Abstract
Background: Prognostic markers have not been extensively studied in plastic and reconstructive surgery. Objective: We aimed to evaluate the prognostic value of preoperative C-reactive protein (CRP)-to-albumin ratio (CAR) in plastic and reconstructive surgery and to compare it with the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and modified Glasgow prognostic score (mGPS). Methods: From January 2011 to July 2019, we identified 2519 consecutive adult patients who were undergoing plastic and reconstructive surgery with available preoperative CRP and albumin levels. The receiver operating characteristic (ROC) curve was generated to evaluate predictability and estimate the threshold. The patients were divided according to this threshold, and the risk was compared. The primary outcome was one-year mortality, and the overall mortality was also analyzed. Results: The one-year mortality was 4.9%. The CAR showed an area under the ROC curve of 0.803, which was higher than those of NLR, PLR, and mGPS. According to the estimated threshold of 1.05, the patients were divided into two groups; 1585 (62.9%) were placed in the low group, and 934 (37.1%) were placed in the high group. After inverse probability weighting, the mortality rate during the first year after plastic and reconstructive surgery was significantly increased in the high group (1.3% vs. 10.9%; hazard ratio, 2.88; 95% confidence interval, 2.17–3.83; p < 0.001). Conclusions: In this study, high CAR was significantly associated with one-year mortality of patients after plastic and reconstructive surgery. Further studies are needed on prognostic markers in plastic and reconstructive surgery.
1. Introduction
Plastic and reconstructive surgery involves many applications and complex procedures. However, these surgeries are generally considered relatively safe, with a low risk of death [1]. Improvements in surgical and anesthetic techniques have increased the safety of plastic and reconstructive surgery. As a result, deaths from these procedures are rare; however, when they do occur, they can be costly. While the risks associated with other types of major surgery have been thoroughly studied, the risk factors for perioperative mortality in plastic and reconstructive surgery have not been as extensively researched [2].
Several simplified markers have been proposed for predicting prognosis, including biologic markers, such as C-reactive protein (CRP)-to-albumin ratio (CAR), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and modified Glasgow prognostic score (mGPS) [3,4,5,6,7]. In fact, NLR has been significantly associated with mortality following plastic and reconstructive surgery in our previous study [8]. Recently, increasing evidence has indicated that combining markers of inflammatory response with nutritional condition could better reflect clinical prognosis. In particular, the CAR has been demonstrated as a prognostic marker of various clinical situations, but it has not been validated in plastic and reconstructive surgery [7]. Therefore, we enrolled patients undergoing plastic and reconstructive surgery with available preoperative CRP and albumin levels and evaluated whether preoperative CAR was associated with postoperative mortality. We also compared the predictive value with biomarkers of NLR, PLR, and mGPS. Our findings may help clinicians to predict postoperative outcomes using a simple index.
2. Materials and Methods
This study was a retrospective observational study that was approved by the Institutional Review Board at Samsung Medical Center (SMC 2020-09-001). The requirement for written informed consent was waived by the Institutional Review Board at Samsung Medical Center because we extracted the data in de-identified form. We conducted this research following the Declaration of Helsinki and reported the results according to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
2.1. Study Population and Data Collection
We enrolled consecutive adult patients who underwent plastic and reconstructive surgery at Samsung Medical Center between January 2011 and July 2019. We excluded patients without preoperative CRP and albumin levels. The data were extracted in a de-identified form from our electronic archive system, Clinical Data Warehouse Darwin-C. This electronic system allows researchers to review and retrieve data for investigation from electronic hospital records and contains more than 2.2 million surgeries, 1 billion laboratory findings, 100 million disease codes, and 200 million prescriptions. The results of blood laboratory tests were automatically extracted for analysis. In this study, the mortality data from other hospitals were regularly checked and updated using the National Population Registry of the Korea National Statistical Office, so there was no missing information on deaths. The medical records were reviewed by investigators who were blind to patient mortality to avoid bias.
2.2. Study Outcomes and Definitions
The primary endpoint in this study was mortality during a one-year follow-up period that began immediately after surgery. The secondary outcome was mortality during the overall follow-up period. The Charlson Comorbidity Index was calculated using the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) [9].
According to our institutional protocol, patients exhibiting clinical symptoms suggestive of infection undergo a comprehensive evaluation, including blood laboratory tests, before surgery. The attending clinician assesses the risks and benefits of surgical procedures, and at their discretion, patients may be referred to the Department of Infectious Diseases for further evaluation.
2.3. Statistical Analysis
The baseline characteristics are described using the mean (±standard deviation) or median (interquartile range) for continuous variables and the number and percentage of categorical variables. To compare differences between the two groups, the t-test or Mann–Whitney U test was used for continuous variables, and the chi-square or Fisher’s exact test was employed for categorical variables. To evaluate the predictability of CAR, NLR, and PLR results and their association with one-year mortality, receiver operating characteristics (ROC) curve analysis was performed, and Youden’s Index was used to estimate the optimal cut-off. We compared the predictability using the comparison between the area under the ROC curve (AUC) using a nonparametric approach [10]. The predictabilities were also evaluated according to surgery type and presence of malignancy. Based on the estimated cut-off value of CAR, the study patients were classified into either a low or a high group. The mortalities were compared with Cox regression analysis. To control for selection bias and other confounding factors, we adjusted for differences in baseline patient characteristics using inverse probability weighting (IPW). This technique involves the use of weights that are the reciprocal of the propensity scores to balance the groups. A difference <10% in absolute standardized difference (ASD) between the groups was considered a balanced comparison [11]. The differences in mortality were reported as hazard ratios (HRs) and 95% confidence intervals (CIs) using Cox proportional hazards analysis with IPW. The power of analysis was evaluated based on sample size [12]. Kaplan–Meier estimates were used to create survival curves, which were compared using the log-rank test. All statistical analyses were performed using R 4.0.2 (Vienna, Austria; http://www.R-project.org/, accessed on 27 February 2024). All tests were two-tailed, and p < 0.05 was considered statistically significant.
3. Results
Of the 7089 patients who underwent plastic and reconstructive surgery from January 2011 to July 2019 at our institution, 2519 patients with available preoperative CRP and albumin levels were enrolled for analysis. The mean values of CRP were 1.33 for head and neck surgery, 1.18 for trunk and extremities surgery, 1.42 for breast surgery, 1.21 for aesthetic surgery, and 1.32 for mass excision surgery. We divided the study patients into survivors and non-survivors during the one-year follow-up period after surgery. The median follow-up period for one-year mortality was 365 (365–365) days, and the median overall follow-up period was 1055 (371–2029) days. The one-year mortality was 4.9%, and the baseline characteristics according to one-year mortality are summarized in Table 1. The median value of CAR was much higher in the non-survivor group (0.38 vs. 7.86), as were NLR (1.93 vs. 4.56) and PLR (7.90 vs. 11.17), respectively.

Table 1.
Baseline characteristics according to one-year mortality.
We generated the ROC curve for each index, and the AUC of CAR, NLR, PLR, and mGPS were 0.803, 0.785, 0.620, and 0.729, respectively (Figure 1). In the AUC comparison, CAR showed no significant difference from NLR (z = 0.84; p = 0.401). Based on the maximum Youden’s Index, the optimal cut-off threshold values of CAR, NLR, and PLR were 1.05, 3.04, and 13.55 for one-year mortality, respectively. The ROC curves were also generated for overall mortality (Figure 1), and the AUC of CAR was significantly higher than that of NLR (0.719 vs. 0.672; z = 2.45; p = 0.01). In the subgroup analysis according to surgery type, the AUC of CAR was 0.827 for head and neck surgery, 0.772 for trunk and extremities surgery, 0.785 for breast surgery, 0.805 for aesthetic surgery, and 0.868 for mass excision surgery. The estimated thresholds were 1.08, 1.69, 1.05, 0.81, and 0.87, respectively (Figure 2). In patients without malignancy, the AUC of CAR, NLR, PLR, and mGPS were 0.819, 0.794, 0.617, and 0.745, respectively.
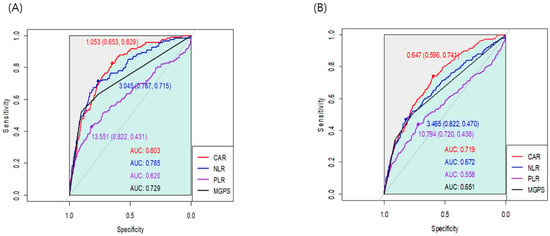
Figure 1.
Receiver operating characteristic curves for the associations of CAR, NLR, PLR, and mGPS with (A) one-year mortality and (B) overall mortality.
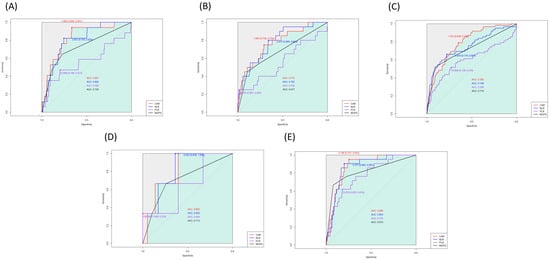
Figure 2.
Receiver operating characteristic curves for the associations of CAR with one-year mortality in (A) head and neck, (B) trunk and extremities, (C) breast, (D) aesthetic surgery, and (E) mass excision.
The patients were divided according to the estimated threshold of CAR associated with one-year mortality. Using a CAR of 1.05, 1585 (62.9%) patients were classified into the low group, and 934 (37.1%) were placed into the high group (Table 2). The high group tended to be older with more habitual risk factors. The incidence rates of the underlying disease and the Charlson Comorbidity Index were also higher in the high group. After an adjustment using an IPW technique, ASD < 10% was suggested to offer a successful balance of all covariates between the groups. The unadjusted analysis showed an increased incidence and risk of mortality in the high group for one year (1.3% vs. 10.9%; HR: 9.04; 95% CI: 5.65–14.45; p < 0.001) and overall (5.4% vs. 17.7%; HR: 3.97; 95% CI: 3.06–5.16; p < 0.001) follow-up periods (Table 3). An IPW analysis revealed similar results (HR: 2.88; 95% CI: 2.17–3.83; p < 0.001 for one year and HR, 5.48; 95% CI: 3.33–9.03; p < 0.001 for overall mortality). The survival curves are presented in Figure 3. Based on the sample size, the power of analysis was 0.99 when HR was >1.2.

Table 2.
Baseline characteristics according to the estimated cut-off point of C-reactive protein/albumin ratio > 1.05.

Table 3.
Mortalities according to the estimated cut-off point of C-reactive protein/albumin ratio > 1.05.
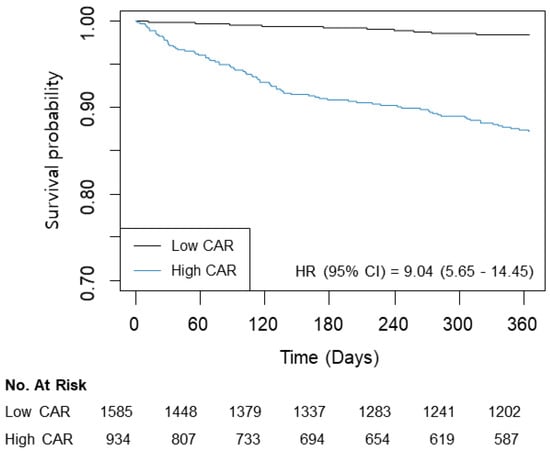
Figure 3.
Kaplan–Meier curves for one-year mortality according to CAR (≤1.05/>1.05).
4. Discussion
The main finding of this study was that preoperative CAR was associated with increased mortality. The estimated threshold of CAR was 1.05 for one-year mortality, and the predictability was comparable with that of NLR. For overall mortality, the AUC of CAR was even higher than that of NLR. Our results suggest that CAR before plastic and reconstructive surgery may be used as a simple biomarker of mortality that is readily available and relatively inexpensive.
Plastic and reconstructive surgeries involve a wide range of procedures and body areas, but patients undergoing these surgeries are generally healthier compared to those who undergo other types of major surgeries. Therefore, not much attention has been given to predicting the risks for patients based on preoperative conditions. Recently, several biomarkers that combine various laboratory tests have been proposed to predict the prognosis of patients preoperatively [3,4,5,6,7]. Our previous study also indicated that NLR was associated with postoperative mortality in patients undergoing plastic and reconstructive surgery [8], but various biomarkers have not been thoroughly studied.
In this study, CAR showed a good predictability for mortality during the one-year follow-up after surgery. CAR is a new and promising biomarker that is determined by the ratio of CRP and albumin levels in the blood. It is a combination of inflammatory- or nutrition-related markers, which have been widely evaluated in various clinical situations [3,7,13,14]. The presence of inflammation and nutritional impairment are well-known risk factors for poor prognosis. The presence of infection, comorbidities, and malnutrition are shown to induce inflammation and deterioration of a patient’s nutritional status [15,16,17], and this is likely to also be found in patients anticipating a scheduled surgery. In surgical patients, pre-existing inflammation can worsen malnutrition and increase the inflammatory response to surgical injuries [18], which can have a significant impact on mortality.
Another notable finding was that CAR showed a significantly higher AUC for overall mortality compared with NLR, which suggests better predictability of a longer follow-up period. By combining CRP with albumin level, CAR reflects both the inflammatory and nutritional status of patients prior to surgery. A reflection of nutritional status may explain why CAR had a greater predictive value compared to other biomarkers, particularly for a long-term follow-up period. In addition, the time required to produce a noticeable decrease in nutritional status may be enough to have a significant effect on patient recovery. Other factors, such as the patient’s comorbidities and response to treatment, can play a more decisive role in their short-term outcome. The estimated thresholds for CAR varied significantly depending on the duration of the follow-up period. Further research is needed before CAR can be adopted into daily practice. The goal of our analysis is not to establish superiority among these variables in predicting mortality. Instead, we emphasize that CAR is independently associated with postoperative mortality. It may prove particularly helpful in clinical situations where the risk is not well-reflected in patient age or comorbidity.
The versatility of the CAR in predicting outcomes in plastic and reconstructive surgery is a noteworthy aspect highlighted in our study. Our subgroup analysis reveals an AUC across a diverse spectrum of surgical procedures. This is particularly significant in plastic and reconstructive surgery, where patients undergo various interventions in diverse medical conditions. Notably, almost half of the patients experiencing one-year mortality underwent wound debridement, indicating a likelihood of infectious complications. This suggests that CAR could serve as a valuable predictor of mortality, offering insights less influenced by age or chronic conditions. Conversely, in aesthetic procedures, more than one-third of patients exhibited elevated CAR, yet only three cases resulted in one-year mortality. This distinction underscores the need for a nuanced discussion regarding the safety of elective aesthetic surgeries. Our analysis identified specific thresholds for CAR based on surgery type, emphasizing the necessity for future studies to establish tailored thresholds for different surgical interventions.
The advantage of CAR in plastic and reconstructive surgeries shown in this study was that it could be used regardless of a wide range of surgical procedures. Our subgroup analysis demonstrated the AUC that suggests that CAR could be used to predict mortality regardless of surgery type. This indeed is a notable finding especially in plastic and reconstructive surgery, because it covers a wide variety of surgical procedures in patients with various medical conditions. In our analysis, almost half of patients with mortality showed one-year mortality. Most of these patients underwent wound debridement and were likely to be infectious. This suggests that CAR could be useful in predicting mortality that is less affected by age or chronic conditions. On the other hand, more than 1/3 of aesthetic patients showed high CAR, while only three patients showed one-year mortality. Moreover, the estimated threshold for CAR was different according to surgery type. So, future studies may need to establish different thresholds for more detailed surgery types.
The mGPS scoring system uses the same laboratory variables as CAR. The difference is that the mGPS assigns patients to a certain category rather than providing a specific value. The system is simple, and the score is considered one of the most effective biomarkers for predicting patient prognosis. Several studies have demonstrated similar prognostic values and performance for mGPS and CAR, particularly in patients with cancer [19,20]. However, our results indicated that CAR had better predictive value than mGPS. This finding may be related to the mGPS score of 0 of most of our study patients. Since the CAR can further stratify patients using a continuous numerical value, it may be a more sensitive marker in relatively healthier populations, such as ours. More research is needed to better understand the relationships between inflammation and nutrition-based markers and the mortality of surgical patients.
There are a few limitations that should be taken into account when interpreting the results of our study. First, it was a retrospective single-center study, so our results may have been influenced by factors that we were unable to control. Although we conducted statistical adjustments, the effects of unknown variables could not be taken into account. Second, preoperative CRP and albumin levels were selectively measured, which could have caused bias. In addition, due to the long study period, changes in surgical techniques and postoperative care may have affected the results. Third, different thresholds were estimated according to surgery type. Considering the wide variety of plastic and reconstructive surgical procedures, we acknowledge that more detailed analyses of specific surgery types may be warranted in future studies to provide a comprehensive understanding. Lastly, our study does not suggest a treatment plan for patients with elevated biomarkers. As a result, the optimal threshold cannot be established from our analysis. Further research in the form of a prospective randomized trial is needed. Despite these limitations, this is the first study to thoroughly examine biomarkers regarding their association with mortality after plastic and reconstructive surgery.
In conclusion, a high CAR value was significantly associated with one-year patient mortality after plastic and reconstructive surgery. Further studies are necessary to explore other potential prognostic markers in plastic and reconstructive surgery.
Author Contributions
All authors met all the criteria for the definition of authorship and contributed substantially to the manuscript. A.R.O. and H.M.S. wrote the draft of the paper. M.J. analyzed and interpreted the data. G.J. and S.M.K. curated the data, and J.P. and S.M.L. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This retrospective cohort study was approved by the Institutional Review Board at Samsung Medical Center (SMC 2020-09-001).
Informed Consent Statement
The requirement for written informed consent was waived because the data were collected retrospectively in de-identified form.
Data Availability Statement
All relevant data are provided within the paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Whizar-Lugo, V.M.; Cárdenas-Maytorena, A.C. Anesthesia Topics for Plastic and Reconstructive Surgery; IntechOpen: Rijeka, Croatia, 2018; Available online: https://www.intechopen.com/chapters/64328 (accessed on 1 February 2024).
- Whizar-Lugo, V.M.; Campos-León, J.; Moreno-Guillen, A. Perioperative Complications in Plastic Surgery. In Anesthesia Topics for Plastic and Reconstructive Surgery; IntechOpen: Rijeka, Croatia, 2018; Available online: https://www.intechopen.com/chapters/64750 (accessed on 1 February 2024).
- Bora Makal, G.; Yıldırım, O. Are the C-reactive protein/albumin ratio (CAR), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (NLR) novel inflammatory biomarkers in the early diagnosis of postoperative complications after laparoscopic sleeve gastrectomy? Obes. Res. Clin. Pract. 2020, 14, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, Y.; Zhou, L.; Dai, Y.; Hu, G. High pretreatment neutrophil-to-lymphocyte ratio as a predictor of poor survival prognosis in head and neck squamous cell carcinoma: Systematic review and meta-analysis. Head Neck 2019, 41, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Noma, D.; Masuda, H.; Masuda, M. Preoperative inflammation-based scores predict early recurrence after lung cancer resection. J. Thorac. Dis. 2021, 13, 2812–2823. [Google Scholar] [CrossRef] [PubMed]
- Mungan, İ.; Dicle, Ç.B.; Bektaş, Ş.; Sarı, S.; Yamanyar, S.; Çavuş, M.; Turan, S.; Bostancı, E.B. Does the preoperative platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio predict morbidity after gastrectomy for gastric cancer? Mil. Med. Res. 2020, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zhao, C.; Lu, J.; Huang, X.; Chen, C.; Lin, X. Evaluation of preoperative inflammation-based prognostic scores in patients with intrahepatic cholangiocarcinoma: A multicenter cohort study. Front. Oncol. 2021, 11, 672607. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.M.; Lee, S.H.; Oh, A.R.; Kim, S.; Kim, J.; Gook, J.; Jang, J.N.; Park, J. Association between preoperative neutrophil-lymphocyte ratio and mortality after plastic and reconstructive surgery. Sci. Rep. 2021, 11, 21541. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, V.; Henderson, T.; Perry, C.; Muggivan, A.; Quan, H.; Ghali, W.A. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J. Clin. Epidemiol. 2004, 57, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef] [PubMed]
- Latouche, A.; Porcher, R.; Chevret, S. Sample size formula for proportional hazards modelling of competing risks. Stat. Med. 2004, 23, 3263–3274. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Chen, C.; Xia, Y.; Bi, X.; Liu, P.; Zhang, F.; Yang, H.; An, X.; Jiang, W.; Wang, F. The Ratio of C-Reactive Protein/Albumin is a Novel Inflammatory Predictor of Overall Survival in Cisplatin-Based Treated Patients with Metastatic Nasopharyngeal Carcinoma. Dis. Markers 2017, 2017, 6570808. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, S.; Zhao, X.; Fang, E.; Zhao, X. The value of C-reactive protein as an independent prognostic indicator for disease-specific survival in patients with soft tissue sarcoma: A meta-analysis. PLoS ONE 2019, 14, e0219215. [Google Scholar] [CrossRef] [PubMed]
- Chiappetta, S.; Jamadar, P.; Stier, C.; Bottino, V.; Weiner, R.A.; Runkel, N. The role of C-reactive protein after surgery for obesity and metabolic disorders. Surg. Obes. Relat. Dis. 2020, 16, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Fedewa, M.V.; Hathaway, E.D.; Ward-Ritacco, C.L. Effect of exercise training on C reactive protein: A systematic review and meta-analysis of randomised and non-randomised controlled trials. Br. J. Sports Med. 2017, 51, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Königsbrügge, O.; Posch, F.; Riedl, J.; Reitter, E.M.; Zielinski, C.; Pabinger, I.; Ay, C. Association between Decreased Serum Albumin with Risk of Venous Thromboembolism and Mortality in Cancer Patients. Oncologist 2016, 21, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Dolan, R.D.; McSorley, S.T.; Horgan, P.G.; Laird, B.; McMillan, D.C. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: Systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2017, 116, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Liu, S.; Feng, J.; Zhao, X. Prognostic Value of Inflammation Biomarkers for Survival of Patients with Neuroblastoma. Cancer Manag. Res. 2020, 12, 2415–2425. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xu, D.; Song, H.; Qiu, B.; Tian, D.; Li, Z.; Ji, Y.; Wang, J. Inflammation and nutrition-based biomarkers in the prognosis of oesophageal cancer: A systematic review and meta-analysis. BMJ Open 2021, 11, e048324. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).