The Interaction between Stress and Inflammatory Bowel Disease in Pediatric and Adult Patients
Abstract
1. Introduction
1.1. Overview of the Epidemiology and Pathophysiology of Inflammatory Bowel Disease
1.2. Stress Definition
2. Objectives of This Study
3. Materials and Methods
3.1. Search Strategy
3.2. Study Selection
4. Stress and Gut Microbiota Brain Axis
5. Stress-Induced Alterations/Inflammation in the Gastrointestinal Mucosa
6. The Role of the Nervous System as an Immune Modulator in Patients with IBD
7. Current Publications on the Interplay between Stress and Psychosocial Disorders in Children and Adults with IBD
8. Anxiety and Depression in Children and Adults with IBD
9. Mendelian Randomization Studies Evaluating the Causal Associations between IBD and Psychological Conditions
10. Quality of Life among Pediatric and Adult Patients with IBD
11. Lifestyle Factors and Psychological Stress in IBD Patients during the Coronavirus Pandemic Period
12. Association between Psychological Stress and IBD Outcomes/Relapses
13. Interventions That Contribute to Stress Reduction in IBD
14. Future Perspectives Regarding the Interaction between Stress and Inflammation
14.1. miR-129-5p—A Significant Controller of Different Pathways
14.2. Fecal Microbiota Transplantation in Inflammatory Bowel Disease
15. Discussion
16. Conclusions
17. The Limitations and Strengths of this Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ho, S.M.; Lewis, J.D.; Mayer, E.A.; Plevy, S.E.; Chuang, E.; Rappaport, S.M.; Croitoru, K.; Korzenik, J.R.; Krischer, J.; Hyams, J.S.; et al. Challenges in IBD Research: Environmental Triggers. Inflamm. Bowel Dis. 2019, 25 (Suppl. S2), S13–S23. [Google Scholar] [CrossRef] [PubMed]
- Yeshi, K.; Ruscher, R.; Hunter, L.; Daly, N.L.; Loukas, A.; Wangchuk, P. Revisiting Inflammatory Bowel Disease: Pathology, Treatments, Challenges and Emerging Therapeutics Including Drug Leads from Natural Products. J. Clin. Med. 2020, 9, 1273. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Jess, T. Implications of the Changing Epidemiology of Inflammatory Bowel Disease in a Changing World. United Eur. Gastroenterol. J. 2022, 10, 1113–1120. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Windsor, J.W. The Four Epidemiological Stages in the Global Evolution of Inflammatory Bowel Disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.Y.; Zhao, M.; Ng, S.C.; Burisch, J. The Epidemiology of Inflammatory Bowel Disease: East Meets West. J. Gastroenterol. Hepatol. 2020, 35, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Kuenzig, M.E.; Fung, S.G.; Marderfeld, L.; Mak, J.W.Y.; Kaplan, G.G.; Ng, S.C.; Wilson, D.C.; Cameron, F.; Henderson, P.; Kotze, P.G.; et al. Twenty-First Century Trends in the Global Epidemiology of Pediatric-Onset Inflammatory Bowel Disease: Systematic Review. Gastroenterology 2022, 162, 1147–1159.e4. [Google Scholar] [CrossRef]
- Yu, Y.R.; Rodriguez, J.R. Clinical Presentation of Crohn’s, Ulcerative Colitis, and Indeterminate Colitis: Symptoms, Extraintestinal Manifestations, and Disease Phenotypes. Semin. Pediatr. Surg. 2017, 26, 349–355. [Google Scholar] [CrossRef]
- Sun, Y.; Li, L.; Xie, R.; Wang, B.; Jiang, K.; Cao, H. Stress Triggers Flare of Inflammatory Bowel Disease in Children and Adults. Front. Pediatr. 2019, 7, 432. [Google Scholar] [CrossRef]
- Piovani, D.; Danese, S.; Peyrin-Biroulet, L.; Nikolopoulos, G.K.; Lytras, T.; Bonovas, S. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-Analyses. Gastroenterology 2019, 157, 647–659.e4. [Google Scholar] [CrossRef]
- Stolzer, I.; Kaden-Volynets, V.; Ruder, B.; Letizia, M.; Bittel, M.; Rausch, P.; Basic, M.; Bleich, A.; Baines, J.F.; Neurath, M.F.; et al. Environmental Microbial Factors Determine the Pattern of Inflammatory Lesions in a Murine Model of Crohn’s Disease-Like Inflammation. Inflamm. Bowel Dis. 2020, 26, 66–79. [Google Scholar] [CrossRef]
- De Lange, K.M.; Moutsianas, L.; Lee, J.C.; Lamb, C.A.; Luo, Y.; Kennedy, N.A.; Jostins, L.; Rice, D.L.; Gutierrez-Achury, J.; Ji, S.G.; et al. Genome-Wide Association Study Implicates Immune Activation of Multiple Integrin Genes in Inflammatory Bowel Disease. Nat. Genet. 2017, 49, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Trindade, I.A.; Pereira, J.; Galhardo, A.; Ferreira, N.B.; Lucena-Santos, P.; Carvalho, S.A.; Oliveira, S.; Skvarc, D.; Rocha, B.S.; Portela, F.; et al. The LIFE with IBD Intervention: Study Protocol for a Randomized Controlled Trial of a Face-to-Face Acceptance and Commitment Therapy and Compassion-Based Intervention Tailored to People with Inflammatory Bowel Disease. Front. Psychiatry 2021, 12, 699367. [Google Scholar] [CrossRef] [PubMed]
- de Dios-Duarte, M.J.; Arias, A.; Durantez-Fernández, C.; Niño Martín, V.; Olea, E.; Barba-Pérez, M.Á.; Pérez-Pérez, L.; Cárdaba-García, R.M.; Barrón, A. Flare-Ups in Crohn’s Disease: Influence of Stress and the External Locus of Control. Int. J. Environ. Res. Public Health 2022, 19, 13131. [Google Scholar] [CrossRef] [PubMed]
- Pinillos Díaz, J.L. Principios de Psicología—El Proceso Activo de Construir Conocimiento: Aprendizaje; Grupo Anaya Publicaciones Generales: Madrid, Spain, 2020. [Google Scholar]
- Kashani, M.; Eliasson, A.; Vernalis, M. Perceived Stress Correlates with Disturbed Sleep: A Link Connecting Stress and Cardiovascular Disease. Stress 2012, 15, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, A.; Kivimäki, M. Stress and Cardiovascular Disease. Nat. Rev. Cardiol. 2012, 9, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Palmero Cantero, F.; Breva, A.; Landeta, Ó. Hostilidad Defensiva y Reactividad Cardiovascular En Una Situación de Estrés Real. Ansiedad y Estrés 2002, 8, 115–142. [Google Scholar]
- Rosenthal, T.; Alter, A. Occupational Stress and Hypertension. J. Am. Soc. Hypertens. 2012, 6, 2–22. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.; García-Vera, M.P.; Espinosa, R.; Inés, M.; Fortún, M.; Segura, J. Psychological Factors Associated with Poor Hypertension Control: Differences in Personality and Stress between Patients with Controlled and Uncontrolled Hypertension. Psychol. Rep. 2010, 107, 923–938. [Google Scholar] [CrossRef]
- Katan, M.; Nigro, N.; Fluri, F.; Schuetz, P.; Morgenthaler, N.G.; Jax, F.; Meckel, S.; Gass, A.; Bingisser, R.; Steck, A.; et al. Stress Hormones Predict Cerebrovascular Re-Events after Transient Ischemic Attacks. Neurology 2011, 76, 563–566. [Google Scholar] [CrossRef]
- Mayer, E.A. Does Stress Damage the Brain? In Understanding Trauma: Integrating Biological, Clinical, and Cultural Perspectives; Cambridge University Press: Cambridge, UK, 2007; pp. 118–141. [Google Scholar] [CrossRef]
- Leza, J.C. Mecanismos de Daño Cerebral Inducido Por Estrés. Ansiedad Estrés 2005, 11, 123–140. [Google Scholar]
- Del Carmen Real Pérez, M.; Alonso, S.R.L. Influencia de Los Factores Psicosociales En Adolescentes Con Diabetes Mellitus Tipo I. Cultura de los Cuidados 2017, 21, 190–198. [Google Scholar] [CrossRef]
- Bitton, A.; Dobkin, P.L.; Edwardes, M.D.; Sewitch, M.J.; Meddings, J.B.; Rawal, S.; Cohen, A.; Vermeire, S.; Dufresne, L.; Franchimont, D.; et al. Predicting Relapse in Crohn’s Disease: A Biopsychosocial Model. Gut 2008, 57, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.N. The Brain-Gut Axis and Stress in Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2017, 46, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Koola, J.; Dulai, P.S.; Prokop, L.J.; Sandborn, W.J.; Singh, S. Rate of Risk Factors for and Interventions to Reduce Hospital Readmission in Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 1939–1948.e7. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Tillisch, K. The Brain-Gut Axis in Abdominal Pain Syndromes. Annu. Rev. Med. 2011, 62, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Galitovskiy, V.; Qian, J.; Chernyavsky, A.I.; Marchenko, S.; Gindi, V.; Edwards, R.A.; Grando, S.A. Cytokine-Induced Alterations of A7 Nicotinic Receptor in Colonic CD4 T-Cells Mediate Dichotomous Response to Nicotine in Murine Models of Th1/Th17 vs. Th2-Mediated Colitis. J. Immunol. 2011, 187, 2677. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; MacRi, J.; McCoy, K.D.; et al. The Intestinal Microbiota Affect Central Levels of Brain-Derived Neurotropic Factor and Behavior in Mice. Gastroenterology 2011, 141, 599–609.e3. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stress and Disorders of the Stress System. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef]
- Stengel, A.; Taché, Y. Corticotropin-Releasing Factor Signaling and Visceral Response to Stress. Exp. Biol. Med. 2010, 235, 1168–1178. [Google Scholar] [CrossRef]
- Bailey, M.T.; Engler, H.; Sheridan, J.F. Stress Induces the Translocation of Cutaneous and Gastrointestinal Microflora to Secondary Lymphoid Organs of C57BL/6 Mice. J. Neuroimmunol. 2006, 171, 29–37. [Google Scholar] [CrossRef]
- Bailey, M.T.; Dowd, S.E.; Galley, J.D.; Hufnagle, A.R.; Allen, R.G.; Lyte, M. Exposure to a Social Stressor Alters the Structure of the Intestinal Microbiota: Implications for Stressor-Induced Immunomodulation. Brain Behav. Immun. 2011, 25, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.T.; Dowd, S.E.; Parry, N.M.A.; Galley, J.D.; Schauer, D.B.; Lyte, M. Stressor Exposure Disrupts Commensal Microbial Populations in the Intestines and Leads to Increased Colonization by Citrobacter Rodentium. Infect. Immun. 2010, 78, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M.; Vulchanova, L.; Brown, D.R. Stress at the Intestinal Surface: Catecholamines and Mucosa-Bacteria Interactions. Cell Tissue Res. 2011, 343, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal Microbial Colonization Programs the Hypothalamic-Pituitary-Adrenal System for Stress Response in Mice. J. Physiol. 2004, 558 Pt 1, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Mourad, F.H.; Saadé, N.E. Neural Regulation of Intestinal Nutrient Absorption. Prog. Neurobiol. 2011, 95, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Mahony, S.M. The Microbiome-Gut-Brain Axis: From Bowel to Behavior. Neurogastroenterol. Motil. 2011, 23, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Clapp, M.; Aurora, N.; Herrera, L.; Bhatia, M.; Wilen, E.; Wakefield, S. Gut Microbiota’s Effect on Mental Health: The Gut-Brain Axis. Clin. Pract. 2017, 7, 987. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.B.; Mazmanian, S.K. The Enteric Network: Interactions between the Immune and Nervous Systems of the Gut. Immunity 2017, 46, 910–926. [Google Scholar] [CrossRef]
- Schneider, S.; Wright, C.M.; Heuckeroth, R.O. Unexpected Roles for the Second Brain: Enteric Nervous System as Master Regulator of Bowel Function. Annu. Rev. Physiol. 2019, 81, 235–259. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the Normal Gut Microbiota. World J. Gastroenterol. WJG 2015, 21, 8787. [Google Scholar] [CrossRef]
- Frank, D.N.; St. Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-Phylogenetic Characterization of Microbial Community Imbalances in Human Inflammatory Bowel Diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [PubMed]
- Haiser, H.J.; Turnbaugh, P.J. Developing a Metagenomic View of Xenobiotic Metabolism. Pharmacol. Res. 2013, 69, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut Biogeography of the Bacterial Microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Guida, F.; Turco, F.; Iannotta, M.; De Gregorio, D.; Palumbo, I.; Sarnelli, G.; Furiano, A.; Napolitano, F.; Boccella, S.; Luongo, L.; et al. Antibiotic-Induced Microbiota Perturbation Causes Gut Endocannabinoidome Changes, Hippocampal Neuroglial Reorganization and Depression in Mice. Brain Behav. Immun. 2018, 67, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Mi, G.L.; Zhao, L.; Qiao, D.D.; Kang, W.Q.; Tang, M.Q.; Xu, J.K. Effectiveness of Lactobacillus reuteri in Infantile Colic and Colicky Induced Maternal Depression: A Prospective Single Blind Randomized Trial. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2015, 107, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut Microbiota and IBD: Causation or Correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sun, K.; Wu, Y.; Yang, Y.; Tso, P.; Wu, Z. Interactions between Intestinal Microbiota and Host Immune Response in Inflammatory Bowel Disease. Front. Immunol. 2017, 8, 942. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Alizadeh-Tabari, S.; Singh, S.; Loomba, R. Meta-Analysis: Prevalence of, and Risk Factors for, Non-Alcoholic Fatty Liver Disease in Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2022, 55, 894–907. [Google Scholar] [CrossRef]
- Takahashi, K.; Nishida, A.; Fujimoto, T.; Fujii, M.; Shioya, M.; Imaeda, H.; Inatomi, O.; Bamba, S.; Andoh, A.; Sugimoto, M. Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn’s Disease. Digestion 2016, 93, 59–65. [Google Scholar] [CrossRef]
- Park, J.H.; Kotani, T.; Konno, T.; Setiawan, J.; Kitamura, Y.; Imada, S.; Usui, Y.; Hatano, N.; Shinohara, M.; Saito, Y.; et al. Promotion of Intestinal Epithelial Cell Turnover by Commensal Bacteria: Role of Short-Chain Fatty Acids. PLoS ONE 2016, 11, e0156334. [Google Scholar] [CrossRef]
- Zheng, L.; Kelly, C.J.; Battista, K.D.; Schaefer, R.; Lanis, J.M.; Alexeev, E.E.; Wang, R.X.; Onyiah, J.C.; Kominsky, D.J.; Colgan, S.P. Microbial-Derived Butyrate Promotes Epithelial Barrier Function through IL-10 Receptor-Dependent Repression of Claudin-2. J. Immunol. 2017, 199, 2976–2984. [Google Scholar] [CrossRef] [PubMed]
- Venegas, D.P.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 424615. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The Microbiome and Butyrate Regulate Energy Metabolism and Autophagy in the Mammalian Colon. Cell Metab. 2011, 13, 517. [Google Scholar] [CrossRef] [PubMed]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-Sensing Receptors GPR43 and GPR109A Facilitate Dietary Fibre-Induced Gut Homeostasis through Regulation of the Inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef] [PubMed]
- Lydiard, R.B. The Role of GABA in Anxiety Disorders. J. Clin. Psychiatry 2003, 64 (Suppl. S3), 21–27. [Google Scholar] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Gephart, S.M.; McGrath, J.M.; Effken, J.A.; Halpern, M.D. Necrotizing Enterocolitis Risk: State of the Science. Adv. Neonatal Care 2012, 12, 77–87. [Google Scholar] [CrossRef]
- D’Agata, A.L.; Wu, J.; Welandawe, M.K.V.; Dutra, S.V.O.; Kane, B.; Groer, M.W. Effects of Early Life NICU Stress on the Developing Gut Microbiome. Dev. Psychobiol. 2019, 61, 650–660. [Google Scholar] [CrossRef]
- Dantzer, R.; Cohen, S.; Russo, S.J.; Dinan, T.G. Resilience and Immunity. Brain Behav. Immun. 2018, 74, 28–42. [Google Scholar] [CrossRef]
- Karl, P.J.; Hatch, A.M.; Arcidiacono, S.M.; Pearce, S.C.; Pantoja-Feliciano, I.G.; Doherty, L.A.; Soares, J.W. Effects of Psychological, Environmental and Physical Stressors on the Gut Microbiota. Front. Microbiol. 2018, 9, 2013. [Google Scholar] [CrossRef]
- Oh, S.; Young, C.; Gravenstein, N.; Islam, S.; Neu, J. Monitoring Technologies in the Neonatal Intensive Care Unit: Implications for the Detection of Necrotizing Enterocolitis. J. Perinatol. 2010, 30, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Thomaidou, A.; Chatziioannou, A.C.; Deda, O.; Benaki, D.; Gika, H.; Mikros, E.; Agakidis, C.; Raikos, N.; Theodoridis, G.; Sarafidis, K. A Pilot Case-Control Study of Urine Metabolomics in Preterm Neonates with Necrotizing Enterocolitis. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2019, 1117, 10–21. [Google Scholar] [CrossRef]
- Sylvester, K.G.; Ling, X.B.; Liu, G.Y.; Kastenberg, Z.J.; Ji, J.; Hu, Z.; Peng, S.; Lau, K.; Abdullah, F.; Brandt, M.L.; et al. A Novel Urine Peptide Biomarker-Based Algorithm for the Prognosis of Necrotising Enterocolitis in Human Infants. Gut 2014, 63, 1284–1292. [Google Scholar] [CrossRef]
- Chatziioannou, A.C.; Wolters, J.C.; Sarafidis, K.; Thomaidou, A.; Agakidis, C.; Govorukhina, N.; Kuivenhoven, J.A.; Bischoff, R.; Theodoridis, G. Targeted LC-MS/MS for the Evaluation of Proteomics Biomarkers in the Blood of Neonates with Necrotizing Enterocolitis and Late-Onset Sepsis. Anal. Bioanal. Chem. 2018, 410, 7163–7175. [Google Scholar] [CrossRef] [PubMed]
- Agakidou, E.; Agakidis, C.; Gika, H.; Sarafidis, K. Emerging Biomarkers for Prediction and Early Diagnosis of Necrotizing Enterocolitis in the Era of Metabolomics and Proteomics. Front. Pediatr. 2020, 8, 602255. [Google Scholar] [CrossRef] [PubMed]
- Huda, S.; Chaudhery, S.; Ibrahim, H.; Pramanik, A. Neonatal Necrotizing Enterocolitis: Clinical Challenges, Pathophysiology and Management. Pathophysiology 2014, 21, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, É.; Thibault, M.P.; Ferretti, E.; Babakissa, C.; Bertelle, V.; Bettolli, M.; Burghardt, K.M.; Colombani, J.F.; Grynspan, D.; Levy, E.; et al. Gene Expression Profiling in Necrotizing Enterocolitis Reveals Pathways Common to Those Reported in Crohn’s Disease. BMC Med. Genom. 2016, 9, 6. [Google Scholar] [CrossRef]
- Chassaing, B.; Srinivasan, G.; Delgado, M.A.; Young, A.N.; Gewirtz, A.T.; Vijay-Kumar, M. Fecal Lipocalin 2, a Sensitive and Broadly Dynamic Non-Invasive Biomarker for Intestinal Inflammation. PLoS ONE 2012, 7, e44328. [Google Scholar] [CrossRef]
- Oikonomou, K.A.; Kapsoritakis, A.N.; Theodoridou, C.; Karangelis, D.; Germenis, A.; Stefanidis, I.; Potamianos, S.P. Neutrophil Gelatinase-Associated Lipocalin (NGAL) in Inflammatory Bowel Disease: Association with Pathophysiology of Inflammation, Established Markers, and Disease Activity. J. Gastroenterol. 2012, 47, 519–530. [Google Scholar] [CrossRef]
- Bin-Nun, A.; Booms, C.; Sabag, N.; Mevorach, R.; Algur, N.; Hammerman, C. Rapid Fecal Calprotectin (FC) Analysis: Point of Care Testing for Diagnosing Early Necrotizing Enterocolitis. Am. J. Perinatol. 2015, 32, 337–342. [Google Scholar] [CrossRef]
- Zhao, T.; Griffith, T.; Zhang, Y.; Li, H.; Hussain, N.; Lester, B.; Cong, X. Early-Life Factors Associated with Neurobehavioral Outcomes in Preterm Infants during NICU Hospitalization. Pediatr. Res. 2022, 92, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- McGowan, E.C.; Hofheimer, J.A.; O’Shea, T.M.; Kilbride, H.; Carter, B.S.; Check, J.; Helderman, J.; Neal, C.R.; Pastyrnak, S.; Smith, L.M.; et al. Analysis of Neonatal Neurobehavior and Developmental Outcomes Among Preterm Infants. JAMA Netw. Open 2022, 5, e2222249. [Google Scholar] [CrossRef] [PubMed]
- Seki, D.; Mayer, M.; Hausmann, B.; Pjevac, P.; Giordano, V.; Goeral, K.; Unterasinger, L.; Klebermaß-Schrehof, K.; De Paepe, K.; Van de Wiele, T.; et al. Aberrant Gut-Microbiota-Immune-Brain Axis Development in Premature Neonates with Brain Damage. Cell Host Microbe 2021, 29, 1558–1572.e6. [Google Scholar] [CrossRef]
- Rozé, J.C.; Ancel, P.Y.; Marchand-Martin, L.; Rousseau, C.; Montassier, E.; Monot, C.; Le Roux, K.; Butin, M.; Resche-Rigon, M.; Aires, J.; et al. Assessment of Neonatal Intensive Care Unit Practices and Preterm Newborn Gut Microbiota and 2-Year Neurodevelopmental Outcomes. JAMA Netw. Open 2020, 3, e2018119. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.C.; Brito, N.H.; Callaghan, B.L. Early Life Stress and the Development of the Infant Gut Microbiota: Implications for Mental Health and Neurocognitive Development. Curr. Psychiatry Rep. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Provenzi, L.; Olson, K.; Giusti, L.; Montirosso, R.; Desantis, A.; Tronick, E. NICU Network Neurobehavioral Scale: 1-Month Normative Data and Variation from Birth to 1 Month. Pediatr. Res. 2018, 83, 1104–1109. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Zhao, T.; Chen, K.; Chen, M.H.; Sun, Z.; Xu, W.; Maas, K.; Lester, B.M.; Cong, X.S. The Impact of Early Life Experiences and Gut Microbiota on Neurobehavioral Development in Preterm Infants: A Longitudinal Cohort Study. Microorganisms 2023, 11, 814. [Google Scholar] [CrossRef] [PubMed]
- Indrio, F.; Neu, J.; Pettoello-Mantovani, M.; Marchese, F.; Martini, S.; Salatto, A.; Aceti, A. Development of the Gastrointestinal Tract in Newborns as a Challenge for an Appropriate Nutrition: A Narrative Review. Nutrients 2022, 14, 1405. [Google Scholar] [CrossRef]
- Al-Turkait, A.; Szatkowski, L.; Choonara, I.; Ojha, S. Review of Drug Utilization Studies in Neonatal Units: A Global Perspective. Int. J. Environ. Res. Public Health 2020, 17, 5669. [Google Scholar] [CrossRef]
- Sun, X.; Zhuan, C.; Xiao, J.; Yao, E.; Chen, L. Impact of Postnatal Exposure to Antibiotics on Intestinal Microbiome in Preterm Infants. Chin. J. Perinat. Med. 2018, 458–464. [Google Scholar] [CrossRef]
- Zou, Z.H.; Liu, D.; Li, H.D.; Zhu, D.P.; He, Y.; Hou, T.; Yu, J.L. Prenatal and Postnatal Antibiotic Exposure Influences the Gut Microbiota of Preterm Infants in Neonatal Intensive Care Units. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Cantey, J.B.; Pyle, A.K.; Wozniak, P.S.; Hynan, L.S.; Sánchez, P.J. Early Antibiotic Exposure and Adverse Outcomes in Preterm, Very Low Birth Weight Infants. J. Pediatr. 2018, 203, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.T.; Lauren Ruoss, J.; de la Cruz, D.; Li, N.; Bazacliu, C.; Patton, L.; McKinley, K.L.; Garrett, T.J.; Polin, R.A.; Triplett, E.W.; et al. Antibiotics and the Developing Intestinal Microbiome, Metabolome and Inflammatory Environment in a Randomized Trial of Preterm Infants. Sci. Rep. 2021, 11, 1943. [Google Scholar] [CrossRef] [PubMed]
- Akagawa, S.; Tsuji, S.; Onuma, C.; Akagawa, Y.; Yamaguchi, T.; Yamagishi, M.; Yamanouchi, S.; Kimata, T.; Sekiya, S.I.; Ohashi, A.; et al. Effect of Delivery Mode and Nutrition on Gut Microbiota in Neonates. Ann. Nutr. Metab. 2019, 74, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Korpela, K. Impact of Delivery Mode on Infant Gut Microbiota. Ann. Nutr. Metab. 2021, 77, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, L.; Jin, B.; Xu, X.; Zuo, X.; Li, Y.; Li, Z. The Effects of Delivery Mode on the Gut Microbiota and Health: State of Art. Front. Microbiol. 2021, 12, 724449. [Google Scholar] [CrossRef]
- Dermyshi, E.; Wang, Y.; Yan, C.; Hong, W.; Qiu, G.; Gong, X.; Zhang, T. The “Golden Age” of Probiotics: A Systematic Review and Meta-Analysis of Randomized and Observational Studies in Preterm Infants. Neonatology 2017, 112, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Van Den Akker, C.H.P.; Van Goudoever, J.B.; Shamir, R.; Domellöf, M.; Embleton, N.D.; Hojsak, I.; Lapillonne, A.; Mihatsch, W.A.; Berni Canani, R.; Bronsky, J.; et al. Probiotics and Preterm Infants: A Position Paper by the European Society for Paediatric Gastroenterology Hepatology and Nutrition Committee on Nutrition and the European Society for Paediatric Gastroenterology Hepatology and Nutrition Working Group for Probiotics and Prebiotics. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 664–680. [Google Scholar] [CrossRef]
- Poindexter, B.; Cummings, J.; Hand, I.; Adams-Chapman, I.; Aucott, S.W.; Puopolo, K.M.; Goldsmith, J.P.; Kaufman, D.; Martin, C.; Mowitz, M. Use of Probiotics in Preterm Infants. Pediatrics 2021, 147, e2021051485. [Google Scholar] [CrossRef]
- Ojima, M.N.; Jiang, L.; Arzamasov, A.A.; Yoshida, K.; Odamaki, T.; Xiao, J.; Nakajima, A.; Kitaoka, M.; Hirose, J.; Urashima, T.; et al. Priority Effects Shape the Structure of Infant-Type Bifidobacterium Communities on Human Milk Oligosaccharides. ISME J. 2022, 16, 2265–2279. [Google Scholar] [CrossRef]
- Lawson, M.A.E.; O’Neill, I.J.; Kujawska, M.; Gowrinadh Javvadi, S.; Wijeyesekera, A.; Flegg, Z.; Chalklen, L.; Hall, L.J. Breast Milk-Derived Human Milk Oligosaccharides Promote Bifidobacterium Interactions within a Single Ecosystem. ISME J. 2020, 14, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Mercer, E.M.; Arrieta, M.C. Probiotics to Improve the Gut Microbiome in Premature Infants: Are We There Yet? Gut Microbes 2023, 15, 2201160. [Google Scholar] [CrossRef] [PubMed]
- Alcon-Giner, C.; Dalby, M.J.; Caim, S.; Ketskemety, J.; Shaw, A.; Sim, K.; Lawson, M.A.E.; Kiu, R.; Leclaire, C.; Chalklen, L.; et al. Microbiota Supplementation with Bifidobacterium and Lactobacillus Modifies the Preterm Infant Gut Microbiota and Metabolome: An Observational Study. Cell Rep. Med. 2020, 1, 100077. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, A.J.; Mannion, C.A.; McDonald, S.W.; Brockway, M.; Tough, S.C. The Impact of Caesarean Section on Breastfeeding Initiation, Duration and Difficulties in the First Four Months Postpartum. BMC Pregnancy Childbirth 2016, 16, 90. [Google Scholar] [CrossRef] [PubMed]
- Pivrncova, E.; Kotaskova, I.; Thon, V. Neonatal Diet and Gut Microbiome Development After C-Section During the First Three Months After Birth: A Systematic Review. Front. Nutr. 2022, 9, 941549. [Google Scholar] [CrossRef] [PubMed]
- Notarbartolo, V.; Giuffre, M.; Montante, C.; Corsello, G.; Carta, M. Composition of Human Breast Milk Microbiota and Its Role in Children’s Health. Pediatr. Gastroenterol. Hepatol. Nutr. 2022, 25, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.Y.; Tan, L.T.H.; Law, J.W.F.; Hong, K.W.; Ratnasingam, V.; Ab Mutalib, N.S.; Lee, L.H.; Letchumanan, V. Exploring the Potential of Human Milk and Formula Milk on Infants’ Gut and Health. Nutrients 2022, 14, 3554. [Google Scholar] [CrossRef]
- Zhang, S.; Li, T.; Xie, J.; Zhang, D.; Pi, C.; Zhou, L.; Yang, W. Gold Standard for Nutrition: A Review of Human Milk Oligosaccharide and Its Effects on Infant Gut Microbiota. Microb. Cell Fact. 2021, 20, 108. [Google Scholar] [CrossRef]
- Ziegler, E.E. Human Milk-a Valuable Tool in the Early Days of Life of Premature Infants. Front. Pediatr. 2019, 7, 411362. [Google Scholar] [CrossRef]
- Ungaro, R.; Bernstein, C.N.; Gearry, R.; Hviid, A.; Kolho, K.L.; Kronman, M.P.; Shaw, S.; Van Kruiningen, H.; Colombel, J.F.; Atreja, A. Antibiotics Associated with Increased Risk of New-Onset Crohn’s Disease but Not Ulcerative Colitis: A Meta-Analysis. Am. J. Gastroenterol. 2014, 109, 1728–1738. [Google Scholar] [CrossRef]
- Ortizo, R.; Lee, S.Y.; Nguyen, E.T.; Jamal, M.M.; Bechtold, M.M.; Nguyen, D.L. Exposure to Oral Contraceptives Increases the Risk for Development of Inflammatory Bowel Disease: A Meta-Analysis of Case-Controlled and Cohort Studies. Eur. J. Gastroenterol. Hepatol. 2017, 29, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Felder, J.B.; Korelitz, B.I.; Rajapakse, R.; Schwarz, S.; Horatagis, A.P.; Gleim, G. Effects of Nonsteroidal Antiinflammatory Drugs on Inflammatory Bowel Disease: A Case-Control Study. Am. J. Gastroenterol. 2000, 95, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Graff, L.A.; Bernstein, C.N. Do NSAIDs, Antibiotics, Infections, or Stress Trigger Flares in IBD? Am. J. Gastroenterol. 2009, 104, 1298–1313. [Google Scholar] [CrossRef] [PubMed]
- Guslandi, M. Exacerbation of Inflammatory Bowel Disease by Nonsteroidal Anti-Inflammatory Drugs and Cyclooxygenase-2 Inhibitors: Fact or Fiction? World J. Gastroenterol. WJG 2006, 12, 1509. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of Psychotropic-like Properties of a Probiotic Formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in Rats and Human Subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef] [PubMed]
- García-Ródenas, C.L.; Bergonzelli, G.E.; Nutten, S.; Schumann, A.; Cherbut, C.; Turini, M.; Ornstein, K.; Rochat, F.; Corthésy-Theulaz, I. Nutritional Approach to Restore Impaired Intestinal Barrier Function and Growth after Neonatal Stress in Rats. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Zareie, M.; Johnson-Henry, K.; Jury, J.; Yang, P.C.; Ngan, B.Y.; McKay, D.M.; Soderholm, J.D.; Perdue, M.H.; Sherman, P.M. Probiotics Prevent Bacterial Translocation and Improve Intestinal Barrier Function in Rats Following Chronic Psychological Stress. Gut 2006, 55, 1553–1560. [Google Scholar] [CrossRef]
- Li, N.; Wang, Q.; Wang, Y.; Sun, A.; Lin, Y.; Jin, Y.; Li, X. Oral Probiotics Ameliorate the Behavioral Deficits Induced by Chronic Mild Stress in Mice via the Gut Microbiota-Inflammation Axis. Front. Behav. Neurosci. 2018, 12, 266. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Rao, S.; Srinivasjois, R.; Patole, S. Prebiotic Supplementation in Full-Term Neonates. Arch. Pediatr. Adolesc. Med. 2009, 163, 755–764. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Marchesi, J.R.; Scully, P.; Codling, C.; Ceolho, A.M.; Quigley, E.M.M.; Cryan, J.F.; Dinan, T.G. Early Life Stress Alters Behavior, Immunity, and Microbiota in Rats: Implications for Irritable Bowel Syndrome and Psychiatric Illnesses. Biol. Psychiatry 2009, 65, 263–267. [Google Scholar] [CrossRef]
- Golubeva, A.V.; Crampton, S.; Desbonnet, L.; Edge, D.; O’Sullivan, O.; Lomasney, K.W.; Zhdanov, A.V.; Crispie, F.; Moloney, R.D.; Borre, Y.E.; et al. Prenatal Stress-Induced Alterations in Major Physiological Systems Correlate with Gut Microbiota Composition in Adulthood. Psychoneuroendocrinology 2015, 60, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Jašarević, E.; Howerton, C.L.; Howard, C.D.; Bale, T.L. Alterations in the Vaginal Microbiome by Maternal Stress Are Associated with Metabolic Reprogramming of the Offspring Gut and Brain. Endocrinology 2015, 156, 3265–3276. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Sun, Y.; Wu, J.; Huang, S.; Jin, G.; Guo, Z.; Zhang, Y.; Liu, T.; Liu, X.; Cao, X.; et al. Maternal High Fat Diet Alters Gut Microbiota of Offspring and Exacerbates Dss-Induced Colitis in Adulthood. Front. Immunol. 2018, 9, 2608. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.T.; Coe, C.L. Maternal Separation Disrupts the Integrity of the Intestinal Microflora in Infant Rhesus Monkeys. Dev. Psychobiol. 1999, 35, 146–155. [Google Scholar] [CrossRef]
- Zijlmans, M.A.C.; Korpela, K.; Riksen-Walraven, J.M.; de Vos, W.M.; de Weerth, C. Maternal Prenatal Stress Is Associated with the Infant Intestinal Microbiota. Psychoneuroendocrinology 2015, 53, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Söderholm, J.D.; Yang, P.C.; Ceponis, P.; Vohra, A.; Riddell, R.; Sherman, P.M.; Perdue, M.H. Chronic Stress Induces Mast Cell-Dependent Bacterial Adherence and Initiates Mucosal Inflammation in Rat Intestine. Gastroenterology 2002, 123, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.; Robbe-Masselot, C.; Ait-Belgnaoui, A.; Mancuso, A.; Mercade-Loubière, M.; Salvador-Cartier, C.; Gillet, M.; Ferrier, L.; Loubière, P.; Dague, E.; et al. Stress Disrupts Intestinal Mucus Barrier in Rats via Mucin O-Glycosylation Shift: Prevention by a Probiotic Treatment. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, 420–429. [Google Scholar] [CrossRef]
- Meddings, J.B.; Swain, M.G. Environmental Stress-Induced Gastrointestinal Permeability Is Mediated by Endogenous Glucocorticoids in the Rat. Gastroenterology 2000, 119, 1019–1028. [Google Scholar] [CrossRef]
- Saunders, P.R.; Santos, J.; Hanssen, N.P.M.; Yates, D.; Groot, J.A.; Perdue, M.H. Physical and Psychological Stress in Rats Enhances Colonic Epithelial Permeability via Peripheral CRH. Dig. Dis. Sci. 2002, 47, 208–215. [Google Scholar] [CrossRef]
- Santos, J.; Yang, P.C.; Söderholm, J.D.; Benjamin, M.; Perdue, M.H. Role of Mast Cells in Chronic Stress Induced Colonic Epithelial Barrier Dysfunction in the Rat. Gut 2001, 48, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cao, Q.; Cheng, Y.; Zhao, D.; Wang, Z.; Yang, H.; Wu, Q.; You, L.; Wang, Y.; Lin, Y.; et al. Chronic Stress Promotes Colitis by Disturbing the Gut Microbiota and Triggering Immune System Response. Proc. Natl. Acad. Sci. USA 2018, 115, E2960–E2969. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced Anxiety-like Behavior and Central Neurochemical Change in Germ-Free Mice. Neurogastroenterol. Motil. 2011, 23, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Donnet-Hughes, A.; Perez, P.F.; Doré, J.; Leclerc, M.; Levenez, F.; Benyacoub, J.; Serrant, P.; Segura-Roggero, I.; Schiffrin, E.J. Potential Role of the Intestinal Microbiota of the Mother in Neonatal Immune Education. Proc. Nutr. Soc. 2010, 69, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal Gut Microbiota Modulates Brain Development and Behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef] [PubMed]
- Marin, I.A.; Goertz, J.E.; Ren, T.; Rich, S.S.; Onengut-Gumuscu, S.; Farber, E.; Wu, M.; Overall, C.C.; Kipnis, J.; Gaultier, A. Microbiota Alteration Is Associated with the Development of Stress-Induced Despair Behavior. Sci. Rep. 2017, 7, srep43859. [Google Scholar] [CrossRef] [PubMed]
- Bharwani, A.; Mian, M.F.; Foster, J.A.; Surette, M.G.; Bienenstock, J.; Forsythe, P. Structural and Functional Consequences of Chronic Psychosocial Stress on the Microbiome and Host; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 63. [Google Scholar] [CrossRef]
- Galley, J.D.; Nelson, M.C.; Yu, Z.; Dowd, S.E.; Walter, J.; Kumar, P.S.; Lyte, M.; Bailey, M.T. Exposure to a Social Stressor Disrupts the Community Structure of the Colonic Mucosa-Associated Microbiota. BMC Microbiol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Noguera, J.C.; Aira, M.; Pérez-Losada, M.; Domínguez, J.; Velando, A. Glucocorticoids Modulate Gastrointestinal Microbiome in a Wild Bird. R. Soc. Open Sci. 2018, 5, 171743. [Google Scholar] [CrossRef]
- Van Der Zaag-Loonen, H.J.; Grootenhuis, M.A.; Last, B.F.; Derkx, H.H.F. Coping Strategies and Quality of Life of Adolescents with Inflammatory Bowel Disease. Qual. Life Res. 2004, 13, 1011–1019. [Google Scholar] [CrossRef]
- Walker, L.S.; Garber, J.; Smith, C.A.; Van Slyke, D.A.; Claar, R.L. The Relation of Daily Stressors to Somatic and Emotional Symptoms in Children with and without Recurrent Abdominal Pain. J. Consult. Clin. Psychol. 2001, 69, 85–91. [Google Scholar] [CrossRef]
- Maes, M.; Kubera, M.; Leunis, J.C.; Berk, M. Increased IgA and IgM Responses against Gut Commensals in Chronic Depression: Further Evidence for Increased Bacterial Translocation or Leaky Gut. J. Affect. Disord. 2012, 141, 55–62. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Gianaros, P.J. Central Role of the Brain in Stress and Adaptation: Links to Socioeconomic Status, Health, and Disease. Ann. N. Y. Acad. Sci. 2010, 1186, 190–222. [Google Scholar] [CrossRef] [PubMed]
- Mawdsley, J.E.; Rampton, D.S. Psychological Stress in IBD: New Insights into Pathogenic and Therapeutic Implications. Gut 2005, 54, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, G.A.; Van De Kar, L.D. Neuroendocrine Pharmacology of Stress. Eur. J. Pharmacol. 2003, 463, 235–272. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A. The Neurobiology of Stress and Gastrointestinal Disease. Gut 2000, 47, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Molecular Mechanisms of Corticosteroids in Allergic Diseases. Allergy 2001, 56, 928–936. [Google Scholar] [CrossRef]
- Joyce, D.A.; Gimblett, G.; Steer, J.H. Targets of Glucocorticoid Action on TNF-Alpha Release by Macrophages. Inflamm. Res. 2001, 50, 337–340. [Google Scholar] [CrossRef]
- Franchimont, D.; Kino, T.; Galon, J.; Meduri, G.U.; Chrousos, G. Glucocorticoids and Inflammation Revisited: The State of the Art. NIH Clinical Staff Conference. Neuroimmunomodulation 2002, 10, 247–260. [Google Scholar] [CrossRef]
- Amsterdam, A.; Tajima, K.; Sasson, R. Cell-Specific Regulation of Apoptosis by Glucocorticoids: Implication to Their Anti-Inflammatory Action. Biochem. Pharmacol. 2002, 64, 843–850. [Google Scholar] [CrossRef]
- Straub, R.H.; Dhabhar, F.S.; Bijlsma, J.W.J.; Cutolo, M. How Psychological Stress via Hormones and Nerve Fibers May Exacerbate Rheumatoid Arthritis. Arthritis Rheum. 2005, 52, 16–26. [Google Scholar] [CrossRef]
- Frank, M.G.; Wieseler Frank, J.L.; Hendricks, S.E.; Burke, W.J.; Johnson, D.R. Age at Onset of Major Depressive Disorder Predicts Reductions in NK Cell Number and Activity. J. Affect. Disord. 2002, 71, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Fortes, C.; Farchi, S.; Forastiere, F.; Agabiti, N.; Pacifici, R.; Zuccaro, P.; Perucci, C.A.; Ebrahim, S. Depressive Symptoms Lead to Impaired Cellular Immune Response. Psychother. Psychosom. 2003, 72, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Schleifer, S.J.; Bartlett, J.A.; Keller, S.E.; Eckholdt, H.M.; Shiflett, S.C.; Delaney, B.R. Immunity in Adolescents with Major Depression. J. Am. Acad. Child. Adolesc. Psychiatry 2002, 41, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Danner, M.; Kasl, S.V.; Abramson, J.L.; Vaccarino, V. Association between Depression and Elevated C-Reactive Protein. Psychosom. Med. 2003, 65, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Sajadieh, A.; Nielsen, O.W.; Rasmussen, V.; Hein, H.O.; Abedini, S.; Hansen, J.F. Increased Heart Rate and Reduced Heart-Rate Variability Are Associated with Subclinical Inflammation in Middle-Aged and Elderly Subjects with No Apparent Heart Disease. Eur. Heart J. 2004, 25, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Ott, G.; Jaeger, B.; Meyer, S.; Stephan, E.; Kapp, A.; Werfel, T. Different Expression of Cytokine and Membrane Molecules by Circulating Lymphocytes on Acute Mental Stress in Patients with Atopic Dermatitis in Comparison with Healthy Controls. J. Allergy Clin. Immunol. 2001, 108, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.A.; Aoki, N.; Homori, M.; Kawano, K.; Maki, A.; Kimura, M.; Yanagisawa, A.; Ohsaki, T.; Takahashi, R.; Shiohara, T.; et al. Mental Stress and Physical Exercise Increase Platelet-Dependent Thrombin Generation. Heart Vessel. 2000, 15, 280–288. [Google Scholar] [CrossRef]
- Steptoe, A.; Magid, K.; Edwards, S.; Brydon, L.; Hong, Y.; Erusalimsky, J. The Influence of Psychological Stress and Socioeconomic Status on Platelet Activation in Men. Atherosclerosis 2003, 168, 57–63. [Google Scholar] [CrossRef]
- Irving, P.M.; Macey, M.G.; Shah, U.; Webb, L.; Langmead, L.; Rampton, D.S. Formation of Platelet-Leukocyte Aggregates in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2004, 10, 361–372. [Google Scholar] [CrossRef]
- O’Connor, T.M.; O’Connell, J.; O’Brien, D.I.; Goode, T.; Bredin, C.P.; Shanahan, F. The Role of Substance P in Inflammatory Disease. J. Cell Physiol. 2004, 201, 167–180. [Google Scholar] [CrossRef]
- Barker, N.; Van Es, J.H.; Kuipers, J.; Kujala, P.; Van Den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of Stem Cells in Small Intestine and Colon by Marker Gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Kaser, A.; Lee, A.H.; Franke, A.; Glickman, J.N.; Zeissig, S.; Tilg, H.; Nieuwenhuis, E.E.S.; Higgins, D.E.; Schreiber, S.; Glimcher, L.H.; et al. XBP1 Links ER Stress to Intestinal Inflammation and Confers Genetic Risk for Human Inflammatory Bowel Disease. Cell 2008, 134, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Gracie, D.J.; Hamlin, P.J.; Ford, A.C. The Influence of the Brain-Gut Axis in Inflammatory Bowel Disease and Possible Implications for Treatment. Lancet Gastroenterol. Hepatol. 2019, 4, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.L.; Bernstein, C.N. Brain-Gut Interactions in Inflammatory Bowel Disease. Gastroenterology 2013, 144, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, F. 99th Dahlem Conference on Infection, Inflammation and Chronic Inflammatory Disorders: Host–Microbe Interactions in the Gut: Target for Drug Therapy, Opportunity for Drug Discovery. Clin. Exp. Immunol. 2010, 160, 92. [Google Scholar] [CrossRef] [PubMed]
- Burgmann, T.; Clara, I.; Graff, L.; Walker, J.; Lix, L.; Rawsthorne, P.; McPhail, C.; Rogala, L.; Miller, N.; Bernstein, C.N. The Manitoba Inflammatory Bowel Disease Cohort Study: Prolonged Symptoms before Diagnosis—How Much Is Irritable Bowel Syndrome? Clin. Gastroenterol. Hepatol. 2006, 4, 614–620. [Google Scholar] [CrossRef]
- Brinkman, D.J.; Ten Hove, A.S.; Vervoordeldonk, M.J.; Luyer, M.D.; de Jonge, W.J. Neuroimmune Interactions in the Gut and Their Significance for Intestinal Immunity. Cells 2019, 8, 670. [Google Scholar] [CrossRef]
- Van Maanen, M.A.; Lebre, M.C.; Van Der Poll, T.; LaRosa, G.J.; Elbaum, D.; Vervoordeldonk, M.J.; Tak, P.P. Stimulation of Nicotinic Acetylcholine Receptors Attenuates Collagen-Induced Arthritis in Mice. Arthritis Rheum. 2009, 60, 114–122. [Google Scholar] [CrossRef]
- Ghia, J.E.; Blennerhassett, P.; Kumar-Ondiveeran, H.; Verdu, E.F.; Collins, S.M. The Vagus Nerve: A Tonic Inhibitory Influence Associated with Inflammatory Bowel Disease in a Murine Model. Gastroenterology 2006, 131, 1122–1130. [Google Scholar] [CrossRef]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus Nerve Stimulation Attenuates the Systemic Inflammatory Response to Endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef]
- Meroni, E.; Stakenborg, N.; Gomez-Pinilla, P.J.; De Hertogh, G.; Goverse, G.; Matteoli, G.; Verheijden, S.; Boeckxstaens, G.E. Functional Characterization of Oxazolone-Induced Colitis and Survival Improvement by Vagus Nerve Stimulation. PLoS ONE 2018, 13, e0197487. [Google Scholar] [CrossRef] [PubMed]
- Verheijden, S.; Boeckxstaens, G.E. Neuroimmune Interaction and the Regulation of Intestinal Immune Homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G75–G80. [Google Scholar] [CrossRef] [PubMed]
- Tracey, K.J. The Inflammatory Reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Straub, R.H.; Grum, F.; Strauch, U.; Capellino, S.; Bataille, F.; Bleich, A.; Falk, W.; Schölmerich, J.; Obermeier, F. Anti-Inflammatory Role of Sympathetic Nerves in Chronic Intestinal Inflammation. Gut 2008, 57, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Vieira-Coelho, M.A.; Fraga, S.; Serräo, M.P.; Veloso, F.T.; Ribeiro, T.; Soares-da-Silva, P. Impaired Synthesis or Cellular Storage of Norepinephrine, Dopamine, and 5-Hydroxytryptamine in Human Inflammatory Bowel Disease. Dig. Dis. Sci. 2002, 47, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Blandizzi, C.; Fornai, M.; Colucci, R.; Baschiera, F.; Barbara, G.; De Giorgio, R.; De Ponti, F.; Breschi, M.C.; Del Tacca, M. Altered Prejunctional Modulation of Intestinal Cholinergic and Noradrenergic Pathways by Alpha2-Adrenoceptors in the Presence of Experimental Colitis. Br. J. Pharmacol. 2003, 139, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Knowles, S.R.; Graff, L.A.; Wilding, H.; Hewitt, C.; Keefer, L.; Mikocka-Walus, A. Quality of Life in Inflammatory Bowel Disease: A Systematic Review and Meta-Analyses—Part I. Inflamm. Bowel Dis. 2018, 24, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Knowles, S.R.; Keefer, L.; Wilding, H.; Hewitt, C.; Graff, L.A.; Mikocka-Walus, A. Quality of Life in Inflammatory Bowel Disease: A Systematic Review and Meta-Analyses—Part II. Inflamm. Bowel Dis. 2018, 24, 966–976. [Google Scholar] [CrossRef]
- Barberio, B.; Zamani, M.; Black, C.J.; Savarino, E.V.; Ford, A.C. Prevalence of Symptoms of Anxiety and Depression in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 359–370. [Google Scholar] [CrossRef]
- Mikocka-Walus, A.A.; Turnbull, D.A.; Moulding, N.T.; Wilson, I.G.; Andrews, J.M.; Holtmann, G.J. Controversies Surrounding the Comorbidity of Depression and Anxiety in Inflammatory Bowel Disease PatientsA Literature Review. Inflamm. Bowel Dis. 2007, 13, 225–234. [Google Scholar] [CrossRef]
- Schoultz, M.; Beattie, M.; Gorely, T.; Leung, J. Assessment of Causal Link between Psychological Factors and Symptom Exacerbation in Inflammatory Bowel Disease: A Systematic Review Utilising Bradford Hill Criteria and Meta-Analysis of Prospective Cohort Studies. Syst. Rev. 2020, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Gracie, D.J.; Irvine, A.J.; Sood, R.; Mikocka-Walus, A.; Hamlin, P.J.; Ford, A.C. Effect of Psychological Therapy on Disease Activity, Psychological Comorbidity, and Quality of Life in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Folkman, S. Stress: Appraisal and Coping; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Lazarus, R.S. Stress and Emotion: A New Synthesis; Springer Pub. Co., Ltd.: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Goodhand, J.R.; Wahed, M.; Rampton, D.S. Management of Stress in Inflammatory Bowel Disease: A Therapeutic Option? Expert Rev. Gastroenterol. Hepatol. 2014, 3, 661–679. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, T.; Ohta, A.; Sherriff-Tadano, R.; Matsuura, E.; Takashima, T.; Iwakiri, R.; Fujimoto, K. Imbalance in the Stress-Adaptation System in Patients with Inflammatory Bowel Disease. Biol. Res. Nurs. 2010, 13, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Nahon, S.; Lahmek, P.; Saas, C.; Durance, C.; Olympie, A.; Lesgourgues, B.; Gendre, J.P. Socioeconomic and Psychological Factors Associated with Nonadherence to Treatment in Inflammatory Bowel Disease Patients: Results of the ISSEO Survey. Inflamm. Bowel Dis. 2011, 17, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N. Epidemiology and Risk Factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Ramos, G.P.; Kane, S. Alcohol Use in Patients with Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2021, 17, 211. [Google Scholar]
- Regueiro, M.; Greer, J.B.; Szigethy, E. Etiology and Treatment of Pain and Psychosocial Issues in Patients with Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 430–439.e4. [Google Scholar] [CrossRef]
- Mittermaier, C.; Dejaco, C.; Waldhoer, T.; Oefferlbauer-Ernst, A.; Miehsler, W.; Beier, M.; Tillinger, W.; Gangl, A.; Moser, G. Impact of Depressive Mood on Relapse in Patients with Inflammatory Bowel Disease: A Prospective 18-Month Follow-Up Study. Psychosom. Med. 2004, 66, 79–84. [Google Scholar] [CrossRef]
- Persoons, P.; Vermeire, S.; Demyttenaere, K.; Fischler, B.; Vandenberghe, J.; Van Oudenhove, L.; Pierik, M.; Hlavaty, T.; Van Assche, G.; Noman, M.; et al. The Impact of Major Depressive Disorder on the Short- and Long-Term Outcome of Crohn’s Disease Treatment with Infliximab. Aliment. Pharmacol. Ther. 2005, 22, 101–110. [Google Scholar] [CrossRef]
- Mikocka-Walus, A.; Pittet, V.; Rossel, J.B.; von Känel, R. Symptoms of Depression and Anxiety Are Independently Associated with Clinical Recurrence of Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 829–835.e1. [Google Scholar] [CrossRef] [PubMed]
- Graff, L.A.; Walker, J.R.; Bernstein, C.N. Depression and Anxiety in Inflammatory Bowel Disease: A Review of Comorbidity and Management. Inflamm. Bowel Dis. 2009, 15, 1105–1118. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.N.; Denson, L.A.; Baldassano, R.N.; Hommel, K.A. Treatment Adherence in Adolescents with Inflammatory Bowel Disease: The Collective Impact of Barriers to Adherence and Anxiety/Depressive Symptoms. J. Pediatr. Psychol. 2012, 37, 282. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Gainer, V.S.; Perez, R.G.; Cai, T.; Cheng, S.C.; Savova, G.; Chen, P.; Szolovits, P.; Xia, Z.; De Jager, P.L.; et al. Psychiatric Co-Morbidity Is Associated with Increased Risk of Surgery in Crohn’s Disease. Aliment. Pharmacol. Ther. 2013, 37, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Fuller-Thomson, E.; Lateef, R.; Sulman, J. Robust Association Between Inflammatory Bowel Disease and Generalized Anxiety Disorder: Findings from a Nationally Representative Canadian Study. Inflamm. Bowel Dis. 2015, 21, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Khalili, H.; Pan, A.; Higuchi, L.M.; de Silva, P.; Richter, J.M.; Fuchs, C.S.; Chan, A.T. Association between Depressive Symptoms and Incidence of Crohn’s Disease and Ulcerative Colitis: Results from the Nurses’ Health Study. Clin. Gastroenterol. Hepatol. 2013, 11, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.R.; Ediger, J.P.; Graff, L.A.; Greenfeld, J.M.; Clara, I.; Lix, L.; Rawsthorne, P.; Miller, N.; Rogala, L.; McPhail, C.M.; et al. The Manitoba IBD Cohort Study: A Population-Based Study of the Prevalence of Lifetime and 12-Month Anxiety and Mood Disorders. Am. J. Gastroenterol. 2008, 103, 1989–1997. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Hitchon, C.A.; Walld, R.; Bolton, J.M.; Sareen, J.; Walker, J.R.; Graff, L.A.; Patten, S.B.; Singer, A.; Lix, L.M.; et al. Increased Burden of Psychiatric Disorders in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 360–368. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Wajda, A.; Svenson, L.W.; MacKenzie, A.; Koehoorn, M.; Jackson, M.; Fedorak, R.; Israel, D.; Blanchard, J.F. The Epidemiology of Inflammatory Bowel Disease in Canada: A Population-Based Study. Am. J. Gastroenterol. 2006, 101, 1559–1568. [Google Scholar] [CrossRef]
- Fiest, K.M.; Bernstein, C.N.; Walker, J.R.; Graff, L.A.; Hitchon, C.A.; Peschken, C.A.; Zarychanski, R.; Abou-Setta, A.; Patten, S.B.; Sareen, J.; et al. Systematic Review of Interventions for Depression and Anxiety in Persons with Inflammatory Bowel Disease. BMC Res. Notes 2016, 9, 404. [Google Scholar] [CrossRef]
- He, Y.; Chen, C.L.; He, J.; De Liu, S. Causal Associations between Inflammatory Bowel Disease and Anxiety: A Bidirectional Mendelian Randomization Study. World J. Gastroenterol. 2023, 29, 5872–5881. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, T.H.; Allin, K.H.; Keefer, L.; Ananthakrishnan, A.N.; Jess, T. Depression and Anxiety in Inflammatory Bowel Disease: Epidemiology, Mechanisms and Treatment. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Cooney, R.; Tang, D.; Barrett, K.; Russell, R.K. Children and Young Adults with Inflammatory Bowel Disease Have an Increased Incidence and Risk of Developing Mental Health Conditions: A UK Population-Based Cohort Study. Inflamm. Bowel Dis. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xu, Z.; Noordam, R.; Van Heemst, D.; Li-Gao, R. Depression and Inflammatory Bowel Disease: A Bidirectional Two-Sample Mendelian Randomization Study. J. Crohns Colitis 2022, 16, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Fairbrass, K.M.; Lovatt, J.; Barberio, B.; Yuan, Y.; Gracie, D.J.; Ford, A.C. Bidirectional Brain–Gut Axis Effects Influence Mood and Prognosis in IBD: A Systematic Review and Meta-Analysis. Gut 2022, 71, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Frolkis, A.D.; Vallerand, I.A.; Shaheen, A.A.; Lowerison, M.W.; Swain, M.G.; Barnabe, C.; Patten, S.B.; Kaplan, G.G. Depression Increases the Risk of Inflammatory Bowel Disease, Which May Be Mitigated by the Use of Antidepressants in the Treatment of Depression. Gut 2019, 68, 1606–1612. [Google Scholar] [CrossRef]
- Agirman, G.; Yu, K.B.; Hsiao, E.Y. Signaling Inflammation across the Gut-Brain Axis. Science 2021, 374, 1087–1092. [Google Scholar] [CrossRef]
- HRQOL Concepts|CDC. Available online: https://www.cdc.gov/hrqol/concept.htm (accessed on 5 July 2023).
- Jones, J.L.; Nguyen, G.C.; Benchimol, E.I.; Bernstein, C.N.; Bitton, A.; Kaplan, G.G.; Murthy, S.K.; Lee, K.; Cooke-Lauder, J.; Otley, A.R. The Impact of Inflammatory Bowel Disease in Canada 2018: Quality of Life. J. Can. Assoc. Gastroenterol. 2019, 2 (Suppl. S1), S42–S48. [Google Scholar] [CrossRef]
- Bernklev, T.; Jahnsen, J.; Lygren, I.; Henriksen, M.; Vatn, M.; Moum, B. Health-Related Quality of Life in Patients with Inflammatory Bowel Disease Measured with the Short Form-36: Psychometric Assessments and a Comparison with General Population Norms. Inflamm. Bowel Dis. 2005, 11, 909–918. [Google Scholar] [CrossRef]
- Hoivik, M.L.; Moum, B.; Solberg, I.C.; Cvancarova, M.; Hoie, O.; Vatn, M.H.; Bernklev, T. Health-Related Quality of Life in Patients with Ulcerative Colitis after a 10-Year Disease Course: Results from the IBSEN Study. Inflamm. Bowel Dis. 2012, 18, 1540–1549. [Google Scholar] [CrossRef]
- Høivik, M.L.; Bernklev, T.; Solberg, I.C.; Cvancarova, M.; Lygren, I.; Jahnsen, J.; Moum, B. Patients with Crohn’s Disease Experience Reduced General Health and Vitality in the Chronic Stage: Ten-Year Results from the IBSEN Study. J. Crohns Colitis 2012, 6, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Casellas, F.; Arenas, J.I.; Baudet, J.S.; Fábregas, S.; García, N.; Gelabert, J.; Medina, C.; Ochotorena, I.; Papo, M.; Rodrigo, L.; et al. Impairment of Health-Related Quality of Life in Patients with Inflammatory Bowel Disease: A Spanish Multicenter Study. Inflamm. Bowel Dis. 2005, 11, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Wilburn, J.; Twiss, J.; Kemp, K.; McKenna, S.P. A Qualitative Study of the Impact of Crohn’s Disease from a Patient’s Perspective. Front. Gastroenterol. 2017, 8, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Pihl-Lesnovska, K.; Hjortswang, H.; Ek, A.C.; Frisman, G.H. Patients’ Perspective of Factors Influencing Quality of Life While Living with Crohn Disease. Gastroenterol. Nurs. 2010, 33, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Rahier, J.F.; Magro, F.; Abreu, C.; Armuzzi, A.; Ben-Horin, S.; Chowers, Y.; Cottone, M.; de Ridder, L.; Doherty, G.; Ehehalt, R.; et al. Second European Evidence-Based Consensus on the Prevention, Diagnosis and Management of Opportunistic Infections in Inflammatory Bowel Disease. J. Crohns Colitis 2014, 8, 443–468. [Google Scholar] [CrossRef] [PubMed]
- Ben-Horin, S.; Bujanover, Y.; Goldstein, S.; Nadler, M.; Lang, A.; Kopylov, U.; Katz, L.; Lahat, A.; Schwartz, E.; Avidan, B. Travel-Associated Health Risks for Patients with Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2012, 10, 160–165.e1. [Google Scholar] [CrossRef] [PubMed]
- Greveson, K.; Shepherd, T.; Mulligan, J.P.; Hamilton, M.; Woodward, S.; Norton, C.; Murray, C. Travel Health and Pretravel Preparation in the Patient with Inflammatory Bowel Disease. Front. Gastroenterol. 2016, 7, 60–65. [Google Scholar] [CrossRef]
- Libby, G.W.; Dawson, A.M. The Social Toll of Crohn’s Disease. Br. Med. J. 1978, 2, 1117–1119. [Google Scholar] [CrossRef]
- Rivière, P.; Zallot, C.; Desobry, P.; Sabaté, J.M.; Vergniol, J.; Zerbib, F.; Peyrin-Biroulet, L.; Laharie, D.; Poullenot, F. Frequency of and Factors Associated with Sexual Dysfunction in Patients with Inflammatory Bowel Disease. J. Crohns Colitis 2017, 11, 1347–1352. [Google Scholar] [CrossRef]
- Bel, L.G.J.; Vollebregt, A.M.; Van der Meulen-de Jong, A.E.; Fidder, H.H.; Ten Hove, W.R.; Vliet-Vlieland, C.W.; ter Kuile, M.M.; de Groot, H.E.; Both, S. Sexual Dysfunctions in Men and Women with Inflammatory Bowel Disease: The Influence of IBD-Related Clinical Factors and Depression on Sexual Function. J. Sex. Med. 2015, 12, 1557–1567. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, J.; Liu, Y.; Luo, L.; Zhu, Z.; Li, E.; Luo, J.; Zhao, Z. Inflammatory Bowel Diseases Were Associated with Risk of Sexual Dysfunction in Both Sexes: A Meta-Analysis. Inflamm. Bowel Dis. 2019, 25, 699–707. [Google Scholar] [CrossRef] [PubMed]
- de Arce, E.P.; Quera, R.; Barros, J.R.; Sassaki, L.Y. Sexual Dysfunction in Inflammatory Bowel Disease: What the Specialist Should Know and Ask. Int. J. Gen. Med. 2021, 14, 2003–2015. [Google Scholar] [CrossRef]
- Büller, H.A. Objectives and Outcomes in the Conventional Treatment of Pediatric Crohn’s Disease. J. Pediatr. Gastroenterol. Nutr. 2001, 33 (Suppl. S1), S11–S18. [Google Scholar] [CrossRef] [PubMed]
- Mackner, L.M.; Crandall, W.V. Brief Report: Psychosocial Adjustment in Adolescents with Inflammatory Bowel Disease. J. Pediatr. Psychol. 2006, 31, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.; Drotar, D.; Palermo, T.M.; McGowan, K.; Arendt, R. Health-Related Quality of Life in Children and Adolescents with Inflammatory Bowel Disease. Child. Health Care 2007, 36, 29–43. [Google Scholar] [CrossRef]
- Kunz, J.H.; Hommel, K.A.; Greenley, R.N. Health-Related Quality of Life of Youth with Inflammatory Bowel Disease: A Comparison with Published Data Using the PedsQL 4.0 Generic Core Scales. Inflamm. Bowel Dis. 2010, 16, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.N.; Boyle, S.L.; Graef, D.M.; Janicke, D.M.; Jolley, C.D.; Denson, L.A.; Baldassano, R.N.; Hommel, K.A. Health-Related Quality of Life in Youth with Crohn Disease: Role of Disease Activity and Parenting Stress. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 749–753. [Google Scholar] [CrossRef]
- Knez, R.; Francisković, T.; Samarin, R.M.; Niksić, M. Parental Quality of Life in the Framework of Paediatric Chronic Gastrointestinal Disease. Coll. Antropol. 2011, 35 (Suppl. S2), 275–280. [Google Scholar]
- Argyriou, K.; Kapsoritakis, A.; Oikonomou, K.; Manolakis, A.; Tsakiridou, E.; Potamianos, S. Disability in Patients with Inflammatory Bowel Disease: Correlations with Quality of Life and Patient’s Characteristics. Can. J. Gastroenterol. Hepatol. 2017, 2017, 6138105. [Google Scholar] [CrossRef]
- Lönnfors, S.; Vermeire, S.; Greco, M.; Hommes, D.; Bell, C.; Avedano, L. IBD and Health-Related Quality of Life -- Discovering the True Impact. J. Crohns Colitis 2014, 8, 1281–1286. [Google Scholar] [CrossRef]
- Nishida, Y.; Hosomi, S.; Fujimoto, K.; Nakata, R.; Itani, S.; Ohminami, M.; Nadatani, Y.; Fukunaga, S.; Otani, K.; Tanaka, F.; et al. Effect of the Coronavirus Disease 2019 Lockdown on Lifestyle Factors in Japanese Patients with Inflammatory Bowel Disease. Intern. Med. 2022, 61, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, L.; Klucker, E.; Le Thi, T.G.; Breiteneicher, S.; Rubio-acero, R.; Neuhaus, L.; Stark, R.G.; Standl, M.; Wieser, A.; Török, H.; et al. Following Pediatric and Adult Ibd Patients through the COVID-19 Pandemic: Changes in Psychosocial Burden and Perception of Infection Risk and Harm over Time. J. Clin. Med. 2021, 10, 4124. [Google Scholar] [CrossRef]
- Conti, C.; Rosa, I.; Zito, L.; Grossi, L.; Efthymakis, K.; Neri, M.; Porcelli, P. Influence of the COVID-19 Outbreak on Disease Activity and Quality of Life in Inflammatory Bowel Disease Patients. Front. Psychiatry 2021, 12, 664088. [Google Scholar] [CrossRef] [PubMed]
- Occhipinti, V.; Pastorelli, L. Challenges in the Care of IBD Patients during the COVID-19 Pandemic: Report from a “Red Zone” Area in Northern Italy. Inflamm. Bowel Dis. 2020, 26, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Corrias, A.; Cortes, G.M.; Bardanzellu, F.; Marcialis, M.A.; Melis, A.; Fanos, V. Risk, Course, and Effect of SARS-CoV-2 Infection in Children and Adults with Chronic Inflammatory Bowel Diseases. Children 2021, 8, 753. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, R.P.; Rodrigues, M. COVID-19 and Pediatric Inflammatory Bowel Disease: How to Manage It? Clinics 2020, 75, e1962. [Google Scholar] [CrossRef]
- Sansotta, N.; Norsa, L.; Zuin, G.; Panceri, R.; Dilillo, D.; Pozzi, E.; De Giacomo, C.; Moretti, C.; Celano, R.; Nuti, F.; et al. Children with Inflammatory Bowel Disease in the COVID-19 Main Endemic Focus: The Lombardy Experience. Front. Pediatr. 2021, 9, 607285. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Huang, Y.; Martín-De-Carpi, J.; Aloi, M.; Focht, G.; Kang, B.; Zhou, Y.; Sanchez, C.; Kappelman, M.D.; Uhlig, H.H.; et al. Corona Virus Disease 2019 and Paediatric Inflammatory Bowel Diseases: Global Experience and Provisional Guidance (March 2020) from the Paediatric IBD Porto Group of European Society of Paediatric Gastroenterology, Hepatology, and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 727–733. [Google Scholar] [CrossRef]
- Brenner, E.J.; Pigneur, B.; Focht, G.; Zhang, X.; Ungaro, R.C.; Colombel, J.F.; Turner, D.; Kappelman, M.D.; Ruemmele, F.M. Benign Evolution of SARS-CoV2 Infections in Children with Inflammatory Bowel Disease: Results From Two International Databases. Clin. Gastroenterol. Hepatol. 2021, 19, 394–396.e5. [Google Scholar] [CrossRef]
- Bezzio, C.; Pellegrini, L.; Manes, G.; Arena, I.; Picascia, D.; Della Corte, C.; Devani, M.; Schettino, M.; Saibeni, S. Biologic Therapies May Reduce the Risk of COVID-19 in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26, E107–E109. [Google Scholar] [CrossRef]
- Arrigo, S.; Alvisi, P.; Banzato, C.; Bramuzzo, M.; Civitelli, F.; Corsello, A.; D’Arcangelo, G.; Dilillo, A.; Dipasquale, V.; Felici, E.; et al. Management of Paediatric IBD after the Peak of COVID-19 Pandemic in Italy: A Position Paper on Behalf of the SIGENP IBD Working Group. Dig. Liver Dis. 2021, 53, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Salvatori, S.; Baldassarre, F.; Mossa, M.; Monteleone, G. Long COVID in Inflammatory Bowel Diseases. J. Clin. Med. 2021, 10, 5575. [Google Scholar] [CrossRef] [PubMed]
- Black, J.; Sweeney, L.; Yuan, Y.; Singh, H.; Norton, C.; Czuber-Dochan, W. Systematic Review: The Role of Psychological Stress in Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2022, 56, 1235–1249. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.N.; Singh, S.; Graff, L.A.; Walker, J.R.; Miller, N.; Cheang, M. A Prospective Population-Based Study of Triggers of Symptomatic Flares in IBD. Am. J. Gastroenterol. 2010, 105, 1994–2002. [Google Scholar] [CrossRef] [PubMed]
- Rozich, J.J.; Holmer, A.; Singh, S. Effect of Lifestyle Factors on Outcomes in Patients with Inflammatory Bowel Diseases. Am. J. Gastroenterol. 2020, 115, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Peppas, S.; Pansieri, C.; Piovani, D.; Danese, S.; Peyrin-Biroulet, L.; Tsantes, A.G.; Brunetta, E.; Tsantes, A.E.; Bonovas, S. The Brain-Gut Axis: Psychological Functioning and Inflammatory Bowel Diseases. J. Clin. Med. 2021, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Kiebles, J.L.; Doerfler, B.; Keefer, L. Preliminary Evidence Supporting a Framework of Psychological Adjustment to Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2010, 16, 1685–1695. [Google Scholar] [CrossRef]
- Sunavsky, A.; Moreau, J.; Tripp, D.A. Understanding Perceived Stress in Adolescent Inflammatory Bowel Disease. J. Can. Assoc. Gastroenterol. 2022, 5, 79–85. [Google Scholar] [CrossRef]
- Ge, L.; Liu, S.; Li, S.; Yang, J.; Hu, G.; Xu, C.; Song, W. Psychological Stress in Inflammatory Bowel Disease: Psychoneuroimmunological Insights into Bidirectional Gut–Brain Communications. Front. Immunol. 2022, 13, 1016578. [Google Scholar] [CrossRef]
- Tavakoli, P.; Vollmer-Conna, U.; Hadzi-Pavlovic, D.; Grimm, M.C. A Review of Inflammatory Bowel Disease: A Model of Microbial, Immune and Neuropsychological Integration. Public Health Rev. 2021, 42, 1603990. [Google Scholar] [CrossRef]
- Jairath, V.; Feagan, B.G. Global Burden of Inflammatory Bowel Disease. Lancet Gastroenterol. Hepatol. 2020, 5, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Abraham, B.P.; Ahmed, T.; Ali, T. Inflammatory Bowel Disease: Pathophysiology and Current Therapeutic Approaches. Handb. Exp. Pharmacol. 2017, 239, 115–146. [Google Scholar] [CrossRef] [PubMed]
- Martin-Subero, M.; Anderson, G.; Kanchanatawan, B.; Berk, M.; Maes, M. Comorbidity between Depression and Inflammatory Bowel Disease Explained by Immune-Inflammatory, Oxidative, and Nitrosative Stress; Tryptophan Catabolite; and Gut-Brain Pathways. CNS Spectr. 2016, 21, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 336468. [Google Scholar] [CrossRef] [PubMed]
- Neuendorf, R.; Harding, A.; Stello, N.; Hanes, D.; Wahbeh, H. Depression and Anxiety in Patients with Inflammatory Bowel Disease: A Systematic Review. J. Psychosom. Res. 2016, 87, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Black, C.J.; Thakur, E.R.; Houghton, L.A.; Quigley, E.M.M.; Moayyedi, P.; Ford, A.C. Efficacy of Psychological Therapies for Irritable Bowel Syndrome: Systematic Review and Network Meta-Analysis. Gut 2020, 69, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, I.; Hewitt, C.; Bell, K.; Phillips, A.; Mikocka-Walus, A. Systematic Review with Meta-Analysis: Online Psychological Interventions for Mental and Physical Health Outcomes in Gastrointestinal Disorders Including Irritable Bowel Syndrome and Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2018, 48, 244–259. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Shorey, S.; Seah, B.; Chan, W.X.; Tam, W.W.S.; Wang, W. The Effectiveness of Psychological Interventions on Self-Care, Psychological and Health Outcomes in Patients with Chronic Heart Failure—A Systematic Review and Meta-Analysis. Int. J. Nurs. Stud. 2018, 78, 16–25. [Google Scholar] [CrossRef]
- Wynne, B.; McHugh, L.; Gao, W.; Keegan, D.; Byrne, K.; Rowan, C.; Hartery, K.; Kirschbaum, C.; Doherty, G.; Cullen, G.; et al. Acceptance and Commitment Therapy Reduces Psychological Stress in Patients With Inflammatory Bowel Diseases. Gastroenterology 2019, 156, 935–945.e1. [Google Scholar] [CrossRef]
- Li, C.; Hou, Z.; Liu, Y.; Ji, Y.; Xie, L. Cognitive-Behavioural Therapy in Patients with Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis. Int. J. Nurs. Pract. 2019, 25, e12699. [Google Scholar] [CrossRef]
- Jordan, C.; Hayee, B.; Chalder, T. Cognitive Behaviour Therapy for Distress in People with Inflammatory Bowel Disease: A Benchmarking Study. Clin. Psychol. Psychother. 2019, 26, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.G.; Loftus, P.; Accardo, M.; Keenan, M.; Cohen, L.; Osterman, M.T. Self-Help Cognitive Behavioral Therapy Improves Health-Related Quality of Life for Inflammatory Bowel Disease Patients: A Randomized Controlled Effectiveness Trial. J. Clin. Psychol. Med. Settings 2020, 27, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Bennebroek Evertsz, F.; Sprangers, M.A.G.; Sitnikova, K.; Stokkers, P.C.F.; Ponsioen, C.Y.; Bartelsman, J.F.W.M.; Van Bodegraven, A.A.; Fischer, S.; Depla, A.C.T.M.; Mallant, R.C.; et al. Effectiveness of Cognitive-Behavioral Therapy on Quality of Life, Anxiety, and Depressive Symptoms among Patients with Inflammatory Bowel Disease: A Multicenter Randomized Controlled Trial. J. Consult. Clin. Psychol. 2017, 85, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Szigethy, E.; Youk, A.O.; Gonzalez-Heydrich, J.; Bujoreanu, S.I.; Weisz, J.; Fairclough, D.; Ducharme, P.; Jones, N.; Lotrich, F.; Keljo, D.; et al. Effect of 2 Psychotherapies on Depression and Disease Activity in Pediatric Crohn’s Disease. Inflamm. Bowel Dis. 2015, 21, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, X.; Sun, Y.; Xie, Y.; Wang, X.; Li, R.; Hesketh, T. The Physiological and Psychological Effects of Cognitive Behavior Therapy on Patients with Inflammatory Bowel Disease before COVID-19: A Systematic Review. BMC Gastroenterol. 2021, 21, 469. [Google Scholar] [CrossRef] [PubMed]
- Hood, M.M.; Jedel, S. Mindfulness-Based Interventions in Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2017, 46, 859–874. [Google Scholar] [CrossRef] [PubMed]
- Berrill, J.W.; Sadlier, M.; Hood, K.; Green, J.T. Mindfulness-Based Therapy for Inflammatory Bowel Disease Patients with Functional Abdominal Symptoms or High Perceived Stress Levels. J. Crohns Colitis 2014, 8, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Ewais, T.; Begun, J.; Kenny, M.; Hay, K.; Houldin, E.; Chuang, K.H.; Tefay, M.; Kisely, S. Mindfulness Based Cognitive Therapy for Youth with Inflammatory Bowel Disease and Depression—Findings from a Pilot Randomised Controlled Trial. J. Psychosom. Res. 2021, 149. [Google Scholar] [CrossRef]
- González-Moret, R.; Cebolla, A.; Cortés, X.; Baños, R.M.; Navarrete, J.; de la Rubia, J.E.; Lisón, J.F.; Soria, J.M. The Effect of a Mindfulness-Based Therapy on Different Biomarkers among Patients with Inflammatory Bowel Disease: A Randomised Controlled Trial. Sci. Rep. 2020, 10, 6071. [Google Scholar] [CrossRef]
- Georgescu, D.; Iurciuc, M.S.; Petre, I.; Georgescu, L.A.; Szasz, F.; Ionita, I.; Ancusa, O.E.; Ionita, M.; Lighezan, D. Chronic Pelvic Pain and Irritable Bowel Syndrome: Is Subclinical Inflammation Bridging the Gap? Rev. Chim. 2019, 70, 3634–3637. [Google Scholar] [CrossRef]
- Keefer, L.; Taft, T.H.; Kiebles, J.L.; Martinovich, Z.; Barrett, T.A.; Palsson, O.S. Gut-Directed Hypnotherapy Significantly Augments Clinical Remission in Quiescent Ulcerative Colitis. Aliment. Pharmacol. Ther. 2013, 38, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Hoekman, D.R.; Vlieger, A.M.; Stokkers, P.C.; Mahhmod, N.; Rietdijk, S.; De Boer, N.K.; De Meij, T.G.; Frankenhuis, C.; D’Haens, G.R.; Benninga, M.A. Hypnotherapy for Irritable Bowel Syndrome-Type Symptoms in Patients with Quiescent Inflammatory Bowel Disease: A Randomized, Controlled Trial. J. Crohns Colitis 2021, 15, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.L.; Muir, J.G.; Gibson, P.R. Review Article: Gut-Directed Hypnotherapy in the Management of Irritable Bowel Syndrome and Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2015, 41, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, O.; Prelipcean, C.C.; Dranga, M.; Soponaru, C.; Mihai, C. The Specialized Educational and Psychological Counseling in Inflammatory Bowel Disease Patients—A Target or a Challenge? Turk. J. Gastroenterol. 2020, 31, 760–766. [Google Scholar] [CrossRef]
- Gerbarg, P.L.; Jacob, V.E.; Stevens, L.; Bosworth, B.P.; Chabouni, F.; Defilippis, E.M.; Warren, R.; Trivellas, M.; Patel, P.V.; Webb, C.D.; et al. The Effect of Breathing, Movement, and Meditation on Psychological and Physical Symptoms and Inflammatory Biomarkers in Inflammatory Bowel Disease: A Randomized Controlled Trial. Inflamm. Bowel Dis. 2015, 21, 2886–2896. [Google Scholar] [CrossRef] [PubMed]
- Arruda, J.M.; Bogetz, A.L.; Vellanki, S.; Wren, A.; Yeh, A.M. Yoga as Adjunct Therapy for Adolescents with Inflammatory Bowel Disease: A Pilot Clinical Trial. Complement. Ther. Med. 2018, 41, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Wilke, E.; Reindl, W.; Thomann, P.A.; Ebert, M.P.; Wuestenberg, T.; Thomann, A.K. Effects of Yoga in Inflammatory Bowel Diseases and on Frequent IBD-Associated Extraintestinal Symptoms like Fatigue and Depression. Complement. Ther. Clin. Pract. 2021, 45, 101465. [Google Scholar] [CrossRef] [PubMed]
- Kavuri, V.; Selvan, P.; Tabesh, A.; Raghuram, N.; Selvan, S.R. Remedial Yoga Module Improves Symptoms of Irritable Bowel Syndrome: Replication in the Wait-List Group and Sustained Improvements at 6 Months. Eur. J. Integr. Med. 2015, 7, 609–616. [Google Scholar] [CrossRef]
- Daghaghzadeh, H.; Naji, F.; Afshar, H.; Sharbafchi, M.R.; Feizi, A.; Maroufi, M.; Tabatabaeeyan, M.; Adibi, P.; Tavakoli, H. Efficacy of Duloxetine Add on in Treatment of Inflammatory Bowel Disease Patients: A Double-Blind Controlled Study. J. Res. Med. Sci. 2015, 20, 595–601. [Google Scholar] [CrossRef]
- Iskandar, H.N.; Cassell, B.; Kanuri, N.; Gyawali, C.P.; Gutierrez, A.; Dassopoulos, T.; Ciorba, M.A.; Sayuk, G.S. Tricyclic Antidepressants for Management of Residual Symptoms in Inflammatory Bowel Disease. J. Clin. Gastroenterol. 2014, 48, 423–429. [Google Scholar] [CrossRef]
- Wichniak, A.; Wierzbicka, A.; Walęcka, M.; Jernajczyk, W. Effects of Antidepressants on Sleep. Curr. Psychiatry Rep. 2017, 19, 63. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.J.; Hamlin, P.J.; Gracie, D.J.; Ford, A.C. The Effect of Antidepressants on the Course of Inflammatory Bowel Disease. Can. J. Gastroenterol. Hepatol. 2018, 2018, 2047242. [Google Scholar] [CrossRef] [PubMed]
- Boicean, A.; Birsan, S.; Ichim, C.; Boeras, I.; Roman-Filip, I.; Blanca, G.; Bacila, C.; Fleaca, R.S.; Dura, H.; Roman-Filip, C. Has-MiR-129-5p’s Involvement in Different Disorders, from Digestive Cancer to Neurodegenerative Diseases. Biomedicines 2023, 11, 2058. [Google Scholar] [CrossRef] [PubMed]
- Ho, V.; Baker, J.R.; Willison, K.R.; Barnes, P.J.; Donnelly, L.E.; Klug, D.R. Single Cell Quantification of MicroRNA from Small Numbers of Non-Invasively Sampled Primary Human Cells. Commun. Biol. 2023, 6, 458. [Google Scholar] [CrossRef] [PubMed]
- Kalita, A.; Sikora-Skrabaka, M.; Nowakowska-Zajdel, E. Role of Some MicroRNA/ADAM Proteins Axes in Gastrointestinal Cancers as a Novel Biomarkers and Potential Therapeutic Targets—A Review. Curr. Issues Mol. Biol. 2023, 45, 2917–2936. [Google Scholar] [CrossRef]
- Ishaq, Y.; Ikram, A.; Alzahrani, B.; Khurshid, S. The Role of MiRNAs, CircRNAs and Their Interactions in Development and Progression of Hepatocellular Carcinoma: An Insilico Approach. Genes 2023, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Balaskas, P.; Goljanek-Whysall, K.; Clegg, P.D.; Fang, Y.; Cremers, A.; Smagul, A.; Welting, T.J.M.; Peffers, M.J. MicroRNA Signatures in Cartilage Ageing and Osteoarthritis. Biomedicines 2023, 11, 1189. [Google Scholar] [CrossRef] [PubMed]
- Angele, P.; Zellner, J.; Pattappa, G.; Ho, P.T.B.; Clark, I.M.; Le, L.T.T. MicroRNA-Based Diagnosis and Therapy. Int. J. Mol. Sci. 2022, 23, 7167. [Google Scholar] [CrossRef]
- Chandrasekera, P.; Perfetto, M.; Lu, C.; Zhuo, M.; Bahudhanapati, H.; Li, J.; Chen, W.C.; Kulkarni, P.; Christian, L.; Liu, J.; et al. Metalloprotease ADAM9 Cleaves Ephrin-B Ligands and Differentially Regulates Wnt and MTOR Signaling Downstream of Akt Kinase in Colorectal Cancer Cells. J. Biol. Chem. 2022, 298, 102225. [Google Scholar] [CrossRef]
- Dobricic, V.; Schilling, M.; Farkas, I.; Gveric, D.O.; Ohlei, O.; Schulz, J.; Middleton, L.; Gentleman, S.M.; Parkkinen, L.; Bertram, L.; et al. Common Signatures of Differential MicroRNA Expression in Parkinson’s and Alzheimer’s Disease Brains. Brain Commun. 2022, 4, fcac274. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Y.; Yue, J.; Shi, Y.; Xiao, B.; Xiao, W.; Luo, Z. Non-Coding RNAs: The Neuroinflammatory Regulators in Neurodegenerative Diseases. Front. Neurol. 2022, 13, 929290. [Google Scholar] [CrossRef] [PubMed]
- Boicean, A.; Birlutiu, V.; Ichim, C.; Anderco, P.; Birsan, S. Fecal Microbiota Transplantation in Inflammatory Bowel Disease. Biomedicines 2023, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M.M. The Microbiome and Inflammatory Bowel Disease. J. Allergy Clin. Immunol. 2020, 145, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Ishimoto, T.; Fu, L.; Zhang, J.; Zhang, Z.; Liu, Y. The Gut Microbiota in Inflammatory Bowel Disease. Front. Cell Infect. Microbiol. 2022, 12, 733992. [Google Scholar] [CrossRef] [PubMed]
- Popa, D.; Neamtu, B.; Mihalache, M.; Boicean, A.; Banciu, A.; Banciu, D.D.; Moga, D.F.C.; Birlutiu, V. Fecal Microbiota Transplant in Severe and Non-Severe Clostridioides Difficile Infection. Is There a Role of FMT in Primary Severe CDI? J. Clin. Med. 2021, 10, 5822. [Google Scholar] [CrossRef] [PubMed]
- Zatorski, H.; Nakov, R. Faecal Microbiota Transplantation in Inflammatory Bowel Disease: Current Concepts and Future Challenges. Curr. Drug Targets 2020, 21, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Waller, K.M.J.; Leong, R.W.; Paramsothy, S. An Update on Fecal Microbiota Transplantation for the Treatment of Gastrointestinal Diseases. J. Gastroenterol. Hepatol. 2022, 37, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Aggarwala, V.; Mogno, I.; Li, Z.; Yang, C.; Britton, G.J.; Chen-Liaw, A.; Mitcham, J.; Bongers, G.; Gevers, D.; Clemente, J.C.; et al. Precise Quantification of Bacterial Strains after Fecal Microbiota Transplantation Delineates Long-Term Engraftment and Explains Outcomes. Nat. Microbiol. 2021, 6, 1309–1318. [Google Scholar] [CrossRef]
- Ianiro, G.; Mullish, B.H.; Kelly, C.R.; Sokol, H.; Kassam, Z.; Ng, S.; Fischer, M.; Allegretti, J.R.; Masucci, L.; Zhang, F.; et al. Screening of Faecal Microbiota Transplant Donors during the COVID-19 Outbreak: Suggestions for Urgent Updates from an International Expert Panel. Lancet Gastroenterol. Hepatol. 2020, 5, 430–432. [Google Scholar] [CrossRef]
- Kelly, C.R.; Yen, E.F.; Grinspan, A.M.; Kahn, S.A.; Atreja, A.; Lewis, J.D.; Moore, T.A.; Rubin, D.T.; Kim, A.M.; Serra, S.; et al. Fecal Microbiota Transplantation Is Highly Effective in Real-World Practice: Initial Results From the FMT National Registry. Gastroenterology 2021, 160, 183–192.e3. [Google Scholar] [CrossRef]
- Michailidis, L.; Currier, A.C.; Le, M.; Flomenhoft, D.R. Adverse Events of Fecal Microbiota Transplantation: A Meta-Analysis of High-Quality Studies. Ann. Gastroenterol. 2021, 34, 802. [Google Scholar] [CrossRef] [PubMed]
- Lopetuso, L.R.; Deleu, S.; Godny, L.; Petito, V.; Puca, P.; Facciotti, F.; Sokol, H.; Ianiro, G.; Masucci, L.; Abreu, M.; et al. The First International Rome Consensus Conference on Gut Microbiota and Faecal Microbiota Transplantation in Inflammatory Bowel Disease. Gut 2023, 72, 1642–1650. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Saperas, E.; Nogueiras, C.; Mourelle, M.; Antolin, M.; Cadahia, A.; Malagelada, J.R. Release of Mast Cell Mediators into the Jejunum by Cold Pain Stress in Humans. Gastroenterology 1998, 114, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.C.; Hatfield, R.A.; Suls, J.M.; Chamberlain, M.J. Psychological and Physical Stress Induce Differential Effects on Human Colonic Motility. Am. J. Gastroenterol. 1998, 93, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Barclay, G.R.; Turnberg, L.A. Effect of Cold-Induced Pain on Salt and Water Transport in the Human Jejunum. Gastroenterology 1988, 94, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Greenwood-Van Meerveld, B.; Gibson, M.S.; Johnson, A.C.; Venkova, K.; Sutkowski-Markmann, D. NK1 Receptor-Mediated Mechanisms Regulate Colonic Hypersensitivity in the Guinea Pig. Pharmacol. Biochem. Behav. 2003, 74, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, M.T.; Targownik, L.E.; Sexton, K.A.; Graff, L.A.; Miller, N.; Walker, J.R. Assessing the Relationship between Sources of Stress and Symptom Changes among Persons with IBD over Time: A Prospective Study. Can. J. Gastroenterol. Hepatol. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Crumbock, S.C.; Loeb, S.J.; Fick, D.M. Physical Activity, Stress, Disease Activity, and Quality of Life in Adults with Crohn Disease. Gastroenterol. Nurs. 2009, 32, 188–195. [Google Scholar] [CrossRef]
- Sullivan, A.J. Psychogenic Factors in Ulcerative Colitis. Am. J. Dig. Dis. 1935, 2, 651–656. [Google Scholar] [CrossRef]
- Duffy, L.C.; Zielezny, M.A.; Marshall, J.R.; Byers, T.E.; Weiser, M.M.; Phillips, J.F.; Calkins, B.M.; Ogra, P.L.; Graham, S. Relevance of Major Stress Events as an Indicator of Disease Activity Prevalence in Inflammatory Bowel Disease. Behav. Med. 1991, 17, 101–110. [Google Scholar] [CrossRef]
- Cámara, R.J.A.; Ziegler, R.; Begré, S.; Schoepfer, A.M.; Von Känel, R. The Role of Psychological Stress in Inflammatory Bowel Disease: Quality Assessment of Methods of 18 Prospective Studies and Suggestions for Future Research. Digestion 2009, 80, 129–139. [Google Scholar] [CrossRef]
- Keefer, L.; Keshavarzian, A.; Mutlu, E. Reconsidering the Methodology of “Stress” Research in Inflammatory Bowel Disease. J. Crohns Colitis 2008, 2, 193–201. [Google Scholar] [CrossRef]
- Szigethy, E.M.; Allen, J.I.; Reiss, M.; Cohen, W.; Perera, L.P.; Brillstein, L.; Cross, R.K.; Schwartz, D.A.; Kosinski, L.R.; Colton, J.B.; et al. White Paper AGA: The Impact of Mental and Psychosocial Factors on the Care of Patients With Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2017, 15, 986–997. [Google Scholar] [CrossRef]

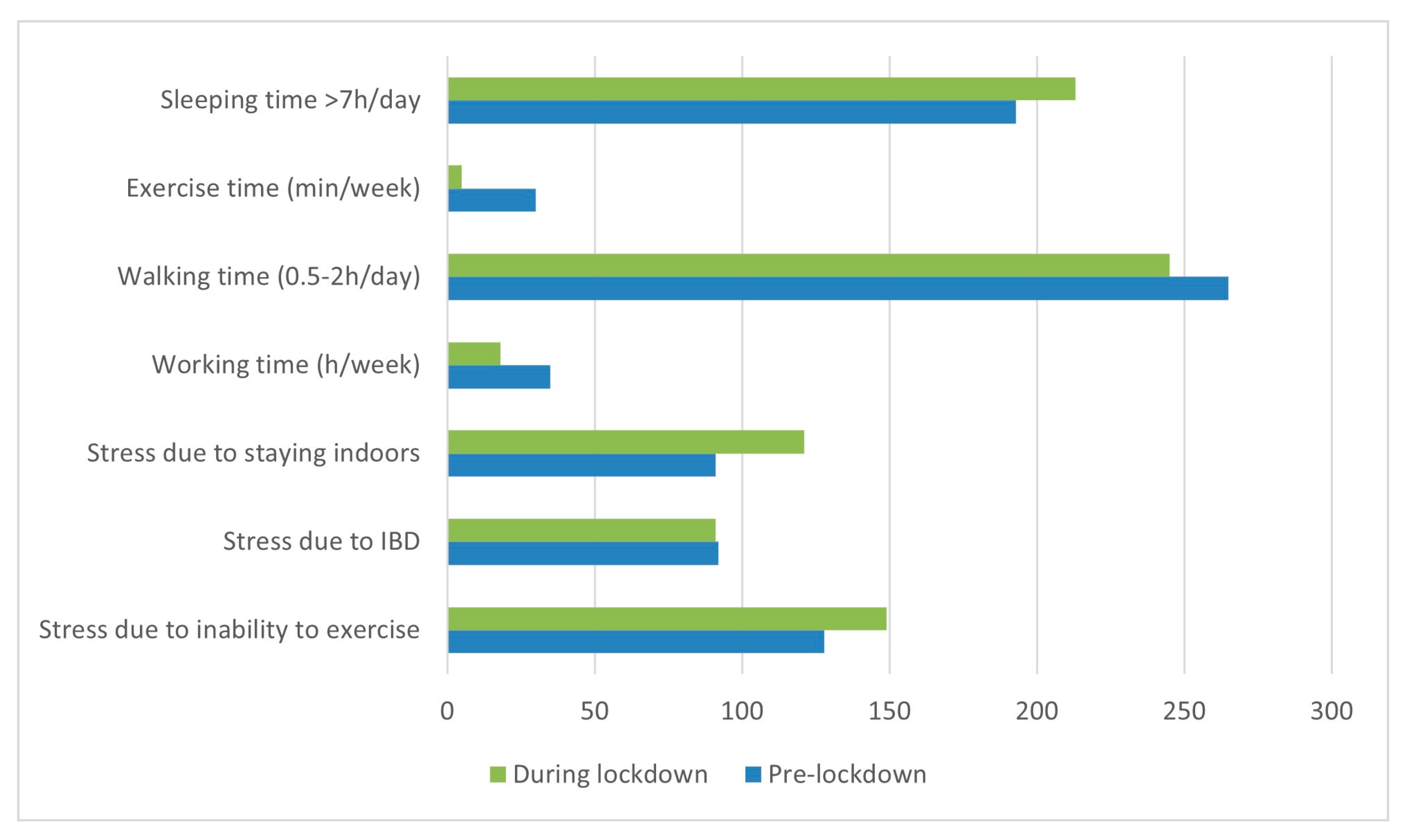
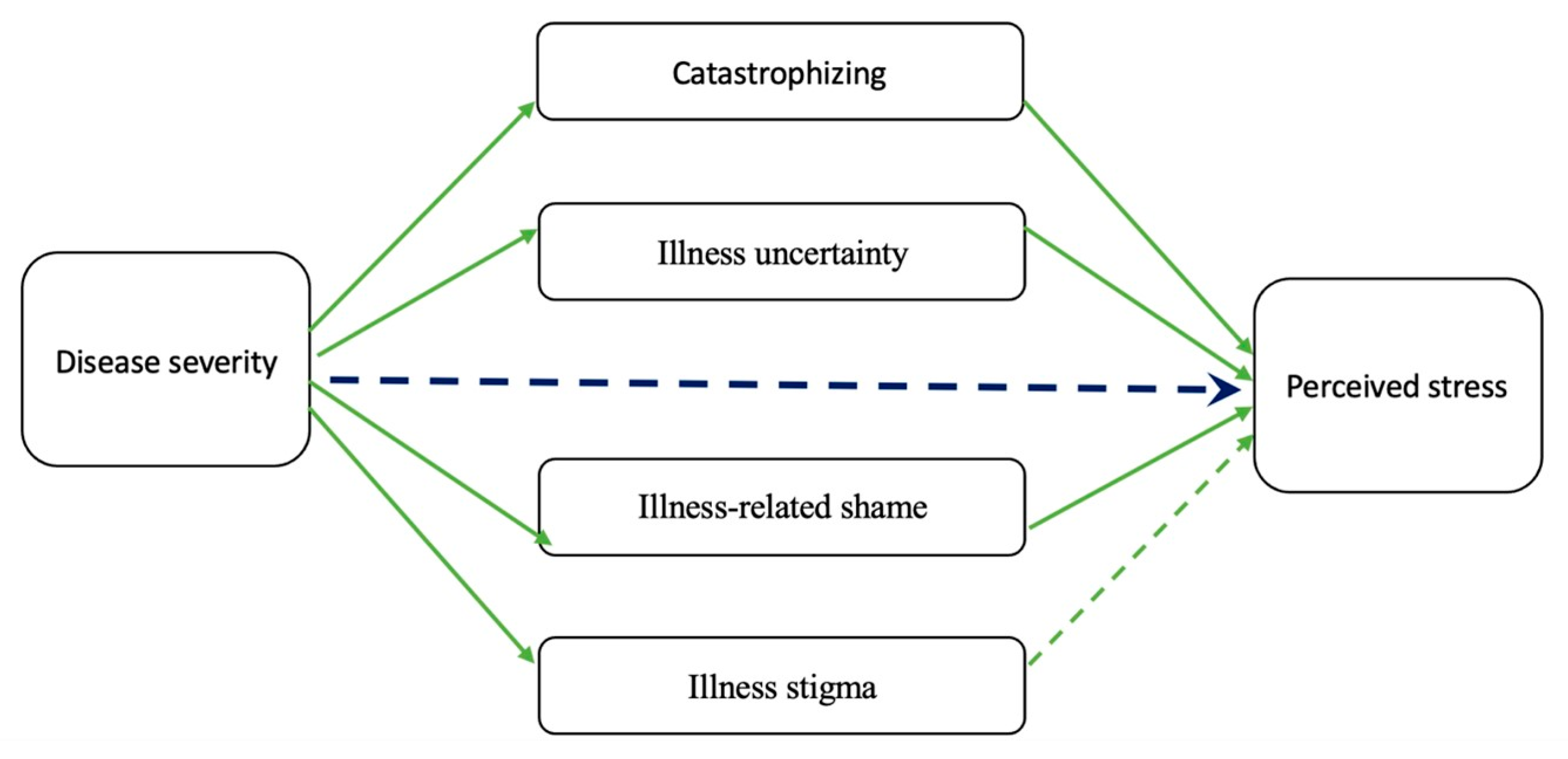
| Factor | Author(s) | Type of Study | N | Intervention/Methodology | Result |
|---|---|---|---|---|---|
| Pro-/prebiotics | Messaoudi, M. et al. [107] | In vivo rat study | 36 rats | Probiotic formulation | Observed anxiolytic-like effects in rat models |
| Garcia-Rodenas, CL. et al. [108] | In vivo rat study | 84 | Maternal separation and prebiotics/probiotics/LC-PUFA | Implementation of a nutritional intervention during the weaning period resulted in the normalization of gut permeability and the restoration of the growth rate | |
| Zareie, M. et al. [109] | In vivo rat study | Four to five rats per group | WAS and probiotics | Probiotics exhibited a preventive effect on chronic stress-induced gastrointestinal abnormalities | |
| Li, N. et al. [110] | In vivo mouse study | Eight mice per group | Chronic mild stress and probiotics | Reduced levels of pro-inflammatory cytokines and modified stress-induced behavioral patterns | |
| Bravo, J.A. et al. [111] | In vivo mouse study | 36 | Probiotic formulation | Highlighted the significance of probiotics in the bidirectional communication between the gut and the brain in stress-related disorders | |
| Messaoudi, M. et al. [107] | Double-blind, placebo-controlled, randomized parallel group study | 66 individuals | Probiotic formulation | Evidenced favorable psychological outcomes in a cohort of healthy human volunteers | |
| Rao, S. et al. [112] | Systematic review | 11 RCTs | Prebiotic supplementation | Demonstrated transient advantageous effects on the composition of the intestinal microbiota in the short term | |
| Prenatal/early life stress | O’Mahony, S.M. et al. [113] | In vivo rat study | 22 | Maternal separation | The effects of early life stress on the gut–brain axis led to modifications that contributed to the manifestation of symptoms in IBD |
| Golubeva, A.V. et al. [114] | In vivo rat study | 6–10 per group | Prenatal stress | Persistent modifications in the composition of the intestinal microbiota over an extended period | |
| Jasarevic, E. et al. [115] | In vivo mouse study | 21–23 mice per group | Prenatal stress | Changes in the vaginal microbiota were implicated in the process of reprogramming the developing brain | |
| Xie, R. et al. [116] | In vivo mouse study | 6–20 mice per group | Maternal high-fat diet | Intestinal dysbiosis and the presence of chronic low-grade inflammation in the gastrointestinal tract | |
| Bailey, M.T. et al. [117] | In vivo primate study | 20 | Maternal separation | Psychological disturbances resulting from maternal separation led to modifications in the composition of the intestinal microflora | |
| Zijlmans, M.A. et al. [118] | Longitudinal clinical study | 192 children | Questionnaire | The presence of prenatal stress was correlated with specific microbial colonization patterns in infants | |
| Chronic/social/environmental stress | Soderholm, J.D. et al. [119] | In vivo rat study | Seven to eight rats per group | WAS | Impaired mucosal defenses against luminal bacteria lead to intestinal inflammation |
| Da Silva, S. et al. [120] | In vivo rat study | 13–14 rats per group | WAS | Modified composition of the intestinal mucus | |
| Meddings, J.B. et al. [121] | In vivo rat study | Not specified | Stress induction | Elevated gastrointestinal permeability facilitates the passage of luminal constituents to the mucosal immune system | |
| Saunders, P.R. et al. [122] | In vivo rat study | 6 | Cold-restraint stress or WAS | Intensified intestinal inflammation resulting from enhanced uptake of immunogenic substances | |
| Santos, J. et al. [123] | In vivo rat study | Four rats per group | WAS | Epithelial mitochondrial damage triggered by stress and activation of mucosal mast cells | |
| Gao, X. et al. [124] | In vivo mouse study | Four to six mice per group | Chronic restraint stress | Disrupted gut microbiota followed by immune system activation resulted in the development of colitis | |
| Neufeld, K.M. et al. [125] | In vivo mouse study | 12 mice per group | Germ-free and specific-pathogen-free | The presence of typical intestinal microbiota played a role in the development of behavior | |
| Donnet-Hughes, A. et al. [126] | In vivo mouse study | 10 mice per group | Lactation | Cellular transfer of bacterial translocation took place in mice during pregnancy and lactation | |
| Heijtz, R. et al. [127] | In vivo mouse study | 7–14 mice per group | Germ-free and specific-pathogen-free | The gut microbiota impacted the development of the mammalian brain and subsequent behavioral patterns in adulthood | |
| Marin, I.A. et al. [128] | In vivo mouse study | 10–12 (three independent experiments) | Unpredictable chronic mild stress | Modified composition of the intestinal microbiota, particularly within the lactobacillus component | |
| Bharwani, A. et al. [129] | In vivo mouse study | 7–20 mice per group | Chronic social defeat | Stress triggered intricate structural alterations in the gut microbiota | |
| Sudo, N. et al. [36] | In vivo mouse study | 18–24 mice per group | Germ-free and specific-pathogen-free; acute restraint stress | The commensal microbiota had the potential to influence the postnatal maturation of the hypothalamic–pituitary–adrenal (HPA) stress response | |
| Galley, J.D. et al. [130] | In vivo mouse study | Five mice per group | SDR | Altered microbial populations that had a close association with the colonic mucosa | |
| Bailey, M.T. et al. [33] | In vivo mouse study | Five mice per group | SDR | Stress resulted in notable alterations in the colonization of the intestinal microbiota | |
| Noguera, J.C. et al. [131] | Field experiment in wild birds | 64 | Corticosterone implant | Modified gut microbiome in birds living in their natural habitat | |
| Van der Zaag-Loonen, H.J. et al. [132] | Clinical study | 65 | Coping style instrument | Adolescents with IBD exhibited a higher utilization of avoidant coping strategies in comparison with healthy individuals | |
| Walker, L.S. et al. [133] | Clinical study | 263 | Daily interview assessment | There was an association between stress and the occurrence of digestive problems and disruptions in gastrointestinal health | |
| Maes, M. et al. [134] | Clinical study | 40 | Depression | Elevated bacterial translocation and heightened immune responses targeting commensal bacteria |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belei, O.; Basaca, D.-G.; Olariu, L.; Pantea, M.; Bozgan, D.; Nanu, A.; Sîrbu, I.; Mărginean, O.; Enătescu, I. The Interaction between Stress and Inflammatory Bowel Disease in Pediatric and Adult Patients. J. Clin. Med. 2024, 13, 1361. https://doi.org/10.3390/jcm13051361
Belei O, Basaca D-G, Olariu L, Pantea M, Bozgan D, Nanu A, Sîrbu I, Mărginean O, Enătescu I. The Interaction between Stress and Inflammatory Bowel Disease in Pediatric and Adult Patients. Journal of Clinical Medicine. 2024; 13(5):1361. https://doi.org/10.3390/jcm13051361
Chicago/Turabian StyleBelei, Oana, Diana-Georgiana Basaca, Laura Olariu, Manuela Pantea, Daiana Bozgan, Anda Nanu, Iuliana Sîrbu, Otilia Mărginean, and Ileana Enătescu. 2024. "The Interaction between Stress and Inflammatory Bowel Disease in Pediatric and Adult Patients" Journal of Clinical Medicine 13, no. 5: 1361. https://doi.org/10.3390/jcm13051361
APA StyleBelei, O., Basaca, D.-G., Olariu, L., Pantea, M., Bozgan, D., Nanu, A., Sîrbu, I., Mărginean, O., & Enătescu, I. (2024). The Interaction between Stress and Inflammatory Bowel Disease in Pediatric and Adult Patients. Journal of Clinical Medicine, 13(5), 1361. https://doi.org/10.3390/jcm13051361








