The Impact of Medial Meniscal Extrusion on Cartilage of the Medial Femorotibial Joint—A Retrospective Analysis Based on Quantitative T2 Mapping at 3.0T
Abstract
1. Introduction
2. Materials and Methods
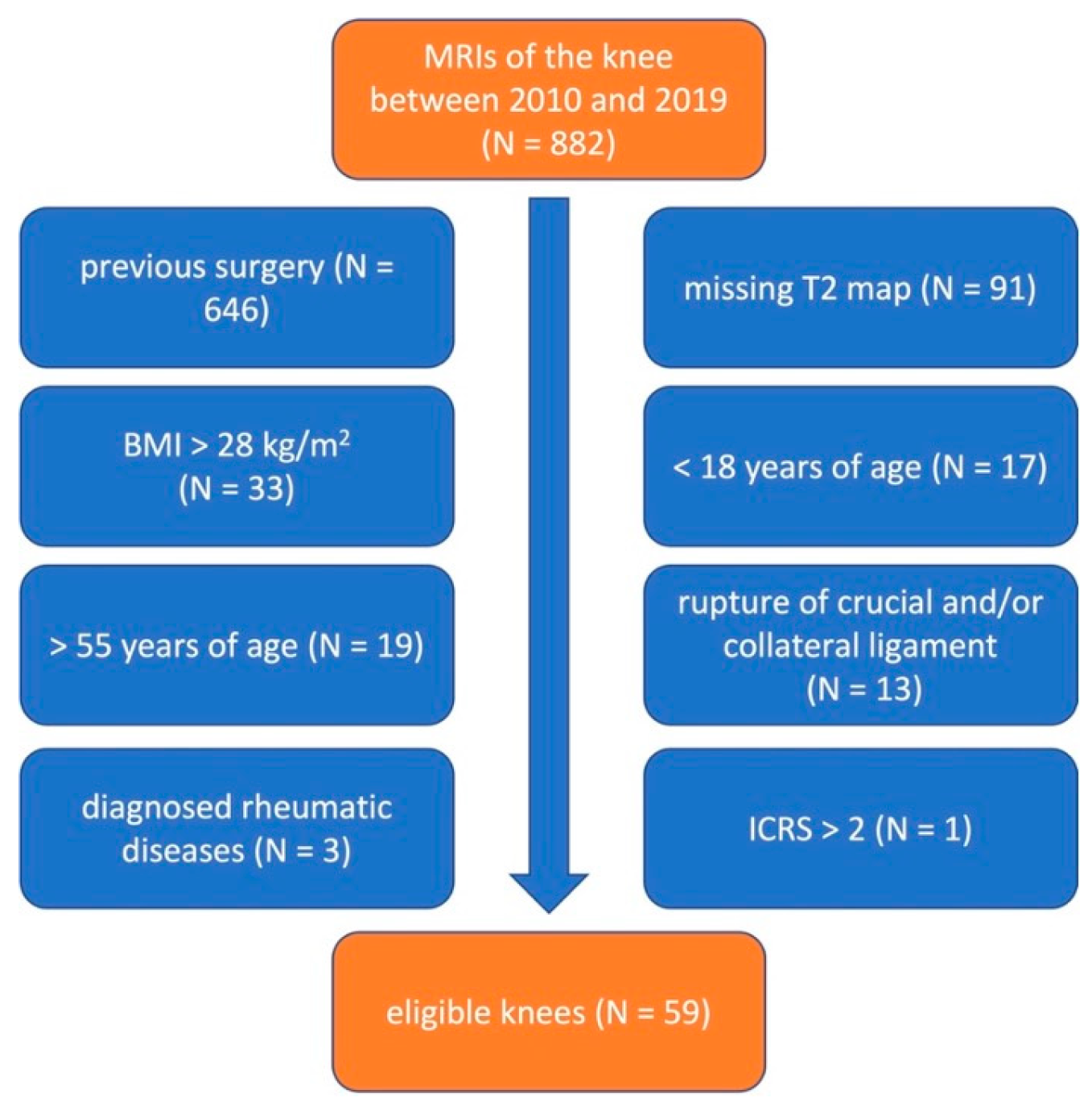
2.1. Patient Population
2.2. Magnetic Resonance Imaging
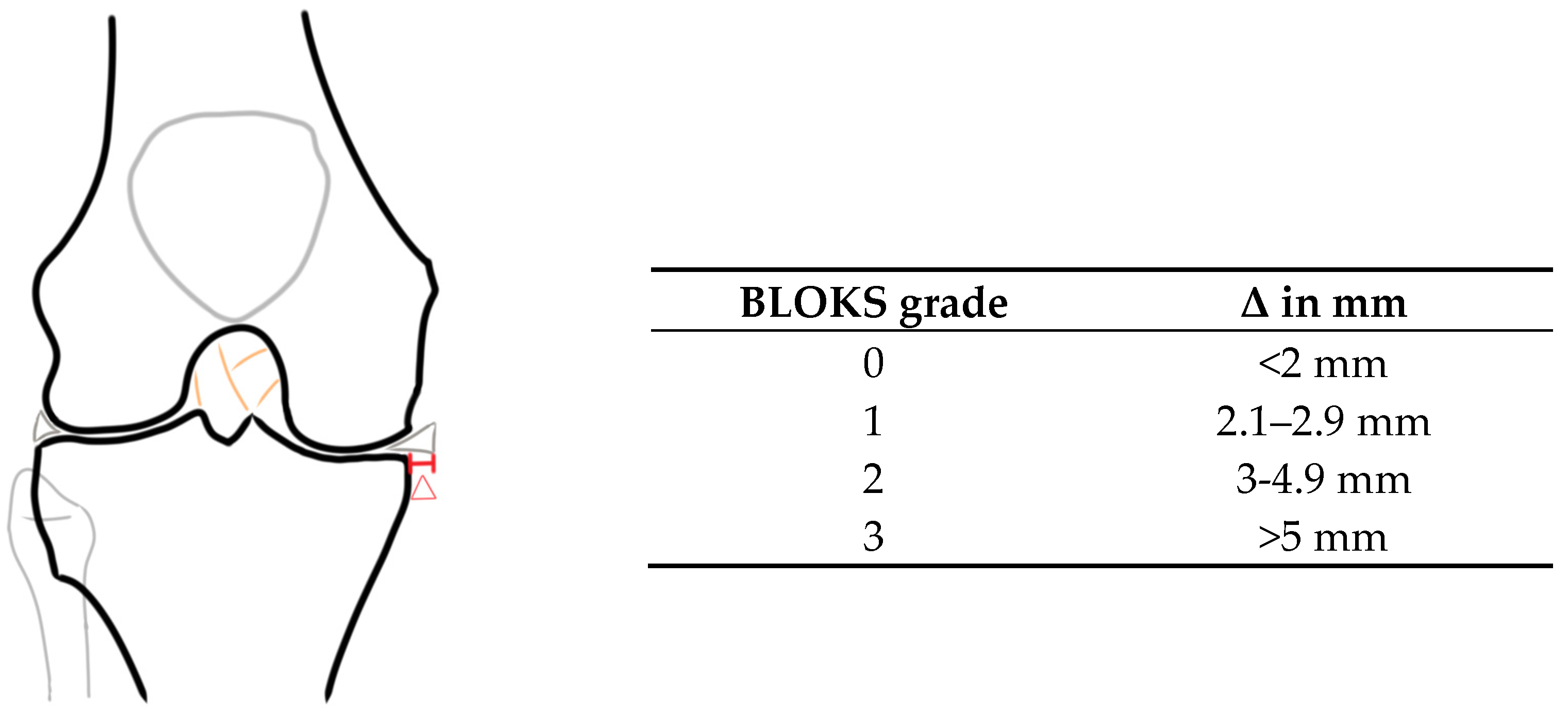
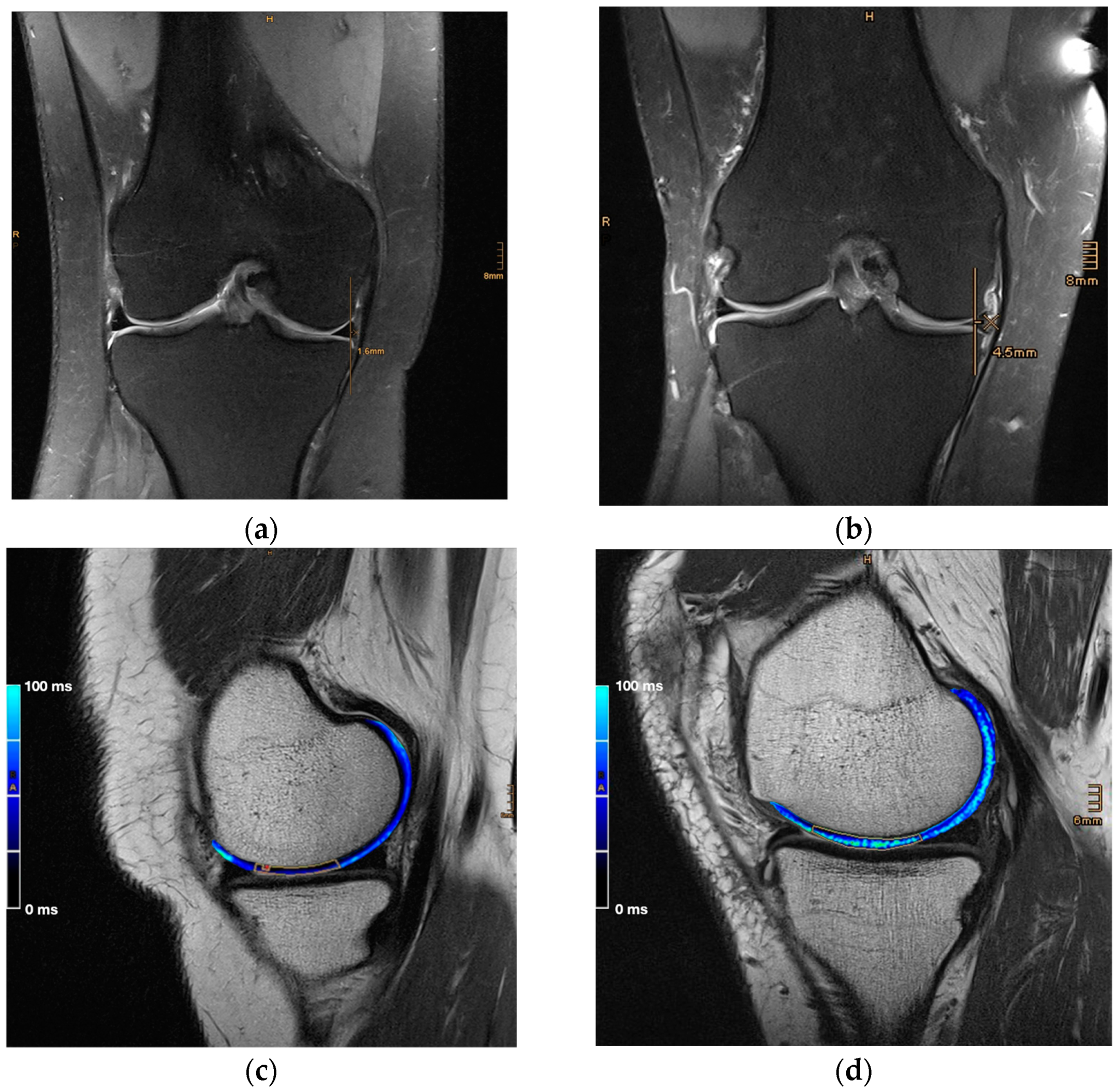
2.3. Image and Data Analysis
2.4. Statistical Analysis
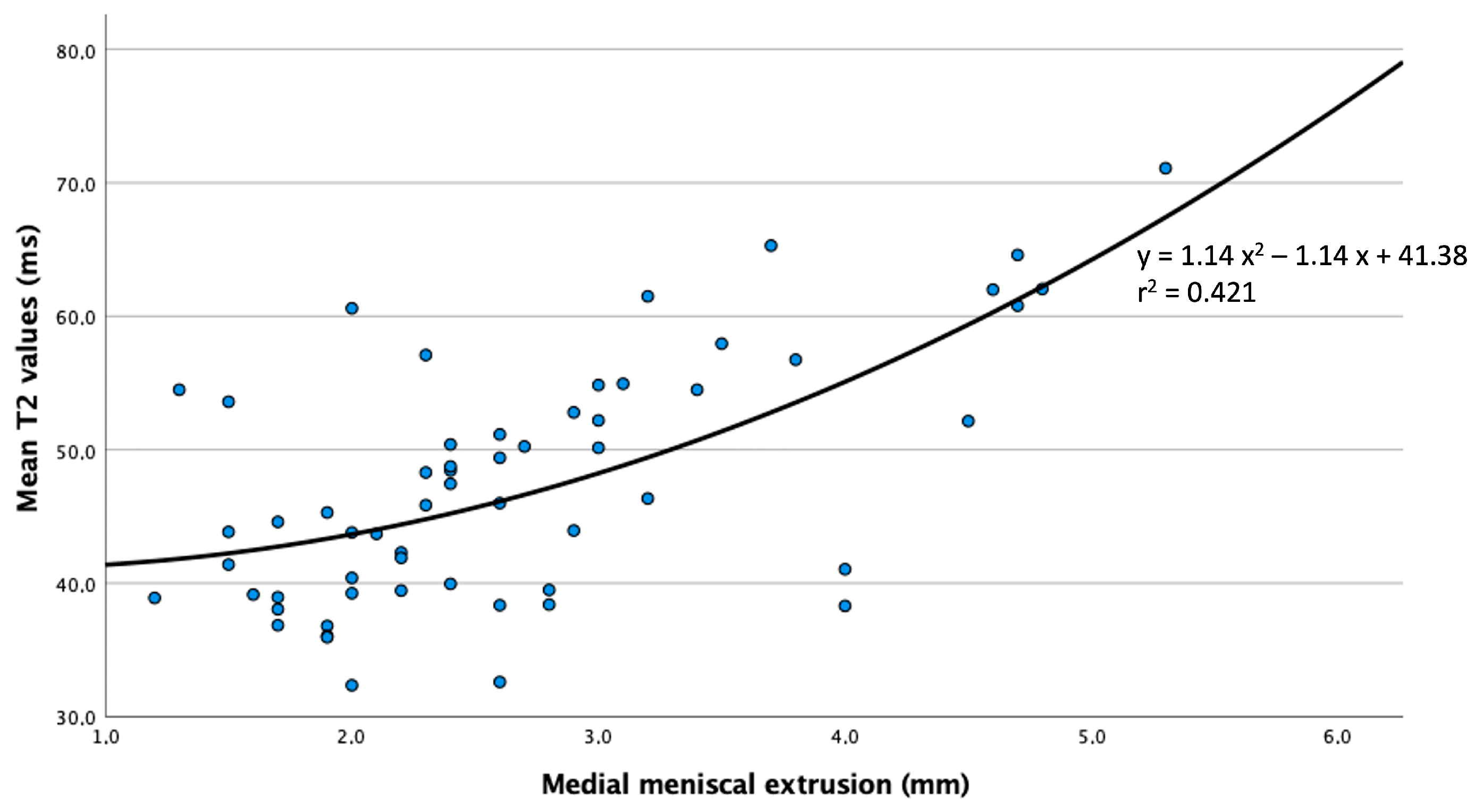
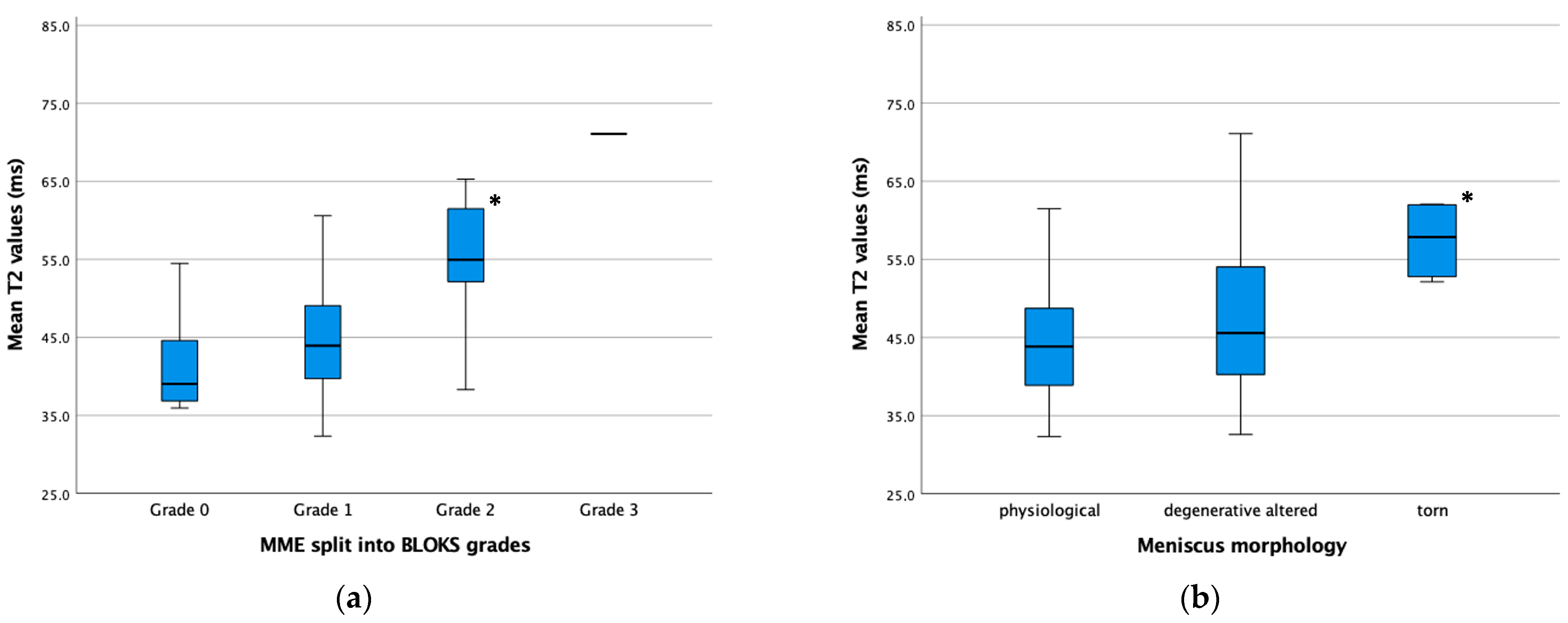
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swamy, N.; Wadhwa, V.; Bajaj, G.; Chhabra, A.; Pandey, T. Medial meniscal extrusion: Detection, evaluation and clinical implications. Eur. J. Radiol. 2018, 102, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Flandry, F.; Hommel, G. Normal anatomy and biomechanics of the knee. Sports Med. Arthrosc. Rev. 2011, 19, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.R.; Morrison, W.B.; Carrino, J.A. Medial meniscus extrusion on knee MRI: Is extent associated with severity of degeneration or type of tear? AJR Am. J. Roentgenol. 2004, 183, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Berthiaume, M.J.; Raynauld, J.P.; Martel-Pelletier, J.; Labonté, F.; Beaudoin, G.; Bloch, D.A.; Choquette, D.; Haraoui, B.; Altman, R.D.; Hochberg, M.; et al. Meniscal tear and extrusion are strongly associated with progression of symptomatic knee osteoarthritis as assessed by quantitative magnetic resonance imaging. Ann. Rheum. Dis. 2005, 64, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Driban, J.B.; Harkey, M.S.; Barbe, M.F.; Ward, R.J.; MacKay, J.W.; Davis, J.E.; Lu, B.; Price, L.L.; Eaton, C.B.; Lo, G.H.; et al. Risk factors and the natural history of accelerated knee osteoarthritis: A narrative review. BMC Musculoskelet. Disord. 2020, 21, 332. [Google Scholar] [CrossRef]
- Springer, E.; Bohndorf, K.; Juras, V.; Szomolanyi, P.; Zbýň, Š.; Schreiner, M.M.; Schmitt, B.; Trattnig, S. Comparison of Routine Knee Magnetic Resonance Imaging at 3 T and 7 T. Investig. Radiol. 2017, 52, 42–54. [Google Scholar] [CrossRef]
- Achtnich, A.; Petersen, W.; Willinger, L.; Sauter, A.; Rasper, M.; Wörtler, K.; Imhoff, A.B.; Diermeier, T. Medial meniscus extrusion increases with age and BMI and is depending on different loading conditions. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2282–2288. [Google Scholar] [CrossRef]
- Link, T.M.; Neumann, J.; Li, X. Prestructural cartilage assessment using MRI. J. Magn. Reson. Imaging 2017, 45, 949–965. [Google Scholar] [CrossRef]
- Baum, T.; Joseph, G.B.; Karampinos, D.C.; Jungmann, P.M.; Link, T.M.; Bauer, J.S. Cartilage and meniscal T2 relaxation time as non-invasive biomarker for knee osteoarthritis and cartilage repair procedures. Osteoarthr. Cartil. 2013, 21, 1474–1484. [Google Scholar] [CrossRef]
- Schooler, J.; Kumar, D.; Nardo, L.; McCulloch, C.; Li, X.; Link, T.M.; Majumdar, S. Longitudinal evaluation of T1ρ and T2 spatial distribution in osteoarthritic and healthy medial knee cartilage. Osteoarthr. Cartil. 2014, 22, 51–62. [Google Scholar] [CrossRef]
- Apprich, S.; Welsch, G.H.; Mamisch, T.C.; Szomolanyi, P.; Mayerhoefer, M.; Pinker, K.; Trattnig, S. Detection of degenerative cartilage disease: Comparison of high-resolution morphological MR and quantitative T2 mapping at 3.0 Tesla. Osteoarthr. Cartil. 2010, 18, 1211–1217. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lansdown, D.A.; Wang, K.; Cotter, E.; Davey, A.; Cole, B.J. Relationship Between Quantitative MRI Biomarkers and Patient-Reported Outcome Measures After Cartilage Repair Surgery: A Systematic Review. Orthop. J. Sports Med. 2018, 6, 2325967118765448. [Google Scholar] [CrossRef] [PubMed]
- Mosher, T.J.; Liu, Y.; Yang, Q.X.; Yao, J.; Smith, R.; Dardzinski, B.J.; Smith, M.B. Age dependency of cartilage magnetic resonance imaging T2 relaxation times in asymptomatic women. Arthritis Rheum. 2004, 50, 2820–2828. [Google Scholar] [CrossRef]
- Baum, T.; Joseph, G.B.; Nardo, L.; Virayavanich, W.; Arulanandan, A.; Alizai, H.; Carballido-Gamio, J.; Nevitt, M.C.; Lynch, J.; McCulloch, C.E.; et al. Correlation of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with body mass index: Thirty-six-month followup data from a longitudinal, observational multicenter study. Arthritis Care Res. 2013, 65, 23–33. [Google Scholar] [CrossRef]
- Mosher, T.J.; Liu, Y.; Torok, C.M. Functional cartilage MRI T2 mapping: Evaluating the effect of age and training on knee cartilage response to running. Osteoarthr. Cartil. 2010, 18, 358–364. [Google Scholar] [CrossRef]
- Apprich, S.; Mamisch, T.C.; Welsch, G.H.; Stelzeneder, D.; Albers, C.; Totzke, U.; Trattnig, S. Quantitative T2 mapping of the patella at 3.0T is sensitive to early cartilage degeneration, but also to loading of the knee. Eur. J. Radiol. 2012, 81, e438–e443. [Google Scholar] [CrossRef]
- Apprich, S.R.; Schreiner, M.M.; Szomolanyi, P.; Welsch, G.H.; Koller, U.K.; Weber, M.; Windhager, R.; Trattnig, S. Potential predictive value of axial T2 mapping at 3 Tesla MRI in patients with untreated patellar cartilage defects over a mean follow-up of four years. Osteoarthr. Cartil. 2020, 28, 215–222. [Google Scholar] [CrossRef]
- Liebl, H.; Joseph, G.; Nevitt, M.C.; Singh, N.; Heilmeier, U.; Subburaj, K.; Jungmann, P.M.; McCulloch, C.E.; Lynch, J.A.; Lane, N.E.; et al. Early T2 changes predict onset of radiographic knee osteoarthritis: Data from the osteoarthritis initiative. Ann. Rheum. Dis. 2015, 74, 1353–1359. [Google Scholar] [CrossRef]
- Jones, L.D.; Mellon, S.J.; Kruger, N.; Monk, A.P.; Price, A.J.; Beard, D.J. Medial meniscal extrusion: A validation study comparing different methods of assessment. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 1152–1157. [Google Scholar] [CrossRef]
- Hunter, D.J.; Lo, G.H.; Gale, D.; Grainger, A.J.; Guermazi, A.; Conaghan, P.G. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston Leeds Osteoarthritis Knee Score). Ann. Rheum. Dis. 2008, 67, 206–211. [Google Scholar] [CrossRef]
- Joseph, G.B.; Baum, T.; Alizai, H.; Carballido-Gamio, J.; Nardo, L.; Virayavanich, W.; Lynch, J.A.; Nevitt, M.C.; McCulloch, C.E.; Majumdar, S.; et al. Baseline mean and heterogeneity of MR cartilage T2 are associated with morphologic degeneration of cartilage, meniscus, and bone marrow over 3 years-data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2012, 20, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Joseph, G.B.; Baum, T.; Carballido-Gamio, J.; Nardo, L.; Virayavanich, W.; Alizai, H.; Lynch, J.A.; McCulloch, C.E.; Majumdar, S.; Link, T.M. Texture analysis of cartilage T2 maps: Individuals with risk factors for OA have higher and more heterogeneous knee cartilage MR T2 compared to normal controls--data from the osteoarthritis initiative. Arthritis Res. Ther. 2011, 13, R153. [Google Scholar] [CrossRef] [PubMed]
- Svensson, F.; Felson, D.T.; Turkiewicz, A.; Guermazi, A.; Roemer, F.W.; Neuman, P.; Englund, M. Scrutinizing the cut-off for "pathological" meniscal body extrusion on knee MRI. Eur. Radiol. 2019, 29, 2616–2623. [Google Scholar] [CrossRef]
- Joseph, G.B.; McCulloch, C.E.; Nevitt, M.C.; Heilmeier, U.; Nardo, L.; Lynch, J.A.; Liu, F.; Baum, T.; Link, T.M. A reference database of cartilage 3 T MRI T2 values in knees without diagnostic evidence of cartilage degeneration: Data from the osteoarthritis initiative. Osteoarthr. Cartil. 2015, 23, 897–905. [Google Scholar] [CrossRef]
- Soellner, S.T.; Goldmann, A.; Muelheims, D.; Welsch, G.H.; Pachowsky, M.L. Intraoperative validation of quantitative T2 mapping in patients with articular cartilage lesions of the knee. Osteoarthr. Cartil. 2017, 25, 1841–1849. [Google Scholar] [CrossRef]
- Crema, M.D.; Guermazi, A.; Li, L.; Nogueira-Barbosa, M.H.; Marra, M.D.; Roemer, F.W.; Eckstein, F.; Le Graverand, M.P.; Wyman, B.T.; Hunter, D.J. The association of prevalent medial meniscal pathology with cartilage loss in the medial tibiofemoral compartment over a 2-year period. Osteoarthr. Cartil. 2010, 18, 336–343. [Google Scholar] [CrossRef]
- Chung, K.S.; Ha, J.K.; Ra, H.J.; Kim, J.G. A meta-analysis of clinical and radiographic outcomes of posterior horn medial meniscus root repairs. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1455–1468. [Google Scholar] [CrossRef]
- Moon, H.S.; Choi, C.H.; Jung, M.; Lee, D.Y.; Hong, S.P.; Kim, S.H. Early Surgical Repair of Medial Meniscus Posterior Root Tear Minimizes the Progression of Meniscal Extrusion: 2-Year Follow-up of Clinical and Radiographic Parameters After Arthroscopic Transtibial Pull-out Repair. Am. J. Sports Med. 2020, 48, 2692–2702. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Kawahara, K.; Samejima, Y.; Kaneko, T.; Ikegami, H.; Musha, Y. Short-term results and surgical technique of arthroscopic centralization as an augmentation for medial meniscus extrusion caused by medial meniscus posterior root tear. Eur. J. Orthop. Surg. Traumatol. 2021, 31, 1235–1241. [Google Scholar] [CrossRef]
- Feucht, M.J.; Kühle, J.; Bode, G.; Mehl, J.; Schmal, H.; Südkamp, N.P.; Niemeyer, P. Arthroscopic Transtibial Pullout Repair for Posterior Medial Meniscus Root Tears: A Systematic Review of Clinical, Radiographic, and Second-Look Arthroscopic Results. Arthroscopy 2015, 31, 1808–1816. [Google Scholar] [CrossRef]
- Kaplan, D.J.; Alaia, E.F.; Dold, A.P.; Meislin, R.J.; Strauss, E.J.; Jazrawi, L.M.; Alaia, M.J. Increased extrusion and ICRS grades at 2-year follow-up following transtibial medial meniscal root repair evaluated by MRI. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2826–2834. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.J.; Bloom, D.; Alaia, E.F.; Walter, W.R.; Meislin, R.J.; Strauss, E.J.; Jazrawi, L.M.; Alaia, M.J. ICRS scores worsen between 2-year short term and 5-year mid-term follow-up after transtibial medial meniscus root repair despite maintained functional outcomes. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 2235–2243. [Google Scholar] [CrossRef] [PubMed]
- Wenger, A.; Wirth, W.; Hudelmaier, M.; Noebauer-Huhmann, I.; Trattnig, S.; Bloecker, K.; Frobell, R.B.; Kwoh, C.K.; Eckstein, F.; Englund, M. Meniscus body position, size, and shape in persons with and persons without radiographic knee osteoarthritis: Quantitative analyses of knee magnetic resonance images from the osteoarthritis initiative. Arthritis Rheum. 2013, 65, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Paproki, A.; Engstrom, C.; Chandra, S.S.; Neubert, A.; Fripp, J.; Crozier, S. Automated segmentation and analysis of normal and osteoarthritic knee menisci from magnetic resonance images--data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2014, 22, 1259–1270. [Google Scholar] [CrossRef]
| Sequences | Coronal 2D PD TSE | Sagittal 2D TSE | Sagittal 2D MESE |
|---|---|---|---|
| Repetition time (ms) | 4250 | 2130 | 1200 |
| Echo time (ms) | 27 | 36 | 6 Echos 11.9–71.4 |
| Field of view (mm) | 150 × 150 | 120 × 120 | 140 × 140 |
| Matrix | 384 × 384 | 448 × 448 | 640 × 640 |
| Pixel size (mm) | 0.36 × 0.36 × 3 | 0.22 × 0.22 × 2 | 0.3 × 0.3 |
| BLOKS | Grade | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Overall | |
| physiological | 6/10.2% | 14/23.7% | 5/8.5% | - | 25/42.4% |
| degenerative altered | 8/13.6% | 12/20.3% | 7/11.9% | 1/1.7% | 28/47.5% |
| torn | - | 1/1.7% | 5/8.5% | - | 6/10.2% |
| Overall | 14/23.7% | 27/45.8% | 17/28.8% | 1/1.7% | 59/100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoppe, P.L.; Priol, M.; Springer, B.; Waldstein-Wartenberg, W.; Böhler, C.; Windhager, R.; Trattnig, S.; Apprich, S. The Impact of Medial Meniscal Extrusion on Cartilage of the Medial Femorotibial Joint—A Retrospective Analysis Based on Quantitative T2 Mapping at 3.0T. J. Clin. Med. 2024, 13, 6628. https://doi.org/10.3390/jcm13226628
Hoppe PL, Priol M, Springer B, Waldstein-Wartenberg W, Böhler C, Windhager R, Trattnig S, Apprich S. The Impact of Medial Meniscal Extrusion on Cartilage of the Medial Femorotibial Joint—A Retrospective Analysis Based on Quantitative T2 Mapping at 3.0T. Journal of Clinical Medicine. 2024; 13(22):6628. https://doi.org/10.3390/jcm13226628
Chicago/Turabian StyleHoppe, Paul Lennart, Moritz Priol, Bernhard Springer, Wenzel Waldstein-Wartenberg, Christoph Böhler, Reinhard Windhager, Siegfried Trattnig, and Sebastian Apprich. 2024. "The Impact of Medial Meniscal Extrusion on Cartilage of the Medial Femorotibial Joint—A Retrospective Analysis Based on Quantitative T2 Mapping at 3.0T" Journal of Clinical Medicine 13, no. 22: 6628. https://doi.org/10.3390/jcm13226628
APA StyleHoppe, P. L., Priol, M., Springer, B., Waldstein-Wartenberg, W., Böhler, C., Windhager, R., Trattnig, S., & Apprich, S. (2024). The Impact of Medial Meniscal Extrusion on Cartilage of the Medial Femorotibial Joint—A Retrospective Analysis Based on Quantitative T2 Mapping at 3.0T. Journal of Clinical Medicine, 13(22), 6628. https://doi.org/10.3390/jcm13226628








