The Use of Tissue Grafts Associated with Immediate Implant Placement to Achieve Better Peri-Implant Stability and Efficacy: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and PICO Strategy
2.2. Eligibility Criteria
2.3. Search Strategy and Data Extraction
2.4. Quality Assessment and Risk of Bias
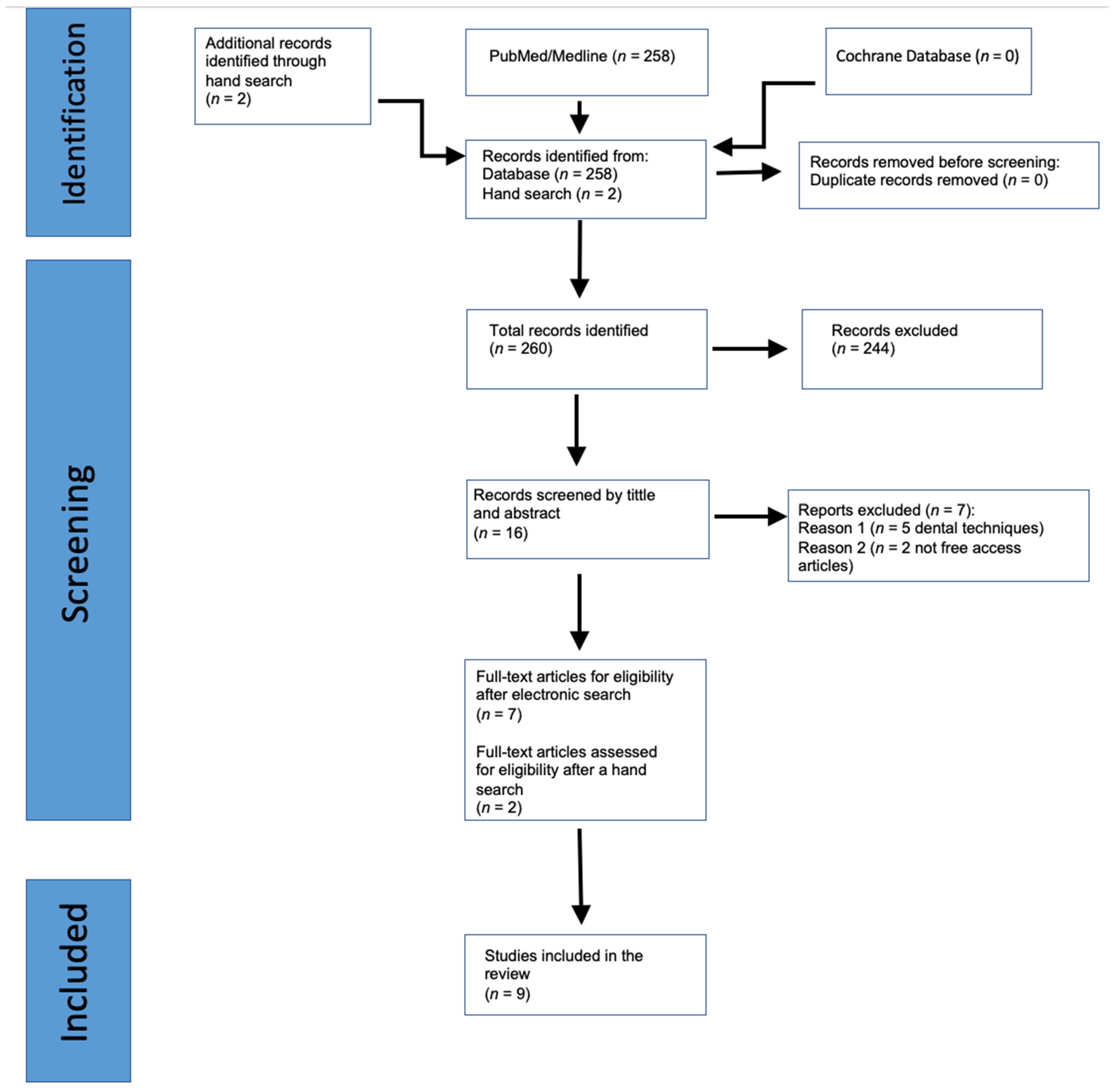
3. Results
3.1. Study Characteristics
3.2. Characteristics and Results of Interventions (Table 4)
3.2.1. Bone Grafting versus Extractive Technique without Bone Grafting
3.2.2. Alloplastic Graft with Membrane versus Extraction Technique (Naji et al., 2021 [22])
3.2.3. Xenograft versus Socket Shield Technique (Atef et al., 2021 [23])
3.2.4. Xenograft with Autogenous Graft and Membrane + VST Technique versus VST Technique without Grafting (Elaskary et al., 2022 [21])
3.2.5. Xenograft with Membrane versus Extraction (Mastrangelo et al., 2018 [24])
3.3. Different Types of Bone Grafting and/or Different Surgical Techniques
3.3.1. Xenograft with Dual-Zone Technique versus Xenograft (Wanis et al., 2022 [25])
3.3.2. Xenograft versus Autogenous Graft (Noelken et al., 2020 [26]; Li et al., 2018 [27])
3.3.3. Xenograft with Autogenous Graft and Connective Tissue Graft (CTG) versus Xenograft with Autogenous Graft (Van Nimwegen et al., 2018 [28])
3.3.4. Xenograft versus Xenograft with Collagen Matrix versus Xenograft with Autogenous CTG (Frizzera et al., 2018 [29])
3.4. Clinical Outcomes
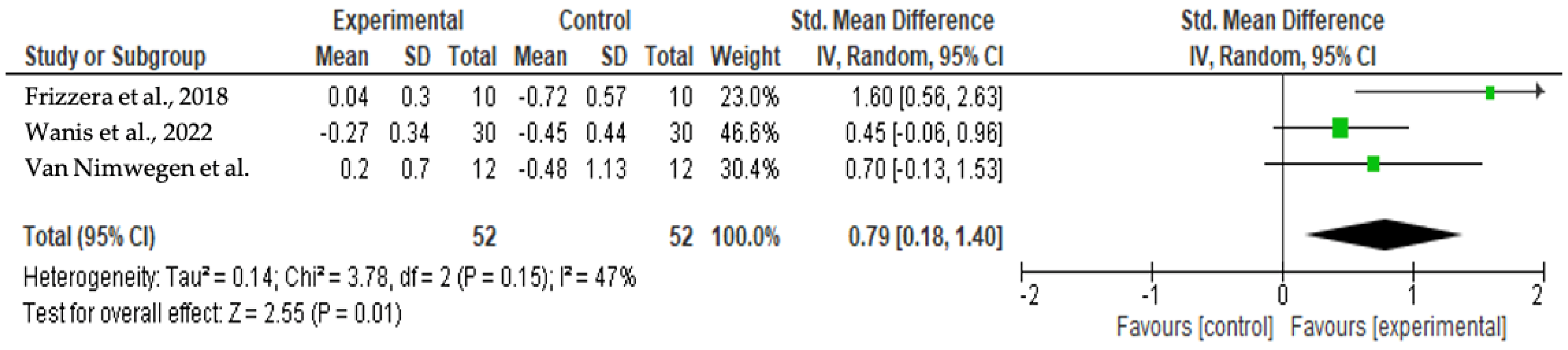
3.4.1. Mid-Facial Mucosa Level at 12 Months
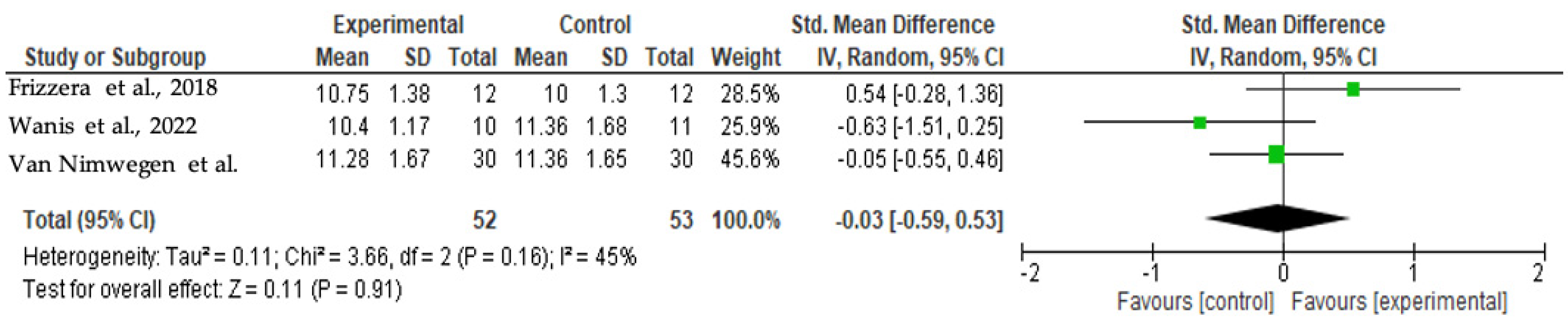
3.4.2. Total PES at 12 Months
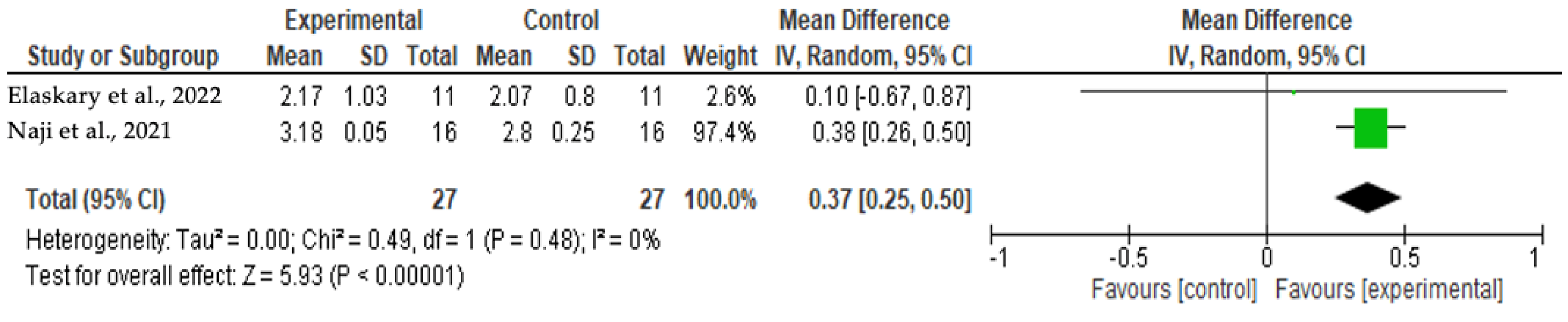
3.4.3. Facial Bone Thickness at 12 Months
3.5. Quality Assessment and Risk of Bias
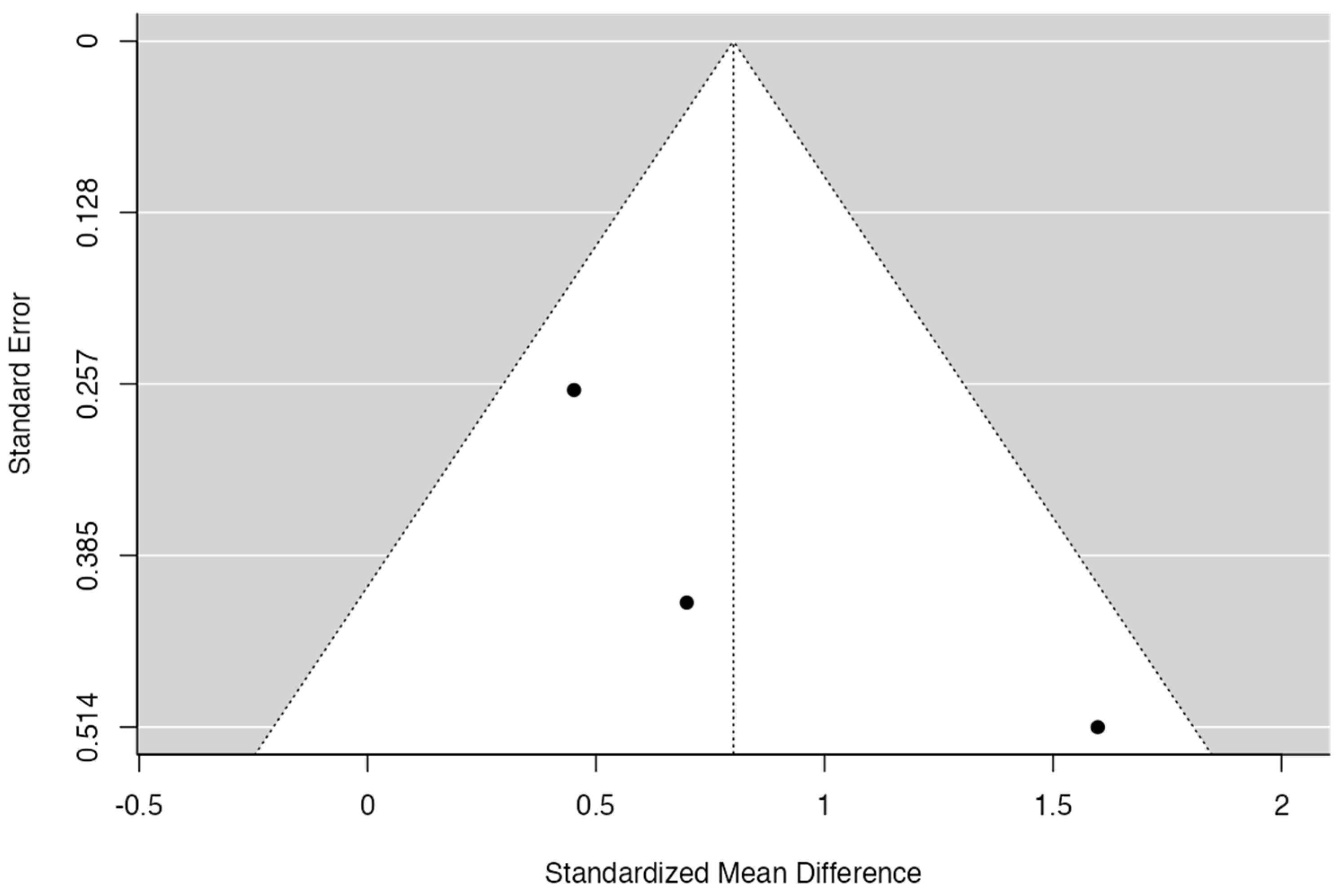
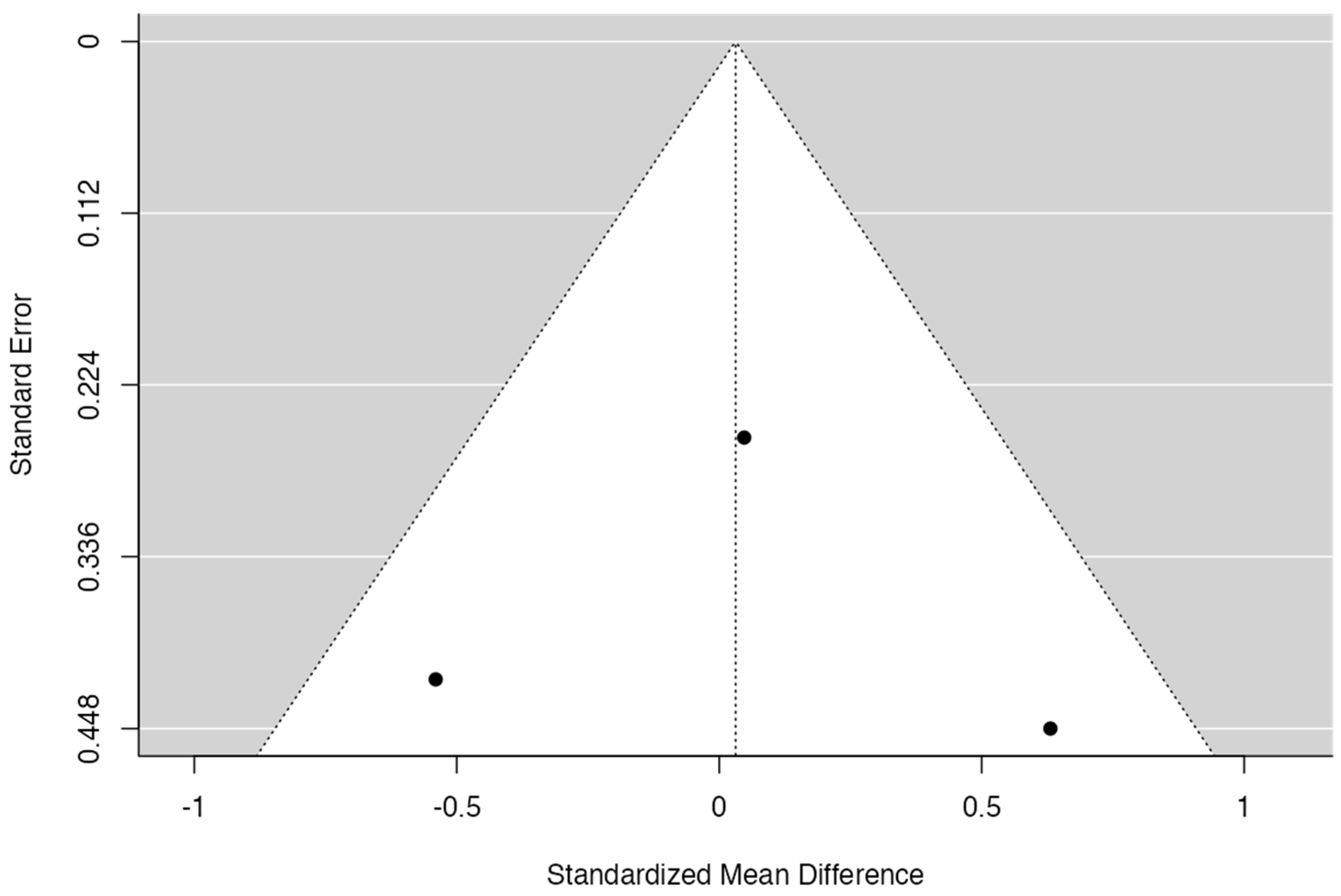
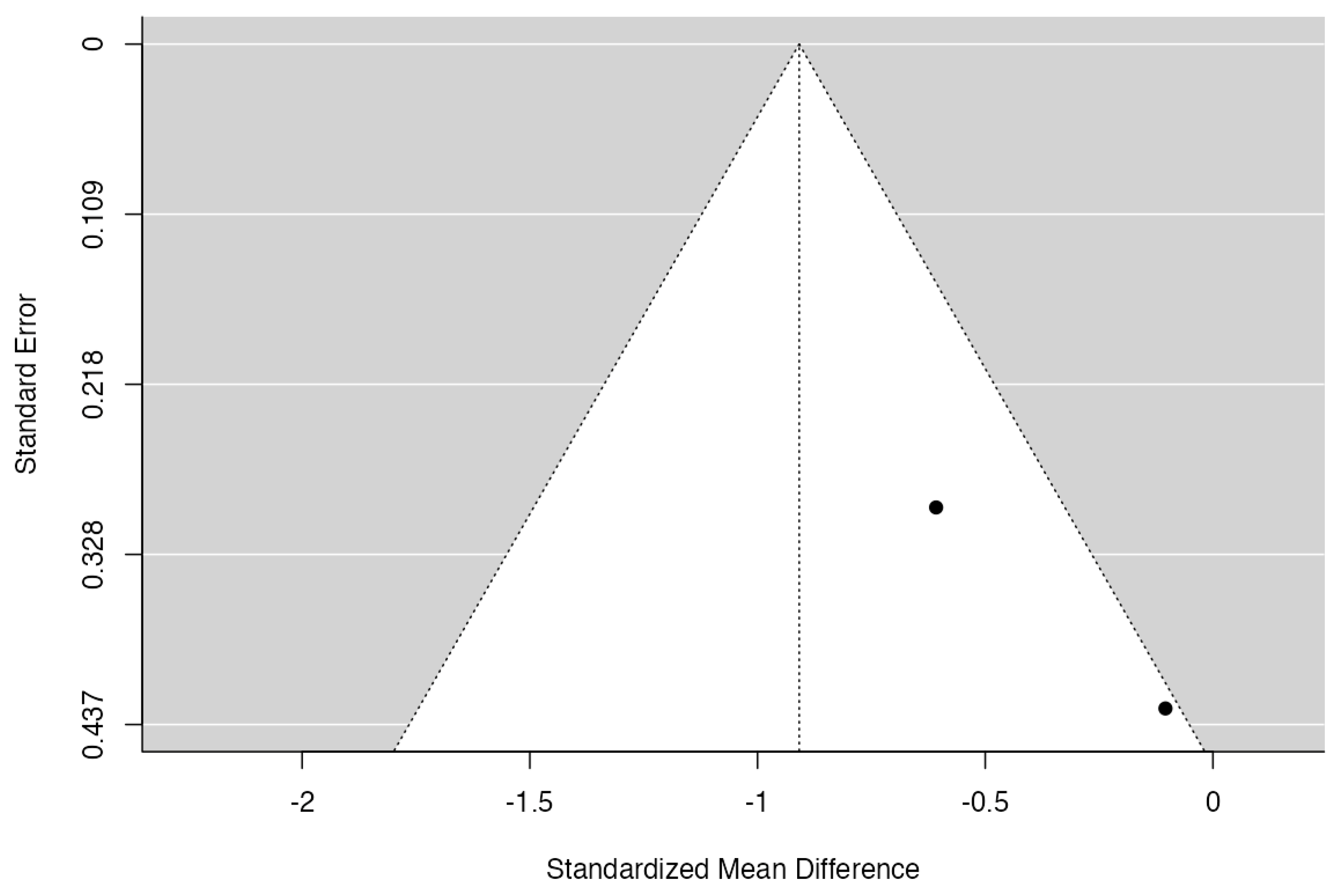
3.6. Meta-Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gasperini, F.M.; Fernandes, G.V.O.; Mitri, F.F.; Calasans-Maia, M.D.; Mavropoulos, E.; Rossi, A.M.; Granjeiro, J.M. Histomorphometric evaluation, SEM, and synchrotron analysis of the biological response of biodegradable and ceramic hydroxyapatite-based grafts: From the synthesis to the bed application. Biomed. Mater. 2023, 18, 065023. [Google Scholar] [CrossRef]
- Bonato, R.S.; Fernandes, G.V.d.O.; Calasans-Maia, M.D.; Mello, A.; Rossi, A.M.; Carreira, A.C.O.; Sogayar, M.C.; Granjeiro, J.M. The Influence of rhBMP-7 Associated with Nanometric Hydroxyapatite Coatings Titanium Implant on the Osseointegration: A Pre-Clinical Study. Polymers 2022, 14, 4030. [Google Scholar] [CrossRef]
- Borges, H.; Correia, A.R.M.; Castilho, R.M.; Fernandes, G.V.O. Zirconia implants and marginal bone loss: A systematic review and meta-analysis of clinical studies. Int. J. Oral Maxillofac. Implants 2020, 35, 707–720. [Google Scholar] [CrossRef]
- Kan, J.Y.; Rungcharassaeng, K. Immediate placement and provisionalization of maxillary anterior single implants: A surgical and prosthodontic rationale. Pract. Periodontics Aesthet. Dent. 2000, 12, 817–824. [Google Scholar]
- Pitman, J.; Seyssens, L.; Christiaens, V.; Cosyn, J. Immediate implant placement with or without immediate provisionalization: A systematic review and meta-analysis. J. Clin. Periodontol. 2022, 49, 1012–1023. [Google Scholar] [CrossRef]
- Ragucci, G.M.; Elnayef, B.; Criado-Cámara, E.; Del Amo, F.S.-L.; Hernández-Alfaro, F. Immediate implant placement in molar extraction sockets: A systematic review and meta-analysis. Int. J. Implant Dent. 2020, 6, 40. [Google Scholar] [CrossRef]
- Seyssens, L.; Eeckhout, C.; Cosyn, J. Immediate implant placement with or without socket grafting: A systematic review and meta-analysis. Clin. Implant Dent. Relat. Res. 2022, 24, 339–351. [Google Scholar] [CrossRef]
- Pitman, J.; Christiaens, V.; Callens, J.; Glibert, M.; Seyssens, L.; Blanco, J.; Cosyn, J.J. Immediate implant placement with flap or flapless surgery: A systematic review and meta-analysis. J. Clin. Periodontol. 2023, 50, 755–764. [Google Scholar] [CrossRef]
- Buser, D.; Chappius, V.; Belser, U.C.; Chen, S. Implant placement post extraction in esthetic single tooth sites: When immediate, when early, when late? Periodontology 2000 2017, 73, 84–102. [Google Scholar] [CrossRef]
- Guarnieri, R.; Ceccherini, A.; Grande, M. Single-tooth replacement in the anterior maxilla by means of immediate implantation and early loading: Clinical and aesthetic results at 5 years. Clin. Imp. Dent. Relat. Res. 2015, 17, 314–326. [Google Scholar] [CrossRef]
- Martins, S.C.R.; da Costa Marques, M.; Gomes Vidal, M.; Tolentino, P.H.M.P.; Dinelli, R.G.; Fernandes, G.V.O.; Shibli, J.A. Is the facial bone wall critical to achieving esthetic outcomes in immediate implant placement with immediate restoration? A systematic review. Adv. Clin. Exp. Med, 2024; ahead of print. [Google Scholar] [CrossRef]
- Tarnow, D.P.; Magner, A.W.; Fletcher, P. The effect of the distance from the contact point to the crest of bone on the presence or absence of the interproximal dental papilla. J. Periodontol. 1992, 63, 995–996. [Google Scholar] [CrossRef]
- Hammerle, C.H.; Araujo, M.G.; Simion, M.; Osteology Consensus, G. Evidence-based knowledge on the biology and treatment of extraction sockets. Clin. Oral Implants Res. 2012, 23 (Suppl. S5), 80–82. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Maffei, S.H.; Fernandes, G.V.O.; Fernandes, J.C.H.; Orth, C.; Joly, J.C. Clinical and histomorphometric soft tissue assessment comparing free gingival graft and a collagen matrix as alveolar-sealer materials: A randomized controlled pilot clinical trial. Quintessence Int. 2023, 54, 756–769. [Google Scholar] [CrossRef]
- Sanz, M.; Cecchinato, D.; Ferrus, J.; Salvi, G.E.; Ramseier, C.; Lang, N.P.; Lindhe, J. Implants placed in fresh extraction sockets in the maxilla: Clinical and radiographic outcomes from 3-year follow-up examination. Clin. Oral Implants Res. 2014, 25, 321–327. [Google Scholar] [CrossRef]
- Blanco, J.; Carral, C.; Argibay, O.; Linares, A. Implant placement in fresh extraction sockets. Periodontology 2000 2019, 79, 151–167. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n712021. [Google Scholar] [CrossRef]
- Methods for Risk of Bias 2 (RoB 2) Tool. Available online: https://methods.cochrane.org/risk-bias-2 (accessed on 3 January 2024).
- Elaskary, A.; Abdelrahman, H.; Elsabagh, H.H.; El-Kimary, G.I. Does grafting the jumping gap in immediately placed anterior implants using vestibular socket therapy influence the labial bone thickness? J. Oral Maxillofac. Surg. 2022, 80, 1398–1407. [Google Scholar] [CrossRef]
- Naji, B.M.; Abdelsameaa, S.S.; Alqutaibi, A.Y.; Said Ahmed, W.M. Immediate dental implant placement with a horizontal gap more than two millimeters: A randomized clinical trial. Int. J. Oral Maxillofac Surg. 2021, 50, 683–690. [Google Scholar] [CrossRef]
- Atef, M.; El Barbary, A.; El-D Dahrous, M.S.; Zahran, A.F. Comparison of the soft and hard peri-implant tissue dimensional changes around single immediate implants in the esthetic zone with socket shield technique versus using xenograft: A randomized controlled clinical trial. Clin. Implant Dent. Res. 2021, 23, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, F.; Gastaldi, G.; Vinci, R.; Troiano, G.; Tettamanti, L.; Gherlone, E.; Lo Muzio, L. Immediate Postextractive Implants With and Without Bone Graft: 3-year Follow-up Results From a Multicenter Controlled Randomized Trial. Imp. Den. 2018, 27, 6. [Google Scholar] [CrossRef]
- Wanis, R.W.; Hosny, M.M.; El Nahass, H. Clinical evaluation of the buccal aspect around immediate implant using dual zone therapeutic concept versus buccal gap fill to bone level: A randomized controlled clinical trial. Clin. Implant Dent. Res. 2022, 24, 307–319. [Google Scholar] [CrossRef]
- Noelken, R.; Pausch, T.; Wagner, W.; Al-Nawas, B. Peri-implant defect grafting with autogenous bone or bone graft material in immediate implant placement in molar extraction sites 1 to 3-year results of a prospective randomized study. Clin. Oral Implants Res. 2020, 31, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhu, H.C.; Huang, D.H. Autogenous DDM versus Bio-Oss granules in GBR for immediate implantation in periodontal postextraction sites: A prospective clinical study. Clin. Implant Dent Relat Res. 2018, 20, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Van Nimwegen, W.; Raghoebar, G.M.; Zuiderveld, E.G.; Jung, R.E.; Meijer, H.J.A.; Mühlemann, S. Immediate placement and provisionalization of implants in the aesthetic zone with or without a connective tissue graft: A 1-year randomized controlled trial and volumetric study. Clin. Oral Impl Res. 2018, 29, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Frizzera, F.; De Freitas, R.M.; Munoz-Chavez, O.F.; Cabral, G.; Marcantonio, E. Impact of soft tissue graft to reduce peri-implant alterations after immediate implant placement and provisionalization in compromised sockets. Int. J. Periodontics Restor. Dent. 2018, 39, 381–389. [Google Scholar] [CrossRef]
- Buser, D.; Martin, W.; Belser, U.C. Optimizing esthetics for implant restorations in the anterior maxilla: Anatomic and surgical considerations. Int. J. Oral Maxillofac. Implants 2004, 19, 43–61. [Google Scholar] [PubMed]
- Bakkali, S.; Rizo-Gorrita, M.; Romero-Ruiz, M.M.; Gutierrez-Perez, J.L.; Torres-Lagares, D.; Serrera-Figallo, M.A. Efficacy of different surgical techniques for peri-implant tissue preservation in immediate implant placement: A systematic review and meta-analysis. Clin. Oral Investig. 2021, 25, 1655–1675. [Google Scholar] [CrossRef]
- Siqueira, G.R.C.; Tavares, J.R.; Pedrosa, R.F.; Siqueira, R.A.C.; Fernandes, G.V.O. Immediate Implant with Provisionalization and Soft Tissue Grafting After 4-Year Follow-up. Clin. Adv. Periodontics 2022, 12, 32–38. [Google Scholar] [CrossRef]
- Tarnow, D.; Chu, S.; Gotta, S.; Saito, H. Flapless postextraction socket implant placement in the esthetic zone: Part 1. The effect of bone grafting and/or provisional restoration on facial–palatal ridge dimensional change—A retrospective cohort study. Int. J. Periodontics Restor. Dent. 2014, 34, 323–331. [Google Scholar] [CrossRef]
- Borges, T.; Fernandes, D.; Almeida, B.; Pereira, M.; Martins, D.; Azevedo, L.; Marques, T. Correlation between alveolar bone morphology and volumetric dimensional changes in immediate maxillary implant placement: A 1-year prospective cohort study. J. Periodontol. 2020, 91, 1167–1176. [Google Scholar] [CrossRef]
- Spray, J.R.; Black, C.G.; Morris, H.F.; Ochi, S. The influence of bone thickness on facial marginal bone response: Stage 1 placement through stage 2 uncovering. Ann. Periodontol. 2000, 5, 119–128. [Google Scholar] [CrossRef]
- Kazor, C.E.; Al-Shammari, K.; Sarment, D.P.; Misch, C.E.; Wang, H.L. Implant plastic surgery: A review and rationale. J. Oral Implantol. 2004, 30, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.G.; Schenk, R.; Buser, D.; Cochran, D. Implants placed in immediate extraction sites: A report of histologic and histometric analyses of human biopsies. Int. J. Oral Maxillofac. Implants 1998, 13, 333–341. [Google Scholar] [PubMed]
- Chu, S.J.; Salama, M.A.; Garber, D.A.; Salama, H.; Sarnachiaro, G.O.; Sarnachiaro, E.; Gotta, S.L.; Reynolds, M.A.; Saito, H.; Tarnow, D.P. Flapless postextraction socket implant placement, part 2: The effects of bone grafting and provisional restoration on peri-implant soft tissue height and thickness—A retrospective study. Int. J. Periodontics Restor. Dent. 2015, 35, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Chappuis, V.; Engel, O.; Reyes, M.; Shahim, K.; Nolte, L.P.; Buser, D. Ridge alterations post-extraction in the esthetic zone: A 3D analysis with CBCT. J. Dent. Res. 2013, 12, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.; Nunes, S.; Lopez-Castro, G.; Marques, T.; Montero, J.; Borges, T. Effect of customized healing abutments on the peri-implant linear and volumetric tissue changes at maxillary immediate implant sites: A 1-year prospective randomized clinical trial. Clin. Implant Dent. Relat. Res. 2021, 23, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elrahman, A.; Shaheen, M.; Askar, N.; Atef, M. Socket shield technique vs conventional immediate implant placement with immediate temporization. Randomized clinical trial. Clin. Implant Dent. Relat Res. 2020, 22, 602–611. [Google Scholar] [CrossRef]
- Siqueira, R.A.C.; Fontao, F.; Sartori, I.A.M.; Santos, P.G.F.; Bernardes, S.R.; Tiossi, R. Effect of different implant placement depths on crestal bone levels and soft tissue behavior: A randomized clinical trial. Clin. Oral Implants Res. 2017, 28, 1227–1233. [Google Scholar] [CrossRef]
- Pardal-Peláez, B.; Flores-Fraile, J.; Pardal-Refoyo, J.L.; Montero, J. Implant loss and crestal bone loss in immediate versus delayed load in edentulous mandibles: A systematic review and meta-analysis. J. Prosthet. Dent. 2021, 125, 437–444. [Google Scholar] [CrossRef]
- Mazzocco, F.; Jimenez, D.; Barallat, L.; Paniz, G.; Del Fabbro, M.; Nart, J. Bone volume changes after immediate implant placement with or without flap elevation. Clin. Oral Implants Res. 2017, 28, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Lindhe, J.; Alcaraz, J.; Sanz-Sanchez, I.; Cecchinato, D. The effect of placing a bone replacement graft in the gap at immediately placed implants: A randomized clinical trial. Clin. Oral Implants Res. 2017, 28, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Tarnow, D.P.; Chu, S.J. Human histologic verification of osseointegration of an immediate implant placed into a fresh extraction socket with excessive gap distance without primary flap closure, graft, or membrane: A case report. Int. J. Periodontics Restor. Dent. 2011, 31, 515–521. [Google Scholar]
- Lops, D.; Chiapasco, M.; Rossi, A.; Bressan, E.; Romeo, E. Incidence of inter-proximal papilla between a tooth and an adjacent immediate implant placed into a fresh extraction socket: 1-year prospective study. Clin. Oral Implant Res. 2008, 19, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Crespi, R.; Capparè, P.; Gherlone, E. Immediate loading of dental implants placed in periodontally infected and non-infected sites: A 4-years follow-up clinical study. J. Periodontol. 2010, 81, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.H.; Chan, H.L.; Wang, H.L. Effects of currently available surgical and restorative interventions on reducing midfacial mucosal recession of immediately placed single-tooth implants: A systematic review. J. Periodontol. 2014, 85, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Braut, V.; Bornstein, M.M.; Belser, U.; Buser, D. Thickness of the anterior maxillary facial bone wall: A retrospective radiographic study using cone-beam computed tomography. Int. J. Periodontics Restor. Dent. 2011, 31, 125–131. [Google Scholar]
- Noelken, R.; Neffe, B.A.; Kunkel, M.; Wagner, W. Maintenance of marginal bone support and soft tissue esthetics at immediately provisionalized OsseoSpeed implants placed into extraction sites: 2-year results. Clin. Oral Implants Res. 2014, 25, 214–220. [Google Scholar] [CrossRef]
- Amid, R.; Kadkhodazadeh, M.; Moscowchi, A. Immediate implant placement in compromised sockets: A systematic review and meta-analysis. J. Prosthet. Dent. 2023, 130, 307–317. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, W.; Liu, Y.; Chung, K.-H. Evaluation of implant placement following ridge preservation in periodontally compromised molar extraction sockets: Three-year results of a prospective cohort study. Clin. Oral Implants Res. 2022, 33, 735–744. [Google Scholar] [CrossRef] [PubMed]
| Author | Year | N | Age Range | Gender (Male/Female) |
|---|---|---|---|---|
| Elaskary et al. [21] | 2022 | 22 | Mean 45 | M: 8 F: 14 |
| Group I (intervention): 11 | Group I: 44.63 | Group I: M: 5 (45.5%) F: 6 (54.5%) | ||
| Group II (control): 11 | Group II: 45.81 | Group II: M: 3 (27.3%) F: 8 (72.7%) | ||
| Naji et al. [22] | 2021 | 48 | 28–55 | F: 30 M: 18 |
| Group I (intervention): 16 | Group I: 40.2 | Group I: M: 5 (31.25%) F: 11 (68.75%) | ||
| Group II (control I): 16 | Group II: 43.3 | Group II: M: 7 (43.75%) F: 9 (56.25%) | ||
| Group III (control II): 16 | Group III: 41.1 | Group III: M: 6 (37.5%) F: 10 (62.5%) | ||
| Atef et al. [23] | 2021 | 42 | >18 | M: 25% F: 75% |
| Intervention Group: 21 | mean 36 | Test group: M: 5 (25%) F: 15 (75%) | ||
| Control group: 21 | Control Group: M: 5 (25%) F: 15 (75%) | |||
| Mastrangelo et al. [24] | 2018 | 102 | 18–72 | M: 63 F: 39 |
| Group A (intervention): 51 | Mean 44 | Group A: M: 31 (60.7%) F: 20 (39.2%) | ||
| Group B (control): 51 | Group B: M: 32 (62.7%) F: 19 (37.2%) | |||
| Wanis et al. [25] | 2022 | 24 | 20–45 | M: 7 F: 17 |
| DZ Group (intervention): 12 | DZ Group: 34.27 | DZ Group: M: 4 (36%) F: 7 (63.4%) | ||
| BCG Group (control): 12 | BCG Group: 30.30 | BCG Group: M: 3 (30%) F: 7 (70%) | ||
| Noelken et al. [26] | 2020 | 50 | 23–73 | M: 18 F: 32 |
| AB Group (control): 25 | Mean 47 | |||
| BBGM Group (intervention): 25 | ||||
| Li et al. [27] | 2018 | 40 | 20–60 | M: 24 F: 16 |
| DDM Group (control): 20 | DDM Group: 36.6 | DDM Group: M: 11 (57.8%) F: 8 (42.10%) | ||
| BIO Group (intervention): 20 | BIO Group: 34.9 | BIO Group: M: 11 (43.75%) F: 8 (56.25%) | ||
| van Nimwegen et al. [28] | 2018 | 60 | 19–82 | M: 28 F: 32 |
| Intervention Group (CTG): 30 | Test Group: 19.5–67.84 (mean 45.5) | Test Group: M: 13 (43.3%) F: 17 (56.5%) | ||
| Control Group: 30 | Control Group: 20.9–82.2 (mean 47.8) | Control Group: M: 15 (50%) F: 15 (50%) | ||
| Frizzera et al. [29] | 2018 | 24 | 23–65 | M: 7 F: 17 |
| CTL Group (control): 8 | ||||
| CM Group (Intervention I): 8 | ||||
| CTG Group (Intervention II): 8 |
| Author | Follow-Up | Intervals | Type of Graft | Implant Placement (Site) | Implant (n) | Outcome |
|---|---|---|---|---|---|---|
| Elaskary et al. [21] | 1 year | T0: baseline preextraction T1: 1 year | Group I: particulate bone graft 75% autogenous bone chips harvested form local surgical sites and 25% deproteinized bovine bone mineral (DBBM) of bovine origin (MinerOss X Cortical Particle Size, 500–1000 microns) (Biohorizons Implant Systems, Birmingham, Alabama, USA) GROUP II: no graft | Esthetic zone | 22 | Buccal bone thickness |
| Naji et al. [22] | 6 months | T0: before extraction T1: immediately after implant placement T2: 6 months | GROUP I: alloplastic nanocrystalline calcium sulphate bone graft (Orthogen LLC, Springfield, New Jersey, USA) and an absorbable collagen membrane (Bioimplon GmbH, Gießen, Germany) GROUP II–III: without graft and membrane | Upper premolar tooth | 52 | CBCT bone examination Pain intensity |
| Atef et al. [23] | 1 year | T0: casts before the extraction T1: CBCT immediately post placement of implant T2: CBCT after 6 months T3: photos, casts and patients satisfaction 12 months | Test group: without graft + collagen plug; Control group: with bovine cancellous xenograft (Tutobone, Tutogen Medical GmbH, Neunkirchen a. Brand, Germany) + collagen plug | 26: premolar tooth 16: upper incisors and canine area | 42 | Peri-implant soft tissue PES Midfacial mucosa alteration Change in the buccal bone I-C (vertical) Change in the buccal bone I-OS (horizontal) Patient satisfaction |
| Mastrangelo et al. [24] | 3 years | Radiographic and clinical periodontal assessment T0: 3 months T1: 1 year T2: 3 years | Group A: granular bone grafting was inserted (BioOss, Geistlich, Germany), which completely covered the pericardium membrane (Osteobiol Evolution, Tecnoss, Italy) Group B: no graft and barrier | Upper premolar: 36: 1.4 26: 1.5 30: 2.4 23: 2.5 | 115 | Implants failure Marginal bone loss PES Pocket depth Biological complications |
| Wanis et al. [25] | 1 year | T0: baseline T1: 6 months T2: 1 year | DZ Group—BCG Group: bone graft cortico-cancellous collagenated bone grafting material of porcine origin pre-hydrated and collagenated cortico-cancellous porcine bone, 250–1000 μm, Gen-Os® (Osteobiol, Technoss Dental S.r.l.) DZ Group: dual technique zone BCG Group: flapless technique | 6: upper central 5: upper lateral 1: canines 5: first premolar 4: second premolar | 24 | PES BBL: Buccal bone changes (horizontal) via probe MFR: The midfacial recession STT: The soft tissue thickness at 2–4–6 mm KTW: The keratinized tissue VAS for POS PS |
| Noelken et al. [26] | 3 years | T0: baseline T1: placement implant T2: 1 year (n = 8 implants) T3: 2 years (n = 16 implants) T4: 3 years (n = 24 implants) | AB Group: autogenous bone grafts were harvested at the mandibular ramus by collecting bone particles with a disposable bone scraper (Micross, META). BBGM Group: a resorbable, biphasic, and anorganic graft material of plant origin derived from red algae (BBGM) (Symbios, Dentsply Sirona). | Molar of the maxilla and the mandible 34: mandibular implants 16: maxillary implants | 50 | Implant survival rate Marginal bone level changes Buccal bone level Buccal width of the alveolar crest Pocket depths Implant success rate Plaque index BoP |
| Li et al. [27] | 18 months | Radiographic T0: baseline T1: 6 months T2: 18 months | DDM Group: autogenous DDM granules from the extracted tooth BIO Group: Bio-Oss (Geistlich Pharma AG, Wolhusen, Switzerland) cancellous granules | Lower premolar: 19 Lower molar: 25 | 45 | ISQ Measurements of marginal bone resorption |
| Van Nimwegen et al. [28] | 1 year | T0: preextraction clinical parameters, photos, and impression T1: 1-year, clinical parameters, photos, and impression | Test and Control Group: a 1:1 mixture of autogenous bone and anorganic bovine bone (Geistlich Bio-Oss®; Geistlich Pharma AG, Wolhusen, Switzerland) Test Group received connective tissue graft (CTG), which was harvested from the tuberosity region | Maxilla Incisor: 47 Maxilla Canine: 10 Maxilla Premolar: 3 | 60 | Volumetric change: thickness Midfacial mucosa recession Gingival biotype Implant probing depths Plaque scores Bleeding scores Mucosa inflammation PES Patient satisfaction: VAS |
| Frizzera et al. [29] | 12 months | T0: baseline T1: 6 months T2: 12 months | CTL Group: no soft tissue graft CM Group: graft of collagen matrix (Mucograft, Geistlich) CTG Group: CTG from palate The facial space was filled with bovine bone mineral containing 10% porcine collagen (Bio-Oss Collagen, Geistlich) placed between the membrane and the dental implant | 13: 1.1/2.1 11: 1.2/2.2 | 24 | MPR Implant success rate Papilla migration PES Soft tissue thickness Bone thickness |
| Author | Surgical Protocol |
|---|---|
| Elaskary et al. [21] | Atraumatic tooth extraction and the VST protocol. Then, a cortical membrane shield was made of heterologous origin and introduced through the tunnel apically. |
| Group I: using the graft | |
| Group II: not using the graft. | |
| Naji et al. [22] | For group I and II a full thickness flap. |
| The junction gap was filled. | |
| Group II was treated without bone graft or membrane. | |
| Group III healing was free. | |
| Atef et al. [23] | Test Group: the socket shield technique. |
| Control Group: atraumatic extraction following implant placement; the junction gap was filled with bovine cancellous xenograft. | |
| A piece of a collagen plug was placed to close the entrance of the extraction socket in both groups. | |
| Mastrangelo et al. [24] | Tooth extraction with mucoperiosteal flap. The immediate implant was inserted. |
| Group A: graft and barrier healing. | |
| Group B: no graft and barrier. | |
| Wanis et al. [25] | A flapless minimally traumatic extraction technique. The immediate implants were inserted. |
| DZ Group: the bone graft filled the junction gap to wall up to the free gingival margin. | |
| BCG Group: the bone graft filled the junction gap; the graft was packed just reaching the buccal bone crestal level. | |
| Noelken et al. [26] | Atraumatic flapeless extraction technique. The immediate implants were inserted. |
| The junction gap was filled with AB or BBGM graft. | |
| The graft was additionally covered with a platelet-rich fibrin (PRF) membrane. | |
| Li et al. [27] | Tooth extraction with a mucoperiosteal flap. Immediate implant was inserted. |
| The junction gap was filled with a graft and injectable PRF and membrane barrier for healing. | |
| Van Nimwegen et al. [28] | Atraumatic flapless extraction technique. The junction gap was filled with xenograft inorganic bovine before the insertion of the immediate implant. In the test group, a connective autogenous graft was utilized. |
| Frizzera et al. [29] | Atraumatic tooth extraction and implant placement with immediate loading of a provisional crown. A bovine graft was utilized in every group. |
| CTL Group: no soft tissue graft. | |
| CM Group: graft of collagen matrix. | |
| CTG Group: tissue autogenous graft from the palate |
| Author | Outcome | ||
|---|---|---|---|
| Elaskary et al. [21] | Comparison of the overall bone thickness: | ||
| Baseline | |||
| Group I: 1.45 ± 0.92 mm | Group II: 0.79 ± 0.49 mm | ||
| 12 months | |||
| Group I: 2.95 ± 0.97 mm | Group II: 1.98 ± 0.56 mm | ||
| Naji et al. [22] | CBCT Bone examinations | ||
| Mean value of the buccal bone plate thickness + horizontal gap width at T1 was: | |||
| Group I: | Group II: | Group III: | |
| T1: 3.56 ± 0.10 mm | T1: 3.71 ± 0.57 mm | T1: 3.43 ± 0.33 mm | |
| T2: 3.18 ± 0.05 mm | T2: 2.80 ± 0.25 mm | T2: 3.19 ± 0.28 mm | |
| T2–T1 = −0.37 ± 0.09 mm | T2–T1 = −0.91 ± 0.54 mm | T2–T1 = −0.24 ± 0.11 mm | |
| PAIN INTENSITY | |||
| Group I: | Group II: | Group III: | |
| 5.14 ± 0.69 | 3.71 ± 0.76 | 0.71 ± 0.49 | |
| Atef et al. [23] | Mid-facial mucosal alteration | ||
| Control group(xenograft) | Test group(socket shield) | ||
| −0.466 ± 0.58 mm | 0.45 ± 0.75 mm | ||
| Radiographic outcomes | |||
| The change in the buccal(I-C): | |||
| Control group | Test group | ||
| 1.71 ± 1.02 mm | 0.36 ± 0.62 mm | ||
| The change in the buccal(I-OS): | |||
| Control group | Test group | ||
| 1.45 ± 0.72 mm | 0.29 ± 0.34 mm | ||
| Patient satisfaction vas score(12 months): | |||
| Control group | Test group | ||
| 9.25 (±0.70) | 9.37 (±0.80) | ||
| PES | |||
| Control group | Test group | ||
| 11.86 ± 0.35 | 12.12 ± 0.64 | ||
| Mastrangelo et al. [24] | Implants failure: | ||
| Group A: 1 | Group B: 1 | ||
| Marginal bone level | |||
| T0–T2 | |||
| Group A: −0.25 ± 0.362 mm | Group B: −0.28 ± 0.3 mm | ||
| PES | |||
| Group A: 8.14 | Group B: 9.7 | ||
| Probing depth | |||
| T0–T2 | |||
| Group A: 1.69 ± 1.34 mm | Group B: 1.4 ± 1.61 mm | ||
| Biological complications like fistulas, mucositis, and periimplantitis: 58 patients | |||
| Wanis et al. [25] | Two implants failed osteointegration after 2 months post-surgery (one from each group). | ||
| PES | |||
| Baseline: | 6 months | 12 months: | |
| DZ Group: 10.82 (±1.54) | DZ Group: 11.09 (±1.58) | DZ Group: 11.36 (±1.69) | |
| BCG Group: 10.10 (±1.20) | BCG Group: 10.40 (±1.17) | BCG Group: 10.80 (±1.55) | |
| BBL (at 0 mm): | |||
| 6 months | 12 months: | ||
| DZ Group: 0.67 (±0.43) mm | DZ Group: 0.88 (±0.41) mm | ||
| BCG Group: 0.84 (±0.26) mm | BCG Group: 1.08 (±0.28) mm | ||
| BBL (at 2 mm): | |||
| 6 months | 12 months: | ||
| DZ Group: 0.59 (±0.32) mm | DZ Group: 0.82 (±0.32) mm | ||
| BCG Group: 0.51 (±0.27) mm | BCG Group: 0.79 (±0.30) mm | ||
| Noelken et al. [26] | Implant survival rate | ||
| AB Group: | BBGM Group: | ||
| 100% | 96% | ||
| Mean interproximal bone level | |||
| AB Group T1: | BBGM Group T1: | ||
| Min: −13.2 mm | Min: −11.86 mm | ||
| Max: −2.19 mm | Max: −3.80 mm | ||
| Mean: −7.36 mm | Mean: −7.6 mm | ||
| AB Group final: | BBGM Group final: | ||
| Min: −0.87 mm | Min: −1.83 mm | ||
| Max: −1.85 mm | Max: 1.93 mm | ||
| Mean: 0.38 ± 0.78 mm | Mean: 0.1 ± 0.78 mm | ||
| Mean vertical distance from implant shoulder to the bottom of the buccal bone defect | |||
| AB Group T1: | BBGM Group T1: | ||
| −7.18 ± 3.43 mm | T1: −6.59 ± 2.65 mm | ||
| Li et al. [27] | ISQ | ||
| DDM Group | |||
| T0: | T1: | T3: | |
| 53.6 ± 11.9 mm | 77.6 ± 7.9 | 79.5 ± 6.0 mm | |
| BIO Group | |||
| T0: | T1: | T3: | |
| 54.1 ± 13.0 mm | 78.1 ± 4.2 | 80.2 ± 4.3 mm | |
| Marginal bone resorption around implant | |||
| DDM Group | |||
| T1: | T2: | ||
| 1.7 ± 0.3 mm | 1.9 ± 0.6 mm | ||
| BIO Group | |||
| T1: | T2: | ||
| 1.8 ± 0.1 mm | 2.0 ± 0.5 mm | ||
| Van Nimwegen et al. [28] | Volumetric change | ||
| A. Thickness (T0–final): | |||
| Control group: | Test group: | ||
| −0.49 ± 0.54 mm | 0.68 ± 0.59 mm | ||
| B. Mid-facial mucosa (T0–final): | |||
| Control group: | Test group: | ||
| −0.48 ± 1.13 mm | 0.20 ± 0.70 mm | ||
| PD at 1 year | |||
| Control group: | Test group: | ||
| 2.44 ± 1.19 mm | 2.28 ± 0.79 mm | ||
| PES | |||
| Control group: | Test group: | ||
| 11.36 ± 1.65 | 11.28 ± 1.67 | ||
| Frizzera et al. [29] | PES | ||
| Baseline: | 12 months: | ||
| (CTL Group) 10.75 (±2.05)mm | (CTL Group) 9.87 (±1.64) mm | ||
| (CM Group) 10.63(±1.84) mm | (CM Group) 10 (±1.3) mm | ||
| (CTG Group) 9.37(±1.9) mm | (CTG Group) 10.75 (±1.38) mm | ||
| MP (mesial papilla migration) | |||
| 6 months: | 12 months: | ||
| (CTL Group) 0.64 (±0.41) mm | (CTL Group) 0.36 (±0.7) mm | ||
| (CM Group) 0.39 (±0.45) mm | (CM Group) 0.41 (±0.47) mm | ||
| (CTG Group) 0.53(±0.28) mm | (CTG Group) 0.56 (±0.57) mm | ||
| DP(distal papilla migration) | |||
| 6 months: | 12 months: | ||
| (CTL Group) 0.69 (±0.62) mm | (CTL Group) 0.74 (±0.68) mm | ||
| (CM Group) 0.64 (±0.80) mm | (CM Group) 0.52 (±0.67) mm | ||
| (CTG Group) 0.44 (±0.79) mm | (CTG Group) 0.47 (±0.53) mm | ||
| MPR(marginal peri-implant recession) | |||
| 6 months: | 12 months: | ||
| (CTL Group) 0.41 (±0.40) mm | (CTL Group) 0.72 (±0.57) mm | ||
| (CM Group) 0.14 (±0.37) mm | (CM Group) 0.42 (±0.60) mm | ||
| (CTG Group) −0.41 (±0.75) mm | (CTG Group) −0.04 (±0.3) mm | ||
| Articles | Randomization Process | Deviations fromThe Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Result | Overall |
|---|---|---|---|---|---|---|
| Naji et al. [22] |  |  |  |  |  |  |
| Atef er al. [23] |  |  |  |  |  |  |
| Wanis et al. [25] |  |  |  |  |  |  |
| Noelken et al. [26] |  |  |  |  |  |  |
| van Nimwegen et al. [28] |  |  |  |  |  |  |
| Elaskary et al. [21] |  |  |  |  |  |  |
| Mastrangelo et al. [24] |  |  |  |  |  |  |
| Li et al. [27] |  |  |  |  |  |  |
| Frizzera et al. [29] |  |  |  |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rondone, E.M.; Leitão-Almeida, B.; Pereira, M.S.; Fernandes, G.V.O.; Borges, T. The Use of Tissue Grafts Associated with Immediate Implant Placement to Achieve Better Peri-Implant Stability and Efficacy: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 821. https://doi.org/10.3390/jcm13030821
Rondone EM, Leitão-Almeida B, Pereira MS, Fernandes GVO, Borges T. The Use of Tissue Grafts Associated with Immediate Implant Placement to Achieve Better Peri-Implant Stability and Efficacy: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(3):821. https://doi.org/10.3390/jcm13030821
Chicago/Turabian StyleRondone, Enrico Maria, Bruno Leitão-Almeida, Miguel Silva Pereira, Gustavo Vicentis Oliveira Fernandes, and Tiago Borges. 2024. "The Use of Tissue Grafts Associated with Immediate Implant Placement to Achieve Better Peri-Implant Stability and Efficacy: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 13, no. 3: 821. https://doi.org/10.3390/jcm13030821
APA StyleRondone, E. M., Leitão-Almeida, B., Pereira, M. S., Fernandes, G. V. O., & Borges, T. (2024). The Use of Tissue Grafts Associated with Immediate Implant Placement to Achieve Better Peri-Implant Stability and Efficacy: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 13(3), 821. https://doi.org/10.3390/jcm13030821