Abstract
Background: The optimum model of perinatal care for low-risk pregnancies has been a topic of debate. Obstetrician-led care tends to perform unnecessary interventions, whereas the quality of midwife-led care has been subject to debate. This review aimed to assess whether midwife-led care reduces childbirth intervention and whether this comes at the expense of maternal and neonatal wellbeing. Methods: PubMed, Scopus, Cochrane Library, and Web of Science were systematically searched for relevant studies. Studies were checked for eligibility by screening the titles, abstracts, and full texts. We performed meta-analyses using the inverse variance method using RevMan software version 5.3. We pooled data using the risk ratio and mean difference with the 95% confidence interval. Results: This review included 44 studies with 1,397,320 women enrolled. Midwife-led care carried a lower risk of unplanned cesarean and instrumental vaginal deliveries, augmentation of labor, epidural/spinal analgesia, episiotomy, and active management of labor third stage. Women who received midwife-led care had shorter hospital stays and lower risks of infection, manual removal of the placenta, blood transfusion, and intensive care unit (ICU) admission. Furthermore, neonates delivered under midwife-led care had lower risks of acidosis, asphyxia, transfer to specialist care, and ICU admission. Postpartum hemorrhage, perineal tears, APGAR score < 7, and other outcomes were comparable between the two models of management. Conclusions: Midwife-led care reduced childbirth interventions with favorable maternal and neonatal outcomes in most cases. We recommend assigning low-risk pregnancies to midwife-led perinatal care in health systems with infrastructure allowing for smooth transfer when complications arise. Further research is needed to reflect the situation in low-resource countries.
1. Introduction
Several models of management are available for women during childbirth. Obstetricians are the primary providers of care for the majority of women during childbirth in North America. Other countries, like New Zealand, Australia, the United Kingdom, Ireland, and the Netherlands, apply additional models of care, including midwife-led care. There has been a constant debate about the optimal model of ante-, intra-, and postpartum care for women [1,2].
The obstetrician-led model of care is hospital-based with access to advanced medical equipment and readiness for various obstetric complications during childbirth. This model suits all pregnancy and childbirth risk levels, with an opportunity for advanced interventions. Although midwives often provide the actual management during childbirth, obstetricians are responsible for the management provided in the obstetrician-led model. This model could be called the biomedical model, as it focuses on the pathology and tries to reverse it [3]. Obstetricians usually apply technology and obstetric skills to minimize the risk of bleeding, infection, and fetal/neonatal adverse outcomes. However, some of the used technologies—such as continuous electronic fetal monitoring—might lead to unnecessary interventions [4]. Women desiring instant access to interventions such as pain management and cesarean section prefer this model of support [5,6]. In high-income countries particularly, this tendency for increased obstetric interventions is an issue of great concern [7,8].
Midwife-led care could be provided at home or in midwifery units (birth centers). Midwifery units are primarily run by midwives who provide perinatal care and transfer complicated cases as appropriate [9,10]. These are either alongside midwifery units (AMUs) located within the hospital or freestanding midwifery units (FMUs) located outside hospitals [11]. Nevertheless, this model of management is only suitable for low-risk pregnancies. Higher-risk pregnancies require a higher level of care, and the presence of an obstetrician and the hospital setting are a must [11,12,13]. For low-risk pregnancies as well, complications that necessitate transfer to obstetrician-led care might arise. In such cases, a smooth transfer system is needed in terms of effective communication along with a vehicle for transportation (for women delivering at home or FMUs). The midwife-led model of care affirms the idea of intervention-less natural childbirth [14]. Both women and midwives tend to believe that spontaneous labor onset, continuous support, no pain relief or labor augmentation, skin-to-skin contact, and exclusive breastfeeding on demand come with the best perinatal outcomes [15]. This tendency for de-medicalization of childbirth, however, should not be at the expense of maternal and fetal/neonatal wellbeing.
For the purpose of this review, low-risk pregnancy is generally defined as an uncomplicated singleton pregnancy in a cephalic-vertex presentation in a healthy woman. This definition, while broadly consistent, can vary slightly across different studies, encompassing factors such as maternal age, absence of pre-existing medical conditions, and a history of uncomplicated pregnancies. Understanding these variations is crucial as they influence the scope and applicability of the findings. It is worth mentioning that the distinction between obstetricians’ and midwives’ care models’ philosophy in regard to the medicalization of care is not exclusive. Many obstetricians advocate for the natural birth process, and many midwives might tend to over-intervene. Several studies were conducted to compare midwife-led and obstetrician-led models of care during childbirth in low-risk pregnancies. However, the inconsistency in studies’ designs and findings makes it difficult to reach a conclusion. Therefore, we conducted this systematic review and meta-analysis to summarize the evidence on whether midwife-led and obstetrician-led models of perinatal care for low-risk pregnancies vary in the intervention rate. Also, we aimed to assess whether there is a difference in maternal and neonatal outcomes between these two models of care.
2. Methods
We conducted this systematic review and meta-analysis in accordance with the Cochrane Handbook for Interventional Reviews [16]. After that, we reported our study following the Preferred Reporting Items for Systematic reviews and Meta-Analysis guidelines [17].
2.1. Information Sources
We conducted an electronic search in PubMed, Cochrane Library, Scopus, and Web of Science using the following strategy: (Midwife OR midwives OR “traditional birth attendant” OR “traditional birth attendants” OR Midwifery OR “Midwifery-Led” OR “midwife-led” OR “Home birth”) AND (physician OR doctor OR clinician OR “general practitioner” OR “medical officer” OR obstetrician OR specialist OR consultant OR “Obstetrician-Led” OR “obstetrician-gynaecologist-led” OR “physician-led”) AND (Birth OR childbirth OR parturition OR delivery OR deliveries OR labor OR pregnancies OR Pregnancy OR pregnant* OR Labour OR Childbearing) AND (“low risk” OR “low-risk”).
Databases were searched from 2000 until 26 June 2024, and no other filters were applied. After that, references of the included studies were manually screened for additional eligible studies.
2.2. Eligibility Criteria and Studies Selection
We included studies that compared midwife-led and obstetrician-led models of perinatal care during childbirth. Only studies enrolling healthy women with low-risk pregnancies were included. We excluded studies not published in English, conference abstracts, theses, books, and unpublished work.
Studies retrieved from database searches were screened after excluding duplicates. We started with title and abstract screening, followed by full-text screening for final eligibility. The screening was double-checked, and a discussion was conducted to resolve any inconsistent judgment.
2.3. Quality Assessment
The included Randomized Clinical Trials (RCTs) quality was evaluated using the Cochrane tool for risk of bias assessment. The tool assesses the quality of RCTs in terms of randomization and allocation process, blinding of participants, staff, and outcomes’ detectors, loss of participants, reporting of the pre-specified outcomes, and other possible sources of bias [18]. The National Institutes of Health tools were used to assess the risk of bias for cohort, cross-sectional, and case-control studies. The studies’ quality was rated as “good”, “fair”, or “poor” according to the score calculated from several questions on the studies’ methodology [19].
2.4. Data Extraction
We extracted data about the studies’ design, country, sample size, women’s eligibility criteria, and the place of birth for each group (midwife-led and obstetrician-led care). Baseline data about participants were also extracted from the studies. In particular, we extracted data about participants’ age, parity, body mass index (BMI), marital status, education, occupation, habits, gestational age, neonate sex, and neonatal birth weight.
The primary outcomes in this meta-analysis are incidences of unplanned cesarean section (CS), instrumental vaginal delivery (including forceps and venous delivery), epidural or spinal analgesia, postpartum hemorrhage (PPH) (defined as blood loss >500 mL for vaginal delivery and >1 L for CS), APGAR score < 7 (at one minute and five minutes), and intrapartum or neonatal mortality. Our secondary outcomes included incidences of birth interventions (CS because of suspected fetal distress, CS of non-progressive labor, augmentation of labor, NO2 or general anesthesia, local analgesia through pudendal nerve block, narcotics use, acupuncture pain relief, hydrotherapy pain relief, no pain relief, episiotomy, and physiological management of the third stage of labor), maternal outcomes (intact perineum, first or second-degree perineal tear, third or fourth-degree perineal tear, vaginal or labial tear, manual removal of the placenta, blood transfusion, maternal infection or fever, severe maternal morbidity, maternal intensive care unit (ICU) admission, the duration of labor (in hours), and the duration of hospital stay (in days)), and neonatal outcomes (one-minute mean APGAR score, five-minute mean APGAR score, umbilical cord arterial pH < 7.1, mean umbilical cord arterial pH, meconium-stained amniotic fluid, asphyxia, need for resuscitation, need for ventilation, shoulder dystocia, transfer to specialist neonatal care, neonatal ICU (NICU) admission, and breastfeeding initiation).
2.5. Quantitative Synthesis Methods and Assessment of Publication Bias
We conducted our meta-analyses using Review Manager (RevMan) software version 5.3 in the inverse variance method. We pooled continuous data using the mean difference (MD) and the 95% confidence interval (CI). For categorical outcomes, we used the risk ratio (RR) and the 95% CI in pooling data. Statistical significance was set at a p-value < 0.05. A chi-square p-value of <0.1 and an I2 value of ≥50% indicated significant heterogeneity in the results. For heterogeneous results, we conducted the meta-analysis via the random effect model instead of the default fixed effect model [20,21]. Moreover, we assessed the presence of publication bias using a funnel plot. The funnel plot is a scatter plot of the effect estimates from individual studies against their standard errors. In the absence of publication bias, the plot should resemble a symmetrical inverted funnel. However, asymmetry in the funnel plot can indicate potential publication bias. If the funnel plot revealed asymmetry, suggesting that smaller studies with negative or non-significant results may be underrepresented. This asymmetry highlights the need for caution when interpreting the results, as the observed effects might be influenced by the selective publication of studies with positive findings. It is important to note that any outcome with fewer than 10 studies could not be assessed for publication bias due to insufficient data.
3. Results
3.1. Studies Selection
Our electronic database search retrieved 1793 results. Following duplicate exclusion, 1261 results remained for the title and abstract screening. Eighty-four full texts were reviewed for final eligibility, and a total of 48 articles reporting 44 studies were involved in this systematic review [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69]. Two studies (Martin-Arribas et al. 2022 and Schroeder et al. 2017) were not included in the quantitative synthesis [25,42] (Figure 1).
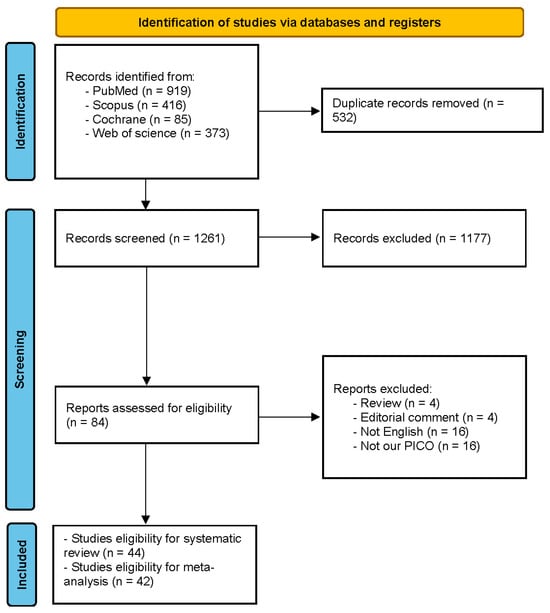
Figure 1.
PRISMA flow diagram.
3.2. Description of the Eligible Studies and Enrolled Women
We included three clinical trials [33,55,56,57,58], 36 cohort studies [22,23,24,27,28,29,30,31,32,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,52,53,59,60,61,62,63,64,65,66,67,68,69], three case-control studies [26,50,54], and two cross-sectional studies [25,51] in this review. These studies were conducted in different countries: Canada, the United States, Ireland, the United Kingdom, France, Germany, Spain, Norway, the Netherlands, Sweden, Belgium, Austria, Denmark, Nepal, Slovenia, Lithuania, China, Japan, Singapore, New Zealand, and Australia. The total number of women enrolled in this review is 1,397,320 (682,908 women received midwife-led care at home, FMU, or AMU, whereas 714,412 received obstetrician-led hospital-based care). Further description of the included studies’ methodology and main outcomes related to this review is available in Table 1.

Table 1.
Description of the methodology of the included studies.
Women enrolled in this review were around 30 years old and were mostly married or partnered. The majority of women had spontaneous onset of labor between 39 and 40 weeks of gestation and gave birth to a newborn weighing 3 to 3.6 kg. Table 2 provides a comprehensive description of the enrolled women’s baseline characteristics.

Table 2.
Baseline characteristics of the enrolled participants.
3.3. Quality Evaluation
The included clinical trials had a low risk of bias in all assessed aspects, with few exceptions. Pérez-Martínez et al. 2019 [33] applied non-random allocation of the participants, which adds a risk of selection bias. In addition, this study did not have a published protocol (Figures S1 and S2). All the included cohort and cross-sectional studies had good quality, indicating minimal risk of bias (Table S1). The included case-control studies were judged to provide fair evidence. Potential sources of bias in these studies were the non-random, non-concurrent selection of the controls and the lack of adjustment for the confounders (Table S2). The inability to blind the enrolled women, study personnel, and outcomes’ detectors might have added a potential source of bias in all the included studies.
3.4. Quantitative Synthesis Results
3.4.1. Primary Outcomes
Unplanned Cesarean Section
Thirty-five studies were included in this synthesis [22,23,24,27,28,29,30,34,35,36,37,38,39,43,44,45,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,65,66,67,68,69], with 1,150,709 women enrolled (510,507 received midwife-led care and 640,202 received obstetrician-led care). Our analysis significantly illustrated a lower risk of unplanned CS with midwife-led care (RR = 0.52; 95% CI [0.40, 0.69], p < 0.001). However, the findings across the studies showed heterogeneity (p < 0.001, I2 = 100%) (Figure 2).
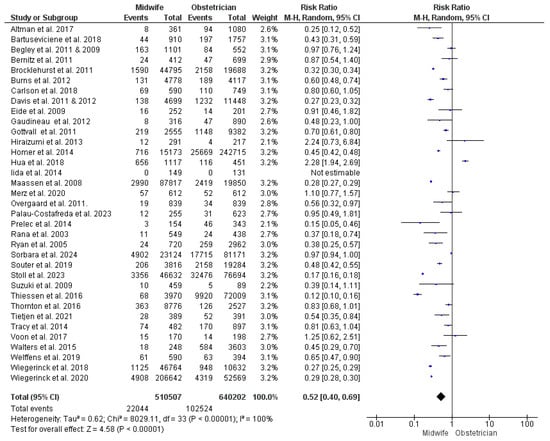
Figure 2.
Forest plot of the analysis; unplanned cesarean section [22,23,24,27,28,29,30,35,36,37,39,43,44,45,47,48,49,50,51,52,53,54,55,56,57,59,60,61,62,63,65,66,67,68,69].
Instrumental Vaginal Delivery
This synthesis was based upon results from 35 studies [22,23,24,26,27,28,29,30,31,34,35,36,38,39,43,44,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,65,66,67,68,69], with 1,139,775 women participating (294,215 received midwife-led care and 586,349 received obstetrician-led care). The risk of instrumental vaginal delivery was found to be significantly lower with midwife-led perinatal care (RR = 0.58; 95% CI [0.52, 0.64], p < 0.00001). Results showed heterogeneity between the studies included in this synthesis (p < 0.001, I2 = 96%) (Figure 3).
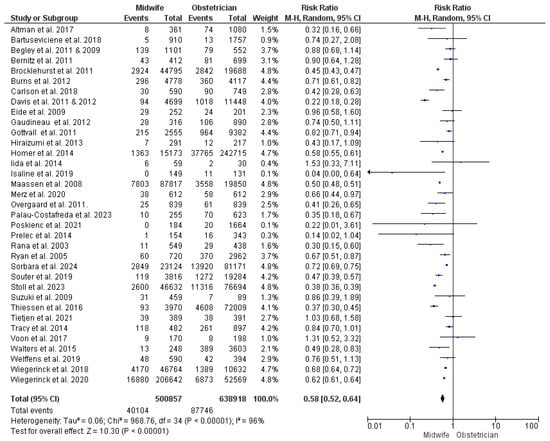
Figure 3.
Forest plot of the analysis; instrumental vaginal delivery [22,23,24,27,28,29,30,35,36,37,39,43,44,45,47,48,49,50,51,52,53,54,55,56,57,59,60,61,62,63,65,66,67,68,69].
Epidural or Spinal Analgesia
Twenty-seven studies contributed data for this synthesis [23,26,27,28,29,31,33,34,35,36,38,39,43,44,47,48,51,53,54,55,56,57,58,59,62,63,64,65,68], with 521,574 women included (132,585 received midwife-led care and 388,989 received obstetrician-led care). Midwife-led perinatal care was detected to carry a significantly lower risk of using epidural or spinal analgesia (RR = 0.54; 95% CI [0.46, 0.63], p < 0.00001). However, heterogeneity across the studies’ findings was significant (p < 0.001, I2 = 99%) (Figure 4).
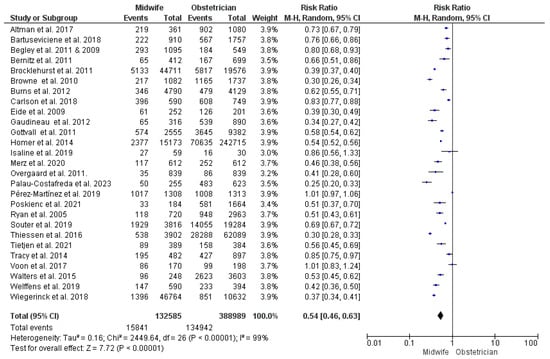
Figure 4.
Forest plot of the analysis; epidural or spinal analgesia [22,23,24,27,28,29,30,35,36,37,39,43,44,45,47,48,49,50,51,52,53,54,55,56,57,59,60,61,62,63,65,66,67,68,69].
PPH
This synthesis was conducted on data from 25 studies [23,27,28,29,32,34,36,38,39,40,41,43,44,45,46,50,52,53,54,55,56,57,58,60,61,62,63,66,68], with 462,684 women participating (255,878 received midwife-led care and 206,806 received obstetrician-led care). The risk of PPH did not differ significantly between the two models of perinatal care (RR = 0.93; 95% CI [0.81, 1.06], p = 0.27). The findings showed heterogeneity across the studies included in this synthesis (p < 0.001, I2 = 91%) (Figure 5).
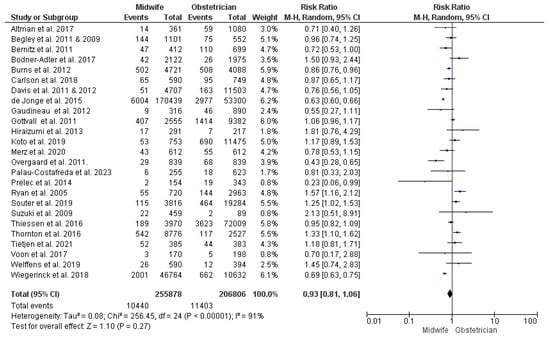
Figure 5.
Forest plot of the analysis; postpartum hemorrhage [22,23,24,27,28,29,30,35,36,37,39,43,44,45,47,48,49,50,51,52,53,54,55,56,57,59,60,61,62,63,65,66,67,68,69].
APGAR Score < 7
At one minute: Eight studies were included in the analysis of one-minute APGAR score < 7 [23,40,41,43,49,50,63,66,68], with 12,027 women participating (4867 received midwife-led care and 7160 received obstetrician-led care). No significant difference was detected between the two studied models, and the findings were heterogeneous (RR = 0.73; 95% CI [0.42, 1.29], p = 0.28), (p < 0.001, I2 = 74%) (Figure 6).
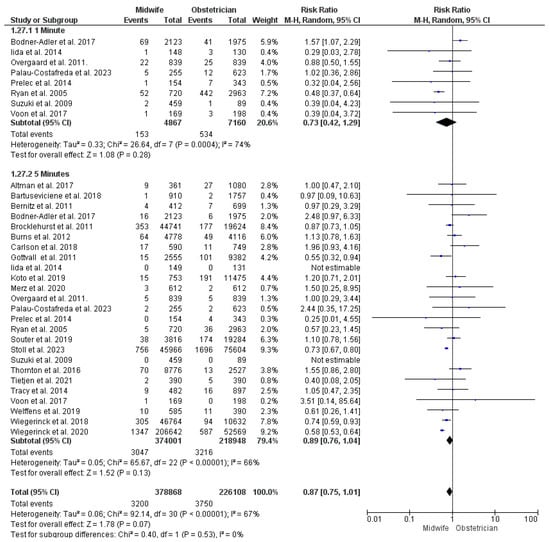
Figure 6.
Forest plot of the analysis; APGAR score < 7 [22,23,24,27,28,29,30,35,36,37,39,43,44,45,47,48,49,50,51,52,53,54,55,56,57,59,60,61,62,63,65,66,67,68,69].
At five minutes: This synthesis was based upon 25 studies [23,24,28,29,30,32,34,35,36,38,39,40,41,43,44,45,49,50,51,53,57,58,59,62,63,66,68], with 592,949 women enrolled (374,001 received midwife-led care and 218,948 received obstetrician-led care). No significant difference was detected between midwife-led and obstetrician-led perinatal care models in the risk of five-minute APGAR score < 7, but the studies’ findings were heterogeneous (RR = 0.89; 95% CI [0.76, 1.04], p = 0.13), (p < 0.001, I2 = 66%) (Figure 6).
Intrapartum or Neonatal Mortality
The intrapartum and neonatal mortality analysis was conducted on the findings of 14 studies [30,32,38,43,44,45,48,55,56,59,60,61,62,63,68,69], with 774,535 enrolled women (336,995 received midwife-led care and 437,540 received obstetrician-led care). The difference between midwife-led and obstetrician-led models of care in the risk of intrapartum or neonatal mortality was not statistically significant (RR = 0.92; 95% CI [0.72, 1.19], p = 0.54). The studies’ findings on this outcome were homogeneous (p = 0.52, I2 = 0%) (Figure 7).
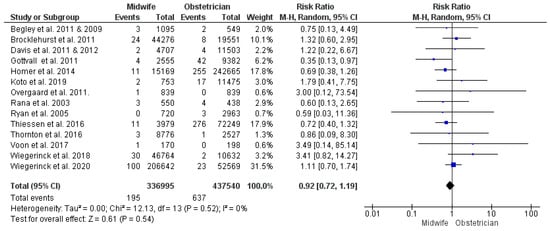
Figure 7.
Forest plot of the analysis; intrapartum or neonatal mortality [35,39,43,45,47,48,49,50,51,53,54,55,56,57,59,60,61,62,63,65,66,67,68,69].
3.4.2. Secondary Outcomes
Birth Interventions
Lower risks of labor augmentation (p < 0.00), episiotomy (p < 0.001), and general anesthesia (p = 0.03) were noticed with midwife-led perinatal care. In addition, women who received midwife-led care had more physiological management of labor in the third stage (p < 0.001) and used more non-pharmacological acupuncture pain relief (p = 0.001) or no pain relief at all (p = 0.02). No significant difference was detected between midwife-led and obstetrician-led models of care in regard to the risk of CS because of suspected fetal distress or non-progressive labor, NO2 anesthesia, pudendal nerve block, narcotics use, or hydrotherapy pain relief (Table 3), (Figures S3–S13).

Table 3.
Secondary outcomes synthesis results.
Maternal Outcomes
The risk of various degrees of perineal tear was not significantly different between the two studied models of perinatal care, except for the risk of vaginal tear, which was higher with the midwife-led model (p = 0.01). Women receiving midwife-led perinatal care had lower risks of manual removal of the placenta (p < 0.001) and blood transfusion (p = 0.03). Moreover, these women had a lower risk of maternal infection or fever (p = 0.01) and maternal ICU admission (p < 0.01), besides shorter hospital stays (p = 0.02). The risk of severe maternal morbidity and the duration of labor did not vary significantly between the midwife-led and obstetrician-led models of care (Table 3), (Figures S14–S24).
Neonatal Outcomes
Mean APGAR score at one minute was higher for neonates born under the midwife-led care model (p = 0.009). However, the five-minute mean score did not differ significantly between the two models. Midwife-led perinatal care was superior to obstetrician-led care in regard to the risk of umbilical cord arterial pH < 7.1 (p = 0.04), asphyxia (p = 0.03), transfer to specialist neonatal care (p = 0.03), and NICU admission (p = 0.01). Mean umbilical cord arterial pH and the risks of needing resuscitation or ventilation, meconium-stained liquor, and shoulder dystocia did not differ significantly between midwife-led and obstetrician-led models of care. Midwife-led care showed superiority with regard to breastfeeding initiation (p < 0.001) (Table 3), (Figures S25–S35).
3.5. Publication Bias
There appears to be some evidence of publication bias in four outcomes: breastfeeding initiation, episiotomy, instrumental vaginal delivery, and NICU admission. The funnel plots revealed asymmetry, suggesting that smaller studies with negative or non-significant results may be underrepresented. This asymmetry highlights the need for caution when interpreting the results, as the observed effects might be influenced by the selective publication of studies with positive findings (Figures S36–S39). However, the rest of the outcomes did not show evidence of publication bias (Figures S40–S49).
3.6. Qualitative Synthesis
3.6.1. Women’s Satisfaction with Care
Bernitz et al. and Begley et al. detected higher satisfaction levels among low-risk women randomized to midwife-led perinatal care. However, intrapartum transfer of care was noticed to influence women’s satisfaction negatively [56,58]. The high satisfaction levels were predominantly noted with the continuity model of midwife care [37,49].
3.6.2. Studies Not Included in the Analysis
Martin-Arribas et al., 2022: The study showed that women under midwife-led care had lower rates of cesarean section, instrumental vaginal delivery, epidural analgesia, and augmentation of labor (p < 0.001 for all outcomes). Women receiving midwife-led care had lower rates of third or fourth-degree perineal tear (p < 0.001), active management of the third stage of labor (p < 0.001), and ICU admissions (p = 0.01). Neonates born under this model of care had lower rates of APGAR score < 7 at five minutes (p = 0.01) and neonatal resuscitation (p < 0.001) and higher rates of breastfeeding initiation (p < 0.001) [25].
Schroeder et al., 2017: Categorical data reported in this study were not presented as events and total to allow for analysis. Cesarean section was less frequent with midwife-led care (p = 0.003). However, the rates of instrumental vaginal delivery, labor augmentation, and manual removal of the placenta did not vary significantly between the two models of care. Using birth pools for pain was more frequent with midwife-led perinatal care (p < 0.001), whereas using pethidine was more frequent with obstetrician-led care (p = 0.02) [42].
4. Discussion
This systematic review summarized the findings of 44 studies comparing midwife-led with obstetrician-led perinatal care for women with low-risk pregnancies. Nearly 1.4 million women (n = 1,397,320) were enrolled in studies conducted in 21 different countries. Our synthesis concluded that the midwife-led model of care carried a lower risk of birth interventions (namely, unplanned CS, instrumental vaginal delivery, augmentation of labor, epidural or spinal analgesia, general anesthesia, and episiotomy). More women in the midwife-led care group received physiological management of the third stage of labor. They did not use any pain relief intervention or used non-pharmacological acupuncture pain relief. This reduction of childbirth interventions by midwives was not noticed to adversely influence maternal and neonatal outcomes. Instead, some maternal and neonatal outcomes were more favorable with the midwife-led perinatal care. The risks of PPH, perineal injuries, and severe maternal morbidity did not differ significantly between the two models of management. However, women who received midwife-led care had shorter hospital stays and lower risks of infection or fever, manual removal of the placenta, blood transfusion, and ICU admission. Conversely, these women had a higher risk of vaginal tears compared with women delivering under obstetrician-led care. The duration of labor did not vary significantly between the two models of care. Regarding neonatal outcomes, the risks of having an APGAR score < 7 (at one minute and five minutes), meconium-stained liquor, shoulder dystocia, needing resuscitation or ventilation, and intrapartum or neonatal mortality did not differ between the two models of care. Yet, newborns delivered under midwife-led care had lower risks of acidosis (umbilical cord arterial pH < 7.1), asphyxia, transfer to specialist care, and NICU admission. These neonates were also more likely to start breastfeeding. Our findings indicate that midwife-led care is associated with fewer medical interventions and favorable maternal and neonatal outcomes in most cases. This supports the potential benefits of midwife-led care for low-risk pregnancies. However, it is important to clarify that our analysis focused on the incidence rates of interventions such as unplanned cesarean delivery and epidural/spinal analgesia, rather than on who performed these interventions. In all the included studies, cesarean deliveries were performed by doctors. Our analysis primarily centered on prenatal care and the overall management provided by midwives versus obstetricians. By focusing on incidence rates, we aimed to provide a comparative analysis of the frequency of these interventions in different care models, irrespective of the practitioner performing them. This approach allows us to highlight the differences in care practices and outcomes between midwife-led and obstetrician-led models, while acknowledging that certain interventions require the involvement of an obstetrician. This distinction is crucial for understanding the scope and limitations of midwife-led care and underscores the importance of integrated care models that facilitate collaboration between midwives and obstetricians to ensure comprehensive care for all patients.
The observed differences in outcomes between midwife-led and obstetrician-led care can be attributed to several factors. Midwife-led care often emphasizes a more holistic and less interventionist approach to childbirth, focusing on the natural birthing process and providing continuous support to the mother. This model of care is associated with lower rates of medical interventions such as labor augmentation, epidural analgesia, and cesarean sections. Midwives are trained to manage normal pregnancies and deliveries, and their approach is often centered on promoting normalcy and minimizing unnecessary interventions. This philosophy aligns with the preferences of many women who seek a more natural childbirth experience. In contrast, obstetrician-led care is typically more medicalized, with a greater emphasis on the use of technology and interventions to manage potential complications. Obstetricians are trained to handle high-risk pregnancies and are more likely to intervene proactively to prevent adverse outcomes. This approach can lead to higher rates of interventions, even in low-risk pregnancies, as a precautionary measure. The differences in training, philosophy, and approach between midwives and obstetricians contribute to the variations in outcomes observed in our study. Additionally, the continuity of care provided by midwives, who often build a strong rapport with their patients, can lead to better communication, increased trust, and a more personalized birthing experience. This continuity and personalized care can positively impact maternal satisfaction and outcomes. On the other hand, obstetrician-led care may involve multiple providers, which can sometimes lead to fragmented care and less personalized attention.
A previous meta-analysis published by Sandall et al. in 2016 compared the hospital-based midwife-led continuity model of care with other models of care during pregnancy and childbirth [70]. This meta-analysis included 15 RCTs with 17,674 women enrolled. Our meta-analysis was not limited to continuity midwife-led care, as we included all midwife-led models of care. Another review of reviews was published in 2012 by Sutcliffe et al. summarizing the findings of 27 studies included in three meta-analyses (Hatem et al. 2008, Villar et al. 2001, and Brown and Grimes 1995) [71,72,73,74]. Sutcliffe et al. compared midwife-led maternity care with physician-led care for low-risk pregnancies. In this review, we focused our comparison on obstetrician-led perinatal care to allow for drawing specific recommendations for implementation in practice. Overall, the findings of Sandall et al. and Sutcliffe et al. were consistent with ours. They concluded that midwife-led perinatal care applied fewer interventions during childbirth without increasing the risk of maternal bleeding or infections and without compromising neonatal outcomes. All previous reviews agreed with us on the reduced risk of instrumental vaginal delivery, episiotomy, and epidural analgesia with midwife-led care. The reviews also found an insignificant difference in the risks of perineal injuries and intrapartum or neonatal mortality between the two models of care [70,71]. The findings of Sutcliffe et al. were consistent with ours regarding the risks of PPH and five-minute Apgar score < 7 [71]. We did not detect a difference in the duration of labor between the two models of care, a finding consistent with Sutcliffe et al. as well [71]. Unlike previous reviews, our analysis revealed a lower risk of cesarean section with midwife-led care [70,71]. Our findings on the risks of labor augmentation, manual removal of the placenta, and NICU admission are also not consistent with those of Sutcliffe et al. [71]. We assume that these differences emerge from our comparison focused on the tertiary obstetrician-led level of care rather than including the primary family physician-led level of care. Tertiary obstetrician-led care has accessibility to the most specialized professionals and advanced care units, which makes interventions like CSs and admissions to NICU more reachable. Although family physicians are doctors, they operate at a primary healthcare level and are not the professionals who perform advanced obstetric interventions.
The American College of Obstetricians and Gynecologists has emphasized the importance of reducing unnecessary interventions during childbirth [75]. Several countries have also advocated for this goal [76]. The obstetrician-led model of care uses medications and obstetric interventions aiming to reduce maternal complications (mainly bleeding and infections) and neonatal adverse outcomes [77,78]. On the contrary, midwives support the normal biological demedicalized process of birth [14]. Our review showed that midwife-led care reduced childbirth interventions without increasing the risk of maternal bleeding. Instead, midwife-led care resulted in a lower risk of maternal infection and more favorable neonatal outcomes. Moreover, midwife-led care has psychological superiority over obstetrician-led care. Previous studies noticed a lower level of maternal anxiety, an accentuated sense of control, and enhanced levels of satisfaction with midwife-led perinatal care [9,13,71,79].
Our findings support shifting low-risk women to give birth under midwives’ care. This shift would allow obstetricians to devote their time to higher-risk women requiring special care for complications. Such a shift was questionable before, particularly when concerns were raised about the quality of midwife-led, un-totally supervised care [80,81]. Our review summarized the studies’ findings, providing answers to these concerns. Shifting low-risk women to midwife-led perinatal care would also minimize the human resources gap in low-income countries and remote areas where obstetricians are not always accessible [82]. This inaccessibility to emergency obstetric services and skilled birth attendants is enormously responsible for high rates of maternal mortality and morbidity [83]. Midwives’ easy training, wide accessibility, and low salary make them ideal practitioners at the primary care level [84]. They can provide high-quality perinatal care for low-risk women besides primary emergency obstetric care and facilitate smooth transfer of complicated, risky cases [81,85,86,87]. A shorter duration and only one-third of the cost of training a physician are sufficient to train a midwife. Furthermore, midwives’ salaries are less than half those of physicians. Previous studies have proved that midwife-led care is a cost-effective alternative to physician-led care [88].
Most institutions define a low-risk pregnancy as an uncomplicated singleton pregnancy in a cephalic-vertex presentation in a healthy woman. However, the criteria for “uncomplicated pregnancy” and “healthy woman” encompass significant variability [77]. Setting unified agreed-upon criteria for low-risk pregnancy is required to identify women eligible for childbirth under midwife-led care. Planning birth should take into consideration the pregnancy risk status and women’s preferences. However, the birth plan could be adjusted at multiple points, from the anomaly scan in the second trimester, the growth scan in the third trimester, late antenatal visits, by the onset of labor, or even during labor. Thus, counseling women about the birth plan and its flexibility is better conveyed in more than one session. Studies reported the highest satisfaction levels when women were involved in planning childbirth [89,90,91].
In this review, data were insufficient to perform a subgroup analysis by the place of birth. However, several previous reviews have revealed an insignificant difference between hospital and community births. Some maternal and neonatal outcomes have even favored community birth [9,79,92,93,94,95,96]. Transfer of care is a substantial part of any primary healthcare service. It should be expected and planned in advance to avoid the three delays causing maternal mortality. Pre-specified guidelines for the criteria on who to transfer should be set by the regulatory bodies. Furthermore, clear, respectful communication between perinatal care providers and birth settings is needed. For births occurring in FMUs and homes, suitable, readily available means of transportation are also required. These three requisites mentioned above make a smoothly articulated transfer system. In low-income countries, the infrastructure is poor for timely transportation from community to hospital. Additionally, communication between community midwives and hospitals is not optimally regulated. Thus, the transfer system is questionable in such settings. The situation in low-resource countries is understated in this review due to insufficient data. Further research from these countries is needed.
It was in the 1990s when a call for reducing unnecessary childbirth interventions emerged. In 1996, the World Health Organization issued a statement about assigning midwives to perinatal care during normal pregnancy and childbirth [76]. After that, regulatory boards in several countries responded to this call. It was around the year 2000 when midwife-led care was officially regulated in several countries [97,98,99]. Therefore, in this review, we included studies published after 2000. This review is strengthened by the large number of included studies representing various nations and health systems. Furthermore, the enormously large number of participants who were enrolled empowered the included studies. It allowed this review to analyze rare outcomes such as the risks of intrapartum or neonatal mortality and third- or fourth-degree perineal tear. Yet, maternal mortality was not reported or did not occur in most of the included studies. Although the gold standard in providing trusted evidence is the RCT, childbirth is a decision that is not generally acceptable to be taken randomly. Conducting an RCT on such a topic is not typically feasible [100,101]. Due to the limited number of published RCTs, we included observational studies in this review. However, the confounding issue was adjusted for in the analysis of most of the observational studies included. Another limitation of this review is the issue of blinding study participants, staff, and outcomes assessors. All the included studies were open-label. Blinding the participants might not be feasible due to the setting in RCTs and their choice in observational studies. Additionally, blinding the study personnel is not possible. Future studies might consider blinding outcomes assessors when feasible, as some outcomes might be influenced, such as evaluating perineal injuries. The same issue was reported in the previous review by Sandall et al. [70]. Another limitation faced by previous reviews is the synthesis of women’s satisfaction [70]. We recommend developing a tool for assessing women’s satisfaction during childbirth that can be used consistently. Such a tool would allow for quantitative synthesis and comparison. Due to the variety in the represented health systems, birth settings, definition of low-risk pregnancy, and studies’ design, heterogeneity across the included studies was significant in most studied outcomes. Yet again, this issue was reported by several previous reviews [70,79,102]. Moreover, the substantial heterogeneity observed in our meta-analysis, indicated by an I² value of 100%, highlights significant variability among the included studies. This high level of heterogeneity can be attributed to several factors, including differences in study design, population characteristics, interventions, and outcome measures. To address this, we conducted subgroup analyses and sensitivity analyses to explore potential sources of heterogeneity. These analyses revealed that variations in study settings, sample sizes, and methodological approaches contributed to the observed heterogeneity. Additionally, we employed a random-effects model to account for the variability among studies, which provides more conservative estimates and acknowledges the diversity of the included studies. Despite these efforts, the high heterogeneity underscores the need for cautious interpretation of the results and suggests that further research is necessary to understand the underlying factors contributing to these variations.
5. Conclusions
Midwife-led care has resulted in a lower risk of birth interventions without increasing the risk of maternal infection, perineal injuries, or bleeding and without compromising neonatal outcomes. Midwives are cost-effective and widely available alternatives to offer primary healthcare services and allow obstetricians to dedicate their efforts to more complex cases. We recommend assigning low-risk women to midwife-led care in nations with an infrastructure system that allows for smooth transfer when needed. Further research from low-resource countries is required to reflect their situation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13226629/s1, Figure S1: Risk of bias graph for clinical trials. Figure S2: Risk of bias summary for clinical trials. Figure S3: Forest plot of the analysis; Cesarean section of suspected fetal distress. Figure S4: Forest plot of the analysis; Cesarean section of non-progressive labor. Figure S5: Forest plot of the analysis; Augmentation of labor. Figure S6: Forest plot of the analysis; NO2 or general anesthesia. Figure S7: Forest plot of the analysis; Local analgesia (pudendal nerve block). Figure S8: Forest plot of the analysis; Narcotics use. Figure S9: Forest plot of the analysis; Acupuncture pain relief. Figure S10: Forest plot of the analysis; Hydrotherapy pain relief. Figure S11: Forest plot of the analysis; No pain relief. Figure S12: Forest plot of the analysis; Episiotomy. Figure S13: Forest plot of the analysis; Physiological management of the third stage of labor. Figure S14: Forest plot of the analysis; Intact perineum. Figure S15: Forest plot of the analysis; First or second-degree perineal tear. Figure S16: Forest plot of the analysis; Third or fourth-degree perineal tear. Figure S17: Forest plot of the analysis; Vaginal or labial tear. Figure S18: Forest plot of the analysis; Manual removal of the placenta Figure S19: Forest plot of the analysis; Blood transfusion. Figure S20: Forest plot of the analysis; Maternal infection or fever. Figure S21: Forest plot of the analysis; Severe maternal morbidity. Figure S22: Forest plot of the analysis; Maternal intensive care unit admission. Figure S23: Forest plot of the analysis; Duration of labor (in hours). Figure S24: Forest plot of the analysis; Duration of hospital stay (in days). Figure S25: Forest plot of the analysis; Mean APGAR score (on-minute and five-minute). Figure S26: Forest plot of the analysis; Umbilical cord arterial pH < 7.1. Figure S27: Forest plot of the analysis; Mean umbilical cord arterial pH. Figure S28: Forest plot of the analysis; Meconium-stained amniotic fluid. Figure S29: Forest plot of the analysis; Asphyxia. Figure S30: Forest plot of the analysis; Need for resuscitation. Figure S31: Forest plot of the analysis; Need for ventilation. Figure S32: Forest plot of the analysis; Shoulder dystocia. Figure S33: Forest plot of the analysis; Transfer to specialist neonatal care. Figure S34: Forest plot of the analysis; Neonatal intensive care unit admission. Figure S35: Forest plot of the analysis; Breastfeeding initiation. Figure S36: Funnel plot of publication bias; Breastfeeding initiation. Figure S37: Funnel plot of publication bias; Episiotomy. Figure S38: Funnel plot of publication bias; Instrumental vaginal delivery. Figure S39: Funnel plot of publication bias; NICU admission. Figure S40: Funnel plot of publication bias; 1st or 2nd degree perineal tear. Figure S41: Funnel plot of publication bias; 3rd or 4th degree perineal tear. Figure S42: Funnel plot of publication bias; Apgar score less than 7. Figure S43: Funnel plot of publication bias; Augmentation of labor. Figure S44: Funnel plot of publication bias; Epidural or Spinal analgesia. Figure S45: Funnel plot of publication bias; Intact perineum. Figure S46: Funnel plot of publication bias; Intrapartum or neonatal mortality. Figure S47: Funnel plot of publication bias; N2O or General analgesia. Figure S48: Funnel plot of publication bias; PPH. Figure S49: Funnel plot of publication bias; Unplanned cesarian delivery. Table S1: Quality assessment of cohort and cross-sectional studies. Table S2: Quality assessment of case-control studies.
Author Contributions
Conceptualization, S.S. and M.A.; methodology, F.M.A., S.S. and M.A.; formal analysis, F.M.A., S.S. and M.A.; data curation, F.M.A., S.S. and M.A.; writing—original draft, F.M.A., S.S. and M.A.; writing—review and editing, F.M.A. and S.S.; supervision, M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are available from the corresponding author upon reasonable request.
Conflicts of Interest
Author Fahad M. Almutairi was employed by Health Holding Company, Ministry of Health, Jeddah, Saudi Arabia. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Walsh, D.; Devane, D. A metasynthesis of midwife-led care. Qual. Health Res. 2012, 22, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.; Revill, P.; Devane, D.; Normand, C. An assessment of the cost-effectiveness of midwife-led care in the United Kingdom. Midwifery 2013, 29, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.J.; Edgman-Levitan, S. Shared decision making--pinnacle of patient-centered care. N. Engl. J. Med. 2012, 366, 780–781. [Google Scholar] [CrossRef]
- Nelson, K.B.; Sartwelle, T.P.; Rouse, D.J. Electronic fetal monitoring, cerebral palsy, and caesarean section: Assumptions versus evidence. BMJ 2016, 355, i6405. [Google Scholar] [CrossRef]
- Cheyney, M.J. Homebirth as systems-challenging praxis: Knowledge, power, and intimacy in the birthplace. Qual. Health Res. 2008, 18, 254–267. [Google Scholar] [CrossRef]
- Houghton, G.; Bedwell, C.; Forsey, M.; Baker, L.; Lavender, T. Factors influencing choice in birth place-An exploration of the views of women, their partners and professionals. Evid. Based Midwifery 2008, 6, 59–64. [Google Scholar]
- Caughey, A.B.; Cahill, A.G.; Guise, J.M.; Rouse, D.J. Safe prevention of the primary cesarean delivery. Am. J. Obstet. Gynecol. 2014, 210, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Abalos, E.; Chamillard, M.; Ciapponi, A.; Colaci, D.; Comandé, D.; Diaz, V.; Geller, S.; Hanson, C.; Langer, A.; et al. Beyond too little, too late and too much, too soon: A pathway towards evidence-based, respectful maternity care worldwide. Lancet 2016, 388, 2176–2192. [Google Scholar] [CrossRef]
- Hodnett, E.D.; Downe, S.; Walsh, D. Alternative versus conventional institutional settings for birth. Cochrane Database Syst. Rev. 2012, 2012, Cd000012. [Google Scholar] [CrossRef]
- Hermus, M.; Wiegers, T.; Hitzert, M.; Boesveld, I.; van den Akker, E.; Akkermans, H.; Bruijnzeels, M.; Franx, A.; de Graaf, H.; Rijnders, M.; et al. The Dutch Birth Centre Study: Study design of a programmatic evaluation of the effect of birth centre care in the Netherlands. BMC Pregnancy Childbirth 2015, 15, 148. [Google Scholar] [CrossRef][Green Version]
- Rocca-Ihenacho, L.; Batinelli, L.; Thaels, E.; Rayment, J.; Newburn, M.; McCourt, C. Midwifery Unit Standards; University of London: London, UK, 2018. [Google Scholar]
- Hermus, M.A.A.; Boesveld, I.C.; Hitzert, M.; Franx, A.; de Graaf, J.P.; Steegers, E.A.P.; Wiegers, T.A.; van der Pal-de Bruin, K.M. Defining and describing birth centres in the Netherlands—A component study of the Dutch Birth Centre Study. BMC Pregnancy Childbirth 2017, 17, 210. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C. Open a Midwifery Center: A Manual for Launching and Operating Midwifery Centers in Global Settings; Goodbirth Network: San Francisco, CA, USA, 2019. [Google Scholar]
- Ten Hoope-Bender, P.; de Bernis, L.; Campbell, J.; Downe, S.; Fauveau, V.; Fogstad, H.; Homer, C.S.; Kennedy, H.P.; Matthews, Z.; McFadden, A.; et al. Improvement of maternal and newborn health through midwifery. Lancet 2014, 384, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Knox, A.; Rouleau, G.; Semenic, S.; Khongkham, M.; Ciofani, L. Barriers and facilitators to birth without epidural in a tertiary obstetric referral center: Perspectives of health care professionals and patients. Birth 2018, 45, 295–302. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions, Version 6.3; Updated February 2022; The Cochrane Collaboration: London, UK, 2022. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration: London, UK, 2019; pp. 205–228.
- NHLBI. Study Quality Assessment Tools; National Institutes of Health: Bethesda, MD, USA, 2018. [Google Scholar]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Sorbara, C.; Ray, J.G.; Darling, E.K.; Chung, H.; Podolsky, S.; Stukel, T.A. Postpartum Emergency Department Use Following Midwifery-Model vs Obstetrics-Model Care. JAMA Netw. Open 2024, 7, e248676. [Google Scholar] [CrossRef]
- Palau-Costafreda, R.; García Gumiel, S.; Eles Velasco, A.; Jansana-Riera, A.; Orus-Covisa, L.; Hermida González, J.; Algarra Ramos, M.; Canet-Vélez, O.; Obregón Gutiérrez, N.; Escuriet, R. The first alongside midwifery unit in Spain: A retrospective cohort study of maternal and neonatal outcomes. Birth 2023, 50, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Stoll, K.; Titoria, R.; Turner, M.; Jones, A.; Butska, L. Perinatal outcomes of midwife-led care, stratified by medical risk: A retrospective cohort study from British Columbia (2008–2018). CMAJ Can. Med. Assoc. J. J. L’association Medicale Can. 2023, 195, e292–e299. [Google Scholar] [CrossRef]
- Martin-Arribas, A.; Escuriet, R.; Borràs-Santos, A.; Vila-Candel, R.; González-Blázquez, C. A comparison between midwifery and obstetric care at birth in Spain: Across-sectional study of perinatal outcomes. Int. J. Nurs. Stud. 2022, 126, 104129. [Google Scholar] [CrossRef]
- Poškienė, I.; Vanagas, G.; Kirkilytė, A.; Nadišauskienė, R.J. Comparison of vaginal birth outcomes in midwifery-led versus physician-led setting: A propensity score-matched analysis. Open Med. 2021, 16, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Tietjen, S.L.; Schmitz, M.T.; Heep, A.; Kocks, A.; Gerzen, L.; Schmid, M.; Gembruch, U.; Merz, W.M. Model of care and chance of spontaneous vaginal birth: A prospective, multicenter matched-pair analysis from North Rhine-Westphalia. BMC Pregnancy Childbirth 2021, 21, 849. [Google Scholar] [CrossRef] [PubMed]
- Merz, W.M.; Tascon-Padron, L.; Puth, M.T.; Heep, A.; Tietjen, S.L.; Schmid, M.; Gembruch, U. Maternal and neonatal outcome of births planned in alongside midwifery units: A cohort study from a tertiary center in Germany. BMC Pregnancy Childbirth 2020, 20, 267. [Google Scholar] [CrossRef] [PubMed]
- Welffens, K.; Derisbourg, S.; Costa, E.; Englert, Y.; Pintiaux, A.; Warnimont, M.; Kirkpatrick, C.; Buekens, P.; Daelemans, C. The “Cocoon”, first alongside midwifery-led unit within a Belgian hospital: Comparison of the maternal and neonatal outcomes with the standard obstetric unit over 2 years. Birth 2020, 47, 115–122. [Google Scholar] [CrossRef]
- Wiegerinck, M.M.J.; Eskes, M.; van der Post, J.A.M.; Mol, B.W.; Ravelli, A.C.J. Intrapartum and neonatal mortality in low-risk term women in midwife-led care and obstetrician-led care at the onset of labor: A national matched cohort study. Acta Obstet. Gynecol. Scand. 2020, 99, 546–554. [Google Scholar] [CrossRef]
- Isaline, G.; Marie-Christine, C.; Rudy, V.T.; Caroline, D.; Yvon, E. An exploratory cost-effectiveness analysis: Comparison between a midwife-led birth unit and a standard obstetric unit within the same hospital in Belgium. Midwifery 2019, 75, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Koto, P.S.; Fahey, J.; Meier, D.; LeDrew, M.; Loring, S. Relative effectiveness and cost-effectiveness of the midwifery-led care in Nova Scotia, Canada: A retrospective, cohort study. Midwifery 2019, 77, 144–154. [Google Scholar] [CrossRef]
- Pérez-Martínez, E.; Sebastián-Viana, T.; Velasco-Vázquez, D.; Del Gallego-Lastra, R. Postpartum complications in women attended by midwives instead of obstetricians. Midwifery 2019, 75, 80–88. [Google Scholar] [CrossRef]
- Souter, V.; Nethery, E.; Kopas, M.L.; Wurz, H.; Sitcov, K.; Caughey, A.B. Comparison of Midwifery and Obstetric Care in Low-Risk Hospital Births. Obstet. Gynecol. 2019, 134, 1056–1065. [Google Scholar] [CrossRef]
- Bartuseviciene, E.; Kacerauskiene, J.; Bartusevicius, A.; Paulionyte, M.; Nadisauskiene, R.J.; Kliucinskas, M.; Stankeviciute, V.; Maleckiene, L.; Railaite, D.R. Comparison of midwife-led and obstetrician-led care in Lithuania: A retrospective cohort study. Midwifery 2018, 65, 67–71. [Google Scholar] [CrossRef]
- Carlson, N.S.; Corwin, E.J.; Hernandez, T.L.; Holt, E.; Lowe, N.K.; Hurt, K.J. Association between provider type and cesarean birth in healthy nulliparous laboring women: A retrospective cohort study. Birth 2018, 45, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Zhu, L.; Du, L.; Li, Y.; Wu, Z.; Wo, D.; Du, W. Effects of midwife-led maternity services on postpartum wellbeing and clinical outcomes in primiparous women under China's one-child policy. BMC Pregnancy Childbirth 2018, 18, 329. [Google Scholar] [CrossRef] [PubMed]
- Wiegerinck, M.M.J.; van der Goes, B.Y.; Ravelli, A.C.J.; van der Post, J.A.M.; Buist, F.C.D.; Tamminga, P.; Mol, B.W. Intrapartum and neonatal mortality among low-risk women in midwife-led versus obstetrician-led care in the Amsterdam region of the Netherlands: A propensity score matched study. BMJ Open 2018, 8, e018845. [Google Scholar] [CrossRef]
- Altman, M.R.; Murphy, S.M.; Fitzgerald, C.E.; Andersen, H.F.; Daratha, K.B. The Cost of Nurse-Midwifery Care: Use of Interventions, Resources, and Associated Costs in the Hospital Setting. Women’s Health Issues Off. Publ. Jacobs Inst. Women’s Health 2017, 27, 434–440. [Google Scholar] [CrossRef]
- Bodner-Adler, B.; Kimberger, O.; Griebaum, J.; Husslein, P.; Bodner, K. A ten-year study of midwife-led care at an Austrian tertiary care center: A retrospective analysis with special consideration of perineal trauma. BMC Pregnancy Childbirth 2017, 17, 357. [Google Scholar] [CrossRef] [PubMed]
- Bodner-Adler, B.; Bodner, K.; Kimberger, O.; Lozanov, P.; Husslein, P.; Mayerhofer, K. Influence of the birth attendant on maternal and neonatal outcomes during normal vaginal delivery: A comparison between midwife and physician management. Wien. Klin. Wochenschr. 2004, 116, 379–384. [Google Scholar] [CrossRef]
- Schroeder, L.; Patel, N.; Keeler, M.; Rocca-Ihenacho, L.; Macfarlane, A.J. The economic costs of intrapartum care in Tower Hamlets: A comparison between the cost of birth in a freestanding midwifery unit and hospital for women at low risk of obstetric complications. Midwifery 2017, 45, 28–35. [Google Scholar] [CrossRef]
- Voon, S.T.; Lay, J.T.S.; San, W.T.W.; Shorey, S.; Lin, S.K.S. Comparison of midwife-led care and obstetrician-led care on maternal and neonatal outcomes in Singapore: A retrospective cohort study. Midwifery 2017, 53, 71–79. [Google Scholar] [CrossRef]
- Thiessen, K.; Nickel, N.; Prior, H.J.; Banerjee, A.; Morris, M.; Robinson, K. Maternity Outcomes in Manitoba Women: A Comparison between Midwifery-led Care and Physician-led Care at Birth. Birth 2016, 43, 108–115. [Google Scholar] [CrossRef]
- Thornton, P.; McFarlin, B.L.; Park, C.; Rankin, K.; Schorn, M.; Finnegan, L.; Stapleton, S. Cesarean Outcomes in US Birth Centers and Collaborating Hospitals: A Cohort Comparison. J. Midwifery Women’s Health 2017, 62, 40–48. [Google Scholar] [CrossRef]
- de Jonge, A.; Mesman, J.A.; Manniën, J.; Zwart, J.J.; Buitendijk, S.E.; van Roosmalen, J.; van Dillen, J. Severe Adverse Maternal Outcomes among Women in Midwife-Led versus Obstetrician-Led Care at the Onset of Labour in the Netherlands: A Nationwide Cohort Study. PLoS ONE 2015, 10, e0126266. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.; Gupta, A.; Nam, A.E.; Lake, J.; Martino, F.; Coyte, P.C. A Cost-Effectiveness Analysis of Low-Risk Deliveries: A Comparison of Midwives, Family Physicians and Obstetricians. Healthc. Policy Polit. Sante 2015, 11, 61–75. [Google Scholar] [CrossRef][Green Version]
- Homer, C.S.; Thornton, C.; Scarf, V.L.; Ellwood, D.A.; Oats, J.J.; Foureur, M.J.; Sibbritt, D.; McLachlan, H.L.; Forster, D.A.; Dahlen, H.G. Birthplace in New South Wales, Australia: An analysis of perinatal outcomes using routinely collected data. BMC Pregnancy Childbirth 2014, 14, 206. [Google Scholar] [CrossRef] [PubMed]
- Iida, M.; Horiuchi, S.; Nagamori, K. A comparison of midwife-led care versus obstetrician-led care for low-risk women in Japan. Women Birth J. Aust. Coll. Midwives 2014, 27, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Prelec, A.; Verdenik, I.; Poat, A. A comparison of frequency of medical interventions and birth outcomes between the midwife led unit and the obstetric unit in low-risk primiparous women. Slov. Nurs. Rev. 2014, 48, 166–176. [Google Scholar] [CrossRef]
- Tracy, S.K.; Welsh, A.; Hall, B.; Hartz, D.; Lainchbury, A.; Bisits, A.; White, J.; Tracy, M.B. Caseload midwifery compared to standard or private obstetric care for first time mothers in a public teaching hospital in Australia: A cross sectional study of cost and birth outcomes. BMC Pregnancy Childbirth 2014, 14, 46. [Google Scholar] [CrossRef]
- Hiraizumi, Y.; Suzuki, S. Perinatal outcomes of low-risk planned home and hospital births under midwife-led care in Japan. J. Obstet. Gynaecol. Res. 2013, 39, 1500–1504. [Google Scholar] [CrossRef]
- Burns, E.E.; Boulton, M.G.; Cluett, E.; Cornelius, V.R.; Smith, L.A. Characteristics, interventions, and outcomes of women who used a birthing pool: A prospective observational study. Birth 2012, 39, 192–202. [Google Scholar] [CrossRef]
- Gaudineau, A.; Sauleau, E.A.; Nisand, I.; Langer, B. Obstetric and neonatal outcomes in a home-like birth centre: A case-control study. Arch. Gynecol. Obstet. 2013, 287, 211–216. [Google Scholar] [CrossRef]
- Begley, C.; Devane, D.; Clarke, M.; McCann, C.; Hughes, P.; Reilly, M.; Maguire, R.; Higgins, S.; Finan, A.; Gormally, S.; et al. Comparison of midwife-led and consultant-led care of healthy women at low risk of childbirth complications in the Republic of Ireland: A randomised trial. BMC Pregnancy Childbirth 2011, 11, 85. [Google Scholar] [CrossRef]
- Begley, C.; Devane, D.; Clarke, M. An Evaluation of Midwifery-Led Care in the Health Service Executive North Eastern Area: The Report of the MiDU Study; Health Service Executive (HSE): Dublin, Ireland, 2009. [Google Scholar]
- Bernitz, S.; Rolland, R.; Blix, E.; Jacobsen, M.; Sjøborg, K.; Øian, P. Is the operative delivery rate in low-risk women dependent on the level of birth care? A randomised controlled trial. Bjog 2011, 118, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Bernitz, S.; Øian, P.; Sandvik, L.; Blix, E. Evaluation of satisfaction with care in a midwifery unit and an obstetric unit: A randomized controlled trial of low-risk women. BMC Pregnancy Childbirth 2016, 16, 143. [Google Scholar] [CrossRef] [PubMed]
- Brocklehurst, P.; Hardy, P.; Hollowell, J.; Linsell, L.; Macfarlane, A.; McCourt, C.; Marlow, N.; Miller, A.; Newburn, M.; Petrou, S.; et al. Perinatal and maternal outcomes by planned place of birth for healthy women with low risk pregnancies: The Birthplace in England national prospective cohort study. BMJ 2011, 343, d7400. [Google Scholar] [PubMed]
- Davis, D.; Baddock, S.; Pairman, S.; Hunter, M.; Benn, C.; Anderson, J.; Dixon, L.; Herbison, P. Risk of severe postpartum hemorrhage in low-risk childbearing women in New Zealand: Exploring the effect of place of birth and comparing third stage management of labor. Birth 2012, 39, 98–105. [Google Scholar] [CrossRef]
- Davis, D.; Baddock, S.; Pairman, S.; Hunter, M.; Benn, C.; Wilson, D.; Dixon, L.; Herbison, P. Planned place of birth in New Zealand: Does it affect mode of birth and intervention rates among low-risk women? Birth 2011, 38, 111–119. [Google Scholar] [CrossRef]
- Gottvall, K.; Waldenström, U.; Tingstig, C.; Grunewald, C. In-hospital birth center with the same medical guidelines as standard care: A comparative study of obstetric interventions and outcomes. Birth 2011, 38, 120–128. [Google Scholar] [CrossRef]
- Overgaard, C.; Møller, A.M.; Fenger-Grøn, M.; Knudsen, L.B.; Sandall, J. Freestanding midwifery unit versus obstetric unit: A matched cohort study of outcomes in low-risk women. BMJ Open 2011, 1, e000262. [Google Scholar] [CrossRef]
- Browne, M.; Jacobs, M.; Lahiff, M.; Miller, S. Perineal injury in nulliparous women giving birth at a community hospital: Reduced risk in births attended by certified nurse-midwives. J. Midwifery Women’s Health 2010, 55, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Eide, B.I.; Nilsen, A.B.; Rasmussen, S. Births in two different delivery units in the same clinic--a prospective study of healthy primiparous women. BMC Pregnancy Childbirth 2009, 9, 25. [Google Scholar] [CrossRef]
- Suzuki, S.; Satomi, M.; Miyake, H. Referrals during labor in midwifery care. J. Nippon Med. Sch. Nippon Ika Daigaku Zasshi 2009, 76, 226–228. [Google Scholar] [CrossRef]
- Maassen, M.S.; Hendrix, M.J.; van Vugt, H.C.; Veersema, S.; Smits, F.; Nijhuis, J.G. Operative deliveries in low-risk pregnancies in The Netherlands: Primary versus secondary care. Birth 2008, 35, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.; Roberts, C. A retrospective cohort study comparing the clinical outcomes of a birth centre and labour ward in the same hospital. Aust. Midwifery 2005, 18, 17–21. [Google Scholar] [CrossRef]
- Rana, T.G.; Rajopadhyaya, R.; Bajracharya, B.; Karmacharya, M.; Osrin, D. Comparison of midwifery-led and consultant-led maternity care for low risk deliveries in Nepal. Health Policy Plan. 2003, 18, 330–337. [Google Scholar] [CrossRef][Green Version]
- Sandall, J.; Soltani, H.; Gates, S.; Shennan, A.; Devane, D. Midwife-led continuity models versus other models of care for childbearing women. Cochrane Database Syst. Rev. 2016, 4, Cd004667. [Google Scholar] [CrossRef]
- Sutcliffe, K.; Caird, J.; Kavanagh, J.; Rees, R.; Oliver, K.; Dickson, K.; Woodman, J.; Barnett-Paige, E.; Thomas, J. Comparing midwife-led and doctor-led maternity care: A systematic review of reviews. J. Adv. Nurs. 2012, 68, 2376–2386. [Google Scholar] [CrossRef]
- Hatem, M.; Sandall, J.; Devane, D.; Soltani, H.; Gates, S. Midwife-led versus other models of care for childbearing women. Cochrane Database Syst. Rev. 2008, 8, Cd004667. [Google Scholar] [CrossRef]
- Villar, J.; Carroli, G.; Khan-Neelofur, D.; Piaggio, G.; Gülmezoglu, M. Patterns of routine antenatal care for low-risk pregnancy. Cochrane Database Syst. Rev. 2001, 4, Cd000934. [Google Scholar]
- Brown, S.A.; Grimes, D.E. A meta-analysis of nurse practitioners and nurse midwives in primary care. Nurs. Res. 1995, 44, 332–339. [Google Scholar] [CrossRef]
- ACOG Committee. Opinion No. 766: Approaches to Limit Intervention During Labor and Birth. Obstet. Gynecol. 2019, 133, e164–e173. [Google Scholar] [CrossRef]
- Care in normal birth: A practical guide. Technical Working Group, World Health Organization. Birth 1997, 24, 121–123.
- Caughey, A.; Cheyney, M. Home and Birth Center Birth in the United States: Time for Greater Collaboration Across Models of Care. Obstet. Gynecol. 2019, 133, 1033–1050. [Google Scholar] [CrossRef] [PubMed]
- Gregg, R. Pregnancy in a High-Tech Age. Paradoxes of Choice; New York University Press: New York, NY, USA, 1995. [Google Scholar]
- Scarf, V.L.; Rossiter, C.; Vedam, S.; Dahlen, H.G.; Ellwood, D.; Forster, D.; Foureur, M.J.; McLachlan, H.; Oats, J.; Sibbritt, D.; et al. Maternal and perinatal outcomes by planned place of birth among women with low-risk pregnancies in high-income countries: A systematic review and meta-analysis. Midwifery 2018, 62, 240–255. [Google Scholar] [CrossRef] [PubMed]
- de Bernis, L.; Dumont, A.; Bouillin, D.; Gueye, A.; Dompnier, J.P.; Bouvier-Colle, M.H. Maternal morbidity and mortality in two different populations of Senegal: A prospective study (MOMA survey). BJOG 2000, 107, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Bouvier-Colle, M.H.; Ouedraogo, C.; Dumont, A.; Vangeenderhuysen, C.; Salanave, B.; Decam, C. Maternal mortality in West Africa. Rates, causes and substandard care from a prospective survey. Acta Obstet. Gynecol. Scand. 2001, 80, 113–119. [Google Scholar] [CrossRef]
- Scheffler, R.M.; Mahoney, C.B.; Fulton, B.D.; dal Poz, M.R.; Preker, A.S. Estimates of health care professional shortages in sub-Saharan Africa by 2015. Health Aff. 2009, 28, w849–w862. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Bertozzi-Villa, A.; Coggeshall, M.S.; Shackelford, K.A.; Steiner, C.; Heuton, K.R.; Gonzalez-Medina, D.; Barber, R.; Huynh, C.; Dicker, D.; et al. Global, regional, and national levels and causes of maternal mortality during 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 980–1004. [Google Scholar] [CrossRef]
- Benatar, S.; Garrett, A.B.; Howell, E.; Palmer, A. Midwifery care at a freestanding birth center: A safe and effective alternative to conventional maternity care. Health Serv. Res. 2013, 48, 1750–1768. [Google Scholar] [CrossRef]
- World Health Organization. Making Pregnancy Safer: The Critical Role of the Skilled Attendant: A Joint Statement by WHO, ICM, and FIGO; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- Graham, W.; Bell, J.; Bullough, C. Can Skilled Attendance at Delivery Reduce Maternal Mortality in Developping Countries? In Studies in Health Services Organisation & Policy; ITGPress: Antwerp, Belgium, 2000; p. 17. [Google Scholar]
- Campbell, O.M.; Graham, W.J. Strategies for reducing maternal mortality: Getting on with what works. Lancet 2006, 368, 1284–1299. [Google Scholar] [CrossRef]
- Friedman, H.S.; Liang, M.; Banks, J.L. Measuring the cost-effectiveness of midwife-led versus physician-led intrapartum teams in developing countries. Women’s Health 2015, 11, 553–564. [Google Scholar] [CrossRef]
- Lindgren, H.; Erlandsson, K. Women’s experiences of empowerment in a planned home birth: A Swedish population-based study. Birth 2010, 37, 309–317. [Google Scholar] [CrossRef]
- Janssen, P.A.; Carty, E.A.; Reime, B. Satisfaction with planned place of birth among midwifery clients in British Columbia. J. Midwifery Women’s Health 2006, 51, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, W.; Bracke, P. Place of birth and satisfaction with childbirth in Belgium and the Netherlands. Midwifery 2009, 25, e11–e19. [Google Scholar] [CrossRef]
- Hutton, E.K.; Reitsma, A.; Simioni, J.; Brunton, G.; Kaufman, K. Perinatal or neonatal mortality among women who intend at the onset of labour to give birth at home compared to women of low obstetrical risk who intend to give birth in hospital: A systematic review and meta-analyses. eClinicalMedicine 2019, 14, 59–70. [Google Scholar] [CrossRef]
- Alliman, J.; Phillippi, J.C. Maternal Outcomes in Birth Centers: An Integrative Review of the Literature. J. Midwifery Women’s Health 2016, 61, 21–51. [Google Scholar] [CrossRef]
- Dixon, L.; Prileszky, G.; Guilliland, K.; Hendry, C.; Miller, S.; Anderson, J. What evidence supports the use of free-standing midwifery led units (primary units) in New Zealand. N. Z. Coll. Midwives 2012, 46, 13–20. [Google Scholar]
- McIntyre, M.J. Safety of non-medically led primary maternity care models: A critical review of the international literature. Aust. Health Rev. A Publ. Aust. Hosp. Assoc. 2012, 36, 140–147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muthu, V.; Fischbacher, C. Free-standing midwife-led maternity units: A safe and effective alternative to hospital delivery for low-risk women? Evid.-Based Healthc. Public Health 2004, 8, 325–331. [Google Scholar] [CrossRef]
- Perinatal Health Report: British Columbia 2019–2020; Perinatal Services: Vancouver, BC, Canada, 2021.
- MacDorman, M.F.; Declercq, E. Trends and state variations in out-of-hospital births in the United States, 2004–2017. Birth 2019, 46, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Bauer, N.; Kehrbach, A.; Krahl, A.; von Rahden, O.; zu Sayn-Wittgenstein-Hohenstein, F. Handbuch Hebammenkreißsaal; Verbund Hebammenforschung: Osnabrück, Germany, 2007. [Google Scholar]
- Hendrix, M.; van Horck, M.; Moreta, D.; Nieman, F.; Nieuwenhuijze, M.; Severens, H.; Nijhuis, J. Why women do not accept randomisation for place of birth: Feasibility of a RCT in The Netherlands. BJOG Int. J. Obstet. Gynaecol. 2009, 116, 537–542, discussion 542. [Google Scholar] [CrossRef]
- Dowswell, T.; Thornton, J.G.; Hewison, J.; Lilford, R.J.; Raisler, J.; Macfarlane, A.; Young, G.; Newburn, M.; Dodds, R.; Settatree, R.S. Should there be a trial of home versus hospital delivery in the United Kingdom? BMJ 1996, 312, 753–757. [Google Scholar] [CrossRef]
- McRae, D.N.; Muhajarine, N.; Stoll, K.; Mayhew, M.; Vedam, S.; Mpofu, D.; Janssen, P.A. Is model of care associated with infant birth outcomes among vulnerable women? A scoping review of midwifery-led versus physician-led care. SSM-Popul. Health 2016, 2, 182–193. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).