Case Reports and Artificial Intelligence Challenges on Squamous Cell Carcinoma Developed on Chronic Radiodermitis
Abstract
1. Introduction
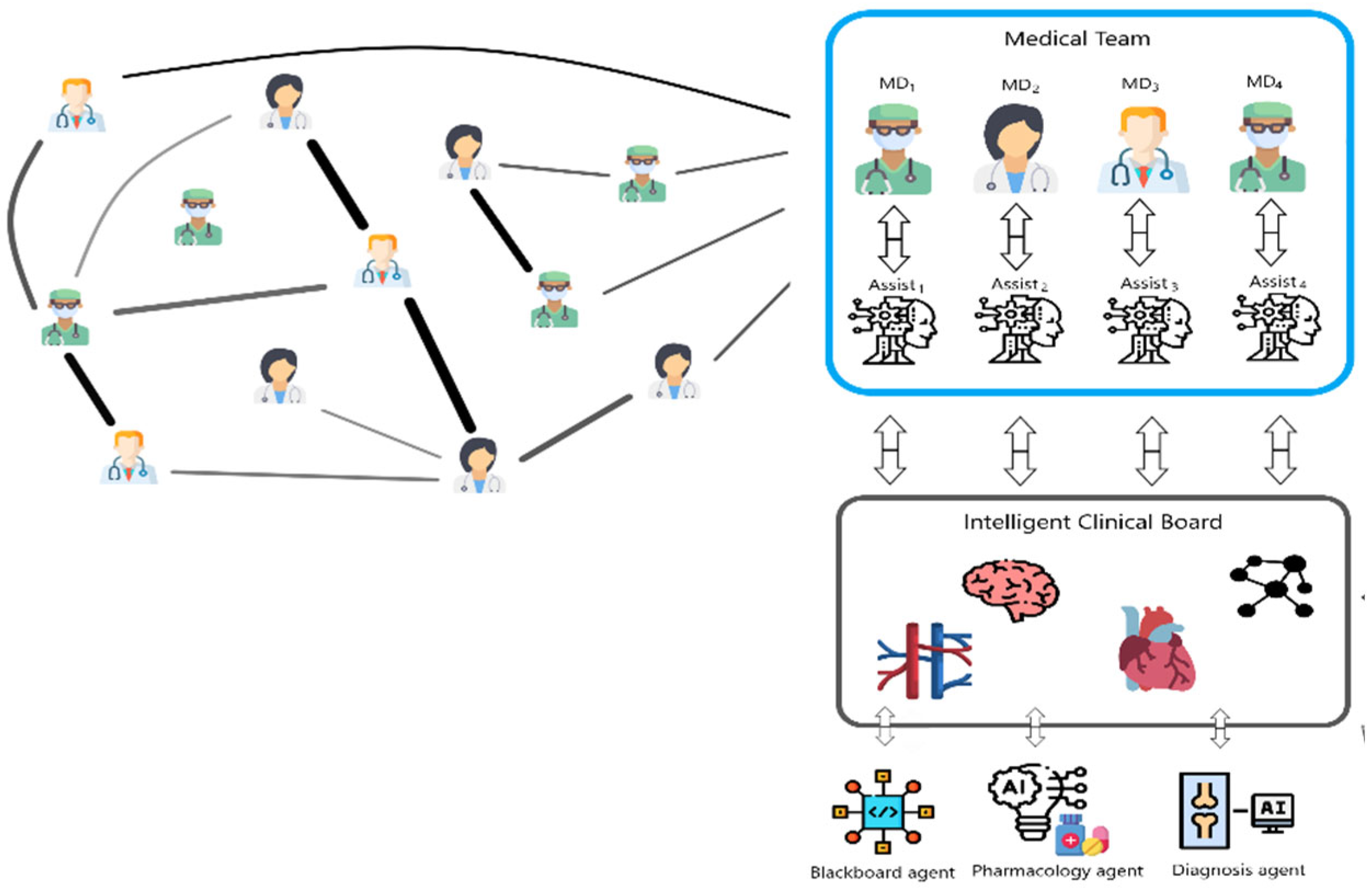
2. Survey on Artificial Intelligence Applications in Decision Support
2.1. AI Methods Applied for the Analysis of External Organs to Provide Decision Support
2.2. AI Methods Applied to Internal Organs to Provide Decision Support
2.3. Concluding Remarks Regarding Research Carried Out Worldwide
3. Cases Presentations
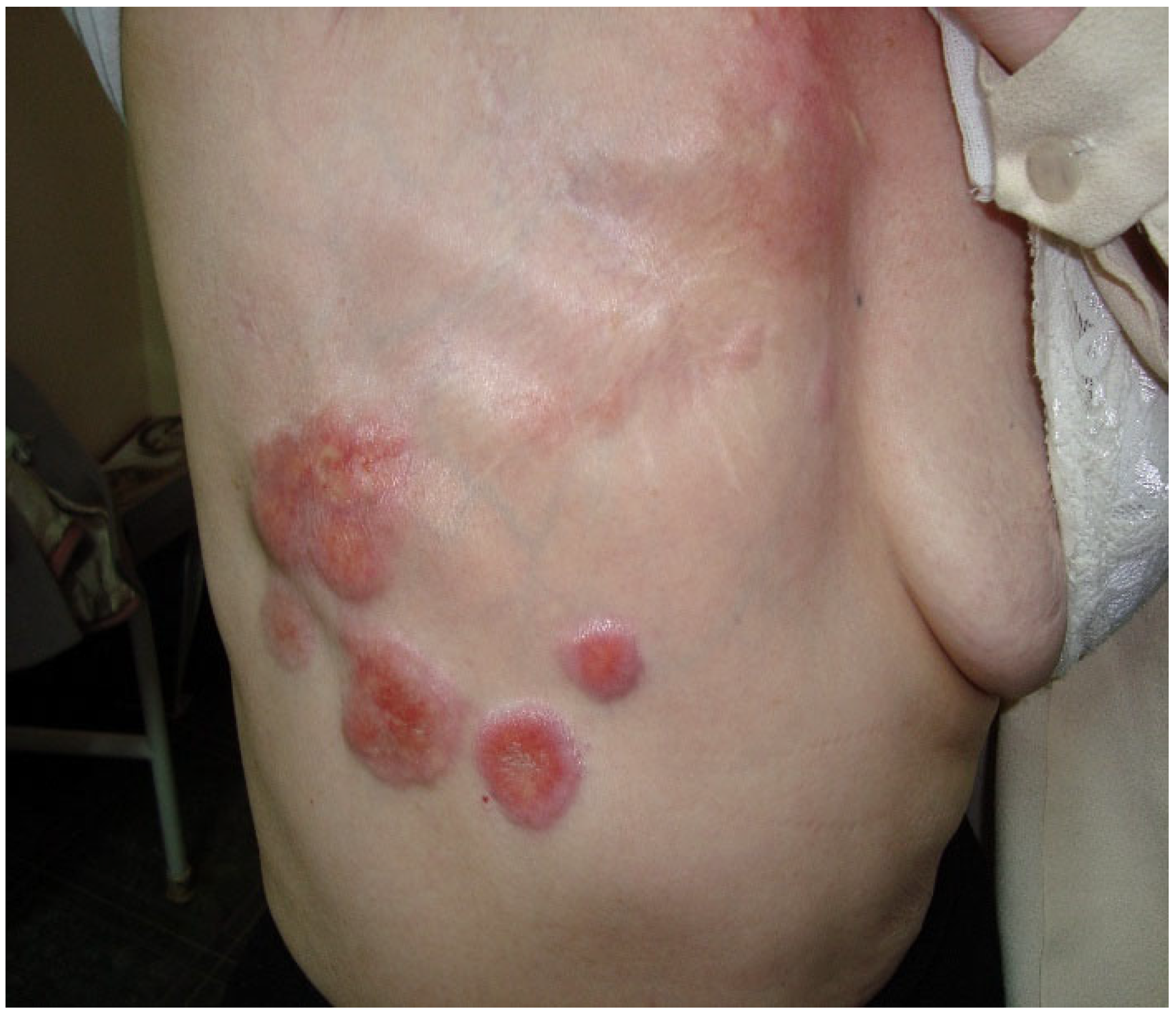
3.1. Case of a 74-Year-Old Female Patient
- Case 1-AI-Suggested AI Further Development
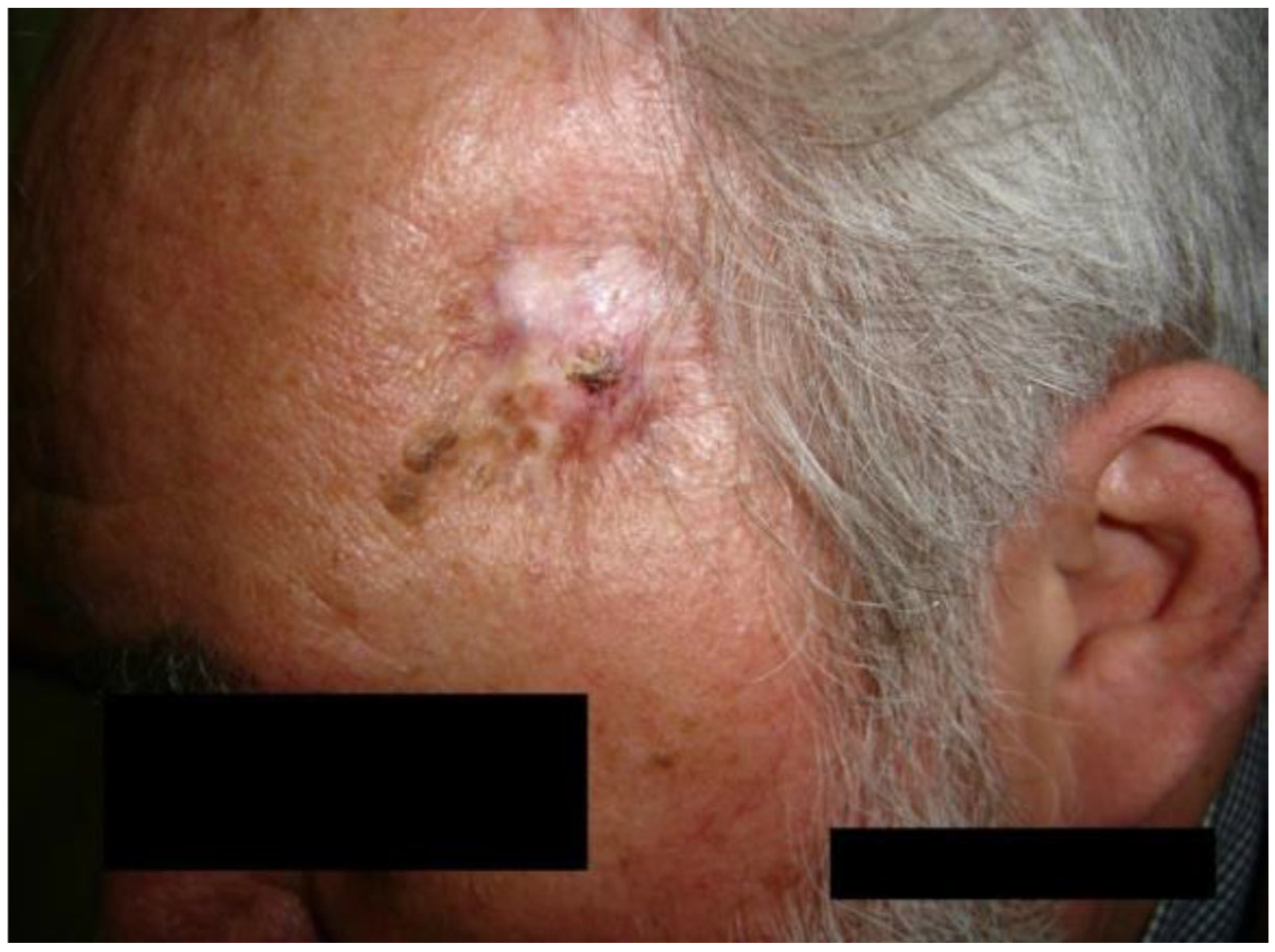
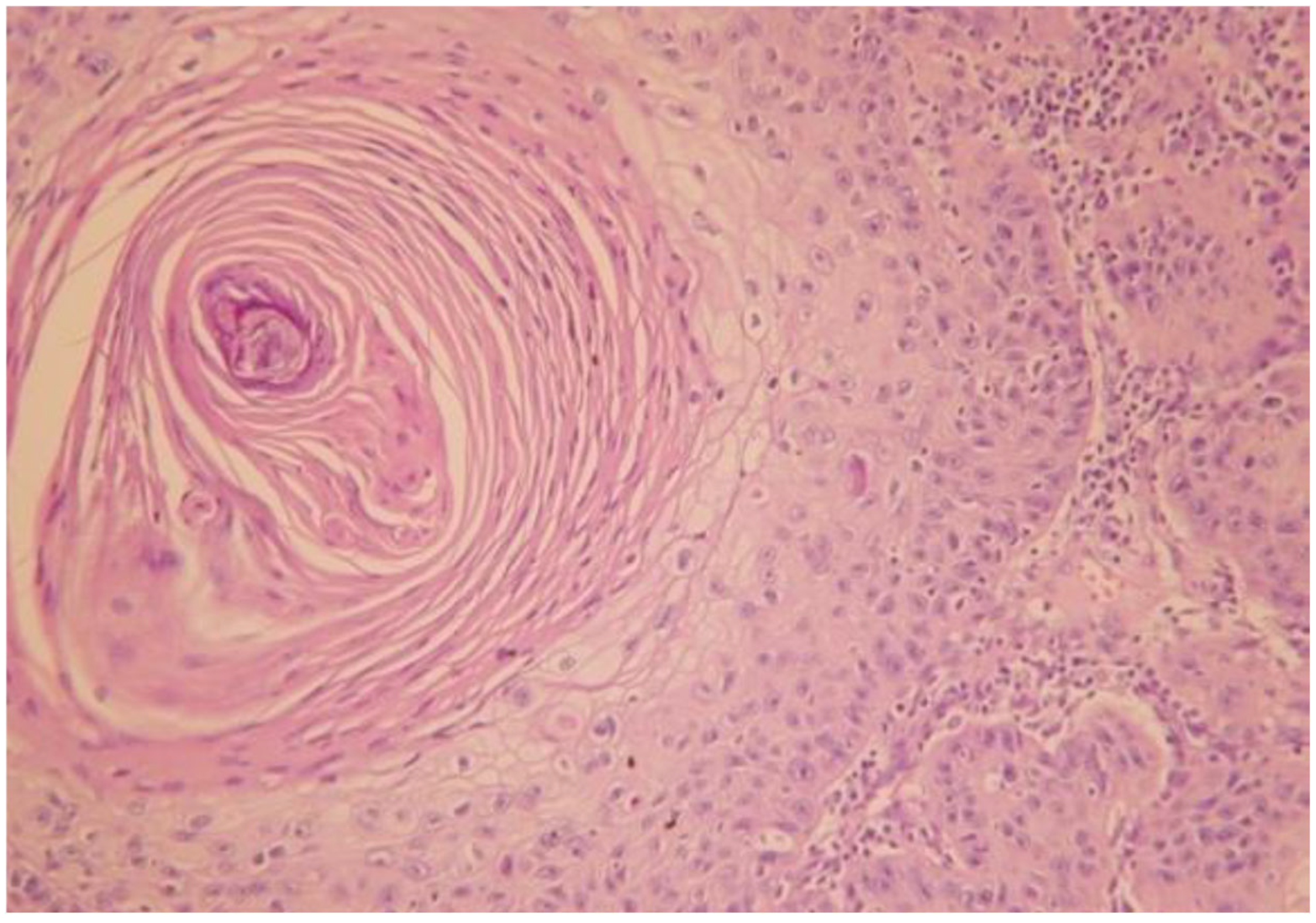
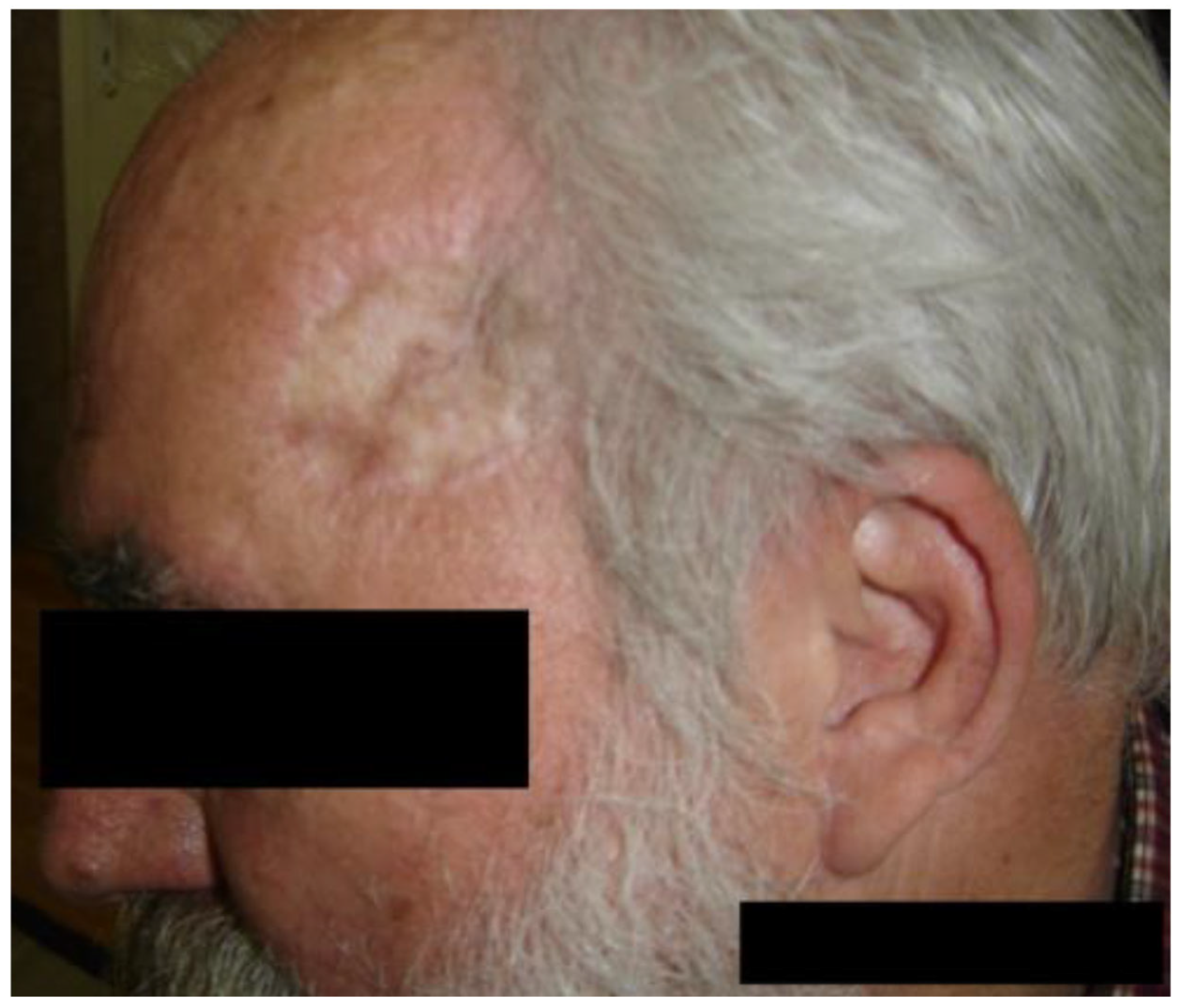
3.2. Case of a 60-Year-Old Male Patient
- Case 2-AI—Suggested AI Future Development
4. Discussion
AI in Decision Support Squamous Cell Carcinoma Developed on Chronic Radiodermitis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malakoutikhah, Z.; Mohaghegh, F.; Derakhshan, M.; Mehdizadeh, M.J. Radio-induced simultaneous development of squamous cell carcinoma and undifferentiated pleomorphic sarcoma of scalp. Clin. Case Rep. 2022, 10, e6058. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Feng, L. Squamous Cell Carcinoma Arising From Chronic Radiodermatitis. J. Cutan. Med. Surg. 2022, 26, 644. [Google Scholar] [CrossRef] [PubMed]
- Ron, E.; Modan, B.; Preston, D.; Alfandary, E.; Stovall, M.; Boice, J.D., Jr. Radiation-induced skin carcinomas of the head and neck. Radiat. Res. 1991, 125, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Hymes, S.R.; Strom, E.A.; Fife, C. Radiation dermatitis: Clinical presentation, pathophysiology, and treatment 2006. J. Am. Acad. Dermatol. 2006, 54, 4616. [Google Scholar] [CrossRef]
- Franchi, A.; Massi, D.; Gallo, O.; Santucci, M.; Porfirio, B. Radiation-induced cutaneous carcinoma of the head and neck: Is there an early role for p53 mutations? Clin. Exp. Dermatol. 2006, 31, 793–798. [Google Scholar] [CrossRef]
- Muller, K.; Meineke, V. Radiation-induced alterations in cytokine production by skin cells. Exp. Hematol. 2007, 35, 96–104. [Google Scholar] [CrossRef]
- Peter, R.U.; Beetz, A.; Ried, C.; van Beuningen, D.; Ruzicka, T.; Michel, G. Increased expression of the epidermal growth factor receptor in human epidermal keratinocytes after exposure to ionizing radiation. Radiat. Res. 1993, 136, 65–70. [Google Scholar] [CrossRef]
- Bernier, J.; Bonner, J.; Vermorken, J.B.; Bensadoun, R.J.; Dummer, R.; Giralt, J.; Kornek, G.; Hartley, A.; Mesia, R.; Robert, C.; et al. Consensus guidelines for the management of radiation dermatitis and coexisting acne-like rash in patients receiving radiotherapy plus EGFR inhibitors for the treatment of squamous cell carcinoma of the head and neck. Ann. Oncol. 2008, 19, 142–149. [Google Scholar] [CrossRef]
- Nedea, E.A.; DeLaney, T.F. Sarcoma and skin radiation oncology. Hematol. Oncol. Clin. N. Am. 2006, 20, 401–429. [Google Scholar] [CrossRef]
- Maalej, M.; Frikha, H.; Kochbati, L.; Bouaouina, N.; Sellami, D.; Benna, F.; Gargouri, W.; Dhraief, S.; Nasr, C.; Daoud, J.; et al. Radio-induced malignancies of the scalp about 98 patients with 150 lesions and literature review. Cancer Radiother. 2004, 8, 81–87. [Google Scholar] [CrossRef]
- Pérez-Baena, M.J.; Mao, J.H.; Pérez-Losada, J.; Santos-Briz, A.; Chang, H.; Cañueto, J. Artificial intelligence-empowered cellular morphometric risk score improves prognostic stratification of cutaneous squamous cell carcinoma. Clin. Exp. Dermatol. 2023, 49, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Schmults, C.D.; Karia, P.S.; Carter, J.B.; Han, J.; Qureshi, A.A. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: A 10-year, single-institution cohort study. JAMA Dermatol. 2013, 149, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Arik, S.; Iantovics, L.B. Next Generation Hybrid Intelligent Medical Diagnosis Systems, 24th International Conference. In Neural Information Processing (ICONIP 2017), Guangzhou, China, November 14–18, 2017, Proceedings, Part III; Liu, D., Xie, S., Li, Y., Zhao, D., El-Alfy, E.S., Eds.; Lecture Notes in Computer Science, Springer Nature: Cham, Switzerland, 2017; Volume 10636, pp. 903–912. [Google Scholar]
- Knuutila, J.S.; Riihilä, P.; Karlsson, A.; Tukiainen, M.; Talve, L.; Nissinen, L.; Kähäri, V.M. Identification of metastatic primary cutaneous squamous cell carcinoma utilizing artificial intelligence analysis of whole slide images. Sci. Rep. 2022, 12, 9876. [Google Scholar] [CrossRef]
- Brorsen, L.F.; McKenzie, J.S.; Tullin, M.F.; Bendtsen, K.M.S.; Pinto, F.E.; Jensen, H.E.; Haedersdal, M.; Takats, Z.; Janfelt, C.; Lerche, C.M. Cutaneous squamous cell carcinoma characterized by MALDI mass spectrometry imaging in combination with machine learning. Sci. Rep. 2024, 14, 11091. [Google Scholar] [CrossRef]
- Karadaghy, O.A.; Shew, M.; New, J.; Bur, A.M. Development and Assessment of a Machine Learning Model to Help Predict Survival Among Patients With Oral Squamous Cell Carcinoma. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Alabi, R.O.; Youssef, O.; Pirinen, M.; Elmusrati, M.; Mäkitie, A.A.; Leivo, I.; Almangush, A. Machine learning in oral squamous cell carcinoma: Current status, clinical concerns and prospects for future—A systematic review. Artif. Intell. Med. 2021, 115, 102060. [Google Scholar] [CrossRef]
- Iantovics, L.B.; Enăchescu, C. Method for Data Quality Assessment of Synthetic Industrial Data. Sensors 2022, 22, 1608. [Google Scholar] [CrossRef]
- Zhang, K.; Ye, B.; Wu, L.; Ni, S.; Li, Y.; Wang, Q.; Zhang, P.; Wang, D. Machine learning?based prediction of survival prognosis in esophageal squamous cell carcinoma. Sci. Rep. 2023, 13, 13532. [Google Scholar] [CrossRef]
- Joon, H.K.; Thalor, A.; Gupta, D. Machine learning analysis of lung squamous cell carcinoma gene expression datasets reveals novel prognostic signatures. Comput. Biol. Med. 2023, 165, 107430. [Google Scholar] [CrossRef]
- Pacheco, B.R.; Tokez, S.; Bramer, E.M.; Venables, Z.C.; van de Werken, H.J.G.; Bellomo, D.; van Klaveren, D.; Mooyaart, A.L.; Hollestein, L.M.; Wakkee, M. Personalised decision making to predict absolute metastatic risk in cutaneous squamous cell carcinoma: Development and validation of a clinico-pathological model. eClinicalMedicine 2023, 63, 102150. [Google Scholar] [CrossRef]
- Li, Z.; Koban, K.C.; Schenck, T.L.; Giunta, R.E.; Li, Q.; Sun, Y. Artificial Intelligence in Dermatology Image Analysis: Current Developments and Future Trends. J. Clin. Med. 2022, 11, 6826. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Amagai, M.; Bruckner, A.L.; Enk, A.H.; Margolis, D.J.; McMichael, A.J.; Orringer, J.S. (Eds.) Fitzpatrick’s Dermatology, 9e; McGraw-Hill Education: New York, NY, USA, 2019; pp. 1901–1919. [Google Scholar]
- Lallas, A.; Apalla, Z.; Lazaridou, E.; Ioannides, D. Dermatoscopy A–Z, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 96–103. [Google Scholar]
- Brinkman, J.N.; Hajder, E.; van der Holt, B.; Den Bakker, M.A.; Hovius, S.E.; Mureau, M.A. The effect of differentiation grade of cutaneous squamous cell carcinoma on excision margins, local recurrence, metastasis, and patient survival: A retrospective follow-up study. Ann. Plast. Surg. 2015, 75, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Bolognia, J.L.; Jorizzo, J.J.; Schaffer, J.V.; Callen, J.P.; Cerroni, L.; Heymann, W.R.; Hruza, G.J.; Mancini, A.J.; McGrath, J.; Patterson, J.W.; et al. Dermatology, 4th ed.; Elsevier: London, UK, 2012; pp. 1858–1892. [Google Scholar]
- Kallini, J.R.; Hamed, N.; Khachemoune, A. Squamous cell carcinoma of the skin: Epidemiology, classification, management, and novel trends. Int. J. Dermatol. 2014, 54, 130–140. [Google Scholar] [CrossRef]
- Fekete, G.L.; Fekete, J.E. Steatocystoma multiplex generalisata partially suppurativa—Case report. Acta Dermatovenerol. Croat. 2010, 18, 114–119. [Google Scholar] [PubMed]
- Rogers, H.W.; Weinstock, M.A.; Harris, A.R.; Hinckley, M.R.; Feldman, S.R.; Fleischer, A.B.; Coldiron, B.M. Incidence estimate of nonmelanoma skin cancer in the UnitedStates, 2006. Arch. Dermatol. 2010, 146, 283–287. [Google Scholar] [CrossRef]
- Yu, H.S.; Liao, W.T.; Chai, C.Y. Arsenic carcinogenesis in the skin. J. Biomed. Sci. 2006, 13, 657–666. [Google Scholar] [CrossRef]
- Davis, M.M.; Hanke, C.W.; Zollinger, T.W.; Montebello, J.F.; Hornback, N.B.; Norins, A.L. Skin cancer in patients with chronic radiation dermatitis. J. Am. Acad. Dermatol. 1989, 20, 608–616. [Google Scholar] [CrossRef]
- Euvrard, S.; Kanitakis, J.; Claudy, A. Skin cancers after organ transplantation. N. Engl. J. Med. 2003, 348, 1681–1691. [Google Scholar] [CrossRef]
- Kivisaari, A.; Kahari, V.M. Squamous cell carcinoma of the skin: Emerging need for novel biomarkers. World J. Clin. Oncol. 2013, 4, 85–90. [Google Scholar] [CrossRef]
- Lichter, M.D.; Karagas, M.R.; Mott, L.A.; Spencer, S.K.; Stukel, T.A.; Greenberg, E.R. Therapeutic ionizing radiation and the incidence of basal cell carcinoma and squamous cell carcinoma. Arch. Dermatol. 2000, 136, 1007–1011. [Google Scholar] [CrossRef]
- Shore, R.E.; Moseson, M.; Xue, X.; Tse, Y.; Harley, N.; Pasternack, B.S. Skin cancer after X-ray treatment for scalp ringworm. Radiat. Res. 2002, 157, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Iantovics, L.B. Agent-Based Medical Diagnosis Systems. Comput. Inform. 2008, 27, 593–625. [Google Scholar]
- Huang, K.A.; Choudhary, H.K.; Kuo, P.C. Artificial Intelligent Agent Architecture and Clinical Decision-Making in the Healthcare Sector. Cureus J. Med. Sci. 2024, 16, e64115. [Google Scholar] [CrossRef] [PubMed]
- Calisto, F.M.; Nunes, N.; Nascimento, J.C. Modeling adoption of intelligent agents in medical imaging. Int. J. Hum. Comput. Stud. 2022, 168, 102922. [Google Scholar] [CrossRef]
- Calisto, F.M.; Santiago, C.; Nunes, N.; Nascimento, J.C. BreastScreening-AI: Evaluating medical intelligent agents for human-AI interactions. Artif. Intell. Med. 2022, 127, 102285. [Google Scholar] [CrossRef]
- Erman, L.D.; Hayes-Roth, F.; Lesser, V.R.; Reddy, D.R. The Hearsay-II Speech-Understanding System: Integrating Knowledge to Resolve Uncertainty. ACM Comput. Surveys. 1980, 12, 213. [Google Scholar] [CrossRef]
- Hayes-Roth, B. A blackboard architecture for control. Artif. Intell. 1985, 26, 251–321. [Google Scholar] [CrossRef]
- Zamfirescu, C.B.; Duta, L.; Iantovics, B. On Investigating the Cognitive Complexity of Designing the Group Decision Process. Stud. Inform. Control 2010, 19, 263–270. [Google Scholar] [CrossRef]
- Smith, R.G. The Contract Net Protocol: High-Level Communication and Control in a Distributed Problem Solver. IEEE Trans. Comput. 1980, C-29, 1104–1113. [Google Scholar] [CrossRef]
- Goktas, P.; Grzybowski, A. Assessing the Impact of ChatGPT in Dermatology: A Comprehensive Rapid Review. J. Clin. Med. 2024, 13, 5909. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fekete, G.L.; Iantovics, L.B.; Fekete, J.E.; Fekete, L. Case Reports and Artificial Intelligence Challenges on Squamous Cell Carcinoma Developed on Chronic Radiodermitis. J. Clin. Med. 2025, 14, 3921. https://doi.org/10.3390/jcm14113921
Fekete GL, Iantovics LB, Fekete JE, Fekete L. Case Reports and Artificial Intelligence Challenges on Squamous Cell Carcinoma Developed on Chronic Radiodermitis. Journal of Clinical Medicine. 2025; 14(11):3921. https://doi.org/10.3390/jcm14113921
Chicago/Turabian StyleFekete, Gyula László, Laszlo Barna Iantovics, Júlia Edit Fekete, and László Fekete. 2025. "Case Reports and Artificial Intelligence Challenges on Squamous Cell Carcinoma Developed on Chronic Radiodermitis" Journal of Clinical Medicine 14, no. 11: 3921. https://doi.org/10.3390/jcm14113921
APA StyleFekete, G. L., Iantovics, L. B., Fekete, J. E., & Fekete, L. (2025). Case Reports and Artificial Intelligence Challenges on Squamous Cell Carcinoma Developed on Chronic Radiodermitis. Journal of Clinical Medicine, 14(11), 3921. https://doi.org/10.3390/jcm14113921







