polyhydramnios (OR 5.06, 95% CI 4.33–5.91), oligohydramnios (OR 1.61, 95% CI 1.19–2.17), neonatal hyperbilirubinemia (OR 3.45, 95% CI 2.51–4.74), neonatal hypoglycemia (OR 19.19, 95% CI 2.78–132.61), neonatal intensive care unit (NICU) admission (OR 4.54, 95% CI 3.87–5.34), congenital malformations (OR 2.44, 95% CI 1.96–3.04), stillbirth (OR 2.87, 95% CI 2.27–3.63) and perinatal mortality (OR 2.94, 95% CI 2.18–3.98). Subgroup analyses indicated a higher risk of neonatal hypoglycemia, stillbirth and perinatal mortality in T1DM pregnancies compared with T2DM pregnancies. Conclusions: This study provides a robust synthesis of evidence underlying the strong association between PGDM and several adverse perinatal outcomes. Early detection, optimal glycemic control during the periconceptional and pregnancy periods, and proper antenatal care are critical to mitigate these risks.
1. Introduction
Diabetes mellitus (DM) seems to be one of the fastest-growing health issues of the 21st century; its global prevalence has more than doubled over the last four decades in the adult population, rising from 4.7% in 1980 to 10.5% in 2021 and it is estimated that the global prevalence will continue increasing, reaching 11.3% by 2030 [1,2]. DM is primarily classified into two types: type 1 diabetes mellitus (T1DM), which often develops in early life and is associated with a genetic predisposition, and type 2 diabetes mellitus (T2DM), which usually develops later in life and is more commonly associated with lifestyle factors. The reasons for the increase in T1DM prevalence are unclear but may involve a combination of environmental changes and altered early life factors (viral infections, gut microbiome) [3]. In contrast, the reasons for the rise in T2DM prevalence are more clearly understood, with increasingly sedentary lifestyles and rising obesity rates being the primary causes [4].
As a result of this trend and the increasing maternal age, more women will likely enter pregnancy with pregestational diabetes mellitus (PGDM) in the future. Compelling evidence demonstrates that hyperglycemia during pregnancy is related to an increased risk of adverse perinatal outcomes, such as preeclampsia, preterm delivery, macrosomia and congenital malformations [5,6,7,8,9,10]. Consequently, the number of pregnancies at risk is expected to rise alongside the increasing prevalence of DM.
Though many adverse perinatal outcomes associated with PGDM may have severe implications, the specific impact of PGDM on these outcomes has not been comprehensively explored in the existing literature. Most current knowledge is derived from single observational studies rather than systematic reviews and meta-analyses [11]. To date, only one systematic review has addressed this topic, but it did not assess crucial outcomes, such as congenital malformations [12]. In contrast, gestational diabetes mellitus (GDM) and its adverse perinatal outcomes have been studied systematically on various occasions [13,14,15].
Given the importance of accurate risk estimation for preconception counseling and the optimization of antenatal care, there is a critical need for robust, comprehensive data on the perinatal risks related to PGDM. To address this gap, the aim of this study is to systematically review the literature and conduct a meta-analysis assessing the association between PGDM and a wide range of adverse perinatal outcomes. By integrating data from an unprecedented number of pregnancies across diverse populations, this study provides highly precise effect estimates with enhanced statistical power. Importantly, it also differentiates between the effects of T1DM and T2DM, allowing for a more detailed understanding of their distinct impact on perinatal risks. This comprehensive approach enhances the generalizability and clinical applicability of the findings, thereby providing healthcare professionals and pregnant women with robust, globally applicable evidence to support evidence-based care and improve maternal and neonatal health.
2. Materials and Methods
The present systematic review and meta-analysis complied with a prespecified protocol registered to the PROSPERO database (International Prospective Register of Systematic Reviews) with registration number CRD42023459730 on 1 September 2023. Additionally, it adheres to the PRISMA guidelines, designed for transparent reporting of systematic reviews and meta-analyses [16].
2.1. Eligibility Criteria
The studies included in this systematic review and meta-analysis were selected based on specific eligibility criteria. We exclusively considered observational studies comparing adverse perinatal outcomes in two distinct groups: pregnancies with PGDM diagnosed before pregnancy (study group) and pregnancies without PGDM or GDM in the current pregnancy (control group). PGDM was defined as DM diagnosed prior to conception. Studies were only included if they clearly differentiated PGDM from gestational diabetes mellitus (GDM). The eligible study period was from 1999 onwards; this period was selected to maximize the homogeneity of results, as at this time, the World Health Organization (WHO) proposed a change in the diagnostic value of fasting glucose concentrations for the diagnosis of DM to 126 mg/dL, with the proposed diagnostic value still being used to this day [17]. Only studies published in English were considered for inclusion. We excluded studies with insufficient data for interpretation, those lacking an appropriate comparison group and those that did not adequately differentiate between PGDM and GDM.
2.2. Outcomes
The outcomes assessed were divided into maternal and fetal/neonatal. Maternal outcomes included gestational hypertension (defined as systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg, presenting after 20 weeks of gestation for the first time without proteinuria or any end-organ dysfunction) [18], preeclampsia (defined as systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg, accompanied by new-onset proteinuria or significant end-organ dysfunction after 20 weeks of gestation) [18], preterm delivery (defined as delivery before completing 37 weeks of gestation) [19], cesarean delivery and induction of labor. Fetal/neonatal outcomes included macrosomia (defined as birth weight > 4000 g) [20], large for gestational age (LGA) neonates (defined as birthweight > 90th percentile for gestational age) [20], small for gestational age (SGA) neonates (defined as birth weight < 10th percentile for gestational age) [21], low 5-min Apgar score (defined as 5-min Apgar score < 7) [22], shoulder dystocia, birth trauma (defined as any physical injury of the neonate during labor, e.g., clavicle fracture, brachial plexus injury), neonatal hyperbilirubinemia, neonatal hypoglycemia, admission to the neonatal intensive care unit (NICU), congenital malformations, stillbirth (defined as delivery of a fetus not exhibiting signs of life at or after 20 weeks of gestation) [23] and perinatal mortality.
2.3. Search Strategy and Information Sources
We aimed to identify observational studies assessing the effect of PGDM on adverse perinatal outcomes compared to control pregnancies without PGDM or GDM. The electronic databases searched were MEDLINE/PubMed, Scopus and Cochrane Library from January 1999 to August 2023. The last search was conducted on the 1st of September 2023. Each search used combinations of free-text and Medical Subject Heading (MeSH) terms combined with Boolean operators. The search syntax utilized in each database can be found in Appendix A. The results were supplemented with a manual search of reference lists of relevant publications and a grey literature search. Although we did not consult a professional librarian during the design of the search strategy, the search terms and strategies were carefully developed and pilot-tested by the authors (medical doctors with experience in systematic reviews) to ensure sensitivity and comprehensiveness.
2.4. Study Selection
All studies derived from the initial search were imported into Systematic Review Accelerator (https://sr-accelerator.com/ accessed on 1 September 2023), an online reference management tool provided by the Institute for Evidence-Based Healthcare of the University of Bond, and duplicates were removed. After deduplication, titles and abstracts of the studies were screened using the same tool by three independent reviewers (DG, AT, GK—medical doctors) to determine the eligibility of the studies against the eligibility criteria. Studies were considered eligible for full-text review and data extraction if they were observational studies with available full-text comparing perinatal outcomes in pregnancies with PGDM and control pregnancies. Full texts of potentially eligible studies were examined independently by two reviewers (DG, AT) to end up with the list of studies to be included in the systematic review and meta-analysis. If two or more studies used the same database for overlapping periods, only data from the study with the largest population were used. Disagreements between the reviewers were resolved by consensus.
2.5. Data Extraction
After the final selection of the eligible studies, data extraction forms were developed independently in Covidence (https://www.covidence.org/ accessed on 10 November 2023), an online tool for systematic review management, by two reviewers (DG, AT). Discrepancies were resolved by consensus. The extracted data included administrative characteristics of the studies, such as authors, year of publication and country where the study took place, design characteristics of the studies, such as type of study and sample size, baseline characteristics of the study populations, such as type of PGDM, as well as intervention characteristics, such as type of treatment during pregnancy. For each outcome of interest, the raw data (number of cases and the total population) were recorded for both the study and control groups.
2.6. Risk of Bias Assessment
The risk of bias for each study included in this systematic review and meta-analysis was assessed independently by two reviewers (DG, AT) using the Newcastle–Ottawa scale. The scale consists of three domains, each addressing distinct aspects of study quality using a “star system” for quality quantification [24]. The first domain assesses the selection of study groups, the second the comparability of these groups and the third domain appraises the ascertainment of either the outcome in cohort studies or the exposure in case-control studies. The studies included in this systematic review and meta-analysis were labeled as having a low risk of bias if they scored four stars for selection, two for comparison and three for outcome/exposure. Any study with a score of one or zero for the selection or outcome/exposure assessment or zero for the comparison assessment was considered to have a high risk of bias. In all other cases, the overall risk of bias was considered “unclear”. Disagreements between the reviewers were resolved by consensus.
2.7. Data Synthesis
The outcome data were dichotomous, so each group’s number of events and total participants were extracted for every outcome available. The odds ratio (OR) with 95% confidence intervals (CIs) was used as the effect measure to determine the likelihood of adverse pregnancy outcomes between PGDM and control pregnancies. Statistical heterogeneity was evaluated with Chi2 and quantified with the I2 statistics test. For outcomes with low heterogeneity (I2 ≤ 50%), the fixed effect model was used, and for outcomes with high heterogeneity (I2 > 50%), the random effect model was used. Potential sources of heterogeneity were explored with subgroup analyses investigating the effect of the different types of PGDM (T1DM and T2DM) on adverse perinatal outcomes. The Review Manager (RevMan) Version 5.4.1 was used for the statistical analysis of the results.
3. Results
3.1. Study Selection and Study Characteristics
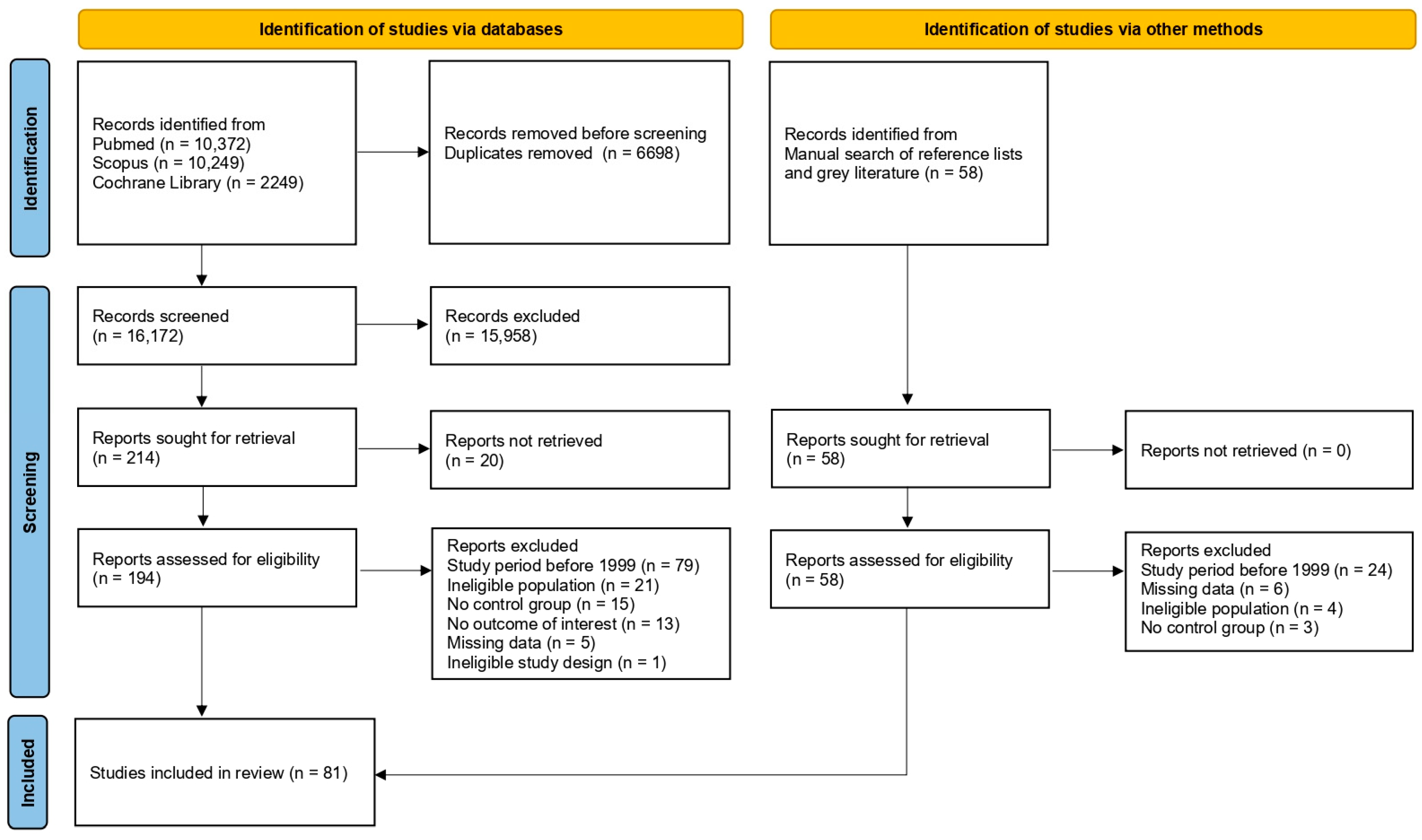
A total of 22,870 records were identified by the initial systematic search (10,372 via PubMed, 10,249 via Scopus and 2249 via Cochrane Library). After removing duplicate records, 16,172 were screened by title and abstract, and 214 were deemed suitable for full-paper appraisal; 194 full-text reports were retrieved, while the remaining 20 could not be obtained, even after a request was sent to the corresponding authors. Following the assessment of eligibility, 134 reports were excluded due to the following reasons: study period before 1999 (n = 79), ineligible population (n = 21), no control group (n = 15), no outcome of interest (n = 13), missing data (n = 5) and ineligible study design (n = 1). A manual search of reference lists of relevant publications and a search of grey literature identified 58 records suitable for full-paper appraisal. Full-text reports were retrieved for all of them. After the assessment of eligibility, 37 reports were excluded for the following reasons: study period before 1999 (n = 24), missing data (n = 6), ineligible population (n = 4) and no control group (n = 3). In total, 81 studies were included in the present meta-analysis [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105]. A flow diagram illustrates the complete review process (Figure 1).
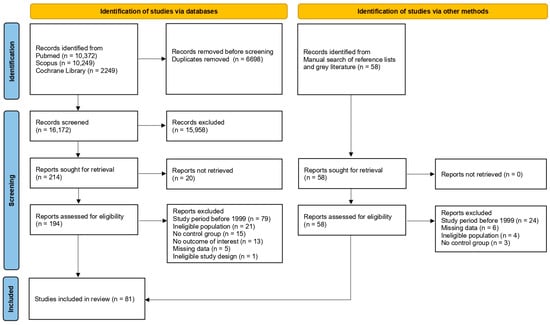
Figure 1.
Study selection flowchart.
All 81 studies included were observational in design. Of these, 71 were cohort studies (60 were retrospective and 11 were prospective), while 10 were case-control studies. A total of 11 studies included only T1DM in their PGDM group, 8 included only T2DM and the remaining 62 included both T1DM and T2DM.
In total, 137,237,640 pregnancies were examined, including 1,151,826 pregnancies with PGDM and 136,085,814 control pregnancies. The studies were conducted at different times and locations, indicating no overlap in study populations. The studies were carried out in North America (26 studies), Europe (25 studies), East Asia (10 studies), the Middle East (8 studies), Oceania (10 studies) and South America (2 studies). A detailed table with the characteristics of the included studies can be found in Appendix A (Table A1).
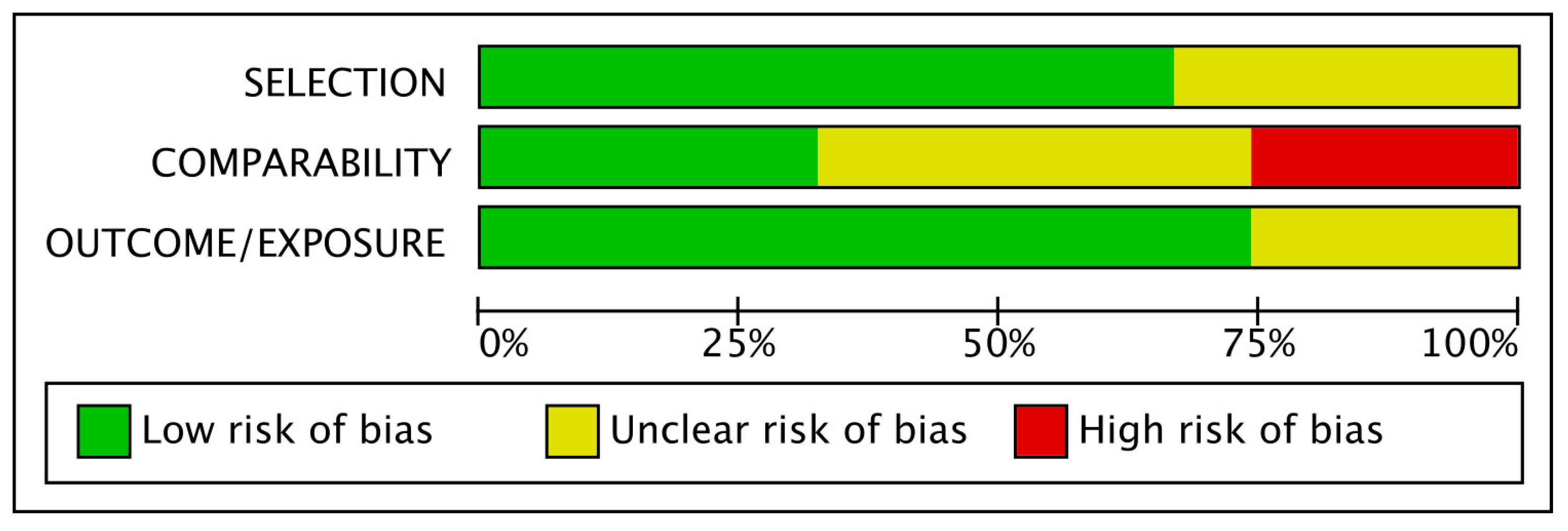
3.2. Risk of Bias Assessment of the Included Studies
Based on the Newcastle–Ottawa scale, out of the 81 included studies, 13 were characterized as low risk, 47 as unclear risk and 21 as high risk. As shown in Figure 2, comparability was the most bias-prone domain among studies. Only a few studies matched their populations and adjusted their results for body mass index (BMI), which is considered the most important confounding factor for adverse perinatal outcomes. In contrast, other confounding factors, such as maternal age, were more frequently used for matches between groups and adjustments of results. At the same time, on many occasions, no confounder was considered.
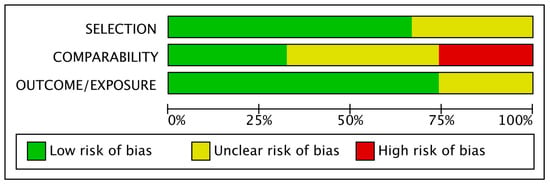
Figure 2.
Risk of bias graph.
3.3. PGDM and Adverse Perinatal Outcomes
The summary of adverse perinatal outcomes and the OR estimates for pregnancies with PGDM compared to control pregnancies are summarized in Table 1.

Table 1.
Summary of findings.
3.3.1. Gestational Hypertension
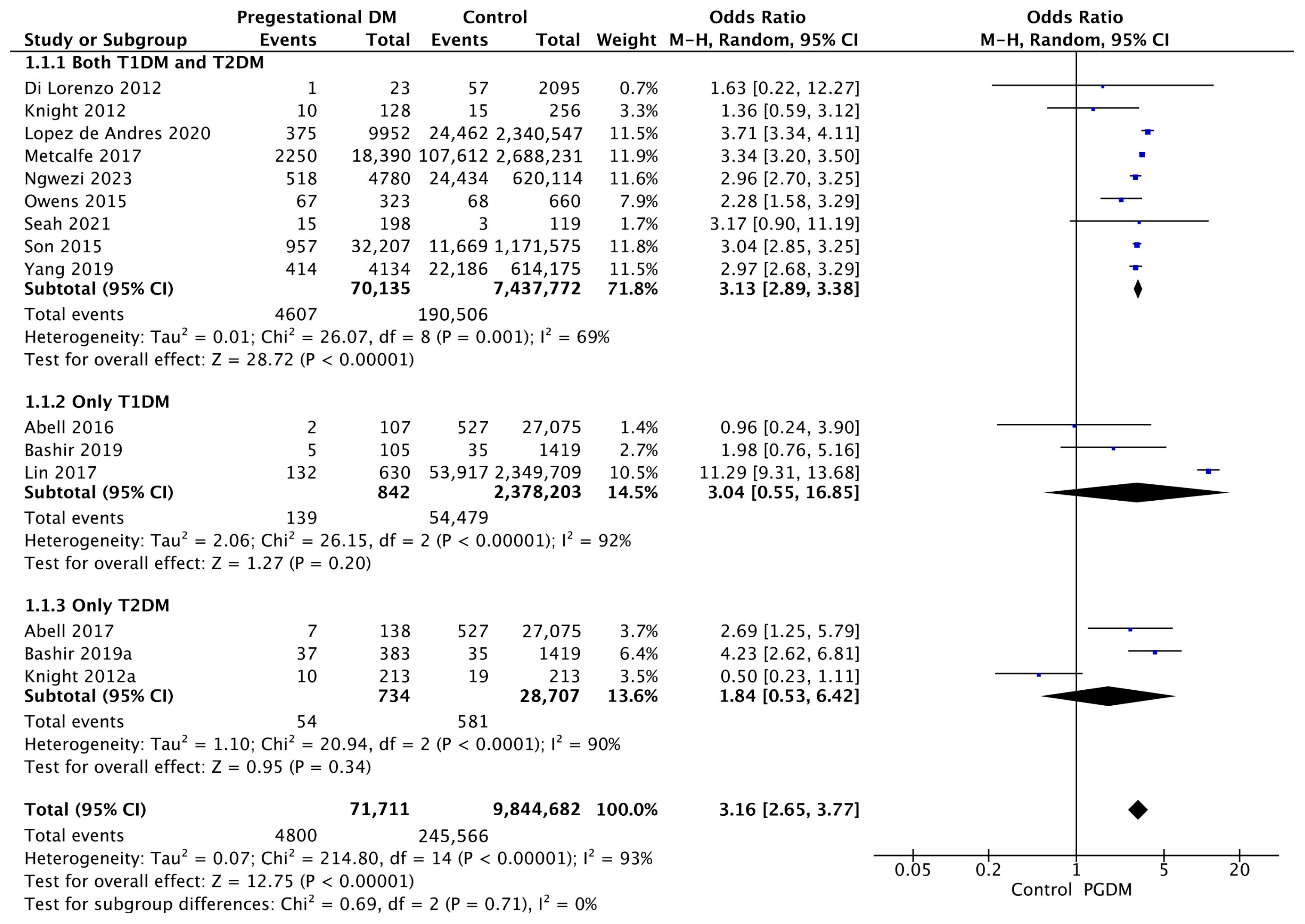
Fifteen studies reported data on gestational hypertension, including 71,711 pregnant women with PGDM and 9,844,682 pregnant women without PGDM or GDM. In pregnancies with PGDM, the risk of gestational hypertension was increased compared to control pregnancies (OR 3.16, 95% CI 2.65–3.77, p < 0.00001, I2 = 93%). The subgroup analysis, including studies reporting data only for one type of PGDM, did not show a statistically significant difference in the risk of gestational hypertension between T1DM and T2DM (p = 0.64) (Figure 3).
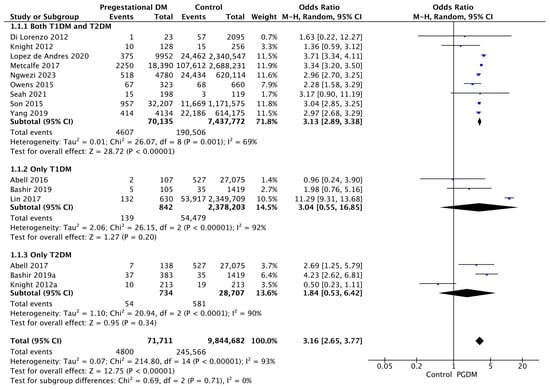
Figure 3.
Forest plot comparing the incidence of gestational hypertension between PGDM and control groups [25,26,30,31,40,60,61,67,70,73,76,77,88,92,102].
3.3.2. Preeclampsia
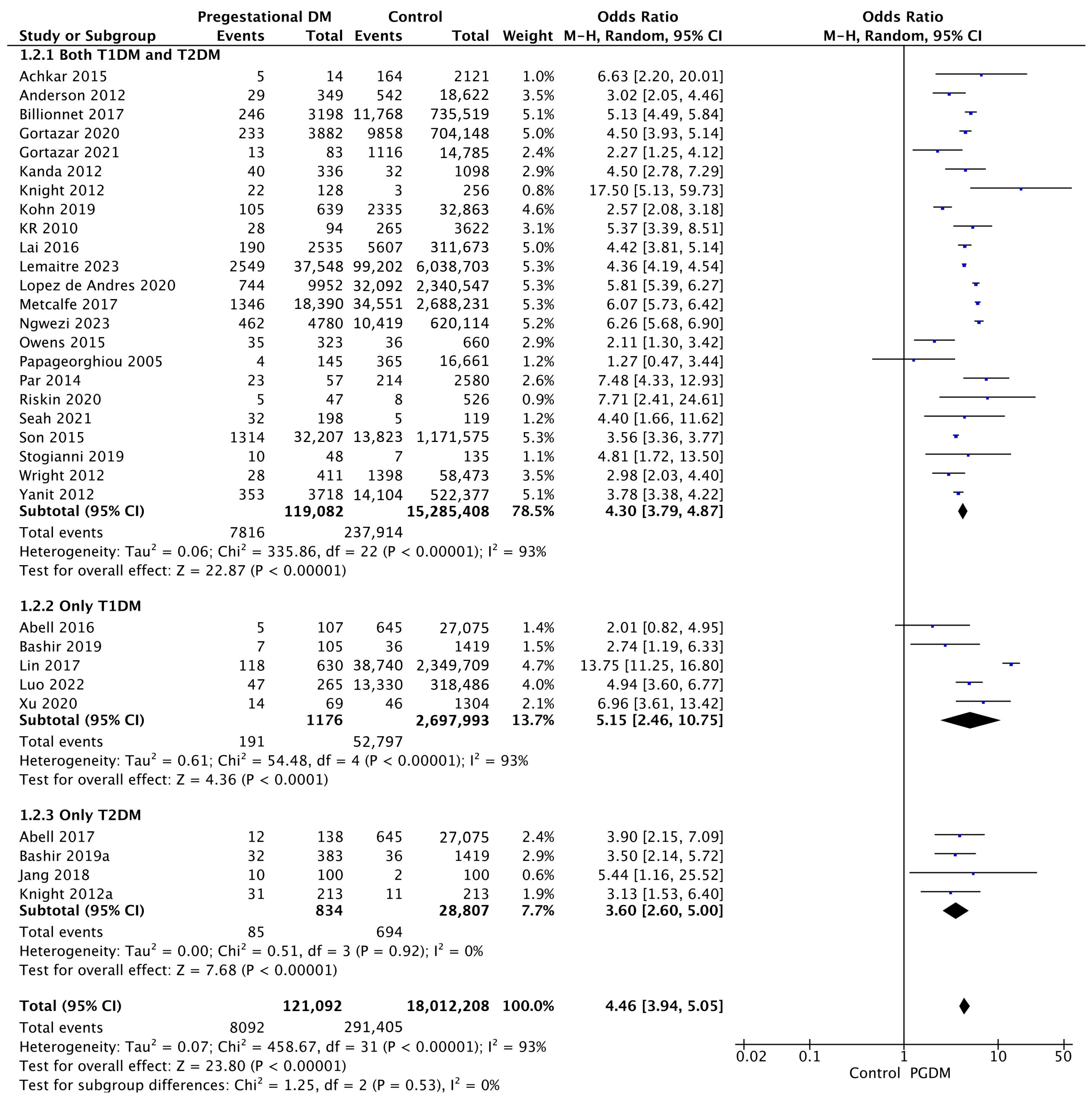
Thirty-two studies reported data on preeclampsia, including 121,092 pregnant women with PGDM and 18,012,208 pregnant women without PGDM or GDM. In pregnancies with PGDM, the risk of preeclampsia was increased compared to control pregnancies (OR 4.46, 95% CI 3.94–5.05, p < 0.00001, I2 = 93%). The subgroup analysis, including studies reporting data only for one type of PGDM, did not show a statistically significant difference in the risk of preeclampsia between T1DM and T2DM (p = 0.39) (Figure 4).
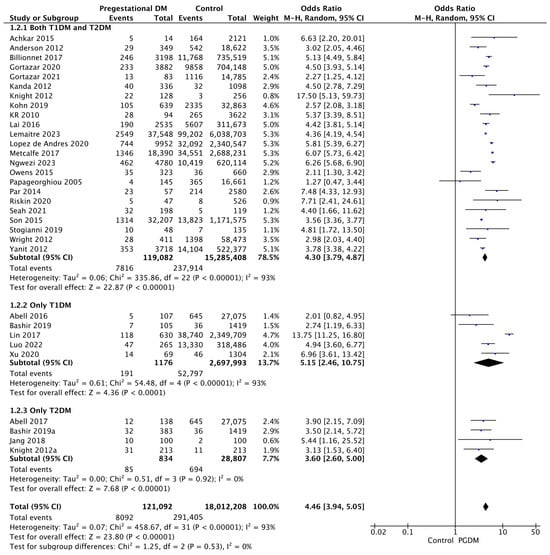
Figure 4.
Forest plot comparing the incidence of preeclampsia between PGDM and control groups [25,26,27,28,30,31,35,47,50,51,55,57,60,61,62,64,66,67,70,72,73,76,77,78,79,86,88,92,94,99,101,103].
3.3.3. Preterm Delivery
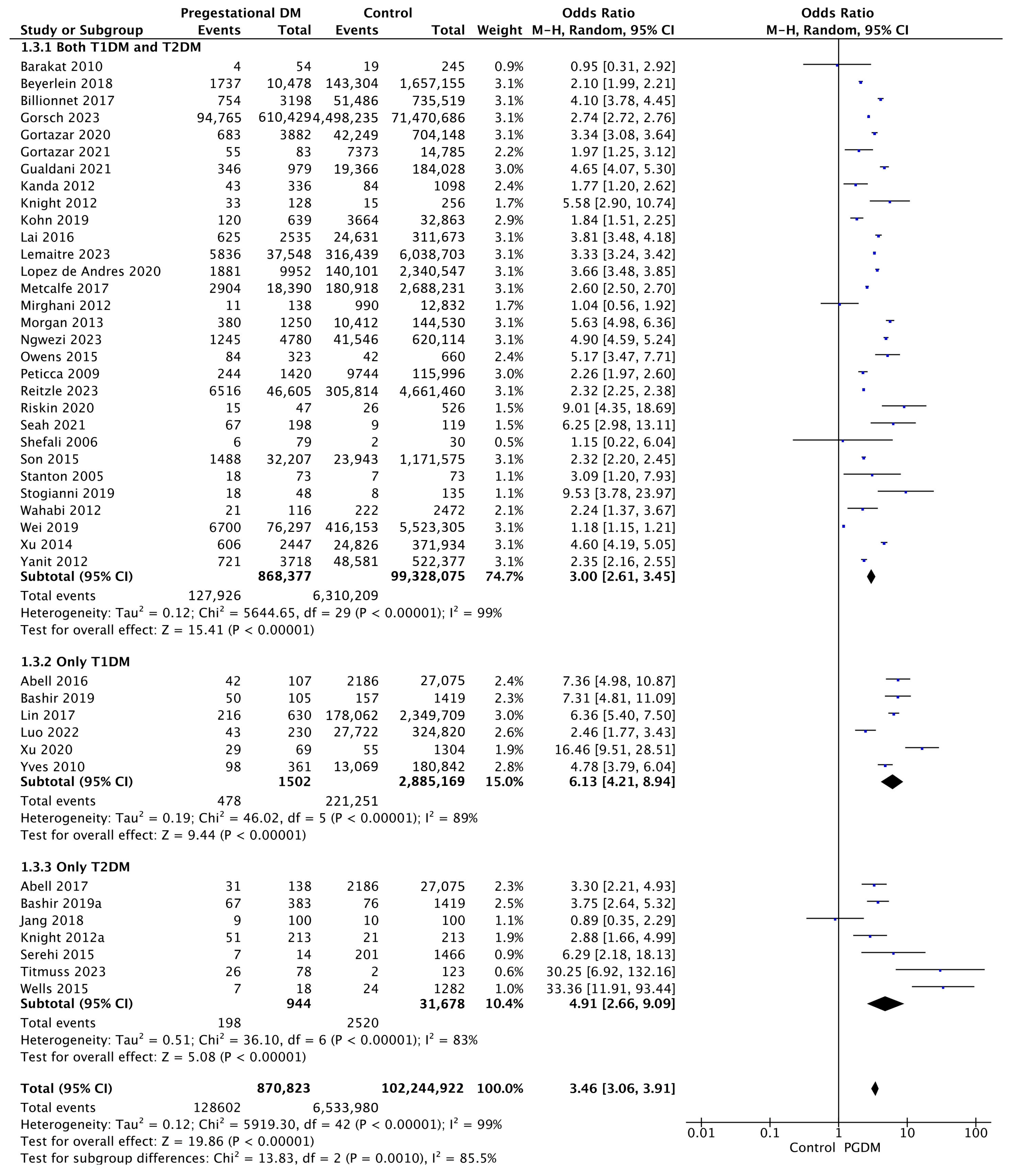
Forty-four studies reported data on preterm delivery, including 870,823 pregnant women with PGDM and 102,244,922 pregnant women without PGDM or GDM. In pregnancies with PGDM, the risk of preterm delivery was increased compared to control pregnancies (OR 3.46, 95% CI 3.06–3.91, p < 0.00001, I2 = 99%). The subgroup analysis, including studies reporting data only for one type of PGDM, did not show a statistically significant difference in the risk of preterm delivery between T1DM and T2DM (p = 0.55) (Figure 5).
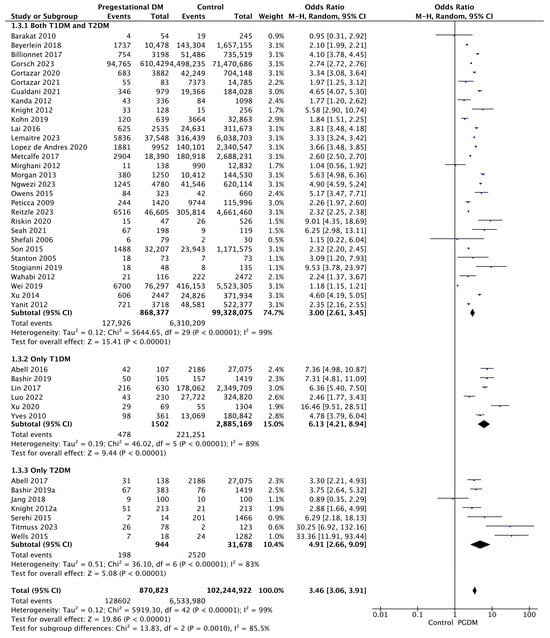
Figure 5.
Forest plot comparing the incidence of preterm delivery between PGDM and control groups [25,26,29,30,31,33,35,49,50,51,52,55,57,60,61,62,64,66,67,70,72,73,74,75,76,77,82,85,86,88,89,90,92,93,94,95,96,97,98,100,101,103,104].
3.3.4. Cesarean Delivery
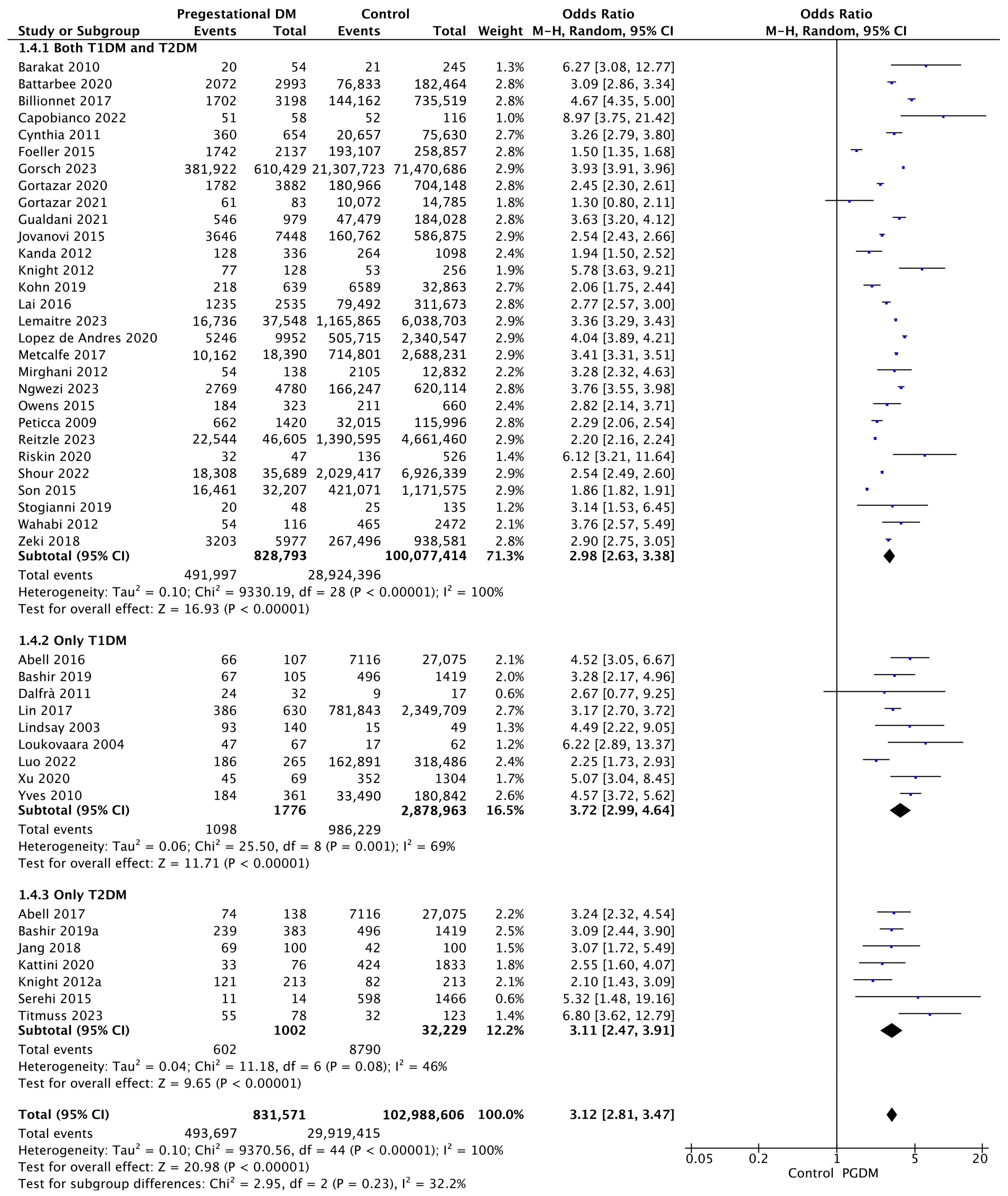
Forty-five studies reported data on cesarean delivery, including 831,571 pregnant women with PGDM and 102,988,606 pregnant women without PGDM or GDM. In pregnancies with PGDM, the risk of cesarean delivery was increased compared to control pregnancies (OR 3.12, 95% CI 2.81–3.47, p < 0.00001, I2 = 100%). The subgroup analysis, including studies reporting data only for one type of PGDM, did not show a statistically significant difference in the risk of cesarean delivery between T1DM and T2DM (p = 0.27) (Figure 6).
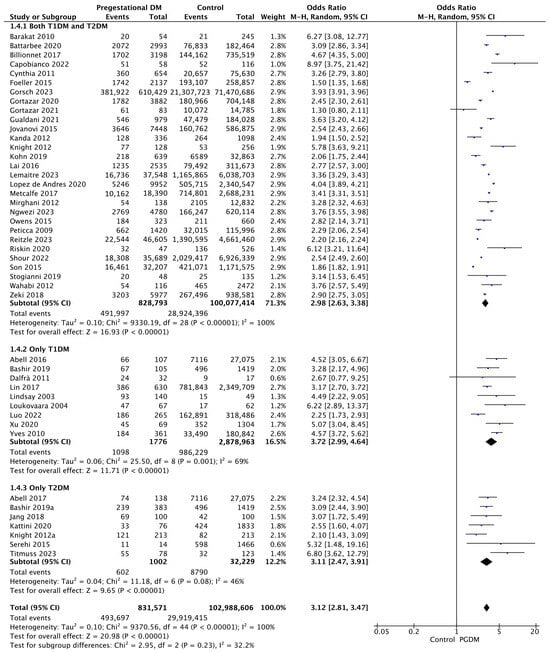
Figure 6.
Forest plot comparing the incidence of cesarean delivery between PGDM and control groups [25,26,29,30,31,32,35,36,38,39,44,49,50,51,52,55,56,57,58,60,61,62,64,66,67,68,70,71,72,73,74,76,77,82,85,86,89,91,92,94,95,96,101,104,105].
3.3.5. Induction of Labor
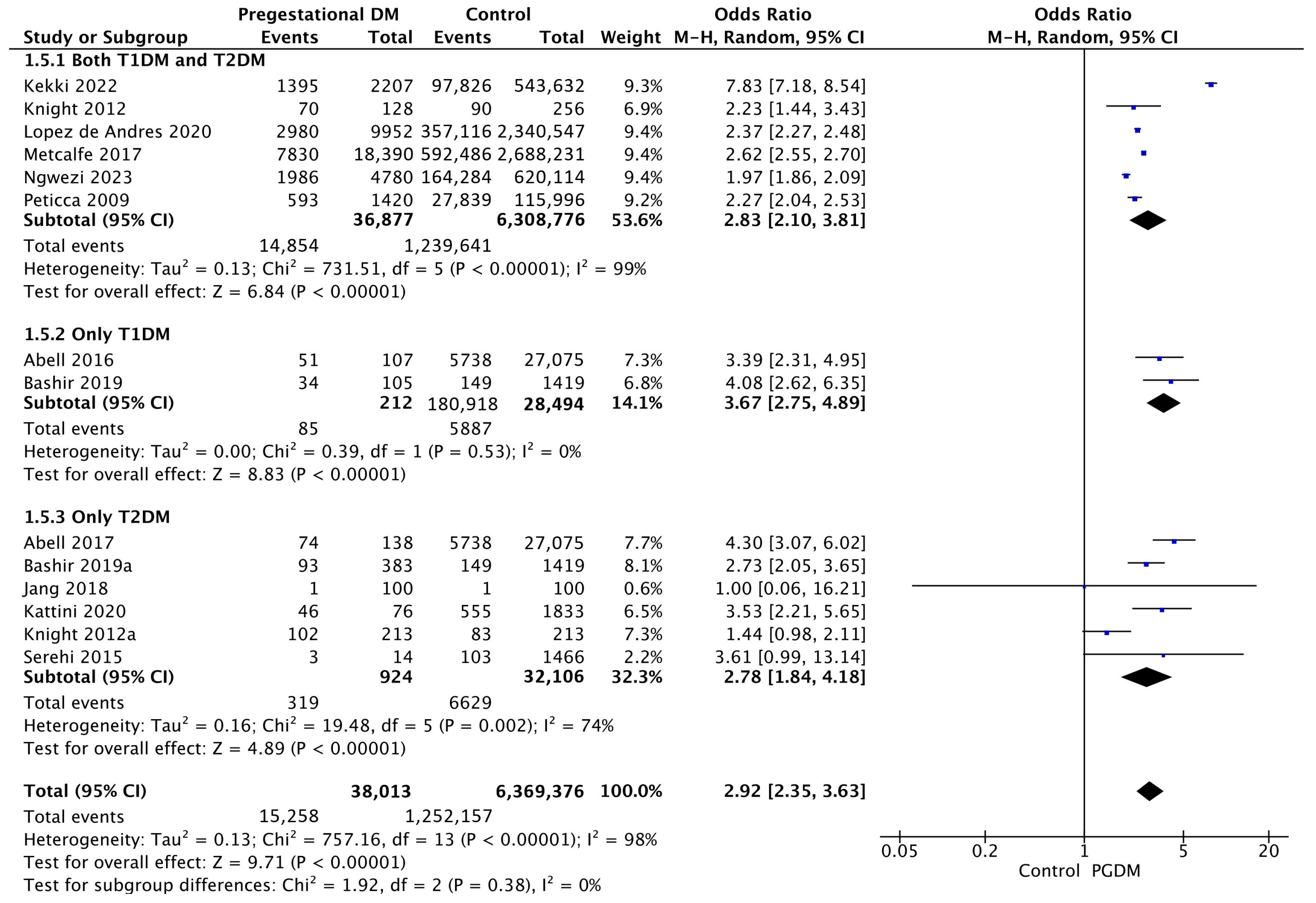
Fourteen studies reported data on the induction of labor, including 38,013 pregnant women with PGDM and 6,369,376 pregnant women without PGDM or GDM. In pregnancies with PGDM, the risk of labor induction was increased compared to control pregnancies (OR 2.92, 95% CI 2.35–3.63, p < 0.00001, I2 = 98%). The subgroup analysis, including studies reporting data only for one type of PGDM, did not show a statistically significant difference in the risk of induction of labor between T1DM and T2DM (p = 0.28) (Figure 7).
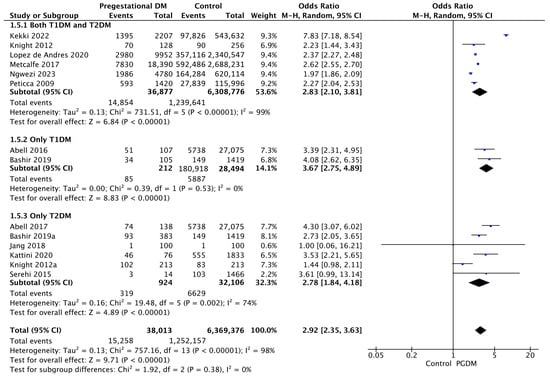
Figure 7.
Forest plot comparing the incidence of induction of labor between PGDM and control groups [25,26,30,31,55,58,59,60,61,70,73,76,82,89].
3.3.6. Macrosomia
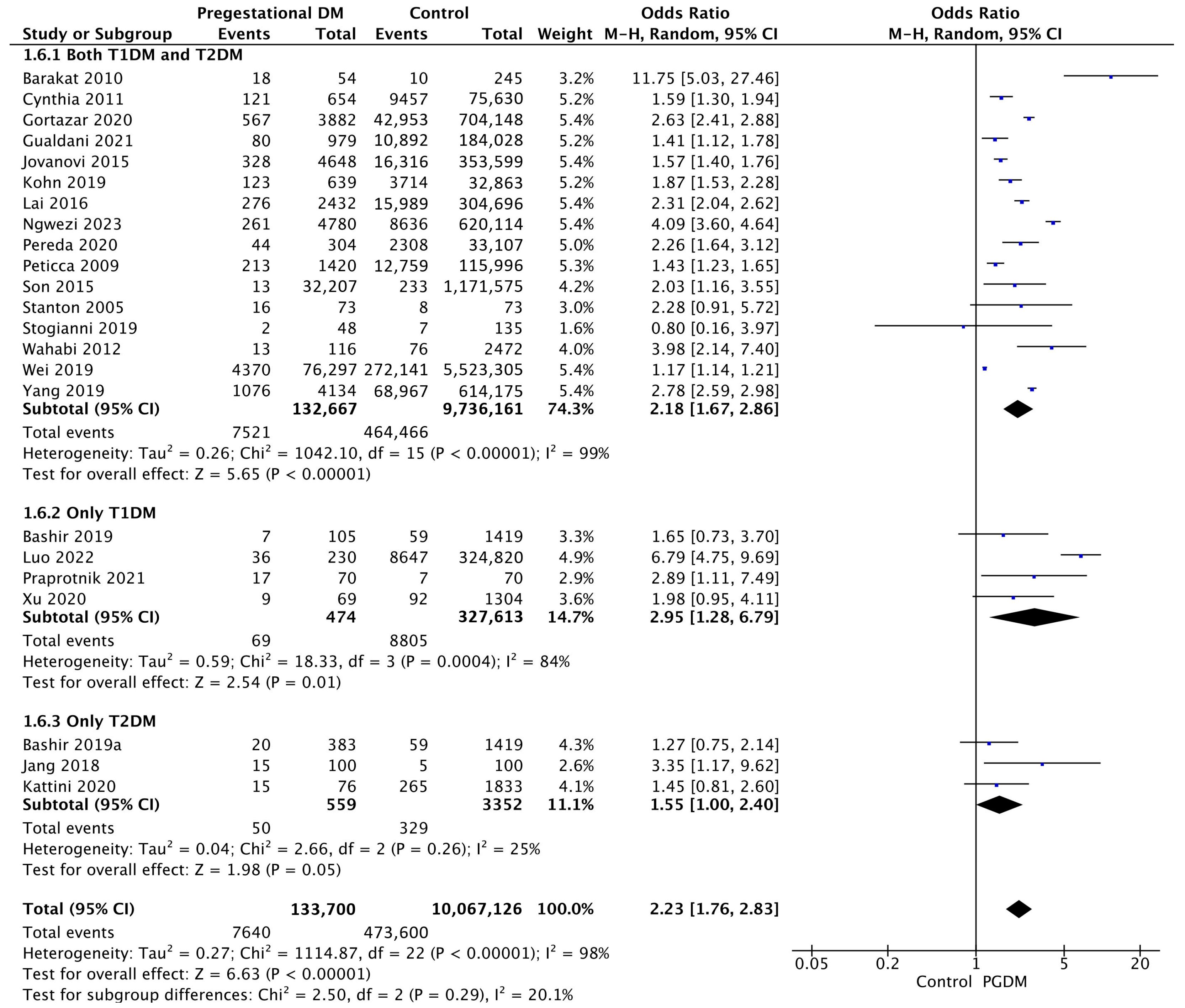
Twenty-three studies reported data on macrosomia, including 133,700 pregnant women with PGDM and 10,067,126 pregnant women without PGDM or GDM. In pregnancies with PGDM, the risk of macrosomia was increased compared to control pregnancies (OR 2.23, 95% CI 1.76–2.83, p < 0.00001, I2 = 98%). The subgroup analysis, including studies reporting data only for one type of PGDM, did not show a statistically significant difference in the risk of macrosomia between T1DM and T2DM (p = 0.18) (Figure 8).
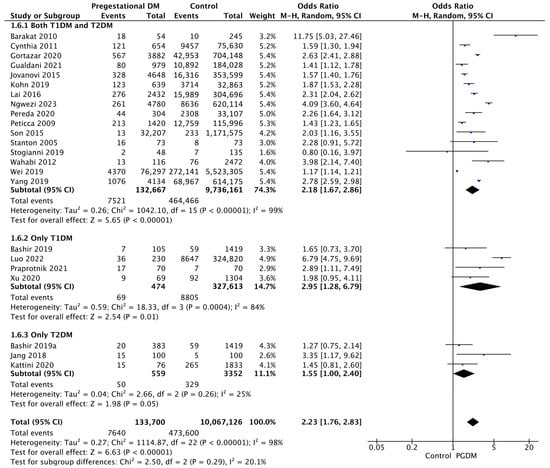
Figure 8.
Forest plot comparing the incidence of macrosomia between PGDM and control groups [29,30,31,38,50,52,55,56,58,62,64,72,76,81,82,83,92,93,94,96,97,101,102].
3.3.7. LGA Neonates
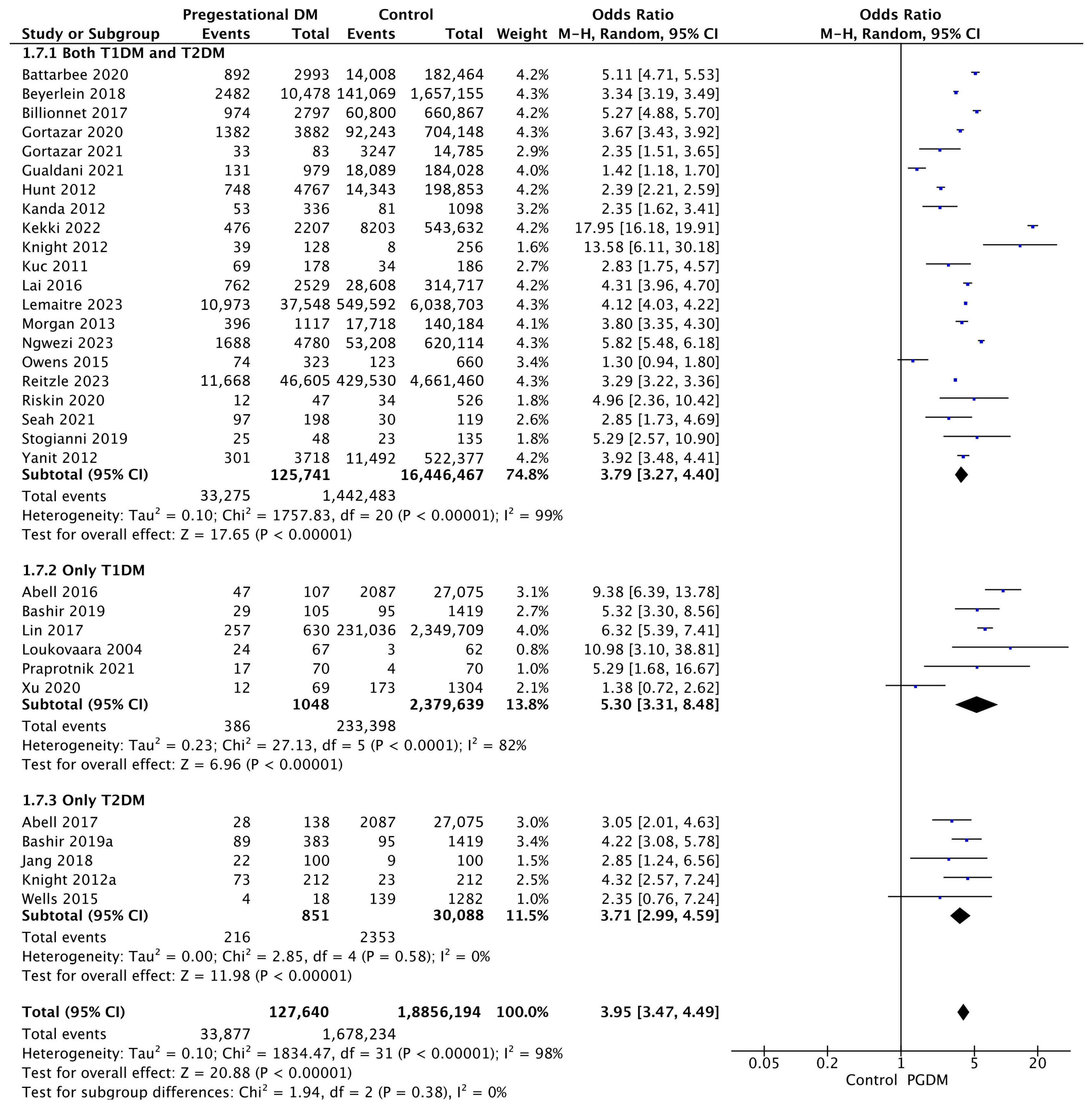
Thirty-two studies reported data on LGA neonates, including 127,640 pregnant women with PGDM and 18,856,194 pregnant women without PGDM or GDM. In pregnancies with PGDM, the risk of LGA neonates was increased compared to control pregnancies (OR 3.95, 95% CI 3.47–4.49, p < 0.00001, I2 = 98%). The subgroup analysis, including studies reporting data only for one type of PGDM, did not show a statistically significant difference in the risk of LGA neonates between T1DM and T2DM (p = 0.17) (Figure 9).
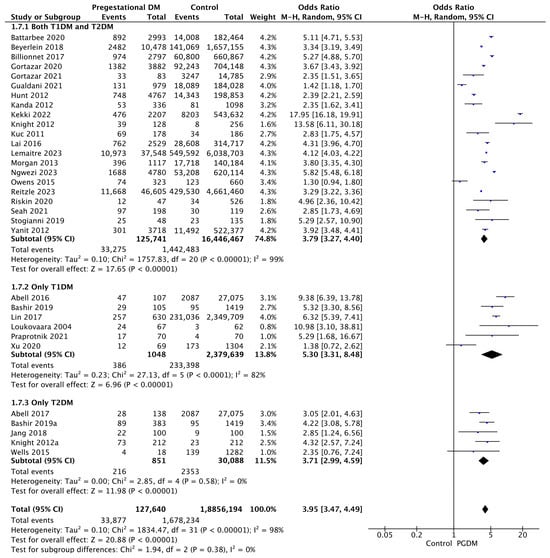
Figure 9.
Forest plot comparing the incidence of LGA neonates between PGDM and control groups [25,26,30,31,32,33,35,50,51,52,54,55,57,59,60,61,63,64,66,67,71,75,76,77,83,85,86,88,94,98,101,103].
3.3.8. SGA Neonates
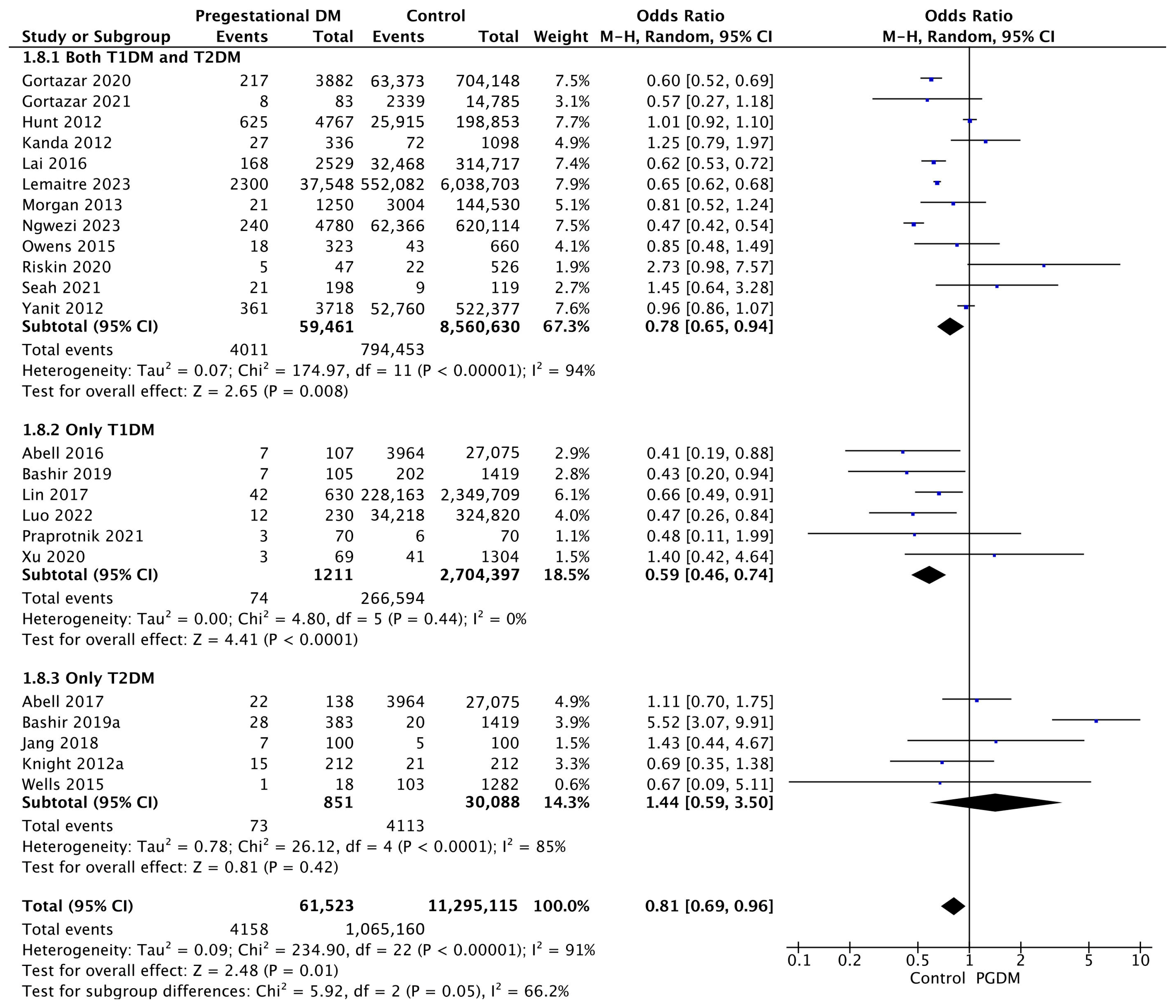
Twenty-three studies reported data on SGA neonates, including 61,523 pregnant women with PGDM and 11,295,115 pregnant women without PGDM or GDM. In pregnancies with PGDM, the risk of SGA neonates was decreased compared to control pregnancies (OR 0.81, 95% CI 0.69–0.96, p = 0.01, I2 = 91%). The subgroup analysis, including studies reporting data only for one type of PGDM, showed a statistically significant difference in the risk of SGA neonates between T1DM and T2DM, with a lower risk in the T1DM group (p = 0.06) (Figure 10).
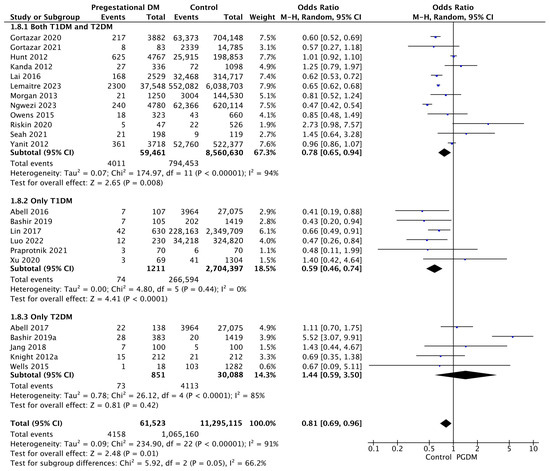
Figure 10.
Forest plot comparing the incidence of SGA neonates between PGDM and control groups [25,26,30,31,50,51,54,55,57,61,64,66,67,72,75,76,77,83,86,88,98,101,103].
3.3.9. Low 5-Min Apgar Score
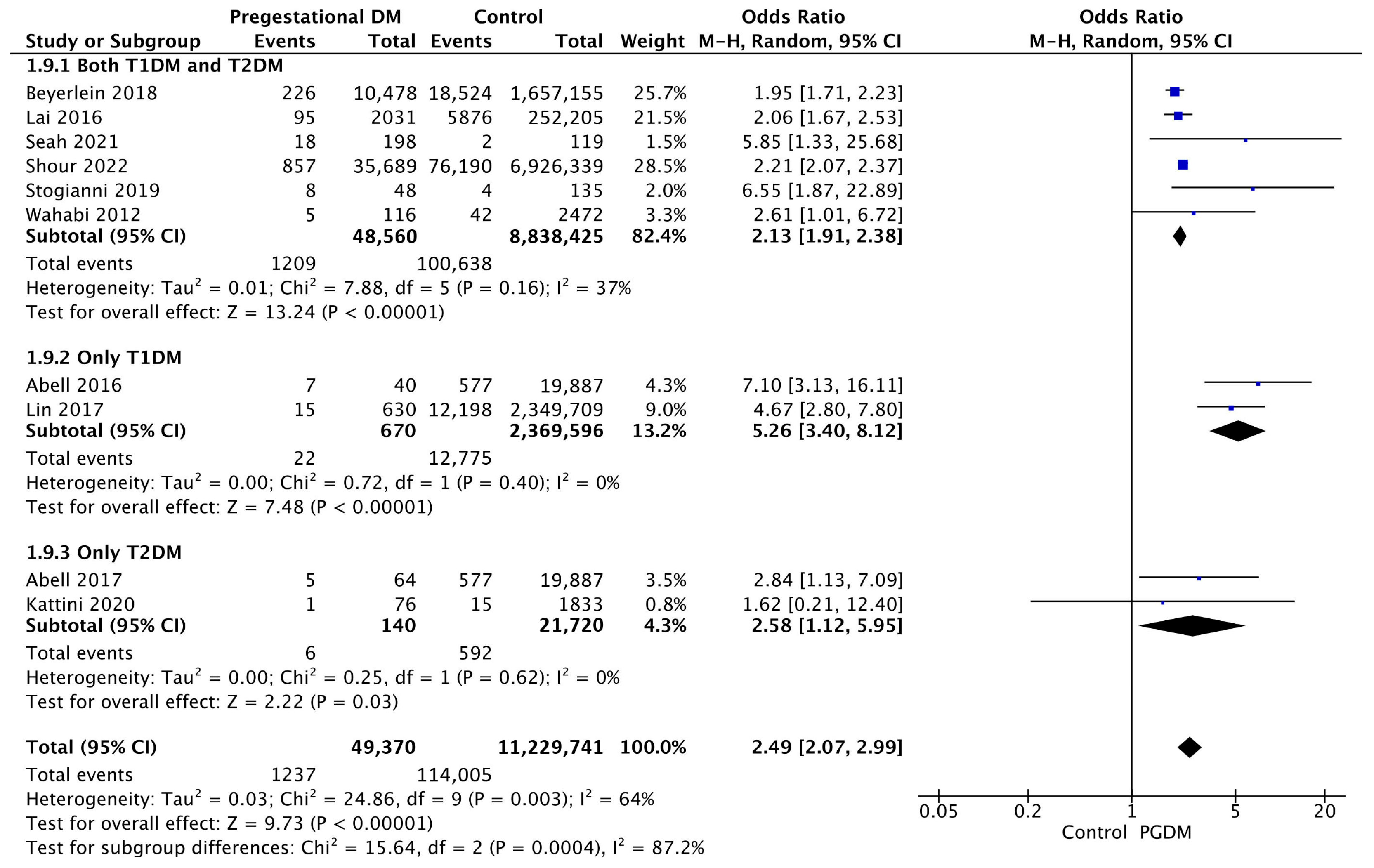
Ten studies reported data on low Apgar scores, including 49,370 pregnant women with PGDM and 11,229,741 pregnant women without PGDM or GDM. In pregnancies with PGDM, the risk of a low 5-min Apgar score was increased compared to control pregnancies (OR 2.49, 95% CI 2.07–2.99, p < 0.00001, I2 = 64%). The subgroup analysis, including studies reporting data only for one type of PGDM, did not show a statistically significant difference in the risk of low Apgar score between T1DM and T2DM (p = 0.14) (Figure 11).
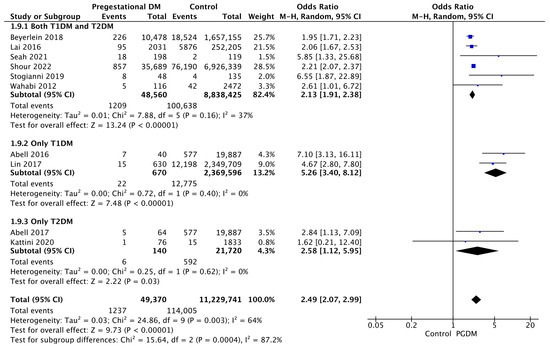
Figure 11.
Forest plot comparing the incidence of low 5-min Apgar score between PGDM and control groups [25,26,33,58,64,67,88,91,94,96].
3.3.10. Shoulder Dystocia
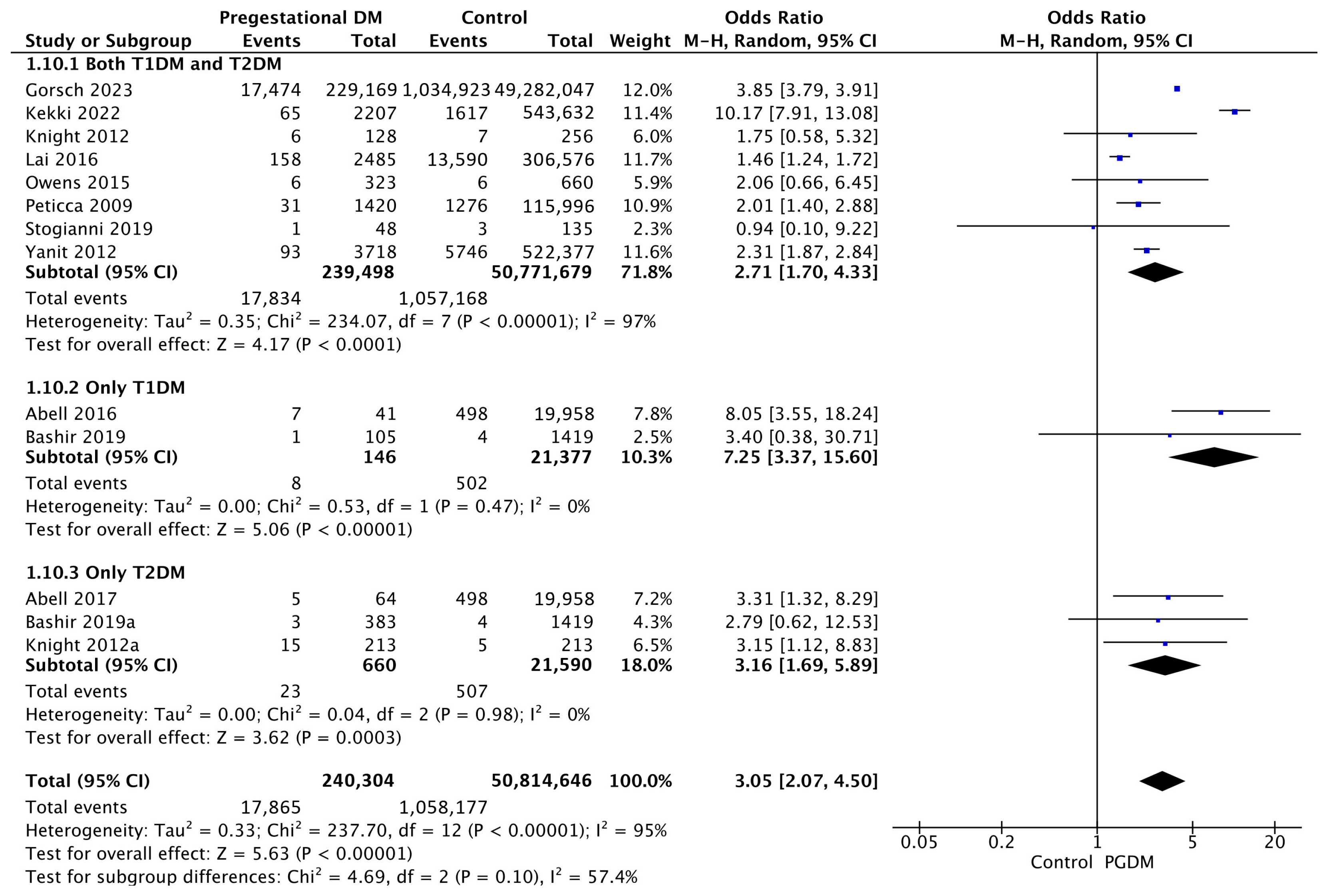
Thirteen studies reported data on shoulder dystocia, including 240,304 pregnant women with PGDM and 50,814,646 pregnant women without PGDM or GDM. In pregnancies with PGDM, the risk of shoulder dystocia was increased compared to control pregnancies (OR 3.05, 95% CI 2.07–4.50, p < 0.00001, I2 = 95%). The subgroup analysis, including studies reporting data only for one type of PGDM, did not show a statistically significant difference in the risk of shoulder dystocia between T1DM and T2DM (p = 0.10) (Figure 12).
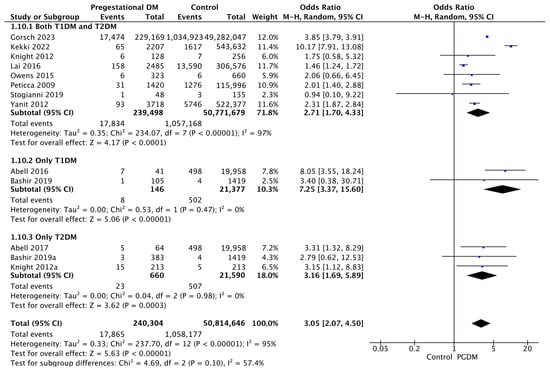
Figure 12.
Forest plot comparing the incidence of shoulder dystocia between PGDM and control groups [25,26,30,31,49,59,60,61,64,77,82,94,103].
3.3.11. Birth Trauma
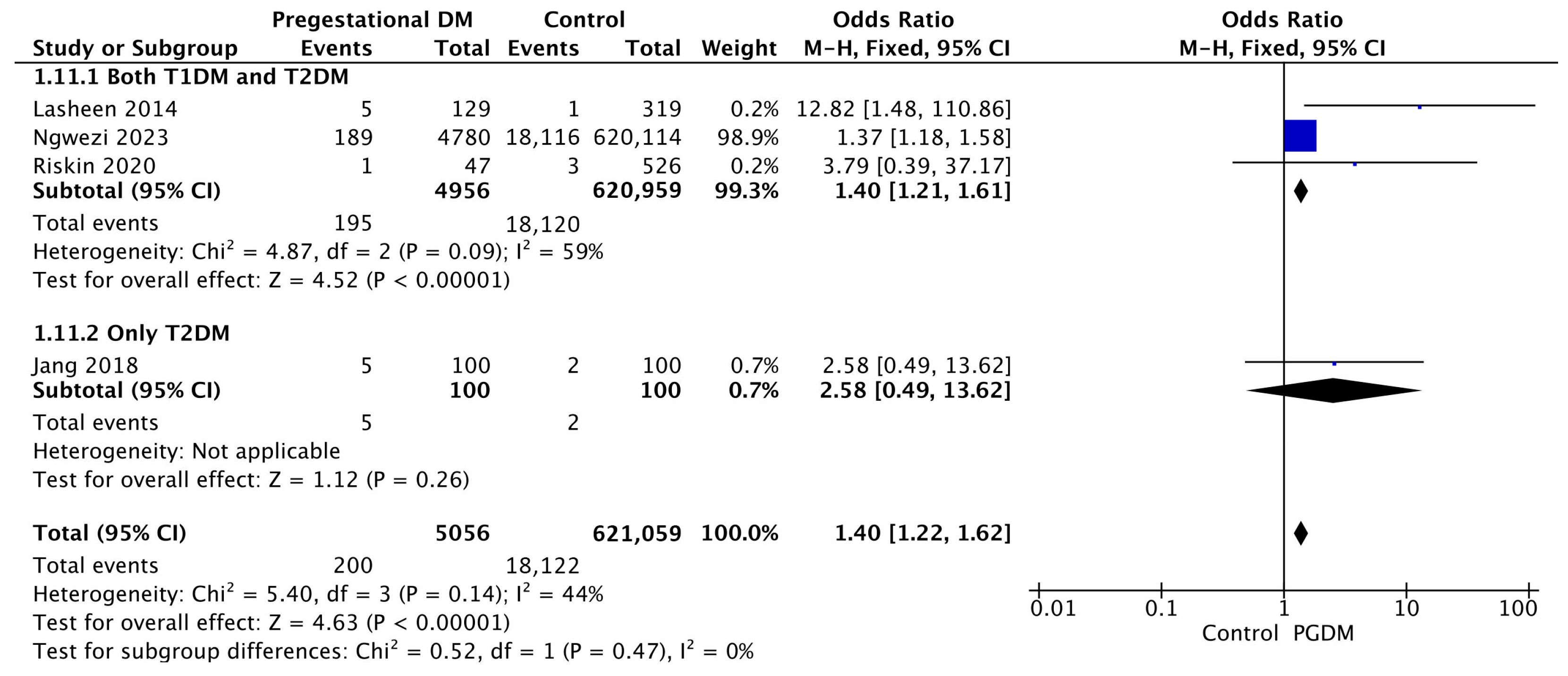
Four studies reported data on birth trauma, including 5056 pregnant women with PGDM and 621,059 pregnant women without PGDM or GDM. In pregnancies with PGDM, the risk of birth trauma was increased compared to control pregnancies (OR 1.40, 95% CI 1.22–1.62, p < 0.00001, I2 = 44%). No studies reported data only for T1DM to allow a subgroup analysis between T1DM and T2DM (Figure 13).
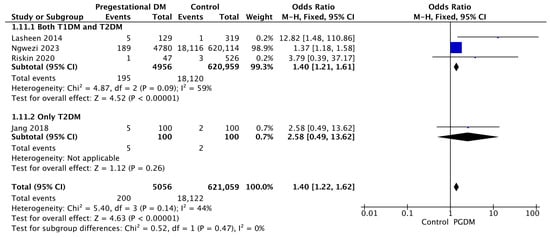
Figure 13.
Forest plot comparing the incidence of birth trauma between PGDM and control groups [55,65,76,86].
3.3.12. Polyhydramnios
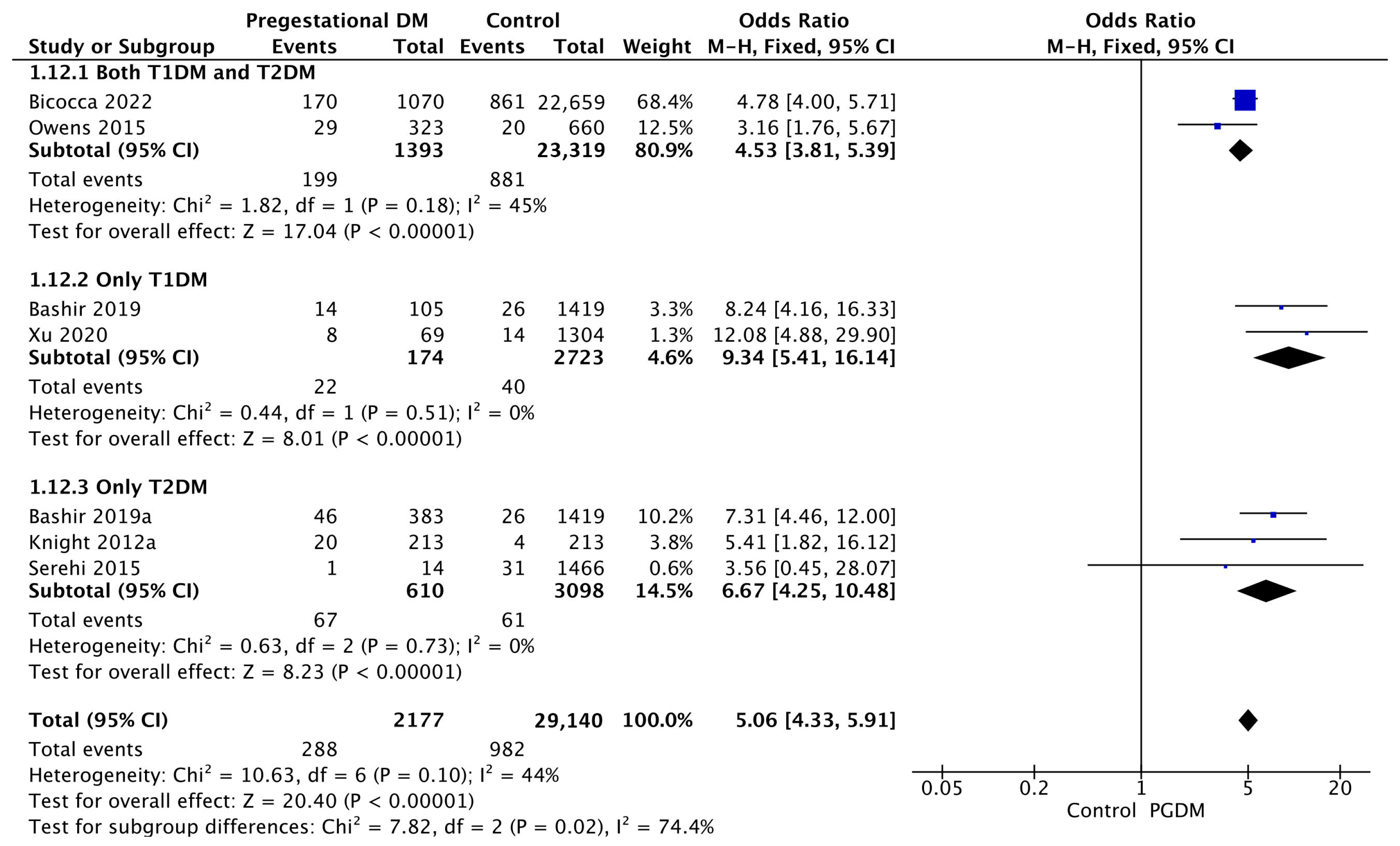
Seven studies reported data on polyhydramnios, including 2177 pregnant women with PGDM and 29,140 pregnant women without PGDM or GDM. In pregnancies with PGDM, the risk of polyhydramnios was increased compared to control pregnancies (OR 5.06, 95% CI 4.33–5.91, p < 0.00001, I2 = 44%). The subgroup analysis, including studies reporting data only for one type of PGDM, did not show a statistically significant difference in the risk of polyhydramnios between T1DM and T2DM (p = 0.35) (Figure 14).
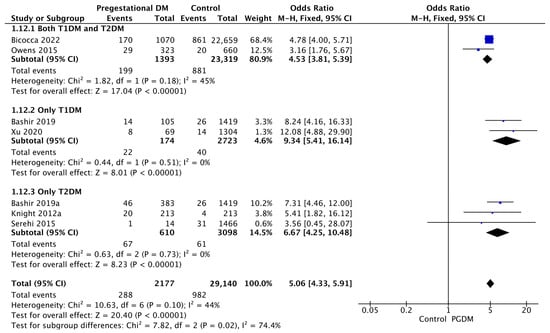
Figure 14.
Forest plot comparing the incidence of polyhydramnios between PGDM and control groups [30,31,34,61,77,89,101].
3.3.13. Oligohydramnios
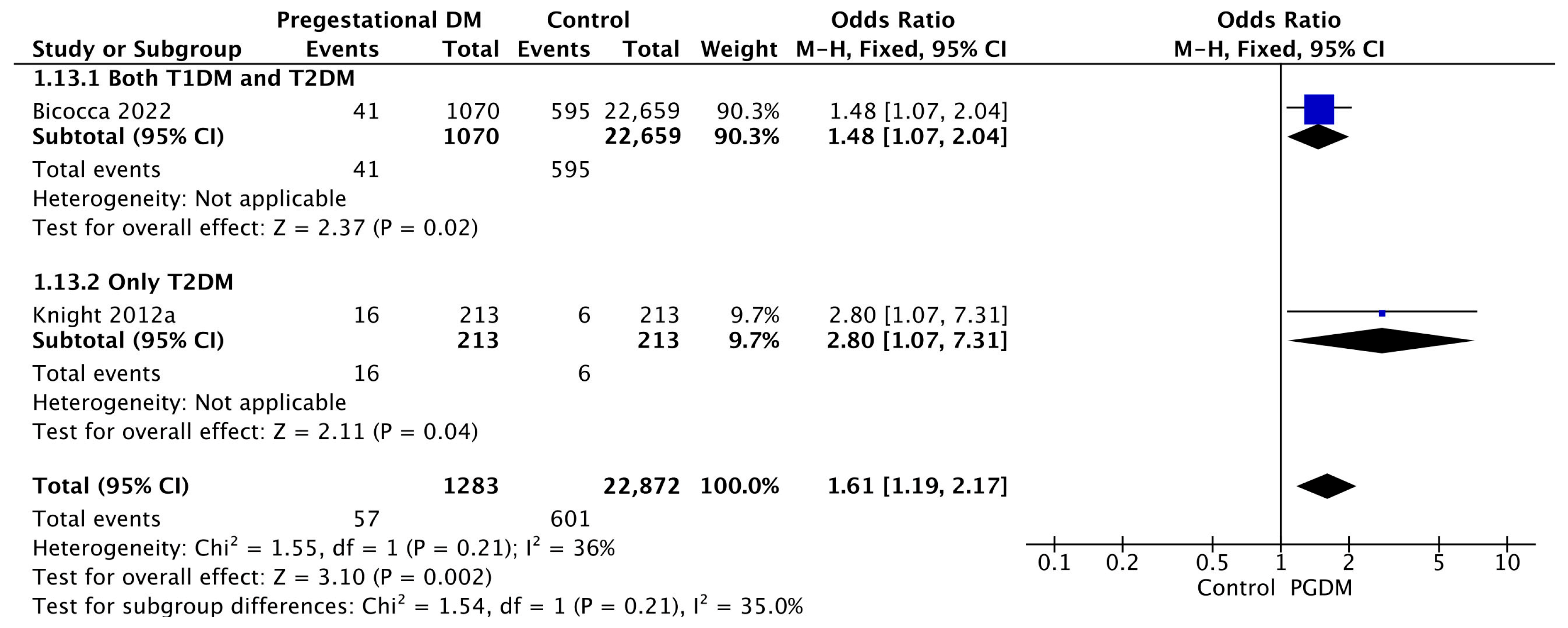
Two studies reported data on oligohydramnios, including 1283 pregnant women with PGDM and 22,872 pregnant women without PGDM or GDM. In pregnancies with PGDM, the risk of oligohydramnios was increased compared to control pregnancies (OR 1.61, 95% CI 1.19–2.17, p = 0.002, I2 = 36%). No studies reported data only for T1DM to allow a subgroup analysis between T1DM and T2DM (Figure 15).
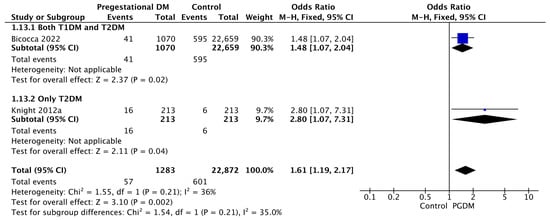
Figure 15.
Forest plot comparing the incidence of oligohydramnios between PGDM and control groups [34,61].
3.3.14. Neonatal Hyperbilirubinemia
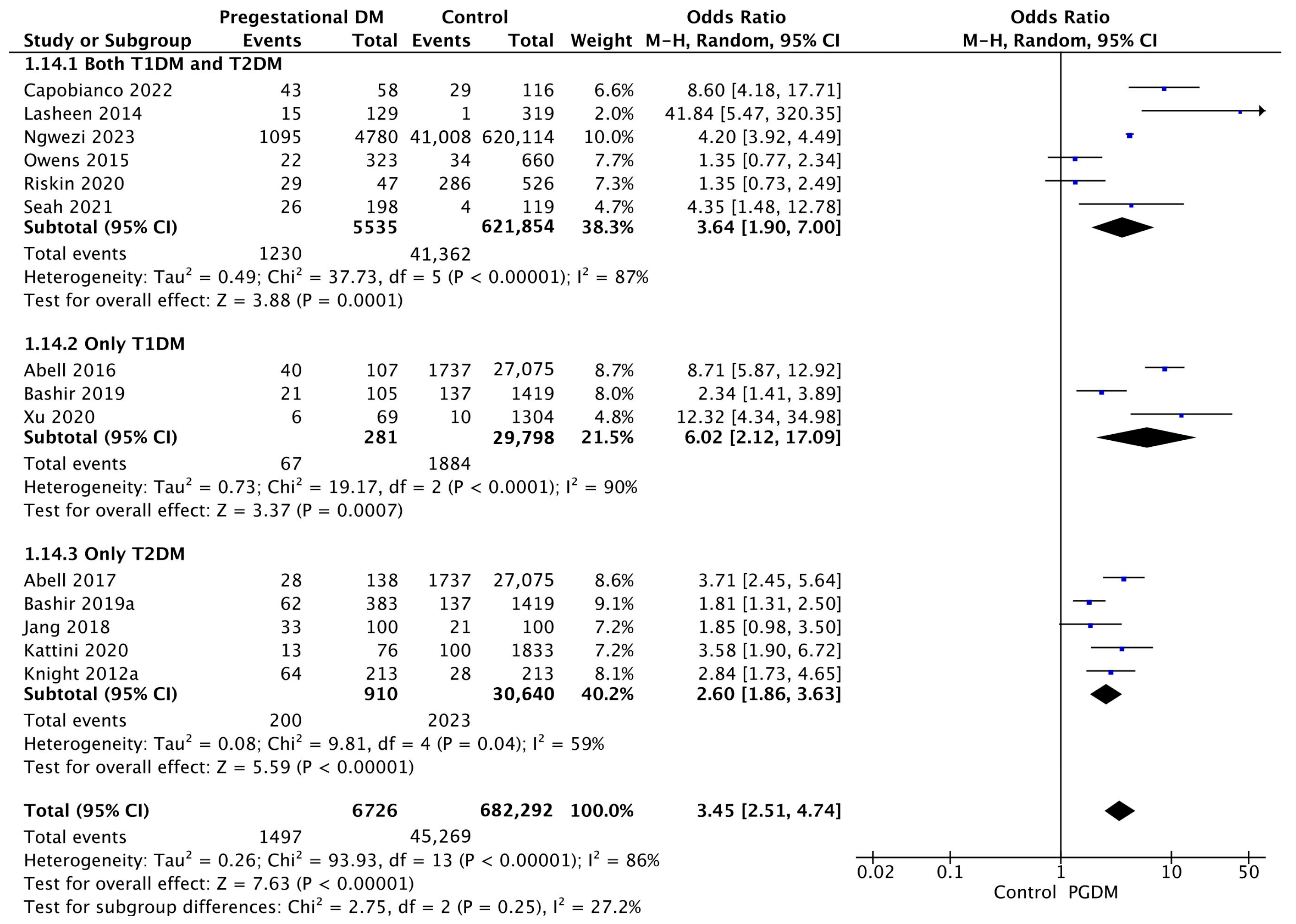
Fourteen studies reported data on neonatal hyperbilirubinemia, including 6726 pregnant women with PGDM and 682,292 pregnant women without PGDM or GDM. In pregnancies with PGDM, the risk of neonatal hyperbilirubinemia was increased compared to control pregnancies (OR 3.45, 95% CI 2.51–4.74, p < 0.00001, I2 = 86%). The subgroup analysis, including studies reporting data only for one type of PGDM, did not show a statistically significant difference in the risk of neonatal hyperbilirubinemia between T1DM and T2DM (p = 0.13) (Figure 16).
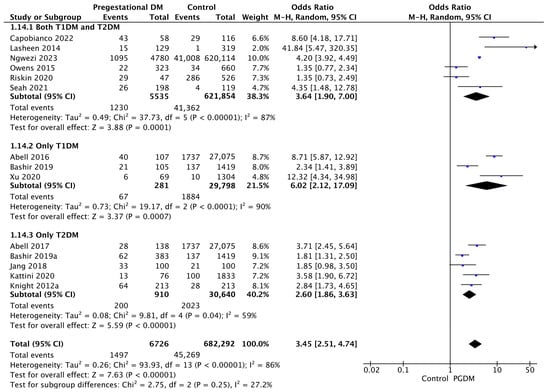
Figure 16.
Forest plot comparing the incidence of neonatal hyperbilirubinemia between PGDM and control groups [25,26,30,31,36,55,58,61,65,76,77,86,88,101].
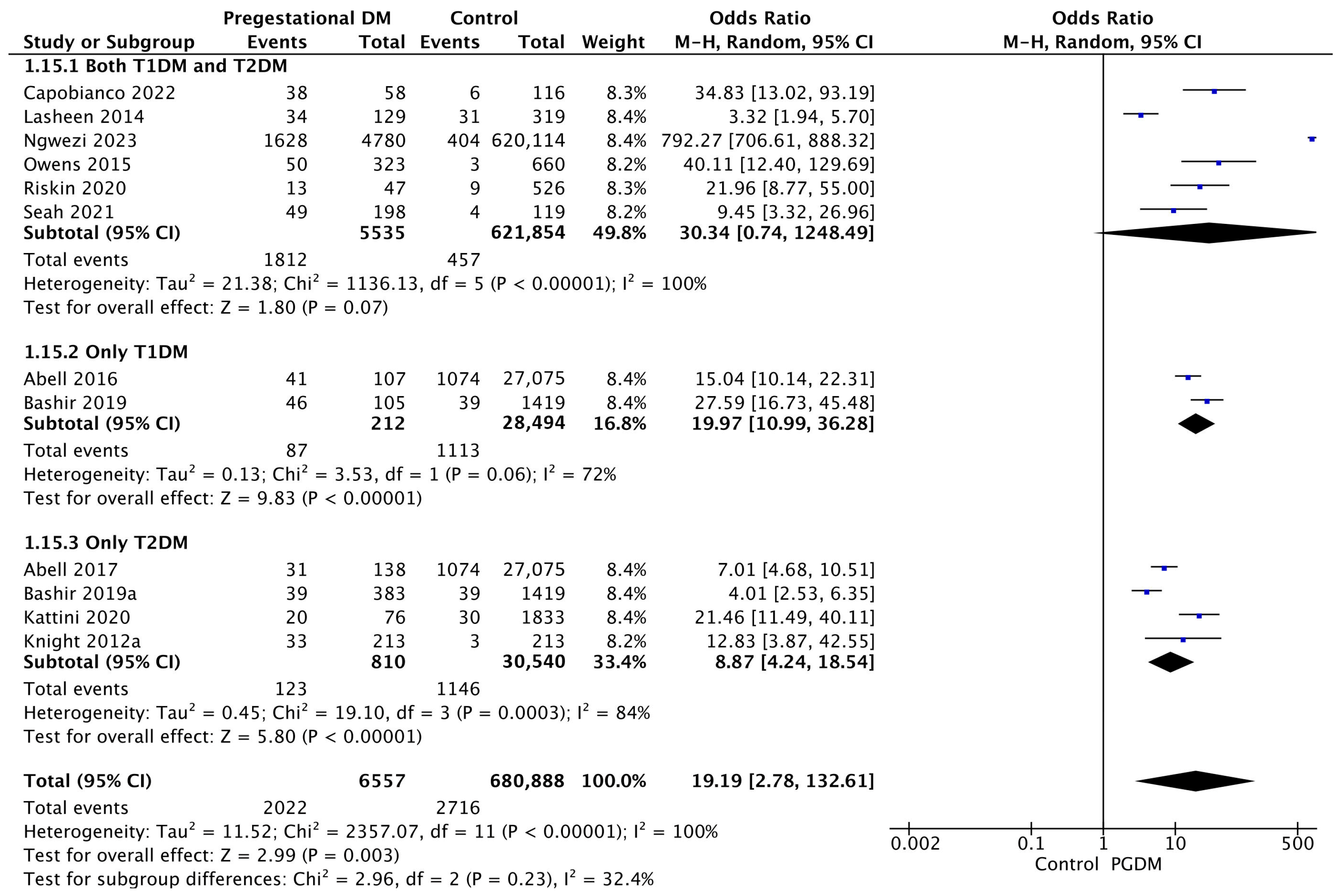
3.3.15. Neonatal Hypoglycemia
Twelve studies reported data on neonatal hypoglycemia, including 6557 pregnant women with PGDM and 680,888 pregnant women without PGDM or GDM. In pregnancies with PGDM, the risk of neonatal hypoglycemia was increased compared to control pregnancies (OR 19.19, 95% CI 2.78–132.61, p = 0.003, I2 = 100%). However, the wide CI and high heterogeneity among the studies indicate significant variability, warranting cautious interpretation of these results. The subgroup analysis, including studies reporting data only for one type of PGDM, showed a statistically significant difference in the risk of neonatal hypoglycemia between T1DM and T2DM, with a higher risk in the T1DM group (p = 0.09) (Figure 17).
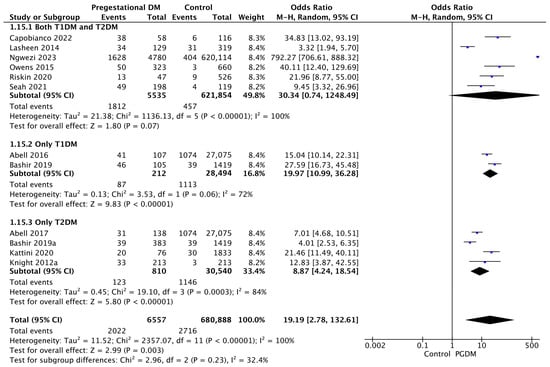
Figure 17.
Forest plot comparing the incidence of neonatal hypoglycemia between PGDM and control groups [25,26,30,31,36,58,61,65,76,77,86,88].
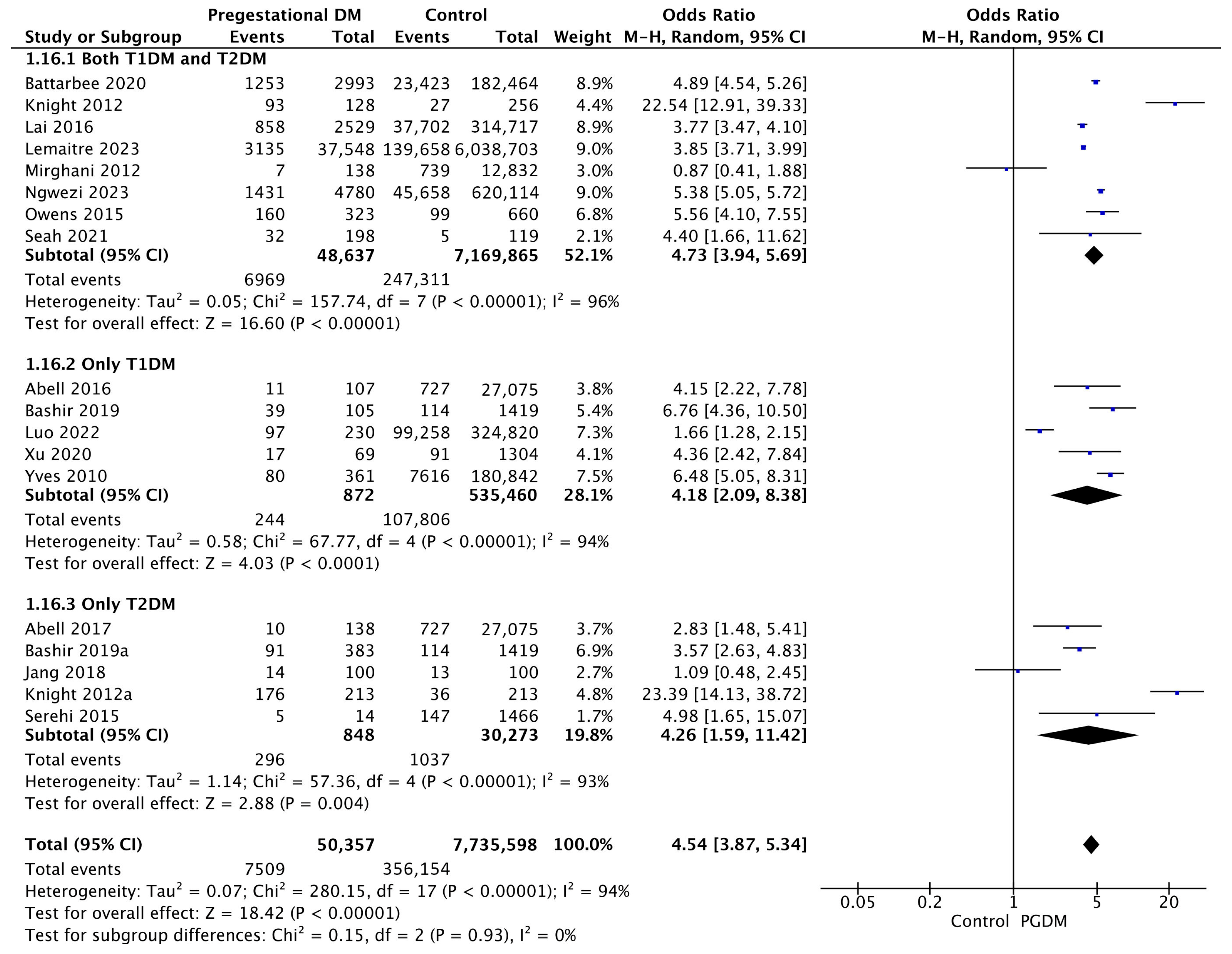
3.3.16. NICU Admission
Eighteen studies reported data on NICU admission, including 50,357 pregnant women with PGDM and 7,735,598 pregnant women without PGDM or GDM. In pregnancies with PGDM, the risk of NICU admission was increased compared to control pregnancies (OR 4.54, 95% CI 3.87–5.34, p < 0.00001, I2 = 94%). The subgroup analysis, including studies reporting data only for one type of PGDM, did not show a statistically significant difference in the risk of NICU admission between T1DM and T2DM (p = 0.98) (Figure 18).
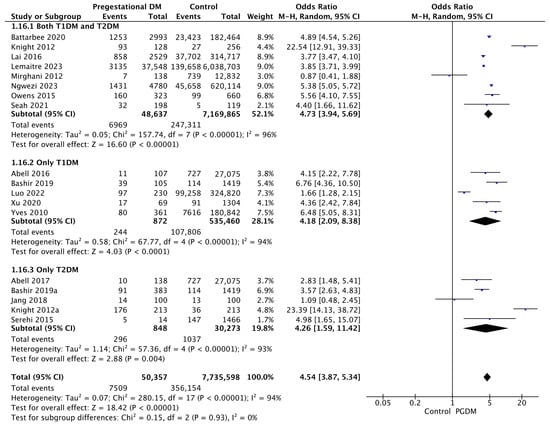
Figure 18.
Forest plot comparing the incidence of NICU admission between PGDM and control groups [25,26,30,31,32,55,60,61,64,66,72,74,76,77,88,89,101,104].
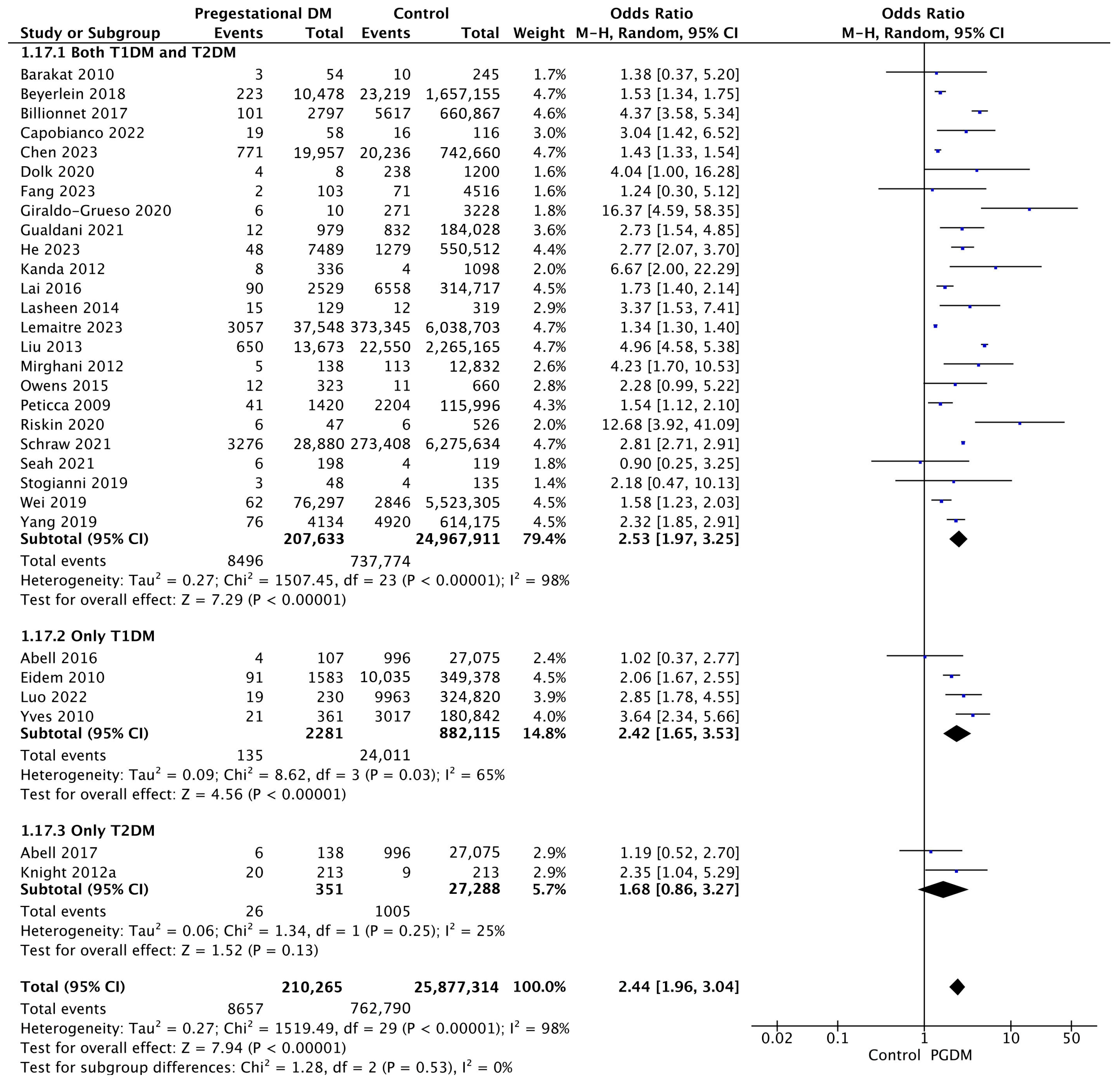
3.3.17. Congenital Malformations
Thirty studies reported data on congenital malformations, including 210,265 pregnant women with PGDM and 25,877,314 pregnant women without PGDM or GDM. In pregnancies with PGDM, the risk of congenital malformation was increased compared to control pregnancies (OR 2.44, 95% CI 1.96–3.04, p < 0.00001, I2 = 98%). The subgroup analysis, including studies reporting data only for one type of PGDM, did not show a statistically significant difference in the risk of congenital malformation between T1DM and T2DM (p = 0.35) (Figure 19).
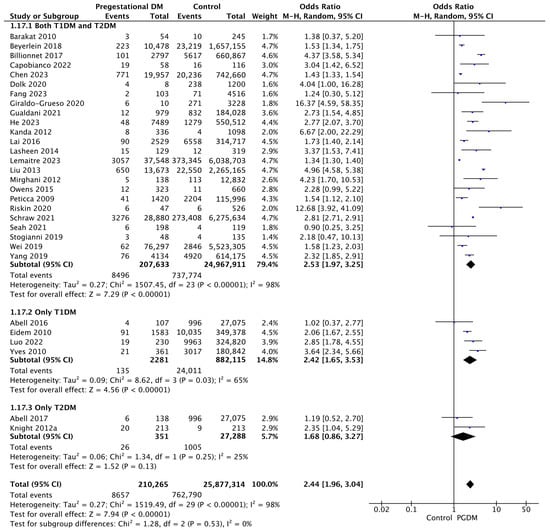
Figure 19.
Forest plot comparing the incidence of congenital malformations between PGDM and control groups [25,26,29,33,35,36,37,41,42,43,46,52,53,57,61,64,65,66,69,72,74,77,82,86,87,88,94,97,102,104].
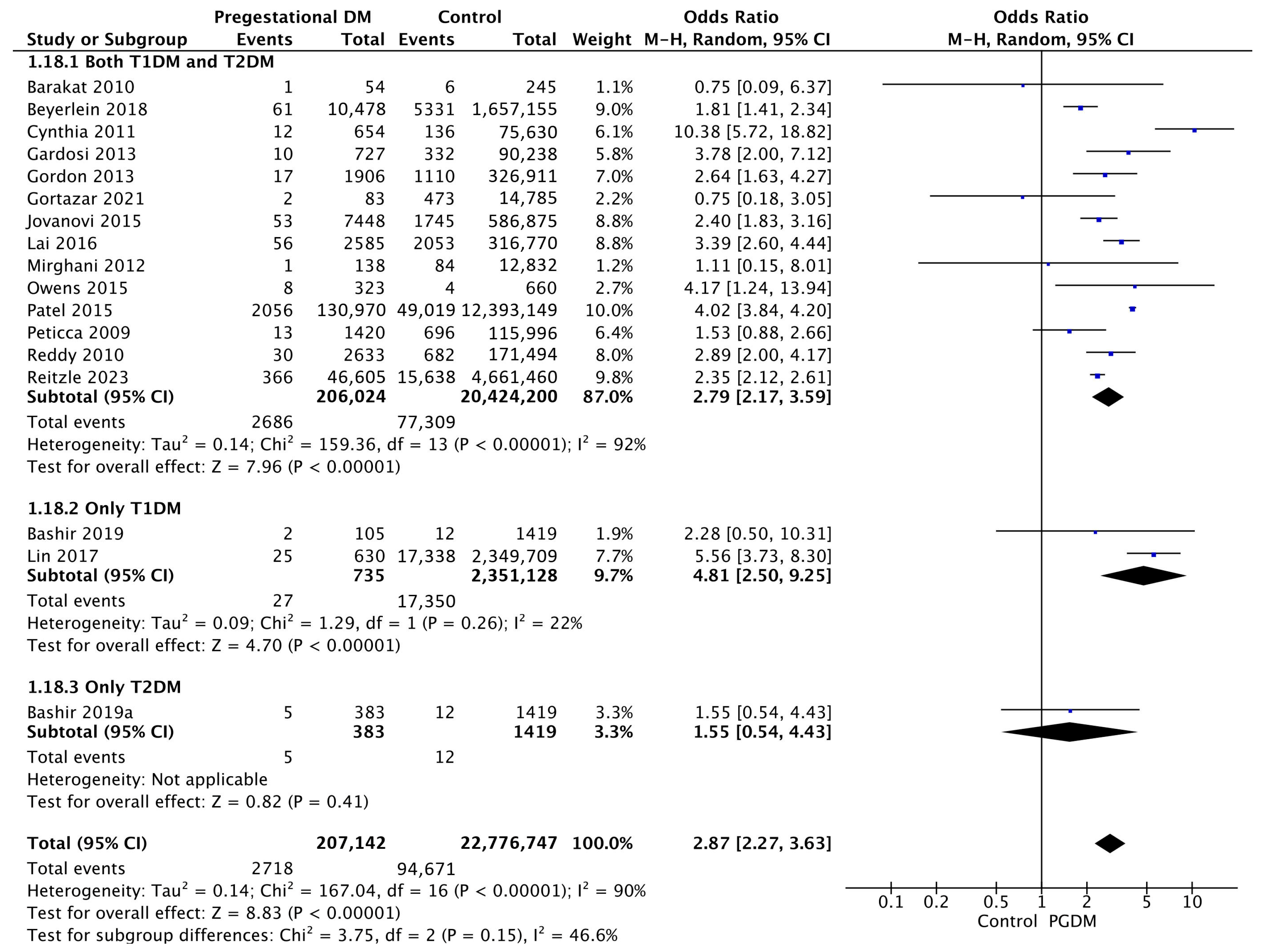
3.3.18. Stillbirth
Seventeen studies reported data on stillbirths, including 207,142 pregnant women with PGDM and 22,776,747 pregnant women without PGDM or GDM. In pregnancies with PGDM, the risk of stillbirth was increased compared to control pregnancies (OR 2.87, 95% CI 2.27–3.63, p < 0.00001, I2 = 90%). The subgroup analysis, including studies reporting data only for one type of PGDM, showed a statistically significant difference in the risk of stillbirth between T1DM and T2DM, with a higher risk in the T1DM group (p = 0.07) (Figure 20).
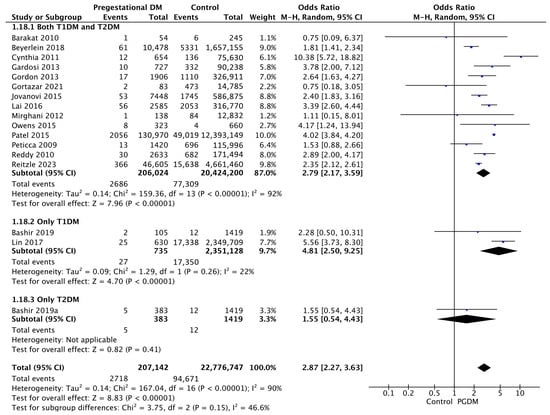
Figure 20.
Forest plot comparing the incidence of stillbirth between PGDM and control groups [29,30,31,33,38,45,48,51,56,64,67,74,77,80,82,84,85].
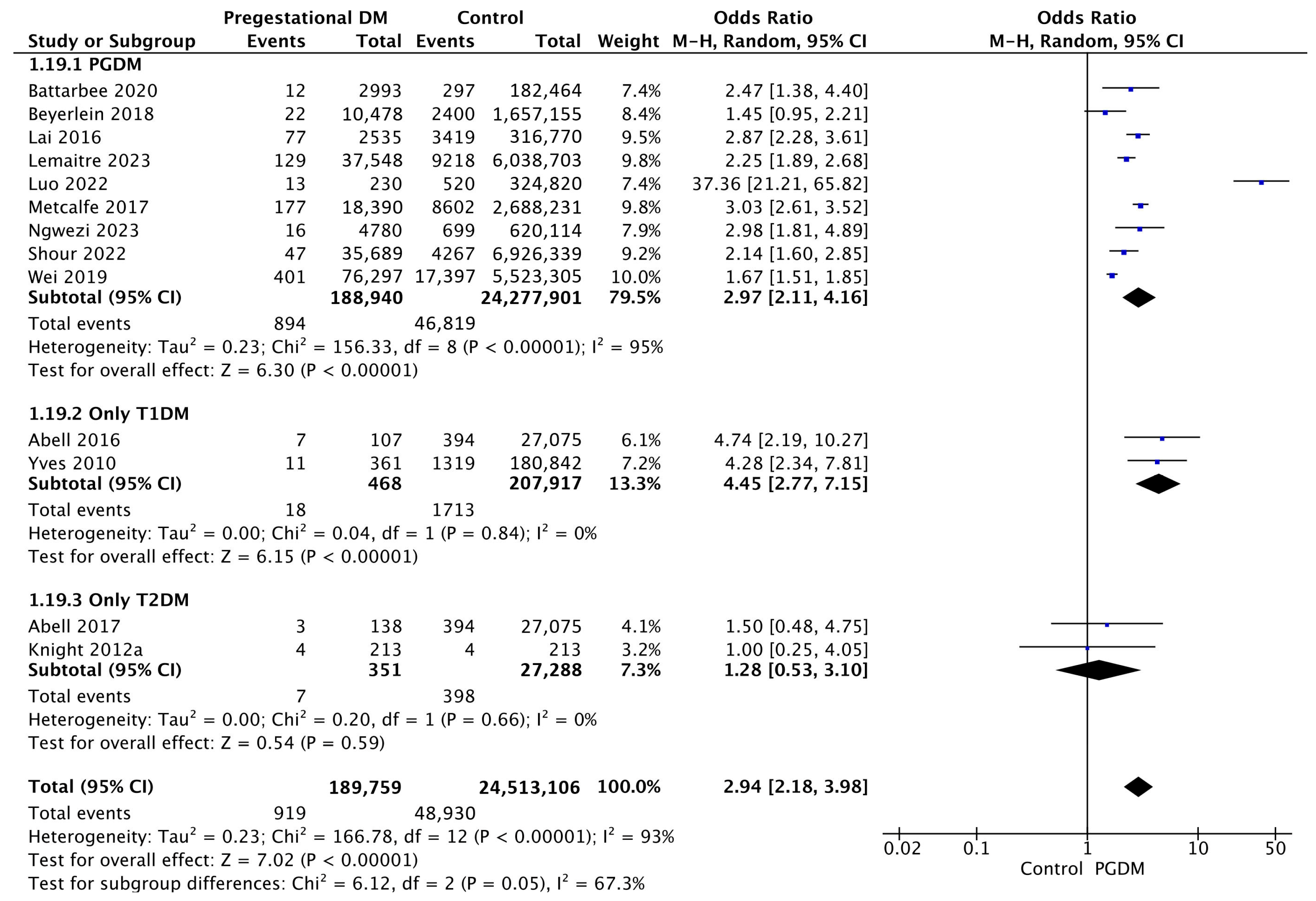
3.3.19. Perinatal Mortality
Thirteen studies reported data on perinatal mortality, including 189,759 pregnant women with PGDM and 24,513,106 pregnant women without PGDM or GDM. In pregnancies with PGDM, the risk of perinatal mortality was increased compared to control pregnancies (OR 2.94, 95% CI 2.18–3.98, p < 0.00001, I2 = 93%). The subgroup analysis, including studies reporting data only for one type of PGDM, showed a statistically significant difference in the risk of perinatal mortality between T1DM and T2DM, with a higher risk in the T1DM group (p = 0.02) (Figure 21).
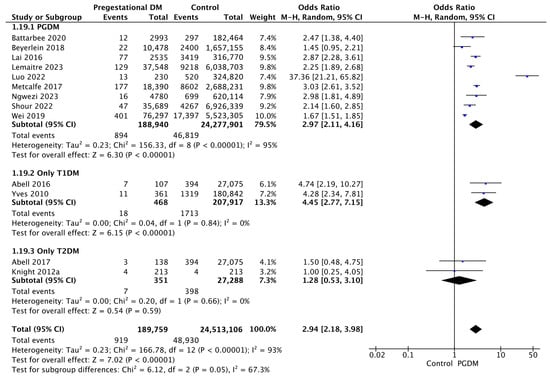
Figure 21.
Forest plot comparing the incidence of perinatal mortality between PGDM and control groups [25,26,32,33,61,64,66,72,73,76,91,97,104].
4. Discussion
4.1. Main Findings
The findings of the present systematic review and meta-analysis reveal that PGDM during pregnancy significantly increases the risk of various adverse perinatal outcomes compared to pregnancies without PGDM or GDM. The risk of each adverse outcome was quantified, allowing a comprehensive understanding of the impact of PGDM. More specifically, with regards to maternal adverse perinatal outcomes, the study demonstrated a significant positive correlation between PGDM and hypertensive disorders of pregnancy, including gestational hypertension and preeclampsia, as well as preterm delivery, cesarean delivery and induction of labor. Regarding fetal/neonatal outcomes, the study revealed that PGDM significantly increases the risk of macrosomia, LGA neonates, low 5-min Apgar score, shoulder dystocia, birth trauma, polyhydramnios, oligohydramnios, neonatal hyperbilirubinemia, neonatal hypoglycemia, NICU admission, congenital malformations, stillbirth and perinatal mortality. Notably, the risk of SGA neonates was found to be decreased in pregnancies with PGDM compared to pregnancies without PGDM or GDM. Subgroup analyses further showed that T1DM conferred a higher risk than T2DM for specific adverse outcomes, including neonatal hypoglycemia, stillbirth and perinatal mortality; for instance, perinatal mortality was quadrupled in T1DM pregnancies but did not increase significantly in those complicated by T2DM. Conversely, T1DM appeared to offer greater protection against SGA births in PGDM pregnancies compared to T2DM. For the remaining outcomes, based on the available data, no statistically significant differences emerged between T1DM and T2DM.
4.2. Comparison with Existing Literature
The findings of this study align with previous research, consistently demonstrating higher rates of adverse perinatal outcomes in pregnancies complicated by PGDM. Studies such as those by Abell et al. and Beyerlein et al. have similarly reported increased risks of adverse perinatal outcomes in pregnancies complicated by diabetes [25,33]. Moreover, studies by Schraw et al. and Lemaitre et al. highlighted the increased risk of congenital anomalies in neonates born to mothers with PGDM [66,87], while a study by Battarbee et al. found a particularly high risk of severe neonatal morbidity and mortality in pregnancies with PGDM [32]. The findings of our study are also in accord with reports by the International Diabetes Federation and the American Diabetes Association, both of which underscore the increased likelihood of poor pregnancy outcomes when PGDM is present [106,107].
To our knowledge, this study is the most comprehensive analysis to date examining the association between PGDM and adverse perinatal outcomes. Only one previous systematic review and meta-analysis by Yu et al., published in 2017, has addressed this topic [12]. It included 100 studies with data on around 40 million individuals and assessed 17 adverse outcomes. While its findings aligned with ours in reporting an increased risk of several adverse perinatal outcomes in pregnancies affected by PGDM, it did not identify an association between PGDM and SGA neonates. In contrast, our analysis demonstrated a decreased risk of SGA neonates in this group (OR 0.81, 95% CI 0.69–0.96). Furthermore, unlike our review, the earlier study did not assess several important outcomes evaluated in our study, such as congenital malformations (OR 2.44, 95% CI 1.96–3.04), induction of labor (OR 2.92, 95% CI 2.35–3.63), birth trauma (OR 1.40, 95% CI 1.22–1.62), polyhydramnios (OR 5.06, 95% CI 4.33–5.91) and oligohydramnios (OR 1.61, 95% CI 1.19–2.17). Additionally, the prior study did not conduct subgroup analyses by diabetes type, which in our review enabled direct comparisons between different forms of PGDM and their respective impacts on perinatal outcomes. Another key difference between the two studies is the sample size. Our study included a significantly larger sample size, incorporating data from over 137 million pregnancies, which enhances the statistical power and generalizability of our findings. We further strengthened our methodology by restricting inclusion to studies conducted from 1999 onwards, ensuring more uniform diagnostic criteria for PGDM and more consistent standards of pregnancy care across studies. These methodological choices position our review as the most current, robust and clinically relevant synthesis in the field.
4.3. Strengths and Limitations
This study has multiple strengths. It examines a broad spectrum of adverse perinatal outcomes across more than 137 million pregnancies, offering robust statistical power. Restricting the eligible study period to 1999 onwards ensured consistency in PGDM diagnostic criteria and allowed inclusion of more recent studies that reflect current care practices. Finally, by registering the protocol in a publicly accessible database before initiating the review, we promoted transparency and minimized bias in our methodology.
This study also has certain limitations. The estimation of risks of adverse perinatal outcomes based on pooled data for pregnancies with PGDM and control pregnancies is subject to the heterogeneity of the primary studies. The heterogeneity could be attributed to differences in study design, population demographics, methods of diagnosing PGDM and specialized pregnancy care for pregnant women with PGDM. The high level of heterogeneity noted for several adverse perinatal outcomes included in this study indicates significant methodological and clinical variation among the included studies, suggesting that the findings should be interpreted cautiously. Nevertheless, adopting a random effects model in the meta-analysis of the results may partially account for the within-study heterogeneity. Another limitation of this study is that there is no universal definition for some of the adverse perinatal outcomes studied (e.g., neonatal hypoglycemia). As a result, heterogeneous outcome definitions were used in the included studies. In addition, minor differences were observed in the offspring exclusion criteria among some studies; for instance, some excluded stillbirths or fetuses with chromosomal abnormalities from their populations, while others did not. Lastly, the restriction of eligibility to studies published only in English could be another limitation of this study. However, although there is a theoretical risk of excluding available evidence from this practice, empirical studies suggest that the impact of language bias on the findings of meta-analyses is likely negligible [108].
5. Conclusions
In conclusion, the present study contributes to a more comprehensive understanding of the strong correlation between PGDM and several adverse perinatal outcomes. The findings of this study underscore the necessity of early detection of PGDM in the preconception period and meticulous management of PGDM during pregnancy while facilitating evidence-based counseling for the affected population. It is well-established that achieving optimal glycemic control before pregnancy is crucial for mitigating the risks of PGDM during pregnancy. Future research should delve deeper into the pathophysiological mechanisms underlying PGDM-related complications and explore interventional strategies to determine the optimal timing and intensity of management. Moreover, large-scale, well-designed studies utilizing standardized outcome measures and controlling for potential confounding factors are required to quantify the risks of adverse perinatal outcomes more precisely.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jcm14134789/s1.
Author Contributions
Conceptualization, D.G., A.T., I.T. and T.D.; methodology, D.G., A.T. and I.T.; software, D.G. and A.T.; validation, A.A. and A.S.; formal analysis, D.G. and A.T.; investigation, D.G., A.T. and G.K.; resources, D.G., A.T. and G.K.; data curation, D.G., A.T. and G.K.; writing—original draft preparation, D.G. and A.T.; writing—review and editing, A.S., A.A., G.K., D.G.G., C.T., I.T. and T.D.; visualization, D.G.; supervision, D.G.G., C.T., I.T. and T.D.; project administration, I.T. and T.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Characteristics of included studies.
Table A1.
Characteristics of included studies.
| Study | Country | Study Design | Types of PGDM Included | PGDM Pregnancies/Controls | Adverse Perinatal Outcomes Studied | Risk of Bias Score (NOS) |
|---|---|---|---|---|---|---|
| Abell 2016 [25] | Australia | Retrospective cohort study | T1DM | 107/27,075 | GH, PE, PD, CD, IoL, LGA, SGA, Low Apgar score, SD, NHB, NHG, NICU admission, CM, Perinatal mortality | 9 |
| Abell 2017 [26] | Australia | Retrospective cohort study | T2DM | 138/27,075 | GH, PE, PD, CD, IoL, LGA, SGA, Low Apgar score, SD, NHB, NHG, NICU admission, CM, Perinatal mortality | 9 |
| Achkar 2015 [27] | Canada | Case-control study | Not specified | 14/2121 | PE | 6 |
| Anderson 2012 [28] | New Zealand | Retrospective cohort study | T1DM, T2DM | 349/18,622 | PE | 8 |
| Barakat 2010 [29] | Oman | Retrospective cohort study | T1DM, T2DM | 54/245 | PD, CD, Macrosomia, CM, Stillbirth | 8 |
| Bashir 2019 [30] | Qatar | Retrospective cohort study | T2DM | 383/1419 | GH, PE, PD, CD, IoL, Macrosomia, LGA, SGA, SD, PH, NHB, NHG, NICU admission, Stillbirth | 7 |
| Bashir 2019 [31] | Qatar | Retrospective cohort study | T1DM | 105/1419 | GH, PE, PD, CD, IoL, Macrosomia, LGA, SGA, SD, PH, NHB, NHG, NICU admission, Stillbirth | 7 |
| Battarbee 2020 [32] | USA | Retrospective cohort study | T1DM, T2DM | 2993/182,464 | CD, LGA, NICU admission, Perinatal mortality | 9 |
| Beyerlein 2018 [33] | Germany | Retrospective cohort study | Not specified | 10,478/1,657,155 | PD, LGA, Low Apgar score, CM, Stillbirth, Perinatal mortality | 9 |
| Bicocca 2022 [34] | USA | Retrospective cohort study | T1DM, T2DM | 1070/22,659 | PH, OH | 7 |
| Billionnet 2017 [35] | France | Retrospective cohort study | T1DM, T2DM | 3198/735,519 | PE, PD, CD, LGA, CM | 8 |
| Capobianco 2022 [36] | Italy | Case-control study | T1DM, T2DM, MODY | 58/116 | CD, NHB, NHG, CM | 7 |
| Chen 2023 [37] | Taiwan | Retrospective cohort study | T1DM, T2DM | 19,957/742,660 | CM | 8 |
| Cynthia 2011 [38] | Australia | Retrospective cohort study | Not specified | 654/75,630 | CD, Macrosomia, Stillbirth | 6 |
| Dalfrà 2011 [39] | Italy | Retrospective cohort study | T1DM | 32/17 | CD | 4 |
| Di Lorenzo 2012 [40] | Italy | Prospective cohort study | Not specified | 23/2095 | GH | 5 |
| Dolk 2020 [41] | UK | Case-control study | Not specified | 8/1200 | CM | 7 |
| Eidem 2010 [42] | Norway | Retrospective cohort study | T1DM | 1583/349,378 | CM | 8 |
| Fang 2023 [43] | Taiwan | Case-control study | Not specified | 103/4516 | CM | 7 |
| Foeller 2015 [44] | USA | Retrospective cohort study | T1DM, T2DM | 2137/258,857 | CD | 8 |
| Gardosi 2013 [45] | UK | Prospective cohort study | Not specified | 727/90,238 | Stillbirth | 9 |
| Giraldo-Grueso 2020 [46] | Colombia | Case-control study | Not specified | 10/3228 | CM | 5 |
| Goetzinger 2010 [47] | USA | Retrospective cohort study | Not specified | 94/3622 | PE | 6 |
| Gordon 2013 [48] | Australia | Prospective cohort study | Not specified | 1906/326,911 | Stillbirth | 8 |
| Gorsch 2023 [49] | USA | Retrospective cohort study | T1DM, T2DM | 610,429/71,470,686 | PD, CD, SD | 8 |
| Gortazar 2020 [50] | Spain | Retrospective cohort study | T1DM, T2DM, other PGDM types | 3882/704,148 | PE, PD, CD, Macrosomia, LGA, SGA | 8 |
| Gortazar 2021 [51] | Spain | Retrospective cohort study | T1DM, T2DM, other PGDM types | 83/14,785 | PE, PD, CD, LGA, SGA, Stillbirth | 8 |
| Gualdani 2021 [52] | Italy | Retrospective cohort study | T1DM, T2DM | 979/184,028 | PD, CD, Macrosomia, LGA, CM | 9 |
| He 2023 [53] | Canada | Retrospective cohort study | Not specified | 7489/550,512 | CM | 9 |
| Hunt 2012 [54] | USA | Retrospective cohort study | Not specified | 4767/198,853 | LGA, SGA | 9 |
| Jang 2018 [55] | Korea | Case-control study | T2DM | 100/100 | PE, PD, CD, IoL, Macrosomia, LGA, SGA, BT, NHB, NICU admission | 6 |
| Jovanovič 2015 [56] | USA | Retrospective cohort study | T1DM, T2DM | 11,261/773,751 | CD, Macrosomia, Stillbirth | 7 |
| Kanda 2012 [57] | Japan | Retrospective cohort study | T1DM, T2DM | 336/1098 | PE, PD, CD, LGA, SGA, CM | 8 |
| Kattini 2020 [58] | Canada | Retrospective cohort study | T2DM | 76/1833 | CD, IoL, Macrosomia, Low Apgar score, NHB, NHG | 7 |
| Kekki 2022 [59] | Finland | Retrospective cohort study | T1DM, T2DM | 2207/543,632 | IoL, LGA, SD | 8 |
| Knight 2012 [60] | USA | Retrospective cohort study | T1DM, T2DM | 128/256 | GH, PE, PD, CD, IoL, LGA, SD, NICU admission | 5 |
| Knight 2012 [61] | USA | Retrospective cohort study | T2DM | 213/213 | GH, PE, PD, CD, IoL, LGA, SGA, SD, PH, OH, NHB, NHG, NICU admission, CM, Perinatal mortality | 9 |
| Kohn 2019 [62] | USA | Retrospective cohort study | Not specified | 639/32,863 | PE, PD, CD, Macrosomia | 8 |
| Kuc 2011 [63] | Netherlands | Case-control study | Not specified | 178/186 | LGA | 6 |
| Lai 2016 [64] | Canada | Retrospective cohort study | T1DM, T2DM | 2535/311,673 | PE, PD, CD, Macrosomia, LGA, SGA, Low Apgar score, SD, NICU admission, CM, Stillbirth, Perinatal mortality | 8 |
| Lasheen 2014 [65] | Saudi Arabia | Prospective cohort study | Not specified | 129/319 | BT, NHB, NHG, CM | 6 |
| Lemaitre 2023 [66] | France | Retrospective cohort study | T1DM, T2DM | 37,548/6,038,703 | PE, PD, CD, LGA, SGA, NICU admission, CM, Perinatal mortality | 8 |
| Lin 2017 [67] | Taiwan | Retrospective cohort study | T1DM | 630/2,349,709 | GH, PE, PD, CD, LGA, SGA, Low Apgar score, Stillbirth | 8 |
| Lindsay 2003 [68] | UK | Case-control study | T1DM | 140/49 | CD | 6 |
| Liu 2013 [69] | Canada | Retrospective cohort study | T1DM, T2DM | 13,673/2,265,165 | CM | 8 |
| Lopez-de-Andres 2020 [70] | Spain | Retrospective cohort study | T1DM, T2DM | 9952/2,340,547 | GH, PE, PD, CD, IoL | 8 |
| Loukovaara 2004 [71] | Finland | Retrospective cohort study | T1DM | 67/62 | CD, LGA | 6 |
| Luo 2022 [72] | China | Retrospective cohort study | T1DM | 265/318,486 | PE, PD, CD, Macrosomia, SGA, NICU admission, CM, Perinatal mortality | 7 |
| Metcalfe 2017 [73] | Canada | Retrospective cohort study | T1DM, T2DM | 18,390/2,688,231 | GH, PE, PD, CD, IoL, Perinatal mortality | 8 |
| Mirghani 2012 [74] | UAE | Prospective cohort study | T1DM, T2DM | 138/12,832 | PD, CD, NICU admission, CM, Stillbirth | 5 |
| Morgan 2013 [75] | UK | Retrospective cohort study | T1DM, T2DM | 1250/144,530 | PD, LGA, SGA | 8 |
| Ngwezi 2023 [76] | Canada | Retrospective cohort study | T1DM, T2DM | 4780/620,114 | GH, PE, PD, CD, IoL, Macrosomia, LGA, SGA, BT, NHB, NHG, NICU admission, Perinatal mortality | 8 |
| Owens 2015 [77] | Ireland | Case-control study | T1DM, T2DM | 323/660 | GH, PE, PD, CD, LGA, SGA, SD, PH, NHB, NHG, NICU admission, CM, Stillbirth | 6 |
| Papageorghiou 2005 [78] | UK | Prospective cohort study | Not specified | 145/16,661 | PE | 6 |
| Paré 2014 [79] | USA | Prospective cohort study | Not specified | 57/2580 | PE | 8 |
| Patel 2015 [80] | USA | Case-control study | Not specified | 130,970/12,393,149 | Stillbirth | 8 |
| Pereda 2020 [81] | Uruguay | Retrospective cohort study | Not specified | 304/33,107 | Macrosomia | 9 |
| Peticca 2009 [82] | Canada | Retrospective cohort study | T1DM, T2DM | 1420/115,996 | PD, CD, IoL, Macrosomia, SD, CM, Stillbirth | 8 |
| Praprotnik 2021 [83] | Croatia | Retrospective cohort study | T1DM | 70/70 | Macrosomia, LGA, SGA | 5 |
| Reddy 2010 [84] | USA | Retrospective cohort study | Not specified | 2633/172,176 | Stillbirth | 8 |
| Reitzle 2023 [85] | Germany | Retrospective cohort study | Not specified | 46,605/4,661,460 | PD, CD, LGA, Stillbirth | 7 |
| Riskin 2020 [86] | Israel | Case-control study | Not specified | 47/526 | PE, PD, CD, LGA, SGA, BT, NHB, NHG, CM | 6 |
| Schraw 2021 [87] | USA | Retrospective cohort study | Not specified | 28,880/6,275,634 | CM | 9 |
| Seah 2021 [88] | Australia | Retrospective cohort study | T1DM, T2DM | 198/119 | GH, PE, PD, LGA, SGA, Low Apgar score, NHB, NHG, NICU admission, CM | 7 |
| Serehi 2015 [89] | Saudi Arabia | Prospective cohort study | T2DM | 14/1466 | PD, CD, IoL, PH, NICU admission | 7 |
| Shefali 2006 [90] | India | Prospective cohort study | T1DM, T2DM | 79/30 | PD | 7 |
| Shour 2022 [91] | USA | Retrospective cohort study | Not specified | 35,689/6,926,339 | CD, Low Apgar score, Perinatal mortality | 9 |
| Son 2015 [92] | Korea | Retrospective cohort study | Not specified | 32,207/1,171,575 | GH, PE, PD, CD, Macrosomia | 8 |
| Stanton 2005 [93] | USA | Retrospective cohort study | Not specified | 73/73 | PD, Macrosomia | 5 |
| Stogianni 2019 [94] | Sweden | Retrospective cohort study | T1DM, T2DM | 48/135 | PE, PD, CD, Macrosomia, LGA, Low Apgar score, SD, CM | 8 |
| Titmuss 2023 [95] | Australia | Prospective cohort study | T2DM | 78/123 | PD, CD | 8 |
| Wahabi 2012 [96] | Saudi Arabia | Retrospective cohort study | T1DM, T2DM | 116/2472 | PD, CD, Macrosomia, Low Apgar score | 8 |
| Wei 2019 [97] | China | Retrospective cohort study | Not specified | 76,297/5,523,305 | PD, Macrosomia, CM, Perinatal mortality | 8 |
| Wells 2015 [98] | Australia | Retrospective cohort study | T2DM | 18/1282 | PD, LGA, SGA | 6 |
| Wright 2012 [99] | UK | Prospective cohort study | T1DM, T2DM | 411/58,473 | PE | 8 |
| Xu 2014 [100] | Australia | Retrospective cohort study | Not specified | 2447/372,954 | PD | 8 |
| Xu 2020 [101] | China | Retrospective cohort study | T1DM | 69/1304 | PE, PD, CD, Macrosomia, LGA, SGA, PH, NHB, NICU admission | 8 |
| Yang 2019 [102] | USA | Retrospective cohort study | Not specified | 4134/614,175 | GH, Macrosomia, CM | 9 |
| Yanit 2012 [103] | USA | Retrospective cohort study | Not specified | 3718/522,377 | PE, PD, LGA, SGA, SD | 8 |
| Yves 2010 [104] | Belgium | Retrospective cohort study | T1DM | 354/177,407 | PD, CD, NICU admission, CM, Perinatal mortality | 7 |
| Zeki 2018 [105] | Australia | Retrospective cohort study | T1DM, T2DM | 5977/938,581 | CD | 8 |
Abbreviations: T1DM = type 1 diabetes mellitus, T2DM = type 2 diabetes mellitus, GH = gestational hypertension, PE = preeclampsia, PD = preterm delivery, CD = cesarean delivery, IoL = induction of labor, LGA = large for gestational age, SGA = small for gestational age, SD = shoulder dystocia, BT = birth trauma, PH = polyhydramnios, OH = oligohydramnios, NHB = neonatal hyperbilirubinemia, NHG = neonatal hypoglycemia, NICU = neonatal intensive care unit, CM = congenital malformation.
Appendix A.1. MEDLINE/PubMed Search Strategy
- MEDLINE/Pubmed search syntax (Advanced search)
- #1: “pregnancy” [All Fields]
- #2: “pregnant” [All Fields]
- #3: #1 OR #2
- #4: “diabetes” [All Fields]
- #5: #3 AND #4
- #6: “pregestational” [All Fields]
- #7: “pre-gestational” [All Fields]
- #8: “preexisting” [All Fields]
- #9: “pre-existing” [All Fields]
- #10: “type 1 diabetes” [All Fields]
- #11: “diabetes type 1” [All Fields]
- #12: type 1 diabetes mellitus [MesH Terms]
- #13: “type 2 diabetes” [All Fields]
- #14: “diabetes type 2” [All Fields]
- #15: type 2 diabetes mellitus [MesH Terms]
- #16: #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15
- #17: #5 AND #16
- Publication date: 1999 onwards
- MEDLINE/PubMed search string
- (((“pregnancy”) OR (“pregnant”)) AND (“diabetes”)) AND ((((((((((“pregestational”) OR (“pre-gestational”)) OR (“preexisting”)) OR (“pre-existing”)) OR (“type 1 diabetes”)) OR (“diabetes type 1”)) OR (type 1 diabetes mellitus [MeSH Terms])) OR (“type 2 diabetes”)) OR (“diabetes type 2”)) OR (type 2 diabetes mellitus [MeSH Terms]))
- Publication date: 1999 onwards
Appendix A.2. Scopus Search Strategy
- Scopus search syntax (Advanced search)
- #1: “pregnancy”
- #2: “pregnant”
- #3: #1 OR #2
- #4: “diabetes” [All Fields]
- #5: #3 AND #4
- #6: “pregestational”
- #7: “pre-gestational”
- #8: “preexisting”
- #9: “pre-existing”
- #10: “type 1 diabetes”
- #11: “diabetes type 1”
- #12: “diabetes mellitus, type 1”
- #13: “type 2 diabetes”
- #14: “diabetes type 2”
- #15: “diabetes mellitus, type 2”
- #16: #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15
- #17: #5 AND #16
- Limited to Subject Area: Medicine
- Limited to English
- Publication date: 1999 onwards
- Scopus search string
- TITLE-ABS-KEY ((“pregnancy” OR “pregnant”) AND “diabetes” AND (“pregestational” OR “pre-gestational” OR “preexisting” OR “pre-existing” OR “type 1 diabetes” OR “diabetes type 1” OR “diabetes mellitus, type 1” OR “type 2 diabetes” OR “diabetes type 2” OR “diabetes mellitus, type 2”)) AND PUBYEAR > 1998 AND PUBYEAR < 2024 AND (LIMIT-TO (SUBJAREA, “MEDI”)) AND (LIMIT-TO (LANGUAGE, “English”))
Appendix A.3. Cochrane Library Search Strategy
- Cochrane Library search syntax
- #1: “pregnancy”
- #2: “pregnant”
- #3: #1 OR #2
- #4: “diabetes”
- #5: #3 AND #4
- #6: “pregestational”
- #7: “pre-gestational”
- #8: “preexisting”
- #9: “pre-existing”
- #10: “type 1 diabetes”
- #11: “diabetes type 1”
- #12: MeSH descriptor: [Diabetes Mellitus, Type 1] explode all trees
- #13: “type 2 diabetes”
- #14: “diabetes type 2”
- #15: MeSH descriptor: [Diabetes Mellitus, Type 2] explode all trees
- #16: #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15
- #17: #5 AND #16
- Publication date: 1999 onwards
References
- World Health Organisation. Global Report on Diabetes; World Health Organisation: Lyon, France, 2016.
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Ogrotis, I.; Koufakis, T.; Kotsa, K. Changes in the Global Epidemiology of Type 1 Diabetes in an Evolving Landscape of Environmental Factors: Causes, Challenges, and Opportunities. Medicina 2023, 59, 668. [Google Scholar] [CrossRef]
- Basu, S.; Yoffe, P.; Hills, N.; Lustig, R.H.; Wagner, B. The Relationship of Sugar to Population-Level Diabetes Prevalence: An Econometric Analysis of Repeated Cross-Sectional Data. PLoS ONE 2013, 8, e57873. [Google Scholar] [CrossRef] [PubMed]
- HAPO Study Cooperative Research Group; Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; et al. Hyperglycemia and Adverse Pregnancy Outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef]
- Sibai, B.M.; Caritis, S.N.; Hauth, J.C.; MacPherson, C.; VanDorsten, J.P.; Klebanoff, M.; Landon, M.; Paul, R.H.; Meis, P.J.; Miodovnik, M.; et al. Preterm delivery in women with pregestational diabetes mellitus or chronic hypertension relative to women with uncomplicated pregnancies. Am. J. Obstet. Gynecol. 2000, 183, 1520–1524. [Google Scholar] [CrossRef]
- Holmes, V.A.; Young, I.S.; Patterson, C.C.; Pearson, D.W.; Walker, J.D.; Maresh, M.J.; McCance, D.R.; For the Diabetes and Pre-eclampsia Intervention Trial Study Group. Optimal Glycemic Control, Pre-eclampsia, and Gestational Hypertension in Women with Type 1 Diabetes in the Diabetes and Pre-eclampsia Intervention Trial. Diabetes Care 2011, 34, 1683–1688. [Google Scholar] [CrossRef] [PubMed]
- Macintosh, M.C.M.; Fleming, K.M.; Bailey, J.A.; Doyle, P.; Modder, J.; Acolet, D.; Golightly, S.; Miller, A. Perinatal mortality and congenital anomalies in babies of women with type 1 or type 2 diabetes in England, Wales, and Northern Ireland: Population based study. BMJ 2006, 333, 177. [Google Scholar] [CrossRef]
- Al-Agha, R.; Firth, R.G.; Byrne, M.; Murray, S.; Daly, S.; Foley, M.; Smith, S.C.; Kinsley, B.T. Outcome of pregnancy in type 1 diabetes mellitus (T1DMP): Results from combined diabetes–obstetrical clinics in Dublin in three university teaching hospitals (1995–2006). Ir. J. Med. Sci. 2012, 181, 105–109. [Google Scholar] [CrossRef]
- Persson, M.; Norman, M.; Hanson, U. Obstetric and Perinatal Outcomes in Type 1 Diabetic Pregnancies. Diabetes Care 2009, 32, 2005–2009. [Google Scholar] [CrossRef]
- Mitanchez, D.; Yzydorczyk, C.; Siddeek, B.; Boubred, F.; Benahmed, M.; Simeoni, U. The offspring of the diabetic mother—Short- and long-term implications. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 256–269. [Google Scholar] [CrossRef]
- Yu, L.; Zeng, X.-L.; Cheng, M.-L.; Yang, G.-Z.; Wang, B.; Xiao, Z.-W.; Luo, X.; Zhang, B.-F.; Xiao, D.-W.; Zhang, S.; et al. Quantitative assessment of the effect of pre-gestational diabetes and risk of adverse maternal, perinatal and neonatal outcomes. Oncotarget 2017, 8, 61048–61056. [Google Scholar] [CrossRef] [PubMed]
- Greco, E.; Calanducci, M.; Nicolaides, K.H.; Barry, E.V.; Huda, M.S.; Iliodromiti, S. Gestational diabetes mellitus and adverse maternal and perinatal outcomes in twin and singleton pregnancies: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2023, 230, 213–225. [Google Scholar] [CrossRef]
- Ye, W.; Luo, C.; Huang, J.; Li, C.; Liu, Z.; Liu, F. Gestational diabetes mellitus and adverse pregnancy outcomes: Systematic review and meta-analysis. BMJ 2022, 377, e067946. [Google Scholar] [CrossRef] [PubMed]
- Mistry, S.K.; Das Gupta, R.; Alam, S.; Kaur, K.; Shamim, A.A.; Puthussery, S. Gestational diabetes mellitus (GDM) and adverse pregnancy outcome in South Asia: A systematic review. Endocrinol. Diabetes Metab. 2021, 4, e00285. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus; World Health Organization: Geneva, Switzerland, 1999.
- Acog Practice Bulletin. Gestational Hypertension and Preeclampsia. Obstet. Gynecol. 2020, 135, e237–e260. [Google Scholar] [CrossRef]
- World Health Organization. Preterm Birth. 2018. Available online: https://www.who.int/en/news-room/fact-sheets/detail/preterm-birth (accessed on 10 October 2023).
- ACOG Committee on Practice Bulletins—Obstetrics. Macrosomia: ACOG Practice Bulletin, Number 216. Obstet. Gynecol. 2020, 135, e18–e35. [Google Scholar] [CrossRef]
- Battaglia, F.C.; Lubchenco, L.O. A practical classification of newborn infants by weight and gestational age. J. Pediatr. 1967, 71, 159–163. [Google Scholar] [CrossRef]
- Committee on Obstetric Practice American Academy of Pediatrics—Committee on Fetus and Newborn. Committee Opinion No. 644. Obstet. Gynecol. 2015, 126, e52–e55. [Google Scholar] [CrossRef]
- Tsakiridis, I.; Giouleka, S.; Mamopoulos, A.; Athanasiadis, A.; Dagklis, T. Investigation and management of stillbirth: A descriptive review of major guidelines. J. Perinat. Med. 2022, 50, 796–813. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 10 October 2023).
- Abell, S.K.; A Boyle, J.; Courten, B.; Knight, M.; Ranasinha, S.; Regan, J.; Soldatos, G.; Wallace, E.M.; Zoungas, S.; Teede, H.J. Contemporary type 1 diabetes pregnancy outcomes: Impact of obesity and glycaemic control. Med. J. Aust. 2016, 205, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Abell, S.K.; Boyle, J.A.; de Courten, B.; Soldatos, G.; Wallace, E.M.; Zoungas, S.; Teede, H.J. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust. N. Z. J. Obstet. Gynaecol. 2017, 57, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Achkar, M.; Dodds, L.; Giguère, Y.; Forest, J.-C.; Armson, B.A.; Woolcott, C.; Agellon, S.; Spencer, A.; Weiler, H.A. Vitamin D status in early pregnancy and risk of preeclampsia. Am. J. Obstet. Gynecol. 2015, 212, 511.e1–511.e7. [Google Scholar] [CrossRef]
- Anderson, N.H.; Sadler, L.C.; Stewart, A.W.; Fyfe, E.M.; McCowan, L.M. Ethnicity, body mass index and risk of pre-eclampsia in a multiethnic New Zealand population. Aust. N. Z. J. Obstet. Gynaecol. 2012, 52, 552–558. [Google Scholar] [CrossRef]
- Barakat, M.N.; Youssef, R.M.; Al-Lawati, J.A. Pregnancy outcomes of diabetic women: Charting Oman’s progress towards the goals of the Saint Vincent Declaration. Ann. Saudi Med. 2010, 30, 265–270. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bashir, M.; Dabbous, Z.; Baagar, K.; Elkhatib, F.; Ibrahim, A.; Brich, S.-A.; Abdel-Rahman, M.E.; Konje, J.C.; Abou-Samra, A.-B. Type 2 diabetes mellitus in pregnancy: The impact of maternal weight and early glycaemic control on outcomes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 233, 53–57. [Google Scholar] [CrossRef]
- Bashir, M.; Naem, E.; Taha, F.; Konje, J.C.; Abou-Samra, A.-B. Outcomes of type 1 diabetes mellitus in pregnancy; effect of excessive gestational weight gain and hyperglycaemia on fetal growth. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 84–88. [Google Scholar] [CrossRef]
- Battarbee, A.N.; Venkatesh, K.K.; Aliaga, S.; Boggess, K.A. The association of pregestational and gestational diabetes with severe neonatal morbidity and mortality. J. Perinatol. 2020, 40, 232–239. [Google Scholar] [CrossRef]
- Beyerlein, A.; Lack, N.; von Kries, R. No further improvement in pregnancy-related outcomes in the offspring of mothers with pre-gestational diabetes in Bavaria, Germany, between 2001 and 2016. Diabet. Med. 2018, 35, 1420–1424. [Google Scholar] [CrossRef]
- Bicocca, M.J.; Qureshey, E.J.; Chauhan, S.P.; Hernandez-Andrade, E.; Sibai, B.M.; Nowlen, C.; Stafford, I. Semiquantitative Assessment of Amniotic Fluid Among Individuals With and Without Diabetes Mellitus. J. Ultrasound Med. 2022, 41, 447–455. [Google Scholar] [CrossRef]
- Billionnet, C.; Mitanchez, D.; Weill, A.; Nizard, J.; Alla, F.; Hartemann, A.; Jacqueminet, S. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia 2017, 60, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Capobianco, G.; Gulotta, A.; Tupponi, G.; Dessole, F.; Virdis, G.; Cherchi, C.; De Vita, D.; Petrillo, M.; Olzai, G.; Antonucci, R.; et al. Fetal Growth and Neonatal Outcomes in Pregestational Diabetes Mellitus in a Population with a High Prevalence of Diabetes. J. Pers. Med. 2022, 12, 1320. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-J.; Chiu, C.-H.; Huang, J.-Y.; Chen, P.-J.; Su, P.-H.; Yang, S.-F.; Chen, J.-Y. Maternal diabetes mellitus and birth defects in Taiwan: A 5-year nationwide population-based cohort study. J. Chin. Med. Assoc. 2023, 86, 589–595. [Google Scholar] [CrossRef]
- Cynthia, P.; Timothy, S.; Isabelle, E. What is the impact of diabetes for Australian Aboriginal women when pregnant? Diabetes Res. Clin. Pract. 2011, 93, e29–e32. [Google Scholar] [CrossRef]
- Dalfrà, M.G.; Sartore, G.; Di Cianni, G.; Mello, G.; Lencioni, C.; Ottanelli, S.; Sposato, J.; Valgimigli, F.; Scuffi, C.; Scalese, M.; et al. Glucose variability in diabetic pregnancy. Diabetes Technol. Ther. 2011, 13, 853–859. [Google Scholar] [CrossRef]
- Di Lorenzo, G.; Ceccarello, M.; Cecotti, V.; Ronfani, L.; Monasta, L.; Brumatti, L.V.; Montico, M.; D’oTtavio, G. First trimester maternal serum PIGF, free β-hCG, PAPP-A, PP-13, uterine artery Doppler and maternal history for the prediction of preeclampsia. Placenta 2012, 33, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Dolk, H.; McCullough, N.; Callaghan, S.; Casey, F.; Craig, B.; Given, J.; Loane, M.; Lagan, B.M.; Bunting, B.; Boyle, B.; et al. Risk Factors for Congenital Heart Disease: The Baby Hearts Study, a Population-Based Case-Control Study. PLoS ONE 2020, 15, e0227908. [Google Scholar] [CrossRef]
- Eidem, I.; Stene, L.C.; Henriksen, T.; Hanssen, K.F.; Vangen, S.; Vollset, S.E.; Joner, G. Congenital anomalies in newborns of women with type 1 diabetes: Nationwide population-based study in Norway, 1999–2004. Acta Obstet. Gynecol. Scand. 2010, 89, 1403–1411. [Google Scholar] [CrossRef]
- Fang, N.-W.; Huang, Y.-S.; Yin, C.-H.; Chen, J.-S.; Chiou, Y.-H. Maternal risk factors in offspring with congenital anomalies of the kidney and urinary tract in Asian women. Pediatr. Nephrol. 2023, 38, 3065–3070. [Google Scholar] [CrossRef]
- E Foeller, M.; Zhao, S.; Szabo, A.; O Cruz, M. Neonatal Outcomes in Twin Pregnancies Complicated by Gestational Diabetes Compared with Non-Diabetic Twins. J. Perinatol. 2015, 35, 1043–1047. [Google Scholar] [CrossRef]
- Gardosi, J.; Madurasinghe, V.; Williams, M.; Malik, A.; Francis, A. Maternal and fetal risk factors for stillbirth: Population based study. BMJ 2013, 346, f108. [Google Scholar] [CrossRef] [PubMed]
- Giraldo-Grueso, M.; Zarante, I.; Mejía-Grueso, A.; Gracia, G. Risk factors for congenital heart disease: A case-control study. Rev. Colomb. Cardiol. 2020, 27, 324–329. [Google Scholar] [CrossRef]
- Goetzinger, K.; Singla, A.; Gerkowicz, S.; Dicke, J.; Gray, D.; Odibo, A. Predicting the risk of pre-eclampsia between 11 and 13 weeks′ gestation by combining maternal characteristics and serum analytes, PAPP-A and free β-hCG. Prenat. Diagn. 2010, 30, 1138–1142. [Google Scholar] [CrossRef]
- Gordon, A.; Raynes-Greenow, C.; McGeechan, K.; Morris, J.; Jeffery, H. Risk factors for antepartum stillbirth and the influence of maternal age in New South Wales Australia: A population based study. BMC Pregnancy Childbirth 2013, 13, 12. [Google Scholar] [CrossRef]
- Gorsch, L.P.; Wen, T.; Lonier, J.Y.; Zork, N.; Mourad, M.; D’aLton, M.E.; Friedman, A.M. Trends in delivery hospitalizations with pregestational and gestational diabetes mellitus and associated outcomes: 2000–2019. Am. J. Obstet. Gynecol. 2023, 229, 63.e1–63.e14. [Google Scholar] [CrossRef] [PubMed]
- Gortazar, L.; Goday, A.; Roux, J.A.F.-L.; Sarsanedas, E.; Payà, A.; Mañé, L.; Pedro-Botet, J.; Benaiges, D. Trends in prevalence of pre-existing diabetes and perinatal outcomes: A large, population-based study in Catalonia, Spain, 2006–2015. BMJ Open Diabetes Res. Care 2020, 8, e001254. [Google Scholar] [CrossRef]
- Gortazar, L.; Roux, J.A.F.-L.; Benaiges, D.; Sarsanedas, E.; Navarro, H.; Payà, A.; Mañé, L.; Pedro-Botet, J.; Goday, A. Trends in Prevalence of Diabetes among Twin Pregnancies and Perinatal Outcomes in Catalonia between 2006 and 2015: The DIAGESTCAT Study. J. Clin. Med. 2021, 10, 1937. [Google Scholar] [CrossRef]
- Gualdani, E.; Di Cianni, G.; Seghieri, M.; Francesconi, P.; Seghieri, G. Pregnancy outcomes and maternal characteristics in women with pregestational and gestational diabetes: A retrospective study on 206,917 singleton live births. Acta Diabetol. 2021, 58, 1169–1176. [Google Scholar] [CrossRef]
- He, R.; Hornberger, L.K.; Kaur, A.; Crawford, S.; Boehme, C.; McBrien, A.; Eckersley, L. Risk of major congenital heart disease in pregestational maternal diabetes is modified by hemoglobin A1c. Ultrasound Obstet. Gynecol. 2023, 63, 378–384. [Google Scholar] [CrossRef]
- Hunt, K.J.; Marlow, N.M.; Gebregziabher, M.; Ellerbe, C.N.; Mauldin, J.; Mayorga, M.E.; Korte, J.E. Impact of maternal diabetes on birthweight is greater in non-Hispanic blacks than in non-Hispanic whites. Diabetologia 2012, 55, 971–980. [Google Scholar] [CrossRef]
- Jang, H.-J.; Kim, H.-S.; Kim, S.-H. Maternal and neonatal outcomes in Korean women with type 2 diabetes. Korean J. Intern. Med. 2018, 33, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Jovanovič, L.; Liang, Y.; Weng, W.; Hamilton, M.; Chen, L.; Wintfeld, N. Trends in the incidence of diabetes, its clinical sequelae, and associated costs in pregnancy. Diabetes/Metab. Res. Rev. 2015, 31, 707–716. [Google Scholar] [CrossRef]
- Kanda, E.; Matsuda, Y.; Makino, Y.; Matsui, H. Risk factors associated with altered fetal growth in patients with pregestational diabetes mellitus. J. Matern. Fetal Neonatal Med. 2012, 25, 1390–1394. [Google Scholar] [CrossRef]
- Kattini, R.; Poirier, J.N.; Kelly, L.F.; Madden, S.N.; Ockenden, H.; Dooley, J.P.; Hummelen, R.B. Outcomes of Pregnancies Affected by Gestational Diabetes and Type 2 Diabetes in a Rural First Nations Obstetrical Program in Northwest Ontario. Can. J. Diabetes 2020, 44, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Kekki, M.; Tihtonen, K.; Salonen, A.; Koukkula, T.; Gissler, M.; Laivuori, H.; Huttunen, T.T. Severe birth injuries in neonates and associated risk factors for injury in mothers with different types of diabetes in Finland. Int. J. Gynecol. Obstet. 2022, 159, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Knight, K.M.; Thornburg, L.L.; Pressman, E.K. Pregnancy outcomes in type 2 diabetic patients as compared with type 1 diabetic patients and nondiabetic controls. J. Reprod. Med. 2012, 57, 397–404. [Google Scholar]
- Knight, K.M.; Pressman, E.K.; Hackney, D.N.; Thornburg, L.L. Perinatal outcomes in type 2 diabetic patients compared with non-diabetic patients matched by body mass index. J. Matern. Neonatal Med. 2012, 25, 611–615. [Google Scholar] [CrossRef]
- Kohn, J.R.; Rajan, S.S.; Kushner, J.A.; Fox, K.A. Outcomes, care utilization, and expenditures in adolescent pregnancy complicated by diabetes. Pediatr. Diabetes 2019, 20, 769–777. [Google Scholar] [CrossRef]
- Kuc, S.; Wortelboer, E.; Koster, M.P.H.; de Valk, H.; Schielen, P.; Visser, G. Prediction of macrosomia at birth in type-1 and 2 diabetic pregnancies with biomarkers of early placentation. BJOG 2011, 118, 748–754. [Google Scholar] [CrossRef]
- Lai, F.Y.; Johnson, J.A.; Dover, D.; Kaul, P. Outcomes of singleton and twin pregnancies complicated by pre-existing diabetes and gestational diabetes: A population-based study in Alberta, Canada, 2005–2011. J. Diabetes 2016, 8, 45–55. [Google Scholar] [CrossRef]
- Lasheen, A.E.; Abdelbasit, O.B.; Seidahmed, M.Z.; Hussein, K.A.; Muqdad, A.M.; Al-Zahrani, M.H.; Farid, J.M.; Badr, H.A. Infants of diabetic mothers. A cohort study. Saudi Med. J. 2014, 35, 572–577. [Google Scholar] [PubMed]
- Lemaitre, M.; Bourdon, G.; Bruandet, A.; Lenne, X.; Subtil, D.; Rakza, T.; Vambergue, A. Pre-gestational diabetes and the risk of congenital heart defects in the offspring: A French nationwide study. Diabetes Metab. 2023, 49, 101446. [Google Scholar] [CrossRef]
- Lin, S.-F.; Kuo, C.-F.; Chiou, M.-J.; Chang, S.-H. Maternal and fetal outcomes of pregnant women with type 1 diabetes, a national population study. Oncotarget 2017, 8, 80679–80687. [Google Scholar] [CrossRef]
- Lindsay, R.S.; Walker, J.D.; Halsall, I.; Hales, C.N.; Calder, A.A.; Hamilton, B.A.; Johnstone, F.D. Insulin and insulin propeptides at birth in offspring of diabetic mothers. J. Clin. Endocrinol. Metab. 2003, 88, 1664–1671. [Google Scholar] [CrossRef][Green Version]
- Liu, S.; Joseph, K.; Lisonkova, S.; Rouleau, J.; Hof, M.V.D.; Sauve, R.; Kramer, M.S. Association between maternal chronic conditions and congenital heart defects: A population-based cohort study. Circulation 2013, 128, 583–589. [Google Scholar] [CrossRef] [PubMed]
- López-De-Andrés, A.; Perez-Farinos, N.; Hernández-Barrera, V.; Palomar-Gallego, M.A.; Carabantes-Alarcón, D.; Zamorano-León, J.J.; De Miguel-Diez, J.; Jimenez-Garcia, R. A Population-Based Study of Diabetes during Pregnancy in Spain (2009–2015): Trends in Incidence, Obstetric Interventions, and Pregnancy Outcomes. J. Clin. Med. 2020, 9, 582. [Google Scholar] [CrossRef]
- Loukovaara, M.; Leinonen, P.; Teramo, K.; Koistinen, R. Cord serum glycodelin concentrations in normal pregnancies and pregnancies complicated by diabetes. Arch. Gynecol. Obstet. 2004, 270, 161–164. [Google Scholar] [CrossRef]
- Luo, S.; Ran, X.; Zhang, M.; Hu, J.; Yang, D.; Zhu, D.; Zhao, J.; Xiao, X.; Guo, X.; Yang, T.; et al. Pregnancy outcomes in women with type 1 diabetes in China during 2004 to 2014: A retrospective study (the CARNATION Study). J. Diabetes 2022, 14, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, A.; Sabr, Y.; A Hutcheon, J.; Donovan, L.; Lyons, J.; Burrows, J.; Joseph, K.S. Trends in Obstetric Intervention and Pregnancy Outcomes of Canadian Women with Diabetes in Pregnancy from 2004 to 2015. J. Endocr. Soc. 2017, 1, 1540–1549. [Google Scholar] [CrossRef]
- Mirghani, H.; Begam, M.; Bekdache, G.; Khan, F. Specialised fetal and maternal service: Outcome of pre-gestational diabetes. J. Obstet. Gynaecol. 2012, 32, 426–429. [Google Scholar] [CrossRef]
- Morgan, K.; Rahman, M.; Atkinson, M.; Zhou, S.-M.; Hill, R.; Khanom, A.; Paranjothy, S.; Brophy, S.; Bruce, A. Association of diabetes in pregnancy with child weight at birth, age 12 months and 5 years—A population-based electronic cohort study. PLoS ONE 2013, 8, e79803. [Google Scholar] [CrossRef]
- Ngwezi, D.P.; Savu, A.; Yeung, R.O.; Butalia, S.; Kaul, P. Temporal Trends in Type 1, Type 2, and Gestational Diabetes in Pregnancy: Impact of Rural Residence, Ethnicity, and Material Deprivation. Can. J. Diabetes 2023, 47, 672–679.e3. [Google Scholar] [CrossRef]
- A Owens, L.; Sedar, J.; Carmody, L.; Dunne, F. Comparing type 1 and type 2 diabetes in pregnancy- similar conditions or is a separate approach required? BMC Pregnancy Childbirth 2015, 15, 69. [Google Scholar] [CrossRef]
- Papageorghiou, A.T.; Yu, C.K.; Erasmus, I.E.; Cuckle, H.S.; Nicolaides, K.H. Assessment of risk for the development of pre-eclampsia by maternal characteristics and uterine artery Doppler. BJOG 2005, 112, 703–709. [Google Scholar] [CrossRef]
- Paré, E.; Parry, S.; McElrath, T.F.; Pucci, D.; Newton, A.; Lim, K.-H. Clinical Risk Factors for Preeclampsia in the 21st Century. Obstet. Gynecol. 2014, 124, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Patel, E.M.; Goodnight, W.H.; James, A.H.; Grotegut, C.A. Temporal trends in maternal medical conditions and stillbirth. Am. J. Obstet. Gynecol. 2015, 212, 673.e1–673.e11. [Google Scholar] [CrossRef] [PubMed]
- Pereda, J.; Bove, I.; Pineyro, M.M. Excessive Maternal Weight and Diabetes Are Risk Factors for Macrosomia: A Cross-Sectional Study of 42,663 Pregnancies in Uruguay. Front. Endocrinol. 2020, 11, 588443. [Google Scholar] [CrossRef] [PubMed]
- Peticca, P.; Keely, E.J.; Walker, M.C.; Yang, Q.; Bottomley, J. Pregnancy Outcomes in Diabetes Subtypes: How Do They Compare? A Province-based Study of Ontario, 2005–2006. J. Obstet. Gynaecol. Can. 2009, 31, 487–496. [Google Scholar] [CrossRef]
- Praprotnik, D.; Meštrović, Z.; Vulić, M.; Radman, M.; Roje, D. Diabetes type 1 and pregnancy outcome at University Hospital Center Split—A retrospective study. Med. Flum. 2021, 57, 190–196. [Google Scholar] [CrossRef]
- Reddy, U.M.; Laughon, S.K.; Sun, L.; Troendle, J.; Willinger, M.; Zhang, J. Prepregnancy Risk Factors for Antepartum Stillbirth in the United States. Obstet. Gynecol. 2010, 116, 1119–1126. [Google Scholar] [CrossRef]
- Reitzle, L.; Heidemann, C.; Baumert, J.; Kaltheuner, M.; Adamczewski, H.; Icks, A.; Scheidt-Nave, C. Pregnancy complications in women with pregestational and gestational diabetes mellitus. Dtsch. Aerzteblatt Int. 2023, 120, 81–86. [Google Scholar] [CrossRef]
- Riskin, A.; Itzchaki, O.; Bader, D.; Iofe, A.; Toropine, A.; Riskin-Mashiah, S. Perinatal Outcomes in Infants of Mothers with Diabetes in Pregnancy. Isr. Med. Assoc. J. 2020, 22, 569–575. [Google Scholar] [PubMed]
- Schraw, J.M.; Langlois, P.H.; Lupo, P.J. Comprehensive assessment of the associations between maternal diabetes and structural birth defects in offspring: A phenome-wide association study. Ann. Epidemiol. 2021, 53, 14–20.e8. [Google Scholar] [CrossRef] [PubMed]
- Seah, J.; Kam, N.M.; Wong, L.; Tanner, C.; Shub, A.; Houlihan, C.; Ekinci, E.I. Risk factors for pregnancy outcomes in Type 1 and Type 2 diabetes. Intern. Med. J. 2021, 51, 78–86. [Google Scholar] [CrossRef]
- Al Serehi, A.; Ahmed, A.M.; Shakeel, F.; Alkhatani, K.; El-Bakri, N.K.; Buhari, B.A.M.; Al Mohareb, U.; Aljohani, N. A comparison on the prevalence and outcomes of gestational versus type 2 diabetes mellitus in 1718 Saudi pregnancies. Int. J. Clin. Exp. Med. 2015, 8, 11502–11507. [Google Scholar]
- Shefali, A.K.; Kavitha, M.; Deepa, R.; Mohan, V. Pregnancy outcomes in pre-gestational and gestational diabetic women in comparison to non-diabetic women—A prospective study in Asian Indian mothers (CURES-35). J. Assoc. Physicians India 2006, 54, 613–618. [Google Scholar]
- Shour, A.; Garacci, E.; Palatnik, A.; Dawson, A.Z.; Anguzu, R.; Walker, R.J.; Egede, L. Association between pregestational diabetes and mortality among appropriate-for-gestational age birthweight infants. J. Matern. Neonatal Med. 2022, 35, 5291–5300. [Google Scholar] [CrossRef]
- Son, K.H.; Lim, N.K.; Lee, J.; Cho, M.; Park, H. Comparison of maternal morbidity and medical costs during pregnancy and delivery between patients with gestational diabetes and patients with pre-existing diabetes. Diabet. Med. 2015, 32, 477–486. [Google Scholar] [CrossRef]
- Stanton, S.G.; Ryerson, E.; Moore, S.L.; Sullivan-Mahoney, M.; Couch, S.C. Hearing Screening Outcomes in Infants of Pregestational Diabetic Mothers. Am. J. Audiol. 2005, 14, 86–93. [Google Scholar] [CrossRef]
- Stogianni, A.; Lendahls, L.; Landin-Olsson, M.; Thunander, M. Obstetric and perinatal outcomes in pregnancies complicated by diabetes, and control pregnancies, in Kronoberg, Sweden. BMC Pregnancy Childbirth 2019, 19, 159. [Google Scholar] [CrossRef]
- Titmuss, A.; Barzi, F.; Barr, E.L.M.; Webster, V.; Wood, A.; Kelaart, J.; Kirkwood, M.; Connors, C.; Boyle, J.A.; Moore, E.; et al. Association between maternal hyperglycemia in pregnancy and offspring anthropometry in early childhood: The pandora wave 1 study. Int. J. Obes. 2023, 47, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- A Wahabi, H.; A Esmaeil, S.; Fayed, A.; Al-Shaikh, G.; Alzeidan, R.A. Pre-existing diabetes mellitus and adverse pregnancy outcomes. BMC Res. Notes 2012, 5, 496. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, Q.; Yang, H.; Yang, Y.; Wang, L.; Chen, H.; Anderson, C.; Liu, X.; Song, G.; Li, Q.; et al. Preconception diabetes mellitus and adverse pregnancy outcomes in over 6.4 million women: A population-based cohort study in China. PLOS Med. 2019, 16, e1002926. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Bleicher, K.; Han, X. Maternal Diabetes, Large-for-Gestational-Age Births, and First Trimester Pregnancy-Associated Plasma Protein-A. J. Clin. Endocrinol. Metab. 2015, 100, 2372–2379. [Google Scholar] [CrossRef]
- Wright, D.; Akolekar, R.; Syngelaki, A.; Poon, L.C.; Nicolaides, K.H. A Competing Risks Model in Early Screening for Preeclampsia. Fetal Diagn. Ther. 2012, 32, 171–178. [Google Scholar] [CrossRef]
- Xu, X.K.; A Wang, Y.; Li, Z.; Lui, K.; A Sullivan, E. Risk factors associated with preterm birth among singletons following assisted reproductive technology in Australia 2007–2009–a population-based retrospective study. BMC Pregnancy Childbirth 2014, 14, 406. [Google Scholar] [CrossRef]
- Xu, Q.; Lu, J.; Hu, J.; Ge, Z.; Zhu, D.; Bi, Y. Perinatal outcomes in pregnancies complicated by type 1 diabetes mellitus. Gynecol. Endocrinol. 2020, 36, 879–884. [Google Scholar] [CrossRef]
- Yang, G.-R.; Dye, T.D.; Li, D. Effects of pre-gestational diabetes mellitus and gestational diabetes mellitus on macrosomia and birth defects in Upstate New York. Diabetes Res. Clin. Pract. 2019, 155, 107811. [Google Scholar] [CrossRef] [PubMed]
- Yanit, K.E.; Snowden, J.M.; Cheng, Y.W.; Caughey, A.B. The impact of chronic hypertension and pregestational diabetes on pregnancy outcomes. Am. J. Obstet. Gynecol. 2012, 207, 333.e1–333.e6. [Google Scholar] [CrossRef]
- Yves, J.; Valerie, V.; Katrien, V.H.; Guy, M.; Grobman, W.A. Birth Weight in Type 1 Diabetic Pregnancy. Obstet. Gynecol. Int. 2010, 2010, 397623. [Google Scholar] [CrossRef]
- Zeki, R.; Oats, J.J.; Wang, A.Y.; Li, Z.; Homer, C.S.; Sullivan, E.A. Cesarean section and diabetes during pregnancy: An NSW population study using the Robson classification. J. Obstet. Gynaecol. Res. 2018, 44, 890–898. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- American Diabetes Association Professional Practice Committee; ElSayed, N.A.; Aleppo, G.; Bannuru, R.R.; Bruemmer, D.; Collins, B.S.; Ekhlaspour, L.; Hilliard, M.E.; Johnson, E.L.; Khunti, K.; et al. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. S1), S282–S294. [Google Scholar] [CrossRef]
- Dobrescu, A.; Nussbaumer-Streit, B.; Klerings, I.; Wagner, G.; Persad, E.; Sommer, I.; Herkner, H.; Gartlehner, G. Restricting evidence syntheses of interventions to English-language publications is a viable methodological shortcut for most medical topics: A systematic review. J. Clin. Epidemiol. 2021, 137, 209–217. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).