New Horizons: The Evolution of Nuclear Medicine in the Diagnosis and Treatment of Pancreatic Neuroendocrine Tumors—A Case Report
Abstract
1. Introduction
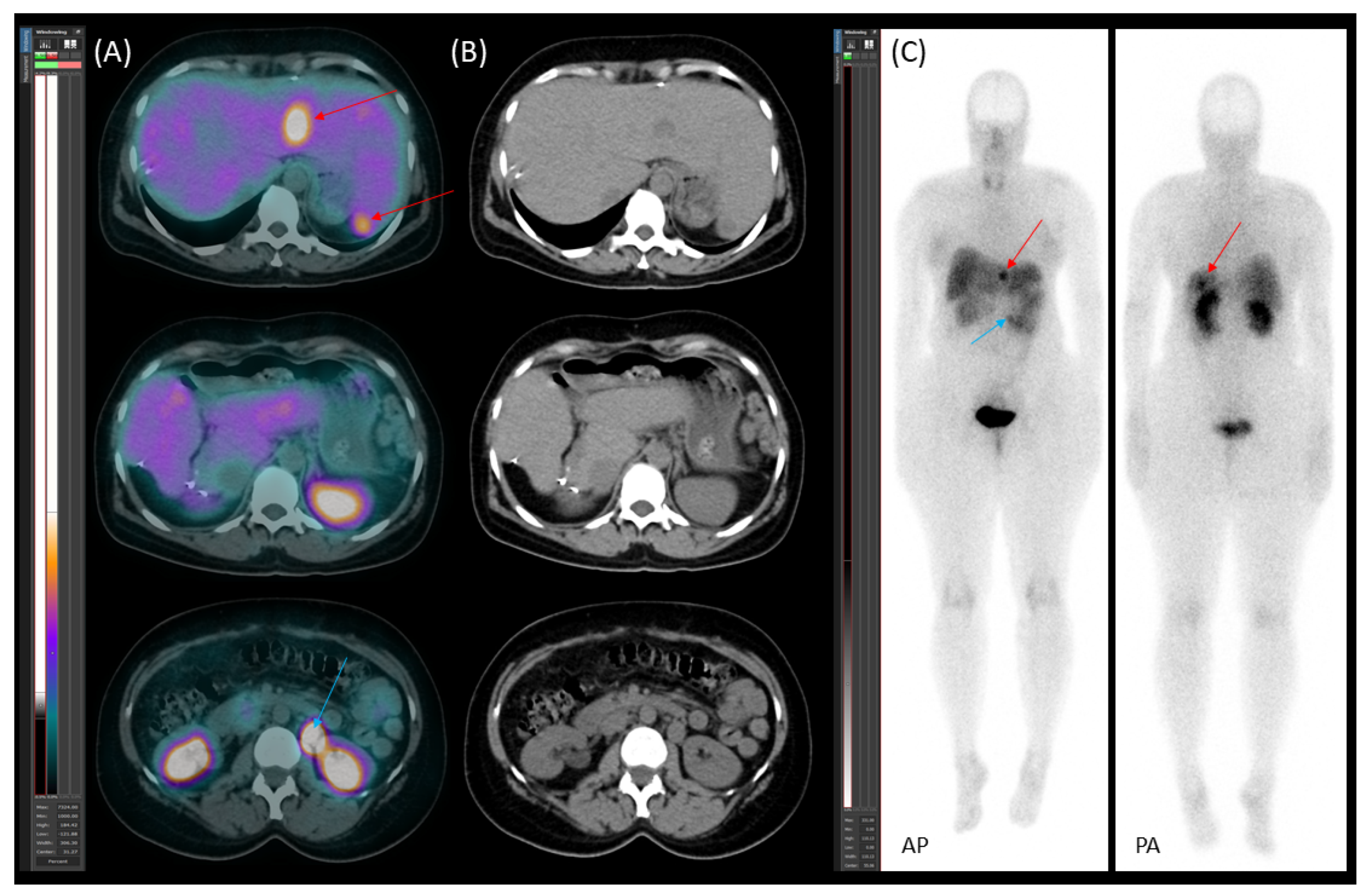
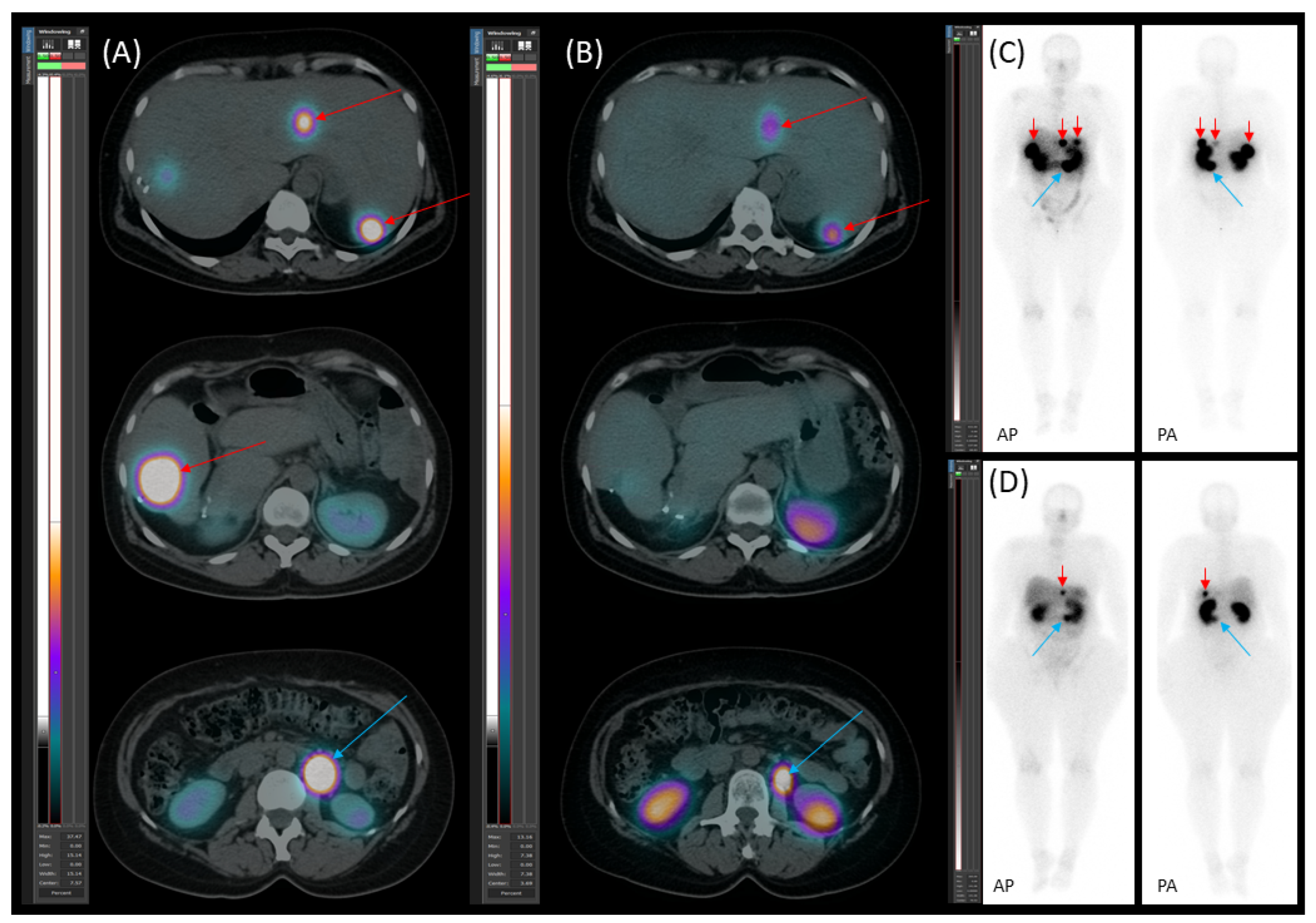
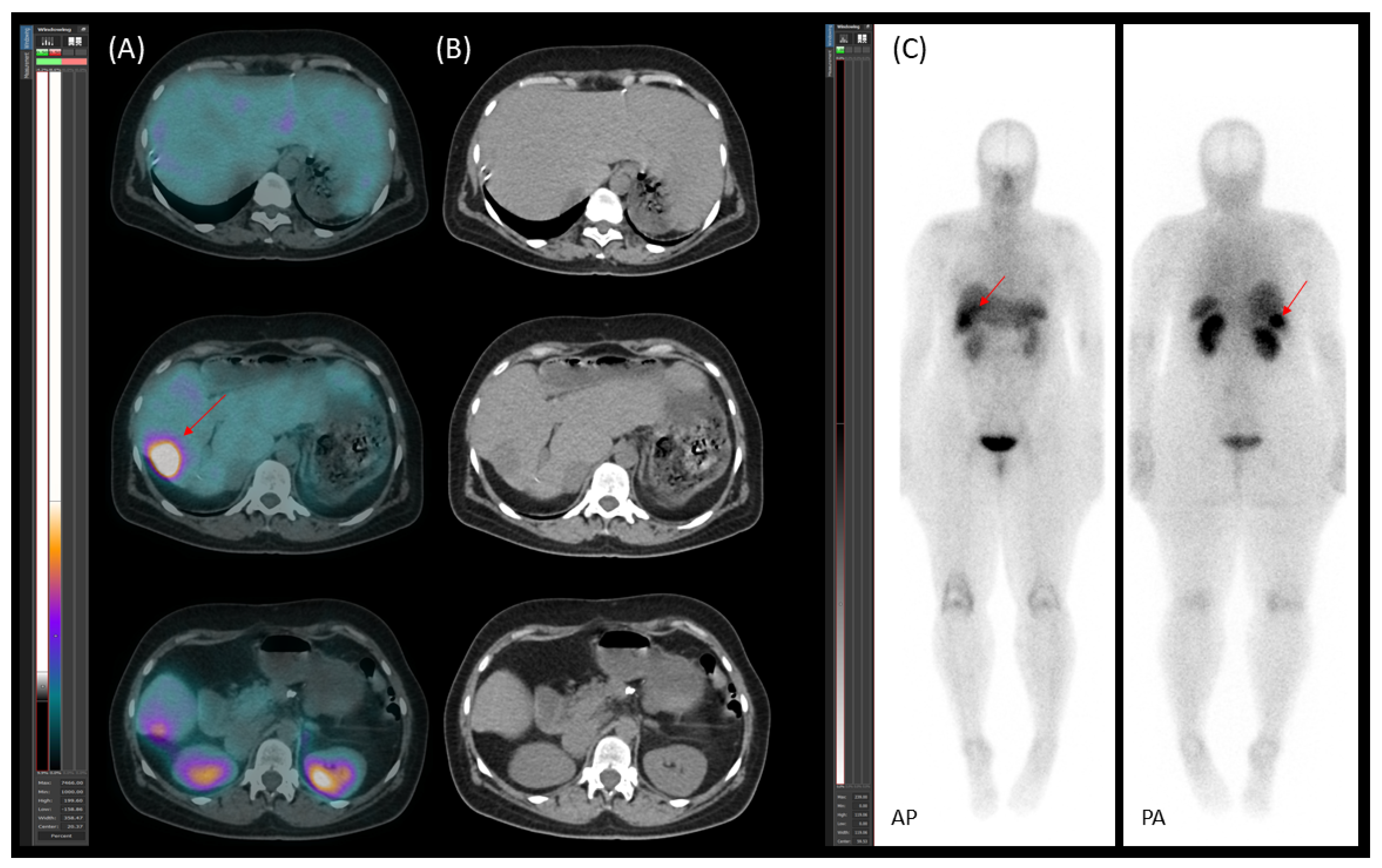
2. The Case
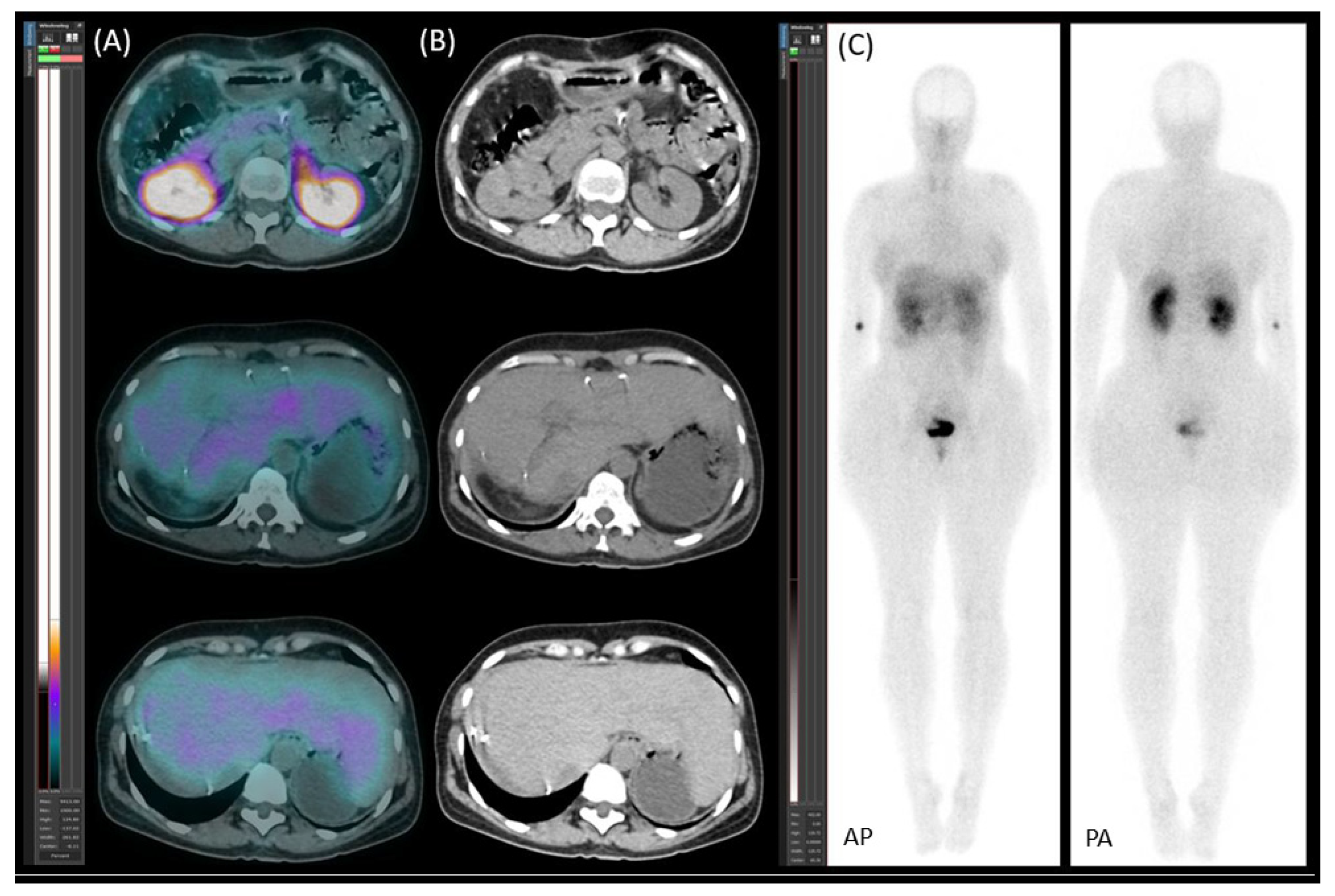
2.1. Patient Presentation and Initial Diagnosis
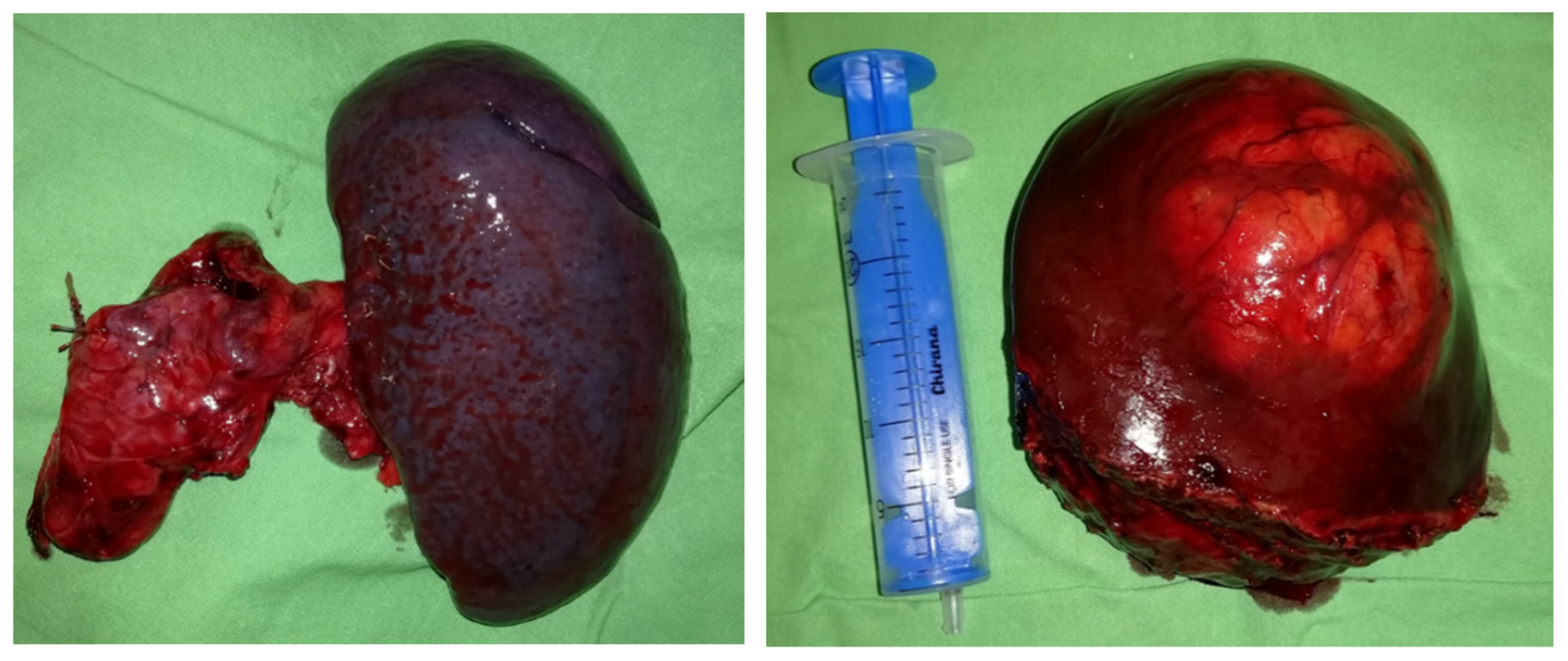
2.2. Preoperative Planning and First-Stage Surgery
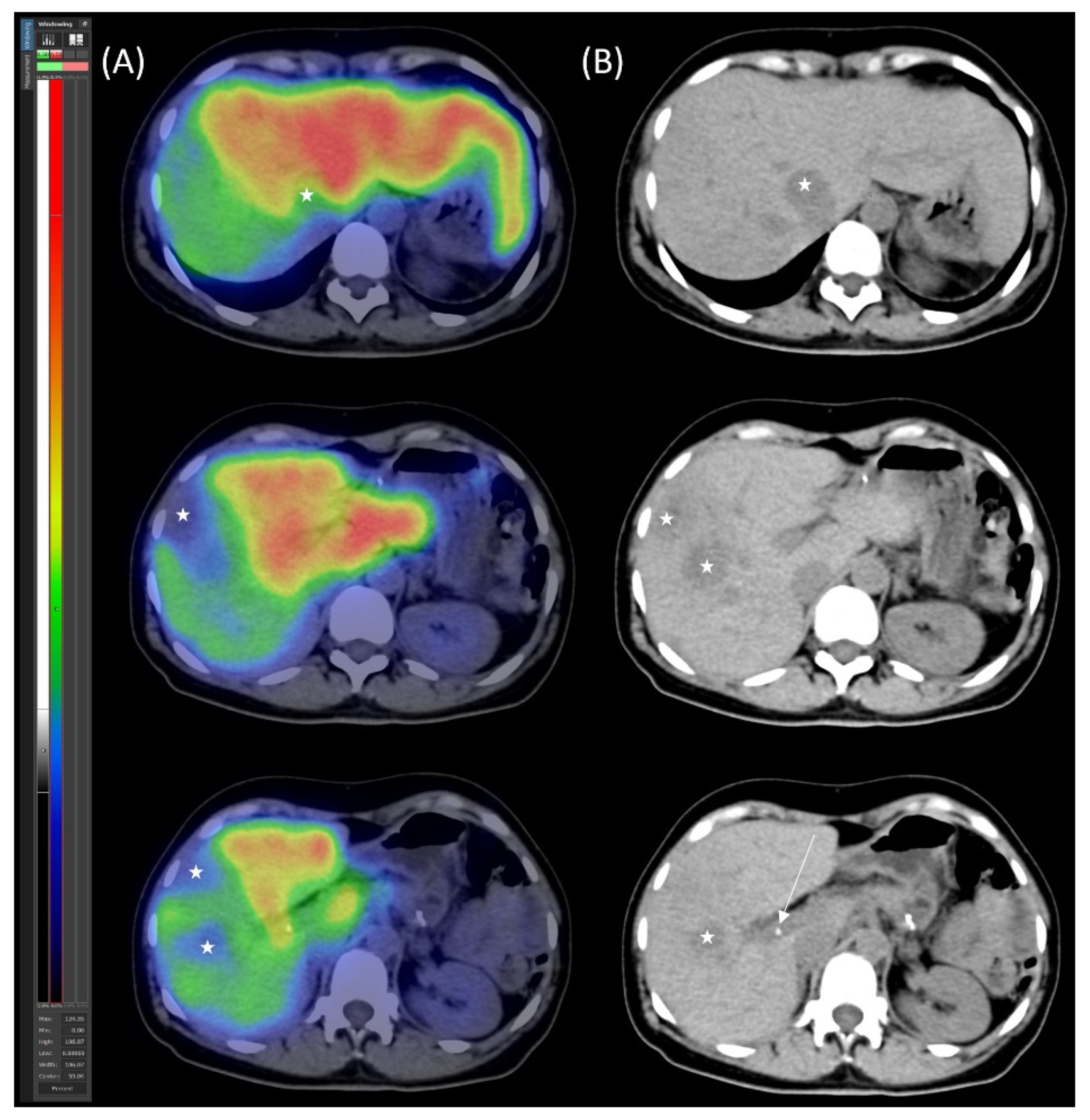
2.3. Preoperative Planning and Second-Stage Surgery
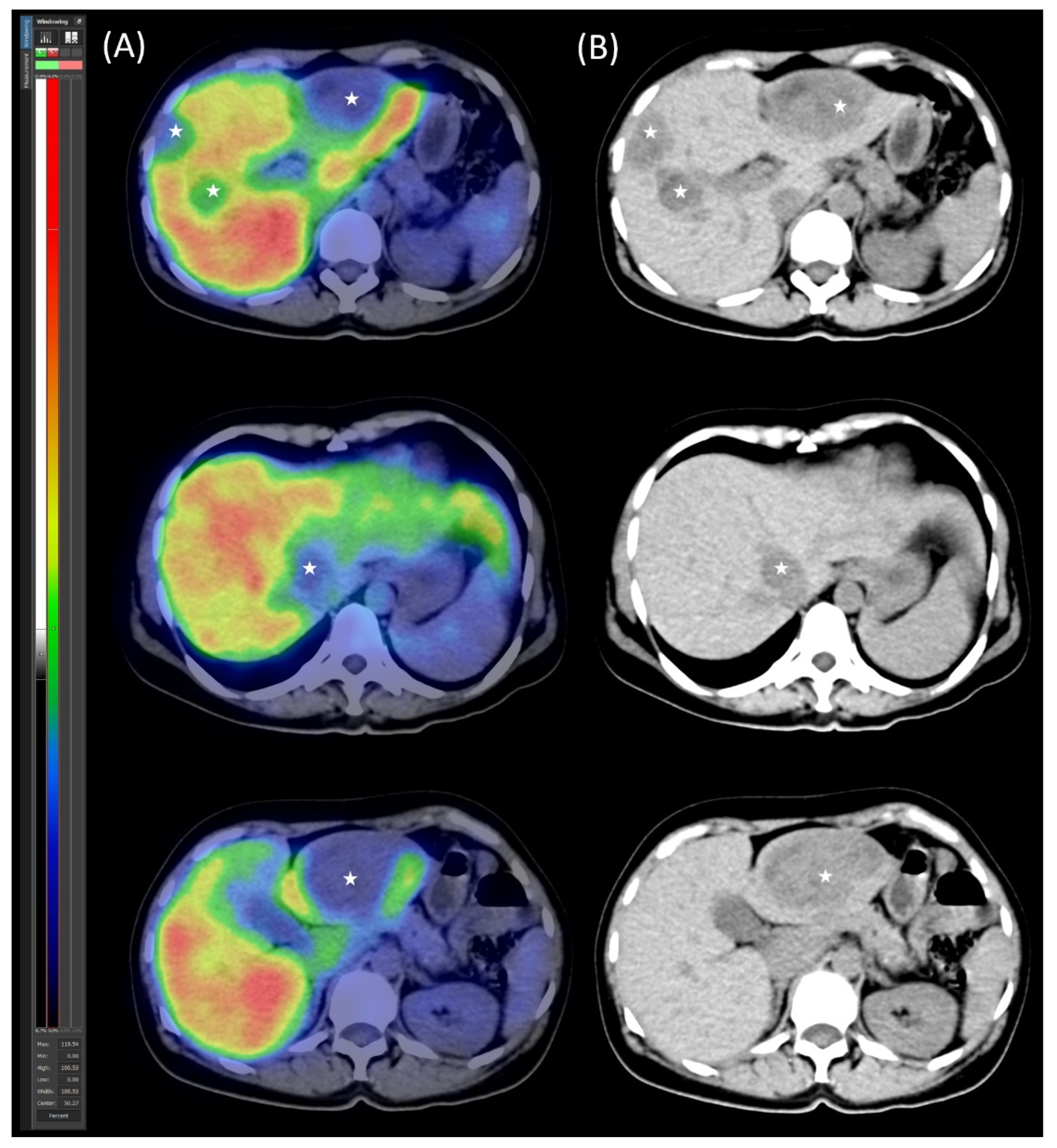
2.4. Follow-Up, Disease Progression, and Treatment
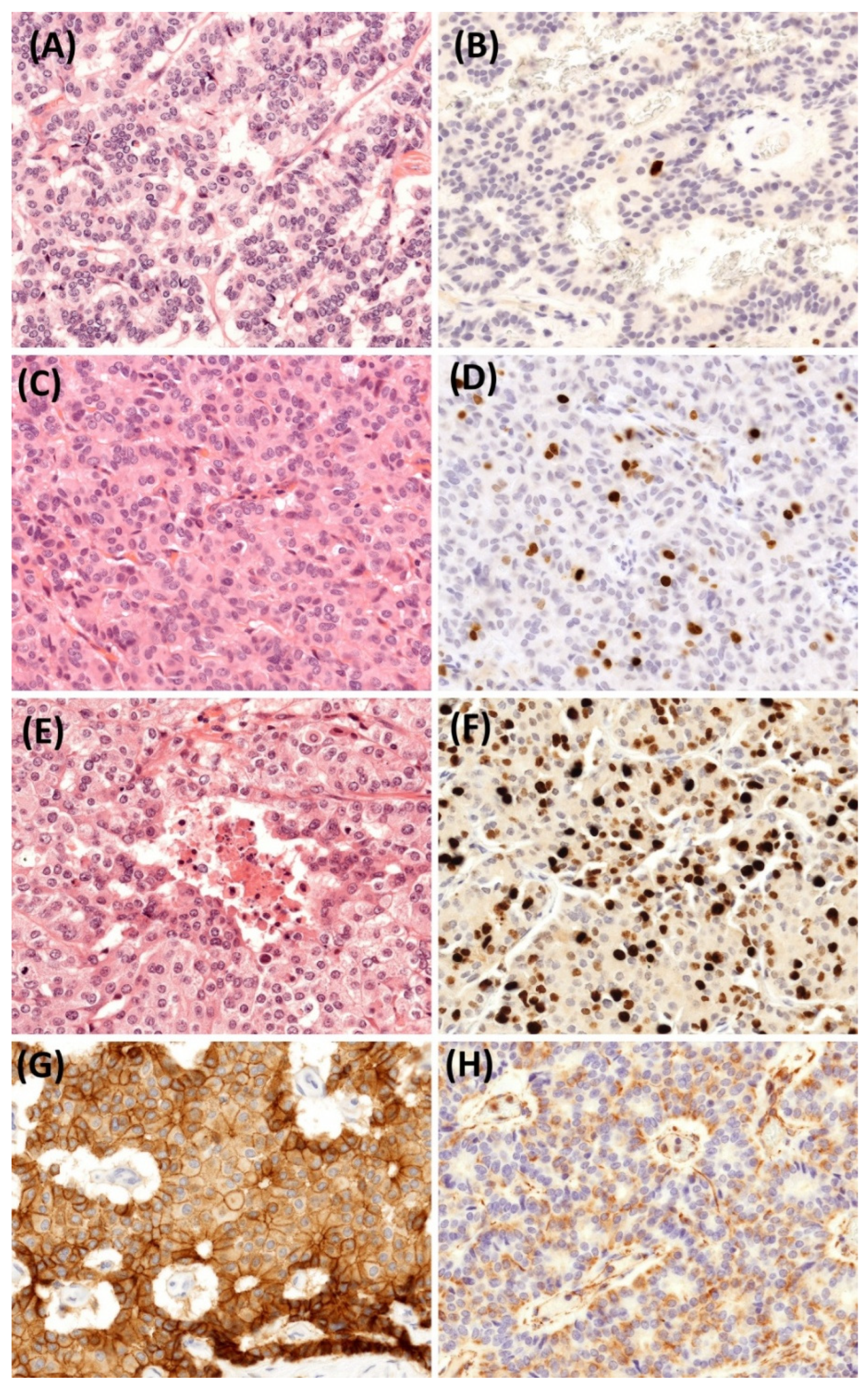
2.5. Histopathological Findings
3. Discussion
3.1. Pathological Aspects and Tumor Grade Progression
3.2. Somatostatin Receptor Imaging and Clinical Relevance
3.3. Therapeutic Considerations
3.3.1. Surgical Treatment
3.3.2. Preoperative Planning
3.3.3. Parenchymal Modulation Technique
3.3.4. Neuroendocrine Tumor-Specific Therapies
3.3.5. Disease Monitoring
4. Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fraenkel, M.; Kim, M.; Faggiano, A.; de Herder, W.W.; Valk, G.D.; Knowledge NETwork. Incidence of gastroenteropancreatic neuroendocrine tumours: A systematic review of the literature. Endocr. Relat. Cancer 2014, 21, R153–R163. [Google Scholar] [CrossRef] [PubMed]
- Leoncini, E.; Boffetta, P.; Shafir, M.; Aleksovska, K.; Boccia, S.; Rindi, G. Increased incidence trend of low-grade and high-grade neuroendocrine neoplasms. Endocrine 2017, 58, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Huguet, I.; Grossman, A.B.; O’Toole, D. Changes in the Epidemiology of Neuroendocrine Tumours. Neuroendocrinology 2017, 104, 105–111. [Google Scholar] [CrossRef]
- Niederle, M.B.; Hackl, M.; Kaserer, K.; Niederle, B. Gastroenteropancreatic neuroendocrine tumours: The current incidence and staging based on the WHO and European Neuroendocrine Tumour Society classification: An analysis based on prospectively collected parameters. Endocr. Relat. Cancer 2010, 17, 909–918. [Google Scholar] [CrossRef]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Fischer, L.; Bergmann, F.; Schimmack, S.; Hinz, U.; Prieß, S.; Müller-Stich, B.P.; Werner, J.; Hackert, T.; Büchler, M.W. Outcome of surgery for pancreatic neuroendocrine neoplasms. Br. J. Surg. 2014, 101, 1405–1412. [Google Scholar] [CrossRef]
- Frilling, A.; Modlin, I.M.; Kidd, M.; Russell, C.; Breitenstein, S.; Salem, R.; Kwekkeboom, D.; Lau, W.-Y.; Klersy, C.; Vilgrain, V.; et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014, 15, e8–e21. [Google Scholar] [CrossRef]
- Primavesi, F.; Maglione, M.; Cipriani, F.; Denecke, T.; E Oberkofler, C.; Starlinger, P.; Dasari, B.V.M.; Heil, J.; Sgarbura, O.; Søreide, K.; et al. E-AHPBA-ESSO-ESSR Innsbruck consensus guidelines for preoperative liver function assessment before hepatectomy. Br. J. Surg. 2023, 110, 1331–1347. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Kumada, T.; Toyoda, H.; Ito, T.; Sone, Y.; Okuda, S.; Tsuji, N.; Imayoshi, Y.; Yasuda, E. Utility of real-time shear wave elastography for assessing liver fibrosis in patients with chronic hepatitis C infection without cirrhosis: Comparison of liver fibrosis indices. Hepatol. Res. 2015, 45, E122–E129. [Google Scholar] [CrossRef]
- Bakos, A.; Libor, L.; Urbán, S.; Géczi, T.; Bukva, M.; Hőhn, J.; Lázár, G.; Nagy, A.; Farkas, I.; Sipka, G.; et al. Dynamic [99mTc]Tc-mebrofenin SPECT/CT in preoperative planning of liver resection: A prospective study. Sci. Rep. 2024, 14, 30305. [Google Scholar] [CrossRef]
- Arntz, P.J.W.; Deroose, C.M.; Marcus, C.; Sturesson, C.; Panaro, F.; Erdmann, J.; Manevska, N.; Moadel, R.; de Geus-Oei, L.-F.; Bennink, R.J. Joint EANM/SNMMI/IHPBA procedure guideline for [99mTc]Tc-mebrofenin hepatobiliary scintigraphy SPECT/CT in the quantitative assessment of the future liver remnant function. HPB 2023, 25, 1131–1144. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, A.; Ruzzenente, A.; Conci, S.; Valdegamberi, A.; Iacono, C. How much remnant is enough in liver resection? Dig. Surg. 2012, 29, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Ekman, M.; Fjälling, M.; Friman, S.; Carlson, S.; Volkmann, R. Liver uptake function measured by IODIDA clearance rate in liver transplant patients and healthy volunteers. Nucl. Med. Commun. 1996, 17, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Mosteller, R.D. Simplified calculation of body-surface area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar] [CrossRef]
- Grillo, F.; Albertelli, M.; Brisigotti, M.P.; Fiocca, R.; Ferone, D.; Mastracci, L. Grade Increases in Gastroenteropancreatic Neuroendocrine Tumor Metastases Compared to the Primary Tumor. Neuroendocrinology 2016, 103, 452–459. [Google Scholar] [CrossRef]
- Raoul, J.L.; Heymann, M.F.; Dumont, F.; Morel, A.; Senellart, H.; Bertucci, F. Case Report: Grade 2 Metastatic Pancreatic Neuroendocrine Tumor With Progression of One Metastasis After Pregnancy to Grade 3 Large-Cell Neuroendocrine Carcinoma: One Case Cured by Resection With Genomic Characterization of the Two Components. Front. Oncol. 2021, 11, 646992. [Google Scholar] [CrossRef]
- Miller, H.C.; Drymousis, P.; Flora, R.; Goldin, R.; Spalding, D.; Frilling, A. Role of Ki-67 proliferation index in the assessment of patients with neuroendocrine neoplasias regarding the stage of disease. World J. Surg. 2014, 38, 1353–1361. [Google Scholar] [CrossRef]
- Singh, S.; Hallet, J.; Rowsell, C.; Law, C.H. Variability of Ki67 labeling index in multiple neuroendocrine tumors specimens over the course of the disease. Eur. J. Surg. Oncol. 2014, 40, 1517–1522. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, Q.; Han, C.; Zhen, D.; Lin, R. Variability of the Ki-67 proliferation index in gastroenteropancreatic neuroendocrine neoplasms—A single-center retrospective study. BMC Endocr. Disord. 2018, 18, 51. [Google Scholar] [CrossRef]
- Yang, Z.; Tang, L.H.; Klimstra, D.S. Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: Implications for prognostic stratification. Am. J. Surg. Pathol. 2011, 35, 853–860. [Google Scholar] [CrossRef]
- Alexandraki, K.I.; Spyroglou, A.; Kykalos, S.; Daskalakis, K.; Kyriakopoulos, G.; Sotiropoulos, G.C.; A Kaltsas, G.; Grossman, A.B. Changing biological behaviour of NETs during the evolution of the disease: Progress on progression. Endocr. Relat. Cancer 2021, 28, R121–R140. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Kidder, W.; Joseph, N.M.; Le, B.K.; Lindsay, S.; Moon, F.; Nakakura, E.K.; Zhang, L.; Bergsland, E.K. Factors associated with grade progression in pancreatic neuroendocrine tumors. Endocr. Relat. Cancer 2025, 32, e240203. [Google Scholar] [CrossRef]
- Feijtel, D.; Doeswijk, G.N.; Verkaik, N.S.; Haeck, J.C.; Chicco, D.; Angotti, C.; Konijnenberg, M.W.; de Jong, M.; Nonnekens, J. Inter and intra-tumor somatostatin receptor 2 heterogeneity influences peptide receptor radionuclide therapy response. Theranostics 2021, 11, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.H.; Basturk, O.; Sue, J.J.; Klimstra, D.S. A Practical Approach to the Classification of WHO Grade 3 (G3) Well-differentiated Neuroendocrine Tumor (WD-NET) and Poorly Differentiated Neuroendocrine Carcinoma (PD-NEC) of the Pancreas. Am. J. Surg. Pathol. 2016, 40, 1192–1202. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, Y.; Rose, J.B.; Nagaraju, G.P.; Jaskula-Sztul, R.; Hjelmeland, A.B.; Beck, A.W.; Chen, H.; Ren, B. Protein Kinase D1 Signaling in Cancer Stem Cells with Epithelial-Mesenchymal Plasticity. Cells 2022, 11, 3885. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Xiang, J.; Jin, M.; Zheng, X.; Li, G.; Yan, S. High vimentin expression with E-cadherin expression loss predicts a poor prognosis after resection of grade 1 and 2 pancreatic neuroendocrine tumors. BMC Cancer 2021, 21, 334. [Google Scholar] [CrossRef]
- Kos-Kudła, B.; Castaño, J.P.; Denecke, T.; Grande, E.; Kjaer, A.; Koumarianou, A.; de Mestier, L.; Partelli, S.; Perren, A.; Stättner, S.; et al. European Neuroendocrine Tumour Society (ENETS) 2023 guidance paper for nonfunctioning pancreatic neuroendocrine tumours. J. Neuroendocrinol. 2023, 35, e13343. [Google Scholar] [CrossRef]
- Hwang, S.; Ha, T.-Y.; Song, G.-W.; Jung, D.-H.; Ahn, C.-S.; Moon, D.-B.; Kim, K.-H.; Lee, Y.-J.; Lee, S.-G. Quantified Risk Assessment for Major Hepatectomy via the Indocyanine Green Clearance Rate and Liver Volumetry Combined with Standard Liver Volume. J. Gastrointest. Surg. 2015, 19, 1305–1314. [Google Scholar] [CrossRef]
- Kim, R.D.; Kim, J.S.; Watanabe, G.; Mohuczy, D.; Behrns, K.E. Liver regeneration and the atrophy-hypertrophy complex. Semin. Interv. Radiol. 2008, 25, 92–103. [Google Scholar] [CrossRef]
- Garcovich, M.; Paratore, M.; Riccardi, L.; Zocco, M.A.; Ainora, M.E.; Mingrone, G.; Gasbarrini, A.; Pompili, M. Correlation between a New Point-Shear Wave Elastography Device (X+pSWE) with Liver Histology and 2D-SWE (SSI) for Liver Stiffness Quantification in Chronic Liver Disease. Diagnostics 2023, 13, 1743. [Google Scholar] [CrossRef]
- Baumgartner, R.; Gilg, S.; Björnsson, B.; Hasselgren, K.; Ghorbani, P.; Sauter, C.; Stål, P.; Sandstöm, P.; Sparrelid, E.; Engstrand, J. Impact of post-hepatectomy liver failure on morbidity and short- and long-term survival after major hepatectomy. BJS Open 2022, 6, zrac097. [Google Scholar] [CrossRef]
- Day, R.W.; Conrad, C.; Vauthey, J.N.; Aloia, T.A. Evaluating surgeon attitudes towards the safety and efficacy of portal vein occlusion and associating liver partition and portal vein ligation: A report of the MALINSA survey. HPB 2015, 17, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Y.; Gu, S.; Tang, K. A systematic review and meta-analysis of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) versus traditional staged hepatectomy. Medicine 2019, 98, e15229. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Schadde, E. Hypertrophy and Liver Function in ALPPS: Correlation with Morbidity and Mortality. Visc. Med. 2017, 33, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Genç, C.G.; Jilesen, A.P.; Partelli, S.; Falconi, M.; Muffatti, F.; van Kemenade, F.J.; van Eeden, S.; Verheij, J.; van Dieren, S.; van Eijck, C.H.J.; et al. A New Scoring System to Predict Recurrent Disease in Grade 1 and 2 Nonfunctional Pancreatic Neuroendocrine Tumors. Ann. Surg. 2018, 267, 1148–1154. [Google Scholar] [CrossRef]
- Sallinen, V.J.; Le Large, T.Y.S.; Tieftrunk, E.; Galeev, S.; Kovalenko, Z.; Haugvik, S.-P.; Antila, A.; Franklin, O.; Martinez-Moneo, E.; Robinson, S.M.; et al. Prognosis of sporadic resected small (≤2 cm) nonfunctional pancreatic neuroendocrine tumors—A multi-institutional study. HPB 2018, 20, 251–259. [Google Scholar] [CrossRef]
- Nanno, Y.; Matsumoto, I.; Zen, Y.; Otani, K.; Uemura, J.; Toyama, H.; Asari, S.; Goto, T.; Ajiki, T.; Okano, K.; et al. Pancreatic Duct Involvement in Well-Differentiated Neuroendocrine Tumors is an Independent Poor Prognostic Factor. Ann. Surg. Oncol. 2017, 24, 1127–1133. [Google Scholar] [CrossRef]
- Wang, W.-Q.; Zhang, W.-H.; Gao, H.-L.; Huang, D.; Xu, H.-X.; Li, S.; Li, T.-J.; Xu, S.-S.; Li, H.; Long, J.; et al. A novel risk factor panel predicts early recurrence in resected pancreatic neuroendocrine tumors. J. Gastroenterol. 2021, 56, 395–405. [Google Scholar] [CrossRef]
- Pavel, M.; Ćwikła, J.B.; Lombard-Bohas, C.; Borbath, I.; Shah, T.; Pape, U.F.; Capdevila, J.; Panzuto, F.; Thanh, X.-M.T.; Houchard, A.; et al. Efficacy and safety of high-dose lanreotide autogel in patients with progressive pancreatic or midgut neuroendocrine tumours: CLARINET FORTE phase 2 study results. Eur. J. Cancer 2021, 157, 403–414. [Google Scholar] [CrossRef]
- Chan, D.L.; Ferone, D.; Albertelli, M.; Pavlakis, N.; Segelov, E.; Singh, S. Escalated-dose somatostatin analogues for antiproliferative effect in GEPNETS: A systematic review. Endocrine 2017, 57, 366–375. [Google Scholar] [CrossRef]
- Strosberg, J.; Wolin, E.; Chasen, B.; Kulke, M.; Bushnell, D.; Caplin, M.; Baum, R.P.; Kunz, P.; Hobday, T.; Hendifar, A.; et al. Health-Related Quality of Life in Patients With Progressive Midgut Neuroendocrine Tumors Treated With 177Lu-Dotatate in the Phase III NETTER-1 Trial. J. Clin. Oncol. 2018, 36, 2578–2584. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Halperin, D.; Myrehaug, S.; Herrmann, K.; Pavel, M.; Kunz, P.L.; Chasen, B.; Tafuto, S.; Lastoria, S.; Capdevila, J.; et al. [177Lu]Lu-DOTA-TATE plus long-acting octreotide versus high-dose long-acting octreotide for the treatment of newly diagnosed, advanced grade 2-3, well-differentiated, gastroenteropancreatic neuroendocrine tumours (NETTER-2): An open-label, randomised, phase 3 study. Lancet 2024, 403, 2807–2817. [Google Scholar] [CrossRef] [PubMed]
- Mariën, L.; Islam, O.; Van Mileghem, L.; Lybaert, W.; Peeters, M.; Vandamme, T. Pathophysiology and Treatment of Pancreatic Neuroendocrine Neoplasms (PNENS): New Developments. In Endotext; Feingold, K.R., Ahmed, S.F., Anawalt, B., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2022. [Google Scholar]
- Nobels, F.R.; Kwekkeboom, D.J.; Coopmans, W.; Schoenmakers, C.H.H.; Lindemans, J.; De Herder, W.W.; Krenning, E.P.; Bouillon, R.; Lamberts, S.W.J. Chromogranin A as serum marker for neuroendocrine neoplasia: Comparison with neuron-specific enolase and the alpha-subunit of glycoprotein hormones. J. Clin. Endocrinol. Metab. 1997, 82, 2622–2628. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakos, A.; Libor, L.; Vasas, B.; Apró, K.; Sipka, G.; Pávics, L.; Valkusz, Z.; Maráz, A.; Besenyi, Z. New Horizons: The Evolution of Nuclear Medicine in the Diagnosis and Treatment of Pancreatic Neuroendocrine Tumors—A Case Report. J. Clin. Med. 2025, 14, 4432. https://doi.org/10.3390/jcm14134432
Bakos A, Libor L, Vasas B, Apró K, Sipka G, Pávics L, Valkusz Z, Maráz A, Besenyi Z. New Horizons: The Evolution of Nuclear Medicine in the Diagnosis and Treatment of Pancreatic Neuroendocrine Tumors—A Case Report. Journal of Clinical Medicine. 2025; 14(13):4432. https://doi.org/10.3390/jcm14134432
Chicago/Turabian StyleBakos, Annamária, László Libor, Béla Vasas, Kristóf Apró, Gábor Sipka, László Pávics, Zsuzsanna Valkusz, Anikó Maráz, and Zsuzsanna Besenyi. 2025. "New Horizons: The Evolution of Nuclear Medicine in the Diagnosis and Treatment of Pancreatic Neuroendocrine Tumors—A Case Report" Journal of Clinical Medicine 14, no. 13: 4432. https://doi.org/10.3390/jcm14134432
APA StyleBakos, A., Libor, L., Vasas, B., Apró, K., Sipka, G., Pávics, L., Valkusz, Z., Maráz, A., & Besenyi, Z. (2025). New Horizons: The Evolution of Nuclear Medicine in the Diagnosis and Treatment of Pancreatic Neuroendocrine Tumors—A Case Report. Journal of Clinical Medicine, 14(13), 4432. https://doi.org/10.3390/jcm14134432




