1. Introduction
As the clinical indications for transcatheter aortic valve implantation (TAVI) procedures have expanded from patients suffering from severe symptomatic aortic stenosis deemed to be at a high risk or too old for conventional surgery to a progressively larger population, further effort is needed to optimize outcomes and reduce complications.
Considering current guidelines on heart valve management [
1], transfemoral (TF)-TAVI is the standard of treatment for patients over 75 years of age suffering from severe symptomatic aortic stenosis.
Among periprocedural complications, access-related complications carry significant morbidity and mortality, and despite the lower profile of latest-generation delivery catheters, their incidence is still high, reported in about 6–20% [
2].
Access-related complications are linked to prolonged hospital stays, increased blood transfusions, and increased intra-hospital mortality.
Currently the transfemoral approach is the preferred access route, utilized in more than 90% of TAVI procedures. A percutaneous femoral vascular access is classically favored over surgical cut-down [
3] because of better outcomes (i.e., faster healing, early deambulation, infection, and lymphorrhagia risk reduction) and a drop in in-hospital stays.
Percutaneous vascular access can be achieved via both traditional blind puncture, using anatomical landmarks (with or without fluoroscopy aid), or using an ultrasound-guided approach, which has gained more consensus in recent years.
In the most recent years, the use of the ultrasound-guided approach for femoral access has not only become established for arterial puncture but also for central venous cannulation. For example, it has been shown that real-time ultrasound-guided central subclavian vein cannulation has greater safety and quality and better first-time success compared with the blind anatomical landmark technique [
4,
5]. Similarly, other studies have observed better efficacy of the peripheral venous cannulation technique in the case of challenging intravenous access [
6]. Poulsen et al. [
7] showed that the use of ultrasound resulted in a three-fold increase in first-attempt success in patients with predicted challenging intravenous access compared to the traditional blind technique.
The main evidence for preferring ultrasound (US)-directed femoral puncture is derived from clinical registries and small clinical studies. In these settings, the systematic use of ultrasound-guided access has been shown to reduce access-related complications such as bleeding and vascular complications, as well as reducing the number of attempts and time to complete the procedure [
8]. The aim of this report is to show our experience with blind versus US-guided femoral artery puncture for the TAVI procedure.
2. Materials and Methods
We have conducted a retrospective observational study on prospectively collected data, with patient follow-up over several years.
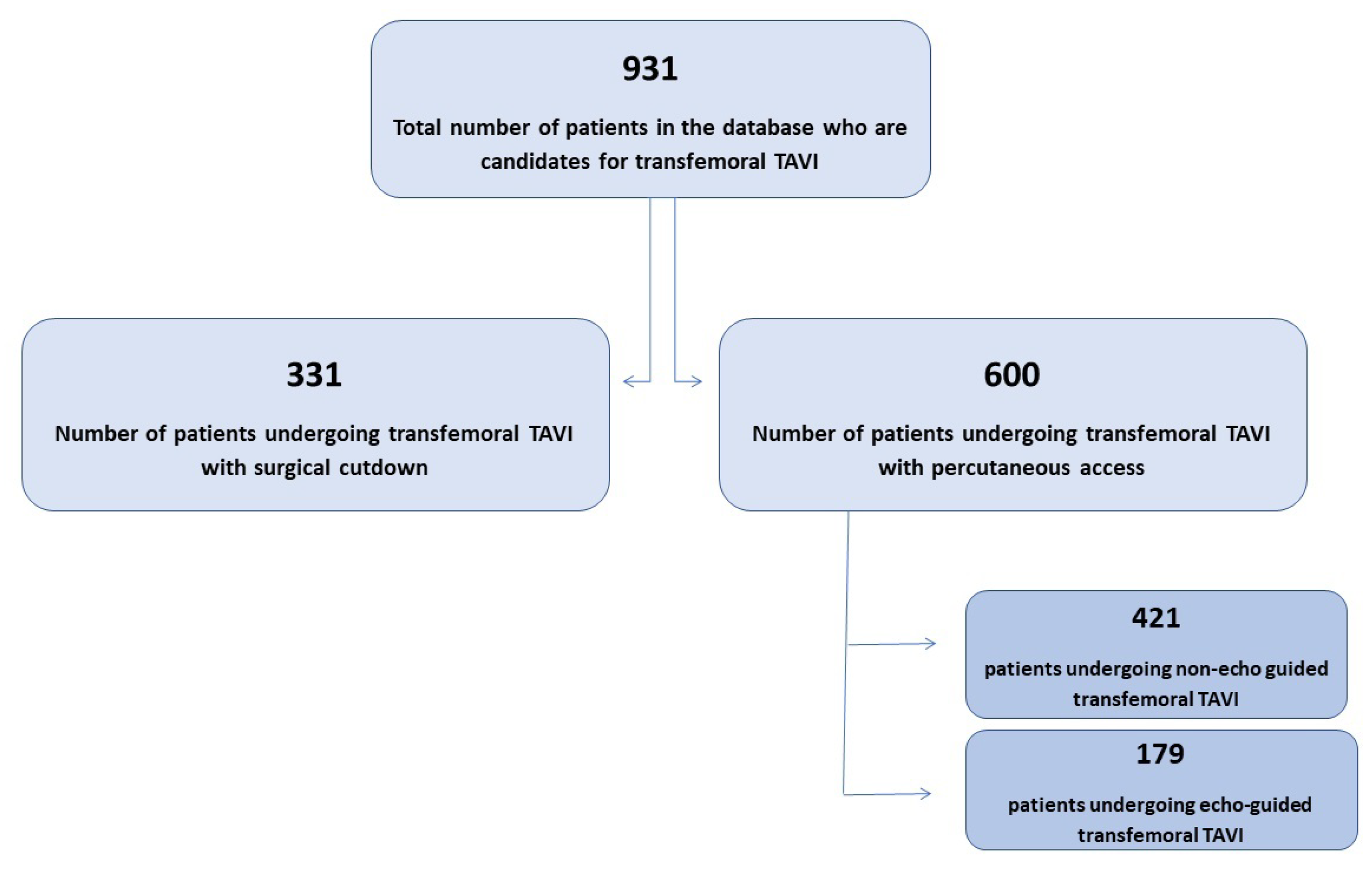
The study was carried out at the Centro Cardiologico Monzino (CCM) in Milan, Italy, from 2013 to 2022. A flowchart of the analysis is shown in
Figure 1.
This study was conducted on 931 consecutive patients who underwent TF-TAVI. Of the overall population, 600 patients had a percutaneous access and were enrolled in the present analysis. This latter group was divided into two subgroups: group A (consisting of 421 patients) included patients having blind arterial puncture, and group B (consisting of 179 patients) had US-guided access.
All patients were approved for TF-TAVI by the local multidisciplinary team. A femoral artery caliber ≥ 5.5 mm was considered mandatory.
The SAPIEN 3 (S3) and SAPIEN 3 Ultra (S3U) (Edwards Lifesciences, Irvine, CA, USA) balloon-expandable transcatheter heart valves (BE-THV) were analyzed in this study, as they were the most frequent devices used at the time of this report.
We compared the perioperative outcomes and follow-up of US-guided and non-US-guided percutaneous transfemoral TAVI. We also compared the odds ratio of a peri-procedural complication between the two groups after adjusting for propensity score.
Follow-up was performed by phone calls, after obtaining verbal informed consent.
2.1. Ethical Committee
The number of the internal protocol approval is CCM1895, made by the “Comitato Etico territoriale Lombardia II”, Italy.
2.2. Echo-Guided Femoral Puncture Protocol
A standard linear vascular ultrasound echo probe is routinely used to identify the common femoral artery of the main access (and its bifurcation) in long-axis view. The echographic characteristics are also used to locate an adequate site of puncture on the common femoral artery, excluding segments with large branches and significant calcifications. After local US-directed anesthesia is performed, we shift the view in short axis and follow the needle by tilting the probe until we see a proper arterial tenting at 12 o’clock at the intended site of puncture, aiming to have the needle oriented at 45° degrees to the skin. In all the cases we have analyzed, we made a pre-closure with two suture-based vascular closure devices (VSCs).
2.3. Statistical Analysis
Numerical variables were reported as mean ± DS, or median and interquartile range (IQR), and categorical variables as number (n) and percentage (%). Continuous variables were compared between the two groups (echo-guided vs. non-echo-guided) using the t-test or Wilcoxon rank sum, as appropriate. Univariate logistic models were applied to investigate the possible association between the incidence of intra-hospital complications and type of procedure (echo-guided or non-echo-guided). A multivariate logistic model was used to eliminate possible confounding factors adjusted for propensity score, created as the following factors: age, sex (M, F), hypertension, diabetes mellitus, peripheral vascular disease, coronary injuries at the time of TAVI, NHYA (1, 2, 3, 4), BMI, EuroSCORE I, and EF (%). Considering the low number of complications, we also performed the logistic model for the incidence of the combined event. This variable was defined as the occurrence of at least one of the following complications: vascular complication access related, VARC-3 mortality, or bleeding sec VARC-3. All tests were 2-coded, and a p-value < 0.05 was considered sufficient for statistical significance. All analyses were performed with SAS Statistical Package v. 9.4 software (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Baseline Characteristics
The baseline characteristics of the population are listed in
Table 1. Body mass index (BMI) and body surface area (BSA) were higher in group B (mean and standard deviation 26.28 ± 4.4 vs. 27.29 ± 5,
p-value = 0.01 and mean and standard deviation 1.8 ± 0.2 vs. 1.83 ± 0.2,
p-value = 0.03, respectively). Interestingly, although this group had a higher BMI and BSA, thus implying a more uncomfortable and complex femoral access, they did not experience higher access-related complications, witnessing a favorable aid of the ultrasound tool.
The EUROSCORE I log. was found to be significantly higher in the non-echo-guided group (11.4 vs. 8.1, p-value < 0.0001). We used this old risk factor calculator, as it was the most adopted at the early stage of the study.
All other cardiovascular risk factors, such as diabetes mellitus, high blood pressure, chronic obstructive pulmonary disease (COPD), and a history of coronary artery disease, showed no significant difference between the two groups.
The only preoperative risk factor that was different between the two groups was the baseline past neurologic dysfunction.
In
Table 2, the patients’ medical histories and pre-operative conditions are shown, with particular attention to their cardiovascular state and the implanted THV. We found differences in coronary artery disease with previous coronary artery bypass grafting (CABG) or percutaneous coronary interventions (PCI) and both mitral and aortic insufficiency that were more frequent in the blind group (group A). Both latter valve diseases were stratified by severity; we found these variables to not have any impact on our study objective.
All other preoperative clinical conditions showed no differences between the two groups.
Regarding the THV, we used S3 and S3U iterations. The diameters implanted were as follows: 23 mm (41.7% in group A, 35.3% in group B), 26 mm (45.7% in group A, 45.1% in group B), and 29 mm (12.6% in group A, 19.7% in group B).
In the blind group, the prosthesis models used were the S3 for 66.1% and the S3U for 33.9%; in the echo-guided group, the S3 was used for 19% and the S3U for 81%.
For the statistical purpose of greater homogeneity of the data, we conducted the analysis on 23 and 26 mm THV, requiring a 14 French (F) sheath. Thus, we excluded the THV requiring 16F sheath (i.e., 29 mm S3) because of the small sample of large annuli we have treated in the period of the study. Nevertheless, we saw a strong tendency to have no increase in vascular injuries using larger sheaths for this larger THV.
3.2. In-Hospital Outcomes
Table 3 depicts the intra-, peri, and postprocedural complications according to VARC-3 criteria [
9]. The overall incidence of intraprocedural or periprocedural complications did not significantly differ between the echo-guided and non-echo-guided groups [30 (7.1%) cases in the non-echo-guided group and 15 (8.5%) in the echo-guided group]. Similarly, no statistically significant periprocedural differences were found between the two groups regarding overall and cardiovascular mortality, conversion to sternotomy, aortic dissection, prosthesis embolization, need for cardiac resuscitation or extra corporeal membrane oxygenation (ECMO), myocardial infarction, or major neurological events according to Valve Academic Research Consortium-3 (VARC-3) criteria.
However, in the non-echo-guided group, there was a clear increase in all bleeding events (life-threatening, major, and minor), with a p-value of 0.00001.
Considering overall vascular complications, there was no statistically significant difference between the two groups, with 36 events (8.6%) in group A and 13 (7.4%) events in group B. Even considering only access-related vascular complications, there was no significant difference between the two groups, although we observed a trend toward fewer access-related vascular complications in the echo-guided route.
Considering the low number of events (only 31 events in the non-echo-guided group and 11 in the echo-guided group), we analyzed the combined risk of periprocedural bleedings and access-related vascular complications and found a statistically significant difference between the two groups, with 73 (17.4%) combined events in group A and 15 (8.6%) events in group B, with a
p-value = 0.005 (
Table 4).
Finally, we have calculated the odds ratio of bleeding events and combined access-related complications (access-related vascular complications and bleeding events) and found a significantly higher risk in the non-ultrasound-guided group (for bleeding events, OR 0.13 with
p-value 0.0007, and for combined access-related events, OR 0.45 with
p-value 0.0069). To eliminate possible confounding factors, we set up a propensity-score analysis using baseline patient characteristics (
Table 5). We found that both categories maintained statistical significance, with an adjusted OR for bleeding events of 0.13 (
p-value = 0.002) and an adjusted OR for combined access-related complications of 0.44 (
p-value = 0.01).
The associations also maintained statistical significance after adjustment for propensity score, with an adjusted OR for bleeding events of 0.13 (p-value = 0.002) and an adjusted OR for combined access-related complications of 0.44 (p-value = 0.01).
4. Discussion
The main findings of this study are as follows:
Ultrasound-guided femoral puncture resulted in fewer vascular-access and bleeding complications in percutaneous TF-TAVI.
Even in obese patients with unfavorable access anatomy, the benefit of the echo-directed puncture over the blind one is strong.
Although larger sheaths (16F) were excluded from the in-depth analysis, we nonetheless found a trend in those benefits, witnessing that larger holes are not per se related to worse vascular outcomes.
Access-related and bleeding events still carry relevant morbidity in TAVI patients, despite tremendous improvements in latest-generation THV delivery systems and increased operator experience [
10]. Although in-depth computed tomography (CT) analysis is routinely pre-operatively performed with dedicated software, the arterial puncture site still poses challenges due to the relevant peripheral morbidity burden of the classic TAVI patient.
In our study (as for our daily practice), we have chosen the femoral puncture site according to the standard pre-operative CT features, focusing most on the absolute diameter of the vessel, tortuosity, calcium burden (anterior versus posterior wall), and femoral bifurcation height [
11].
There was no specific bias in the selection of the puncture site between groups A and B.
Even if the femoral route has been established as the gold standard for TAVI procedures and the ultrasound-guided approach has started to gain widespread popularity in recent years, there is currently a lack of clinical guidelines recommending the use of the echo-guided approach [
12]. However, multiple retrospective studies and registries have shown a safer profile when the access is achieved without a blind puncture [
13].
Kotronias et al. [
14] found that systematic routine use of ultrasound-guided access puncture was associated with a significant reduction in access-related vascular complications as well as the need for post-procedural blood transfusion compared to a fluoro-assisted access.
In our center, we started to systematically perform ultrasound-guided punctures in December 2019, and our retrospective review confirms the literature trend, showing fewer bleeding events in the echo-guided group (11.9% versus 1.7%).
As previously shown, we found a statistically relevant difference even after VARC-3 stratification, demonstrating that the echo-guided group had fewer life-threatening (2.9% vs. 0%), major (6% vs. 1.1%), and minor bleedings (3.1% vs. 0.6%). In our study, vascular complications were not statistically different, probably due to the low number of events in both groups. In group A, vascular complications were 36 (8.6%), and in group B, 13 (7.4%).
Third- and fourth-generation transcatheter heart valve systems present substantial improvements in delivery profile, diameters, and navigability. Despite this, the rate of access-related complications remains high. Much recent evidence suggests that vascular complications may be as high as 20%, with severe complications up to 9% [
15].
A significant morbidity is associated with access-related complications, as these patients required longer hospital stays and had a higher need for blood transfusions.
All this also translates into prolonged in-hospital stays and higher overall procedural costs [
16].
Even if, in our series, the vascular complications rate was not statistically lower in the ultrasound-guided group, if we consider only vascular complications related to the access site (such as pseudoaneurysms, hematomas, arteriovenous fistulas, dissection of the femoral or iliac artery, and local stenoses, thus excluding other vascular districts), the combined number of patients experiencing an access-related complication was significantly lower in the echo-guided group (17.4% versus 8.6%).
Obese patients (i.e., those having the body mass index (BMI) greater than 25) are a particularly challenging subset for percutaneous femoral access. As reported in
Table 6, we have observed strong evidence favoring the ultrasound-guided access in the overweight subsets, with a significant reduction of both bleeding and vascular-access related complications in the adjusted and unadjusted population. This witnesses once again that a precise, anterior femoral wall away from calcifications puncture allows the commonly used VCDs to perform properly, reducing local complications and hemostasis time.
In our experience, the use of ultrasound-guided access has lowered the number of overall access-related problems, and, as it does not have a steep learning curve or significantly lengthen the duration of the procedure, its widespread adoption could significantly improve the clinical benefits. This favorable trend is still evident in the subgroup of obese patients, who classically pose challenges in the percutaneous approach due to the unfavorable anatomy.
5. Conclusions
Among the complications of the TAVI procedures, the most frequent are the access-related and bleeding ones. There is still no clear consensus in the literature on the role of ultrasound-guided puncture in reducing access-related complications, but an increasing number of studies and experiences demonstrate how this technique can contribute to the reduction of these undesirable events.
Our results also move in this direction, demonstrating how, in our series of patients, bleeding events—both combined and stratified by severity—and the overall number of access-related complications (access-related vascular complications plus bleedings) were significantly lower in the ultrasound-guided arterial puncture.
A widespread use of ultrasound could, along with a rigorous pre-operative screening of potential vascular risk factors such as small vessel diameter and/or high calcific burden, be determinant in further reducing the number of access-related TAVI complications.
We need further multicenter studies on larger patient samples to analyze the impact of the US-guided percutaneous puncture technique in reducing access-related complications in transfemoral TAVI and to affirm the role of this technique as a gold standard in clinical practice.
6. Limitations
The main limitation of this study is its single-center retrospective observational characteristic. Although the overall sample size was adequate to obtain statistically relevant results, the number of events, including vascular complications and bleedings, was small in the two groups, making a proper comparison difficult.
Author Contributions
Conceptualization, M.G. and M.A.; Methodology, M.A., M.G. and A.B.; Software, A.B. and N.C.; Validation, M.G., M.A., F.D.M. and G.P., Formal Analysis, A.B. and N.C.; Data Curation, M.M. and L.F.; Writing—Original Draft Preparation, M.G., A.M., G.S. and V.I.; Writing—Review & Editing, M.G., F.D.M. and M.A.; Visualization, M.G.; Supervision, F.D.M. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Italian Ministry of Health-Ricerca Corrente to Centro Cardiologico Monzino IRCCS.
Institutional Review Board Statement
The study was approved by the Ethics Committee of Comitato Etico territoriale Lombardia II (protocol code CCM1895 and date of approval 11 November 2022).
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author (privacy).
Conflicts of Interest
The authors state no conflicts of interests for this study.
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, M.; Pighi, M.; Banning, A.; Reimers, B.; Castriota, F.; Tomai, F.; Venturi, G.; Pesarini, G.; Scarsini, R.; Kotronias, R.; et al. Vascular complications after transcatheter aortic valve implantation: Treatment modalities and long-term clinical impact. Eur. J. Cardiothorac. Surg. 2022, 61, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Gennari, M.; Rigoni, M.; Mastroiacovo, G.; Trabattoni, P.; Roberto, M.; Bartorelli, A.L.; Fabbiocchi, F.; Tamborini, G.; Muratori, M.; Fusini, L.; et al. Proper Selection Does Make the Difference: A Propensity-Matched Analysis of Percutaneous and Surgical Cut-Down Transfemoral TAVR. J. Clin. Med. 2021, 10, 909. [Google Scholar] [CrossRef] [PubMed]
- Zawadka, M.; La Via, L.; Wong, A.; Olusanya, O.; Muscarà, L.; Continella, C.; Andruszkiewicz, P.; Sanfilippo, F. Real-Time Ultrasound Guidance as Compared with Landmark Technique for Subclavian Central Venous Cannulation: A Systematic Review and Meta-Analysis with Trial Sequential Analysis. Crit. Care Med. 2023, 51, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Brass, P.; Hellmich, M.; Kolodziej, L.; Schick, G.; Smith, A.F. Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization. Cochrane Database Syst. Rev. 2015, 1, CD006962. [Google Scholar] [CrossRef] [PubMed]
- Kleidon, T.M.; Schults, J.; Paterson, R.; Rickard, C.M.; Ullman, A.J. Comparison of ultrasound-guided peripheral intravenous catheter insertion with landmark technique in paediatric patients: A systematic review and meta-analysis. J. Paediatr. Child Health 2022, 58, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, E.; Aagaard, R.; Bisgaard, J.; Sørensen, H.T.; Juhl-Olsen, P. The effects of ultrasound guidance on first-attempt success for difficult peripheral intravenous catheterization: A systematic review and meta-analysis. Eur. J. Emerg. Med. 2023, 30, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Potluri, S.P.; Hamandi, M.; Basra, S.S.; Shinn, K.V.; Tabachnick, D.; Vasudevan, A.; Filardo, G.; DiMaio, J.M.; Brinkman, W.T.; Harrington, K.; et al. Comparison of Frequency of Vascular Complications with Ultrasound-Guided Versus Fluroscopic Roadmap-Guided Femoral Arterial Access in Patients Who Underwent Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2020, 132, 93–99. [Google Scholar] [CrossRef] [PubMed]
- VARC-3 Writing Committee; Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J. Am. Coll. Cardiol. 2021, 77, 2717–2746. [Google Scholar] [CrossRef] [PubMed]
- Raju, S.; Eisenberg, N.; Montbriand, J.; Cusimano, R.J.; Feindel, C.; Ouzounian, M.; Horlick, E.; Osten, M.; Tsang, W.; Roche-Nagle, G. Vascular Complications and Procedures Following Transcatheter Aortic Valve Implantation. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Koren, O.; Patel, V.; Tamir, Y.; Koseki, K.; Kaewkes, D.; Sanders, T.; Naami, R.; Naami, E.; Cheng, D.E.; Natanzon, S.S.; et al. Predicting the risk of iliofemoral vascular complication in complex transfemoral-TAVR using new generation transcatheter devices. Front. Cardiovasc. Med. 2023, 10, 1167212. [Google Scholar] [CrossRef] [PubMed]
- Toggweiler, S.; Leipsic, J.; Binder, R.K.; Freeman, M.; Barbanti, M.; Heijmen, R.H.; Wood, D.A.; Webb, J.G. Management of vascular access in transcatheter aortic valve replacement: Part 1: Basic anatomy, imaging, sheaths, wires, and access routes. JACC Cardiovasc. Interv. 2013, 6, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Kotronias, R.A.; Scarsini, R.; De Maria, G.L.; Rajasundaram, S.; Sayeed, R.; Krasopoulos, G.; Grebenik, C.; Keiralla, A.; Newton, J.D.; Banning, A.P.; et al. Ultrasound guided vascular access site management and left ventricular pacing are associated with improved outcomes in contemporary transcatheter aortic valve replacement: Insights from the OxTAVI registry. Catheter. Cardiovasc. Interv. 2020, 96, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Kotronias, R.A.; Bray, J.J.H.; Rajasundaram, S.; Vincent, F.; Delhaye, C.; Scarsini, R.; Marin, F.; Terentes-Printzios, D.; Halcox, J.P.J.; Mamas, M.A.; et al. Ultrasound-Versus Fluoroscopy-Guided Strategy for Transfemoral Transcatheter Aortic Valve Replacement Access: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Interv. 2021, 14, e010742. [Google Scholar] [CrossRef] [PubMed]
- Mach, M.; Okutucu, S.; Kerbel, T.; Arjomand, A.; Fatihoglu, S.G.; Werner, P.; Simon, P.; Andreas, M. Vascular Complications in TAVR: Incidence, Clinical Impact, and Management. J. Clin. Med. 2021, 10, 5046. [Google Scholar] [CrossRef] [PubMed]
- Hanna, G.; Macdonald, D.; Bittira, B.; Horlick, E.; Ali, N.; Atoui, R.; Alqahtani, A.; Fam, N.; Shurrab, M.; Spadafore, J.; et al. The Safety of Early Discharge Following Transcatheter Aortic Valve Implantation Among Patients in Northern Ontario and Rural Areas Utilizing the Vancouver 3M TAVI Study Clinical Pathway. CJC Open 2022, 4, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).