The Use of Medical Hypnosis to Prevent and Treat Acute and Chronic Pain: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
- Direct techniques: These practices correspond to the eye fixation method, where the patient focuses on an image, leading to muscle fatigue and relaxation, which helps achieve hypnosis.
- Indirect techniques: These methods are based on the use of visual imagery, such as asking the patient to imagine a moving screen in their mind to facilitate the hypnotic state.
- Mechanical techniques: These practices utilize repetitive mechanical, visual, or tactile stimuli, often involving tools like metronomes or rhythmic electronic instruments.
2. Materials and Methods
2.1. Eligibility Criteria
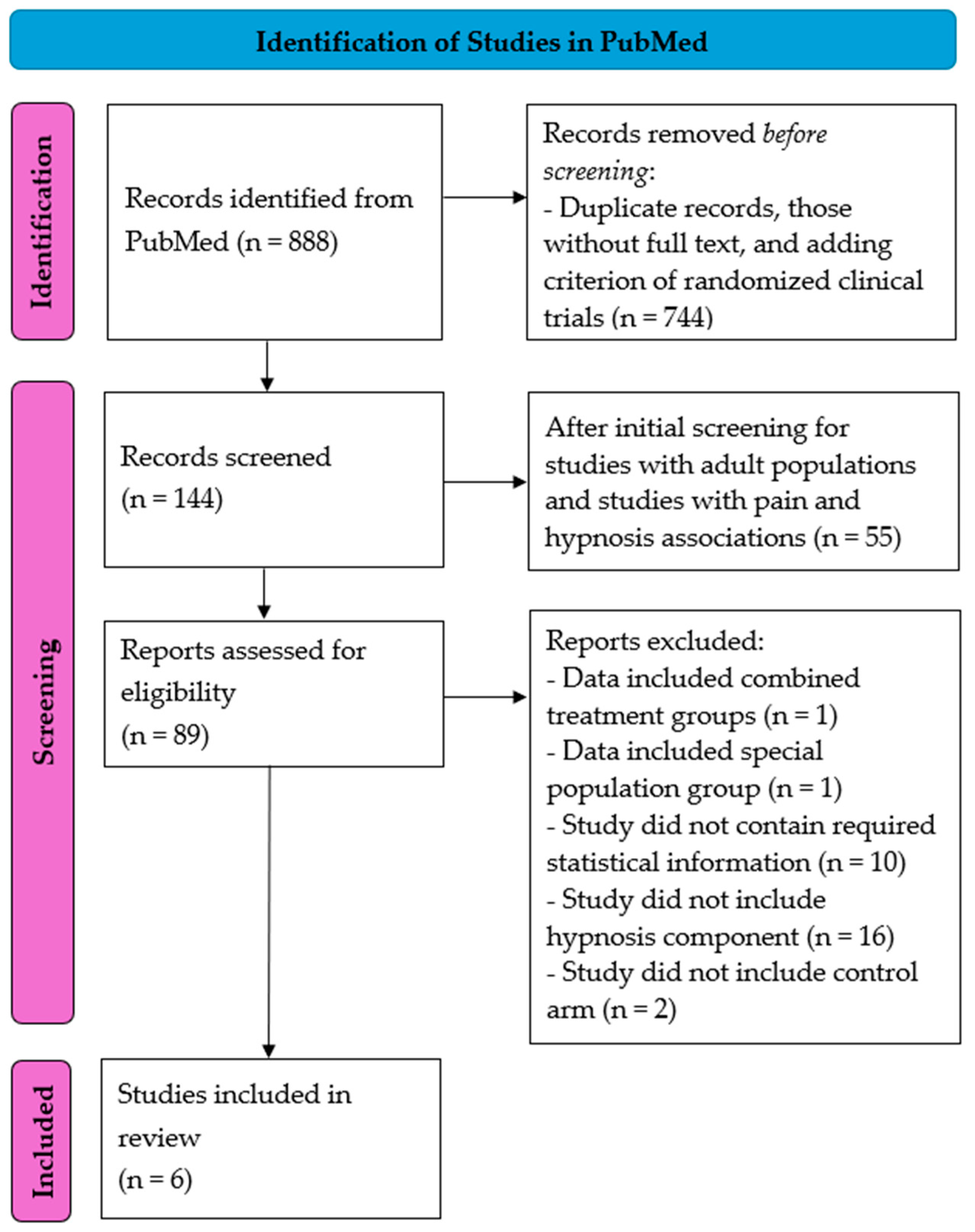
2.2. Search Strategy
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
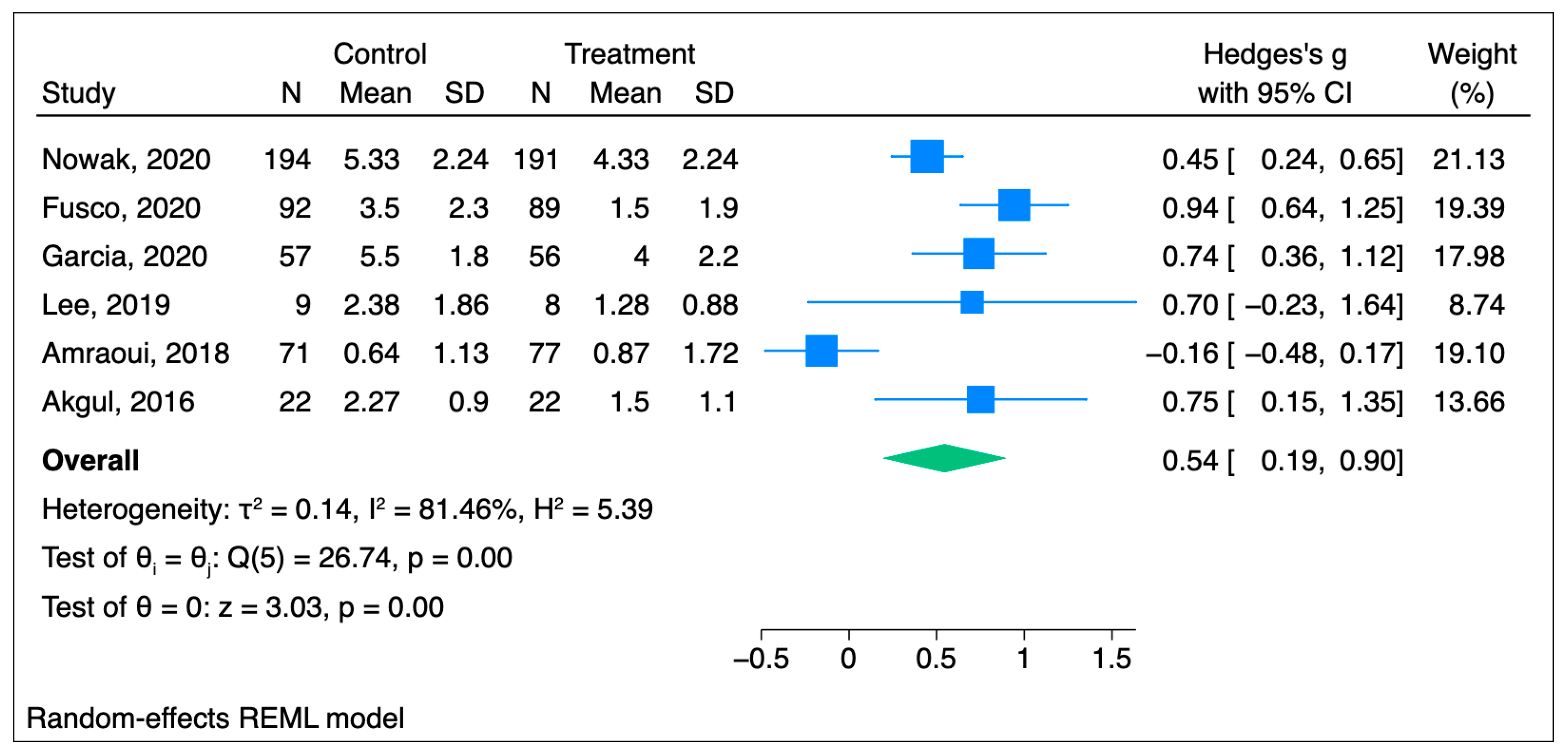
3.1. Acute Pain
3.1.1. Primary Outcome: Acute Pain Scores
3.1.2. Secondary Outcome: Oral Morphine Equivalents
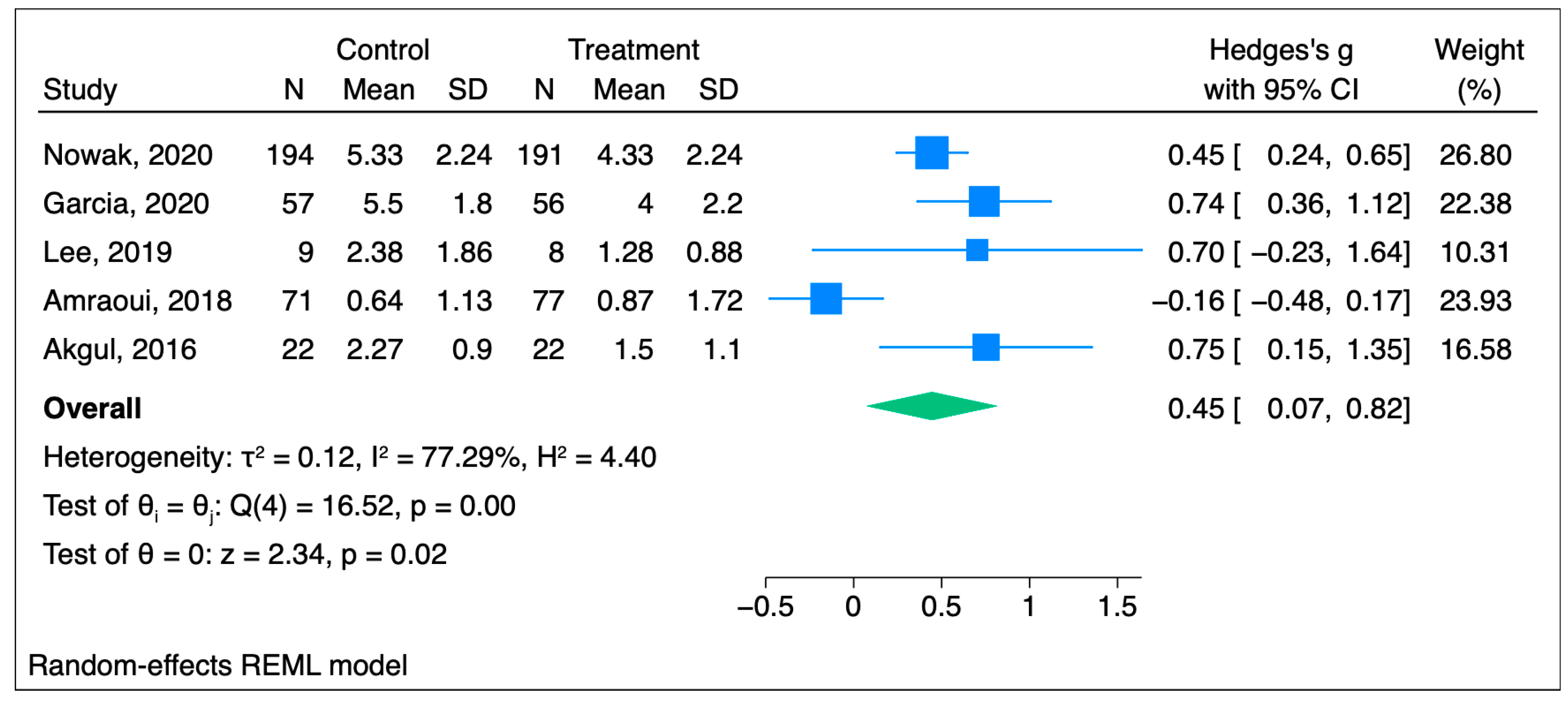
3.1.3. Subgroup Analysis
3.1.4. Sensitivity Analysis
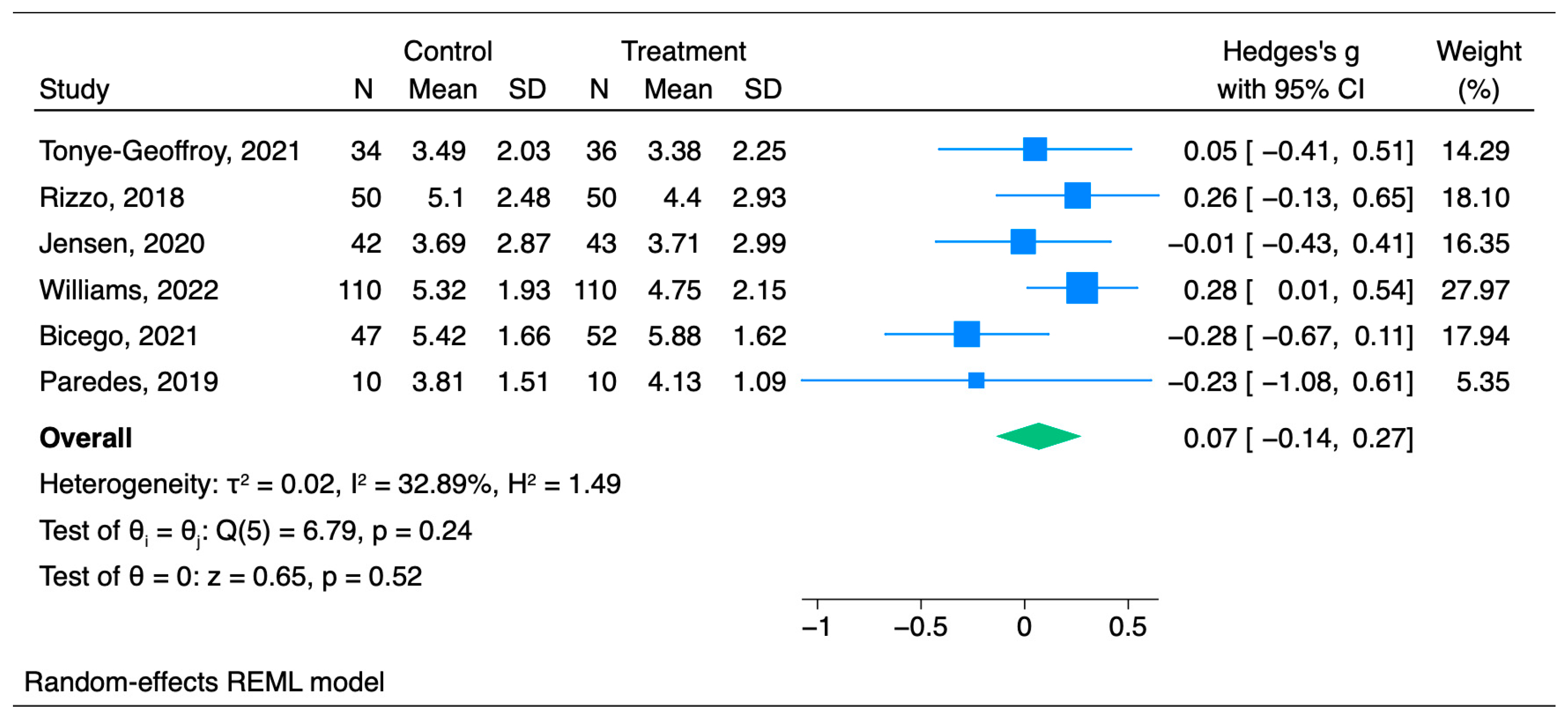
3.2. Chronic Pain
Primary Outcome: Chronic Pain Scores
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BPI | brief pain inventory |
| CAM | complementary and alternative medicine |
| CI | confidence interval |
| NRS | numeric rating scale |
| OME | oral morphine equivalents |
| POD | post-operative day |
| POH | post-operative hour |
| POM | post-operative month |
| POW | post-operative week |
| REML | restricted maximum likelihood |
| RCT | randomized controlled trial |
| SD | standard deviation |
| TIS | tonic immobility state |
| VAS | visual analogue scale |
| VR | virtual reality |
References
- Mayden, K.D. Mind-body therapies: Evidence and implications in advanced oncology practice. J. Adv. Pract. Oncol. 2012, 3, 357–373. [Google Scholar]
- Rengin Güzel, O. History of Hypnosis; Dursun, R., Haspolat, Y.K., Eds.; Orient Publications: Ankara, Turkey, 2003; pp. 5–16. [Google Scholar]
- Riskin, J.D.; Frankel, F.H. A history of medical hypnosis. Psychiatr. Clin. N. Am. 1994, 17, 601–609. [Google Scholar] [CrossRef]
- Perry, C.; Laurence, J.-R. Hypnosis, surgery, and mind–body interaction: An historical evaluation. Can. J. Behav. Sci./Rev. Can. Des Sci. Du Comport. 1983, 15, 351–372. [Google Scholar] [CrossRef]
- Hammond, D.C. A review of the history of hypnosis through the late 19th century. Am. J. Clin. Hypn. 2013, 56, 174–191. [Google Scholar] [CrossRef]
- Patterson, D.R.; Jensen, M.P. Hypnosis and clinical pain. Psychol. Bull. 2003, 129, 495–521. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.; Terhune, D.B.; Oram, C.; Sharangparni, J.; Rouf, R.; Solmi, M.; Veronese, N.; Stubbs, B. The effectiveness of hypnosis for pain relief: A systematic review and meta-analysis of 85 controlled experimental trials. Neurosci. Biobehav. Rev. 2019, 99, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, M.E.; Capafons, A. Efficacy of clinical hypnosis: A summary of its empirical evidence. Papeles Del Psicólogo 2009, 30, 98–116. [Google Scholar]
- Rosenbloom, B.N.; Slepian, P.M.; Azam, M.A.; Aternali, A.; Birnie, K.A.; Curtis, K.; Thaker, S.; Ladak, S.; Waisman, A.; Clarke, H.; et al. A Randomized Controlled Trial of Clinical Hypnosis as an Opioid-Sparing Adjunct Treatment for Pain Relief in Adults Undergoing Major Oncologic Surgery. J. Pain Res. 2024, 17, 45–59. [Google Scholar] [CrossRef]
- Garland, E.L.; Brintz, C.E.; Hanley, A.W.; Roseen, E.J.; Atchley, R.M.; Gaylord, S.A.; Faurot, K.R.; Yaffe, J.; Fiander, M.; Keefe, F.J. Mind-Body Therapies for Opioid-Treated Pain: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2020, 180, 91–105. [Google Scholar] [CrossRef]
- Gay, M.C.; Philippot, P.; Luminet, O. Differential effectiveness of psychological interventions for reducing osteoarthritis pain: A comparison of Erickson hypnosis and Jacobson relaxation. Eur. J. Pain 2002, 6, 1–16. [Google Scholar] [CrossRef]
- Elkins, G.R. Handbook of Medical and Psychological Hypnosis: Foundations, Applications, and Professional Issues; Springer Publishing Company, LLC: New York, NY, USA, 2017. [Google Scholar]
- UpToDate; Greenlee, H. Overview of Complementary, Alternative, and Integrative Medicine Practices in Oncology Care, and Potentialrisks and Harm. 2023. Available online: https://www.uptodate.com/contents/overview-of-complementary-alternative-and-integrative-medicine-practices-in-oncology-care-and-potential-risks-and-harm (accessed on 1 October 2024).
- Lynn, S.J.; Green, J.P. An introduction to the practice of clinical hypnosis. In Evidence-Based Practice in Clinical Hypnosis; Milling, L.S., Ed.; American Psychological Association: Washington, DC, USA, 2023; pp. 3–27. [Google Scholar] [CrossRef]
- Kroger, W.S. Techniques of hypnosis. J. Am. Med. Assoc. 1960, 172, 675. [Google Scholar] [CrossRef] [PubMed]
- Zeig, J.; Tanev, K.S. Advancing hypnotic inductions: An Ericksonian perspective. Eur. J. Psychother. Couns. 2022, 24, 457–472. [Google Scholar] [CrossRef]
- Barnier, A.J.; Nash, M.R. (Eds.) The Oxford Handbook of Hypnosis: Theory, Research, and Practice; Oxford Academic: Oxford, UK, 2012. [Google Scholar] [CrossRef]
- Castiglioni, J.A.; Russell, M.I.; Setlow, B.; Young, K.A.; Welsh, J.C.; Steele-Russell, I. An animal model of hypnotic painattenuation. Behav. Brain Res. 2009, 197, 198–204. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Nowak, H.; Zech, N.; Asmussen, S.; Rahmel, T.; Tryba, M.; Oprea, G.; Grause, L.; Schork, K.; Moeller, M.; Loeser, J.; et al. Effect of therapeutic suggestions during general anaesthesia on postoperative pain and opioid use: Multicentre randomised controlled trial. Br. Med. J. (Clin. Res. Ed.) 2020, 371, m4284. [Google Scholar] [CrossRef] [PubMed]
- Fusco, N.; Bernard, F.; Roelants, F.; Watremez, C.; Musellec, H.; Laviolle, B.; Beloeil, H. Effect of Language and Confusion on Pain During Peripheral Intravenous Catheterization (KTHYPE) group. Hypnosis and communication reduce pain and anxiety in peripheral intravenous cannulation: Effect of Language and Confusion on Pain During Peripheral Intravenous Catheterization (KTHYPE), a multicentre randomised trial. Br. J. Anaesth. 2020, 124, 292–298. [Google Scholar] [CrossRef]
- Garcia, R.; Bouleti, C.; Li, A.; Frasca, D.; El Harrouchi, S.; Marechal, J.; Roumegou, P.; Corbi, P.; Christiaens, L.; Le Gal, F.; et al. Hypnosis Versus Placebo During Atrial Flutter Ablation: The PAINLESS Study: A Randomized Controlled Trial. JACC Clin. Electrophysiol. 2020, 6, 1551–1560. [Google Scholar] [CrossRef]
- Lee, J.K.; Zubaidah, J.O.; Fadhilah, I.S.I.; Normala, I.; Jensen, M.P. Prerecorded hypnotic peri-surgical intervention to alleviate risk of chronic postsurgical pain in total knee replacement: A randomized controlled pilot study. Int. J. Clin. Exp. Hypn. 2019, 67, 217–245. [Google Scholar] [CrossRef]
- Amraoui, J.; Pouliquen, C.; Fraisse, J.; Dubourdieu, J.; Rey Dit Guzer, S.; Leclerc, G.; de Forges, H.; Jarlier, M.; Gutowski, M.; Bleuse, J.P.; et al. Effects of a Hypnosis Session Before General Anesthesia on Postoperative Outcomes in Patients Who Underwent Minor Breast Cancer Surgery: The HYPNOSEIN Randomized Clinical Trial. JAMA Netw. Open 2018, 1, e181164. [Google Scholar] [CrossRef]
- Akgul, A.; Guner, B.; Çırak, M.; Çelik, D.; Hergünsel, O.; Bedirhan, S. The Beneficial Effect of Hypnosis in Elective Cardiac Surgery: A Preliminary Study. Thorac. Cardiovasc. Surg. 2016, 64, 581–588. [Google Scholar] [CrossRef]
- Tonye-Geoffroy, L.; Mauboussin Carlos, S.; Tuffet, S.; Fromentin, H.; Berard, L.; Leblanc, J.; Laroche, F. Efficacy of a combination of hypnosis and transcutaneous electrical nerve stimulation for chronic non-cancer pain: A randomized controlled trial. J. Adv. Nurs. 2021, 77, 2875–2886. [Google Scholar] [CrossRef]
- Rizzo, R.R.N.; Medeiros, F.C.; Pires, L.G.; Pimenta, R.M.; McAuley, J.H.; Jensen, M.P.; Costa, L.O.P. Hypnosis Enhances the Effects of Pain Education in Patients With Chronic Nonspecific Low Back Pain: A Randomized Controlled Trial. J. Pain 2018, 19, 1103.e1–1103.e9. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.P.; Mendoza, M.E.; Ehde, D.M.; Patterson, D.R.; Molton, I.R.; Dillworth, T.M.; Gertz, K.J.; Chan, J.; Hakimian, S.; Battalio, S.L.; et al. Effects of hypnosis, cognitive therapy, hypnotic cognitive therapy, and pain education in adults with chronic pain: A randomized clinical trial. Pain 2020, 161, 2284–2298. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.M.; Day, M.A.; Ehde, D.M.; Turner, A.P.; Ciol, M.A.; Gertz, K.J.; Patterson, D.; Hakimian, S.; Suri, P.; Jensen, M.P. Effects of hypnosis vs mindfulness meditation vs education on chronic pain intensity and secondary outcomes in veterans: A randomized clinical trial. Pain 2022, 163, 1905–1918. [Google Scholar] [CrossRef] [PubMed]
- Bicego, A.; Monseur, J.; Collinet, A.; Donneau, A.F.; Fontaine, R.; Libbrecht, D.; Malaise, N.; Nyssen, A.S.; Raaf, M.; Rousseaux, F.; et al. Complementary treatment comparison for chronic pain management: A randomized longitudinal study. PLoS ONE 2021, 16, e0256001. [Google Scholar] [CrossRef]
- Paredes, A.C.; Costa, P.; Roque, S.; Fernandes, S.; Lopes, M.; Carvalho, M.; Mateus, A.; Almeida, A.; Pinto, P.R. Effectiveness of hypnosis for pain management and promotion of health-related quality-of-life among people with haemophilia: A randomised controlled pilot trial. Complement. Ther. Clin. Pract. 2021, 45, 101486. [Google Scholar] [CrossRef]
- Degenhardt, L.; Grebely, J.; Stone, J.; Hickman, M.; Vickerman, P.; Marshall, B.D.L.; Bruneau, J.; Altice, F.L.; Henderson, G.; Rahimi-Movaghar, A.; et al. Global patterns of opioid use and dependence: Harms to populations, interventions, and future action. Lancet 2019, 394, 1560–1579. [Google Scholar] [CrossRef]
- Kamper, S.J. Interpreting Outcomes 2—Statistical Significance and Clinical Meaningfulness: Linking Evidence to Practice. J. Orthop. Sports Phys. Ther. 2019, 49, 559–560. [Google Scholar] [CrossRef]
- Milling, L.S.; Valentine, K.E.; LoStimolo, L.M.; Nett, A.M.; McCarley, H.S. Hypnosis and the Alleviation of Clinical Pain: A Comprehensive Meta-Analysis. Int. J. Clin. Exp. Hypn. 2021, 69, 297–322. [Google Scholar] [CrossRef]
- Merz, A.E.; Campus, G.; Abrahamsen, R.; Wolf, T.G. Hypnosis on acute dental and maxillofacial pain relief: A systematic review and meta-analysis. J. Dent. 2022, 123, 104184. [Google Scholar] [CrossRef]
- Langlois, P.; Perrochon, A.; David, R.; Rainville, P.; Wood, C.; Vanhaudenhuyse, A.; Pageaux, B.; Ounajim, A.; Lavallière, M.; Debarnot, U.; et al. Hypnosis to manage musculoskeletal and neuropathic chronic pain: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2022, 135, 104591. [Google Scholar] [CrossRef]
- Jones, H.G.; Rizzo, R.R.N.; Pulling, B.W.; Braithwaite, F.A.; Grant, A.R.; McAuley, J.H.; Jensen, M.P.; Moseley, G.L.; Rees, A.; Stanton, T.R. Adjunctive use of hypnosis for clinical pain: A systematic review and meta-analysis. Pain Rep. 2024, 9, e1185. [Google Scholar] [CrossRef] [PubMed]
- Alimi, D. Xerostomia induced by radiotherapy. Ther. Clin. Risk Manag. 2015, 11, 1149–1152. [Google Scholar] [CrossRef][Green Version]
- Ganry, L.; Hersant, B.; Sidahmed-Mezi, M.; Dhonneur, G.; Meningaud, J.P. Using virtual reality to control preoperative anxiety in ambulatory surgery patients: A pilot study in maxillofacial and plastic surgery. J. Stomatol. Oral Maxillofac. Surg. 2018, 119, 257–261. [Google Scholar] [CrossRef]
- Ong, T.L.; Ruppert, M.M.; Akbar, M.; Rashidi, P.; Ozrazgat-Baslanti, T.; Bihorac, A.; Suvajdzic, M. Improving the Intensive Care Patient Experience With Virtual Reality—A Feasibility Study. Crit. Care Explor. 2020, 2, e0122. [Google Scholar] [CrossRef]
- Rousseaux, F.; Dardenne, N.; Massion, P.B.; Ledoux, D.; Bicego, A.; Donneau, A.F.; Faymonville, M.E.; Nyssen, A.S.; Vanhaudenhuyse, A. Virtual reality and hypnosis for anxiety and pain management in intensive care units: A prospective randomised trial among cardiac surgery patients. Eur. J. Anaesthesiol. 2022, 39, 58–66. [Google Scholar] [CrossRef]
- Vickers, A.J.; Vertosick, E.A.; Lewith, G.; MacPherson, H.; Foster, N.E.; Sherman, K.J.; Irnich, D.; Witt, C.M.; Linde, K.; Acupuncture Trialists’ Collaboration. Acupuncture for Chronic Pain: Update of an Individual Patient Data Meta-Analysis. J. Pain 2018, 19, 455–474. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Guo, X.; May, B.H.; Zhang, A.L.; Liu, Y.; Lu, C.; Mao, J.J.; Xue, C.C.; Zhang, H. Clinical Evidence for Association of Acupuncture and Acupressure With Improved Cancer Pain: A Systematic Review and Meta-Analysis. JAMA Oncol. 2020, 6, 271–278. [Google Scholar] [CrossRef]
- Giovanardi, C.M.; Gonzalez-Lorenzo, M.; Poini, A.; Marchi, E.; Culcasi, A.; Ursini, F.; Faldini, C.; Di Martino, A.; Mazzanti, U.; Campesato, E. Acupuncture as an alternative or in addition to conventional treatment for chronic non-specific low back pain: A systematic review and meta-analysis. Integr. Med. Res. 2023, 12, 100972. [Google Scholar] [CrossRef] [PubMed]
- Chelly, J.E.; Orebaugh, S.L.; Rodosky, M.W.; Groff, Y.J.; Norton, B.E.; Monroe, A.L.; Alimi, D.; Sadhasivam, S.K.; Vogt, K.M. Auriculotherapy for Prolonged Postoperative Pain Management Following Rotator Cuff Surgery: A Randomized, Placebo-Controlled Study. J. Med. Acupunct. 2025; in press. [Google Scholar] [CrossRef]
- Nascimento, J.C.; Gonçalves, V.S.S.; Souza, B.R.S.; Nascimento, L.C.; Carvalho, B.M.R.; Ziegelmann, P.K.; Goes, T.C.; Guimarães, A.G. New approaches to the effectiveness of inhalation aromatherapy in controlling painful conditions: A systematic review with meta-analysis. Complement. Ther. Clin. Pract. 2022, 49, 101628. [Google Scholar] [CrossRef]
- Lakhan, S.E.; Sheafer, H.; Tepper, D. The Effectiveness of Aromatherapy in Reducing Pain: A Systematic Review and Meta-Analysis. Pain Res. Treat. 2016, 2016, 8158693. [Google Scholar] [CrossRef] [PubMed]
- Chelly, J.E.; Klatt, B.; O’Malley, M.; Groff, Y.; Kearns, J.; Khetarpal, S.; Sadhasivam, S. The Role of Inhalation Aromatherapy, Lavender and Peppermint in the Management of Perioperative Pain and Opioid Consumption Following Primary Unilateral Total Hip Arthroplasty: A Prospective, Randomized and Placebo-Controlled Study. J. Pain Relief 2023, 12 (Suppl. S1), 003. [Google Scholar] [PubMed]
- Hassan, S.; Zheng, Q.; Rizzolo, E.; Tezcanli, E.; Bhardwaj, S.; Cooley, K. Does Integrative Medicine Reduce Prescribed Opioid Use for Chronic Pain? A Systematic Literature Review. Pain Med. 2020, 21, 836–859. [Google Scholar] [CrossRef] [PubMed]
- Urits, I.; Schwartz, R.H.; Orhurhu, V.; Maganty, N.V.; Reilly, B.T.; Patel, P.M.; Wie, C.; Kaye, A.D.; Mancuso, K.F.; Kaye, A.J.; et al. A Comprehensive Review of Alternative Therapies for the Management of Chronic Pain Patients: Acupuncture, Tai Chi, Osteopathic Manipulative Medicine, and Chiropractic Care. Adv. Ther. 2021, 38, 76–89. [Google Scholar] [CrossRef]
- Kekecs, Z.; Nagy, T.; Varga, K. The effectiveness of suggestive techniques in reducing postoperative side effects: A meta-analysis of randomized controlled trials. Anesth. Analg. 2014, 119, 1407–1419. [Google Scholar] [CrossRef]
- Montgomery, G.H.; David, D.; Winkel, G.; Silverstein, J.H.; Bovbjerg, D.H. The effectiveness of adjunctive hypnosis with surgical patients: A meta-analysis. Anesth. Analg. 2002, 94, 1639–1645. [Google Scholar] [CrossRef]

| Study | Hypnosis Patients (n) | Control Patients (n) | Hypnosis Description | Pain Measure | Outcomes in Mean (SD) [Range] | Surgery/ Procedure |
|---|---|---|---|---|---|---|
| Nowak et al., 2020 [20] | 191 | 194 (blank tape) | Length: 20 min of play with 10 min of silence, repeated throughout the surgery Induction: Indirect—audiotape through earphones Role of hypnotherapist: Authors developed and recorded the text based on hypnotherapeutic principles Background music: Trancemusik CD (Hypnos Verlag, Stuttgart, Germany) Hypnosis susceptibility: Five-item modified Harvard group scale | NRS | Pain scores were reduced by an average of 25% in the hypnosis group within 24 h after surgery POH2 Intervention = 2 (1.49) Control = 2.67 (2.24) p-value < 0.001 POH24 Intervention = 4.33 (2.24) Control = 5.33 (2.24) p < 0.001 OME: Intervention = 4.0 (5.98) Control = 6.43 (7.47) | Elective surgery requiring general anesthesia with a planned duration of 1–3 h |
| Fusco et al., 2020 [21] | 89 | 92 | Length: Five minutes once during the procedure Induction: Indirect—verbal and nonverbal suggestions Role of hypnotherapist: Anesthesiologist or nurse with a diploma in therapeutic and hypnotic communication and at least one year of experience | NRS | Hypnosis group pain was lower compared to the neutral group Hypnosis = 1.5 [1.9]; 0–9 Neutral = 3.5 [2.3]; 0–9 p < 0.0001 OME: not reported | 20G peripheral intravenous cannulation on the dorsal surface of the hand before a scheduled surgery |
| Garcia et al., 2020 [22] | 56 | 57 | Length: Throughout the procedure Induction: Direct—eye-fixation Maintenance: Through headphones (Ericksonian and traditional conversational hypnosis) Role of hypnotherapist: Practitioners trained by the French Hypnosis Association | VAS | Pain during the whole procedure was significantly lower in the hypnosis group compared to the placebo group Hypnosis = 4.0 ± 2.2 Placebo = 5.5 ± 1.8 p < 0.001 OME: Intervention = 1.3 ± 1.3 Control = 3.6 ± 1.8 | Atrial flutter ablation |
| Lee et al., 2019 [23] | 8 | 9 | Length: 35 min presurgical recording and postsurgical recording that needs to be listened to at least 24 h after surgery Induction: Indirect—audio with hypnotic recordings with background music Role of hypnotherapist: Psychologist experienced in pain management and clinical hypnosis | NRS | Pain in the hypnosis group was lower to a small extent compared to the control group. Baseline Hypnosis = 4.58 (1.86) Placebo = 4.60 (1.49) POH24 (before second treatment) Hypnosis = 2.28 (1.75) Placebo = 3.15 (2.39) POH24 (after second treatment) Hypnosis = 1.25 (0.88) Placebo = 2.38 (1.86) POD3 Hypnosis = 1.77 (0.83) Placebo = 2.59 (1.47) p = 0.316 OME: not reported | Total knee arthroplasty |
| Amraoui et al., 2018 [24] | 77 | 71 | Length: 15 min before surgery Induction: Indirect—personalized wording or nonverbal communication Role of hypnotherapist: Trained anesthesiologist with more than one year experience | VAS | No significant difference between the two groups. Baseline Hypnosis = 1.45 (2.05) Placebo = 1.44 (1.78) Post-anesthesia care unit, p = 0.77 Hypnosis = 1.00 (1.57) Placebo = 0.75 (1.19) Evening, p = 0.87 Hypnosis = 0.88 (1.45) Placebo = 0.88 (1.57) POD1, p = 0.88 Hypnosis = 0.87 (1.72) Placebo = 0.64 (1.13) 95% CI all OME: not reported | Breast cancer tumor removal |
| Akgul et al., 2016 [25] | 22 | 22 | Length: 30 min before the surgery Induction: Indirect—verbal Role of hypnotherapist: Anesthesiologist with experience in clinical hypnosis | VAS | Hypnosis group pain was lower compared with the control group Baseline, p = 0.31 Hypnosis = 0.05 ± 0.2 Placebo = 0.0 ± 00 POH2, p = 0.41 Hypnosis = 0.50 ± 0.9 Placebo = 0.73 ± 1.1 POH4, p = 0.39 Hypnosis = 0.91 ± 1.3 Placebo = 1.14 ± 1.2 POH6, p = 0.91 Hypnosis = 1.73 ± 1.1 Placebo = 1.77 ± 1.3 POH8, p = 0.001 Hypnosis = 1.64 ± 1.1 Placebo = 3.00 ± 1.3 POH10, p = 0.001 Hypnosis = 1.27 ± 1.1 Placebo = 2.95 ± 1.7 POH12, p = 0.002 Hypnosis = 1.73 ± 1.2 Placebo = 2.82 ± 0.9 POH24, p = 0.01 Hypnosis = 1.50 ± 1.1 Placebo = 2.27 ± 0.9 99%CI OME: Intervention = 4.9 (3.3) Control = 13.6 (2.7) | Coronary artery bypass grafting |
| Placebo Treatment | Hypnosis Treatment | Total Number of Patients | |||||
|---|---|---|---|---|---|---|---|
| Study | Mean | SD | Number of Patients | Mean | SD | Number of Patients | |
| Overall total number of patients | 888 | ||||||
| Total number of patients for studies reporting NRS scores | 583 | ||||||
| Nowak et al., 2020 [20] | 5.33 | 2.24 | 194 | 4.33 | 2.24 | 191 | 385 |
| Fusco et al., 2020 [21] | 3.5 | 2.3 | 92 | 1.5 | 1.9 | 89 | 181 |
| Lee et al., 2019 [23] | 2.38 | 1.86 | 9 | 1.25 | 0,88 | 8 | 17 |
| Total number of patients for studies reporting VAS scores | 305 | ||||||
| Garcia et al., 2020 [22] | 5.5 | 1.8 | 57 | 4 | 2.2 | 56 | 113 |
| Amraoui et al., 2018 [24] | 0.64 | 1.13 | 71 | 0.87 | 1.72 | 77 | 148 |
| Akgul et al., 2016 [25] | 2.27 | 0.9 | 22 | 1.5 | 1.1 | 22 | 44 |
| Study | OME, mg Mean (SD) | |
|---|---|---|
| Intervention | Control | |
| Nowak et al., 2020 [20] | 4.0 (5.98) | 6.43 (7.47) |
| Garcia et al., 2020 [22] | 1.3 (1.3) | 3.6 (1.8) |
| Akgul et al., 2016 [25] | 4.9 (3.3) | 13.6 (2.7) |
| Study | Hypnosis Patients (n) | Control Patients (n) | Description of Hypnosis | Pain Measure | Outcomes in Mean (SD) [Range] | Chronic Pain Type |
|---|---|---|---|---|---|---|
| Tonye-Geoffroy et al., 2021 [26] | 36 | 34 | Length: 30 min at each visit POD0, POD7, POD21, POD30, POD42, POD56, POM3, POM6 Induction: Indirect—verbally Role of hypnotherapist: Qualified pain and anesthesiology nurse | VAS | No significant difference was observed between the control and intervention groups; Baseline Hypnosis = 5.63 (1.97) Placebo = 5.84 (2.23) POM3 Hypnosis = 3.38 (2.25) Placebo = 3.49 (2.03) | Chronic non-cancer nociceptive and neuropathic pain |
| Rizzo et al., 2018 [27] | 50 | 50 (Pain education) | Length: Four sessions twice a week in a group of 1–7 participants. Also, home hypnosis workbook was given for self-practice Induction: Indirect—verbally Role of hypnotherapist: Physical therapist certified in hypnotherapy | NRS | No significant differences between the groups in average pain intensity Baseline Hypnosis = 6.63 (1.57) Placebo = 7.20 (1.61) POW2 Hypnosis = 4.4 (2.14) Placebo = 5.6 (2.21) POM3 Hypnosis = 4.4 (2.93) Placebo = 5.1 (2.48) | Chronic nonspecific lower back pain |
| Jensen et al., 2020 [28] | 43 | 42 | Length: Once a day Induction: Indirect—audio (adapted from an existing protocol utilized in a series of trials of self-hypnosis for chronic pain) | NRS | No significant differences between the groups Pretreatment Hypnosis = 4.47 (1.72) Placebo = 4.63 (1.82) POM3: Mean (95% CI) Hypnosis = 3.71 (2.802–4.618) Placebo = 3.69 (2.8–4.59) | Chronic lower back pain or chronic pain secondary to one of the following chronic conditions: multiple sclerosis, spinal cord injury, acquired amputation, or muscular dystrophy |
| Williams et al., 2022 [29] | 110 | 110 (Pain education) | Length: 15–30 min, three interventions over 8–10 weeks Induction: Indirect—audio Role of hypnotherapist: Two-day in-person trained clinicians | NRS | No significant difference in pain in the hypnosis group in comparison with the control group Pretreatment: Mean (SD) Hypnosis = 5.7 (1.8) Placebo = 5.8 (1.6) POM3: Mean (95% CI) Hypnosis = 4.75 (4.344—5.156) Placebo = 5.32 (4.951–5.679) | Chronic pain |
| Bicego et al., 2021 [30] | 52 | 47 | Length: 20 min hetero-hypnosis exercise and daily self-hypnosis Induction: Direct—visual fixation and/or breathing attention focalization; five different exercises aimed to increase comfort and sleep quality and to decrease pain sensations Role of hypnotherapist: Clinical hypnosis specialists | NRS | No significant difference was observed between groups Baseline: Mean (SD) Hypnosis = 6.19 ± 1.45 Placebo = 5.74 ± 1.51 Posttreatment Hypnosis = 5.88 ± 1.62 Placebo = 5.42 ± 1.66 POM6 Hypnosis = 5.75 ± 1.91 Placebo = 5.51 ± 1.82 POM12 Hypnosis = 5.92 ± 1.86 Placebo = 5.57 ± 1.37 | Chronic pain |
| Paredes et al., 2021 [31] | 10 | 10 | Length: 60 min four times a week Induction: Indirect—verbal communication Role of hypnotherapist: Certified clinicians | NRS | The differences in pain intensity from pre- to post-intervention were not significant between the groups. Baseline: Mean (SD) Hypnosis = 4.22 (1.99) Placebo = 4.27 (1.77) Posttreatment Hypnosis = 4.13 (1.09) Placebo = 3.81 (1.51) | Joint pain |
| Placebo Treatment | Hypnosis Treatment | Total Number of Patients | |||||
|---|---|---|---|---|---|---|---|
| Study | Mean | SD | Number of Patients | Mean | SD | Number of Patients | |
| Tonye-Geoffroy et al., 2021 [26] | * 3.49 | 2.03 | 34 | * 3.38 | 2.25 | 36 | 70 |
| Rizzo et al., 2018 [27] | 5.1 | 2.48 | 50 | 4.4 | 2.93 | 50 | 100 |
| Jensen et al., 2020 [28] | 3.69 | 2.87 | 42 | 3.71 | 2.99 | 43 | 86 |
| Williams et al., 2022 [29] | 5.32 | 1.93 | 110 | 4.75 | 2.15 | 110 | 220 |
| Bicego et al., 2021 [30] | 5.74 | 1.66 | 47 | 6.19 | 1.62 | 52 | 99 |
| Paredes et al., 2021 [31] | 3.81 | 1.51 | 10 | 4.13 | 1.09 | 10 | 20 |
| Overall total number of patients | 595 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yerzhan, A.; Ayazbekova, A.; Lavage, D.R.; Chelly, J.E. The Use of Medical Hypnosis to Prevent and Treat Acute and Chronic Pain: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 4661. https://doi.org/10.3390/jcm14134661
Yerzhan A, Ayazbekova A, Lavage DR, Chelly JE. The Use of Medical Hypnosis to Prevent and Treat Acute and Chronic Pain: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(13):4661. https://doi.org/10.3390/jcm14134661
Chicago/Turabian StyleYerzhan, Adina, Akbota Ayazbekova, Danielle R. Lavage, and Jacques E. Chelly. 2025. "The Use of Medical Hypnosis to Prevent and Treat Acute and Chronic Pain: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 13: 4661. https://doi.org/10.3390/jcm14134661
APA StyleYerzhan, A., Ayazbekova, A., Lavage, D. R., & Chelly, J. E. (2025). The Use of Medical Hypnosis to Prevent and Treat Acute and Chronic Pain: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(13), 4661. https://doi.org/10.3390/jcm14134661