Abstract
Background/Objectives: Heart failure worsens the prognosis of patients with infective endocarditis (IE) and is mainly caused by severe valvular regurgitation. The aim of our investigation is to describe the clinical, epidemiological, microbiological, and echocardiographic characteristics of patients with native left-sided infective endocarditis (NLSIE) with severe valvular regurgitation; to describe the prognosis according to the therapeutic approach; and to determine the prognostic factors of in-hospital mortality. Methods: We prospectively recruited all episodes of possible or definite NLSIE diagnosed at three tertiary hospitals between 2005 and 2022. Patients were divided into two groups: patients with severe valvular regurgitation at the time of admission or during hospitalization and patients without severe valvular regurgitation. We analyzed up to 85 variables concerning epidemiological, clinical, analytical, microbiological, and echocardiographic data. Results: We recovered 874 patients with NLSIE, 564 (65%) of them with severe valvular regurgitation. There were no differences in mortality among patients with and without severe regurgitation (30.2% vs. 26.5%, p = 0.223). However, mortality increased when patients with severe regurgitation developed heart failure (33% vs. 11.4%, p < 0.001). Independent factors related to heart failure were age (OR 1.02 [1.01–1.034], p = 0.001), anemia (OR 1.2 [1.18–3.31], p = 0.01), atrial fibrillation (OR 2.3 [1.08–4.89], p = 0.03), S. viridans-related IE (OR 0.47 [0.3–0.73], p = 0.001), and mitroaortic severe regurgitation (OR 2.4 [1.15–5.02], p = 0.019). Conclusions: Severe valvular regurgitation is very frequent among patients with NLSIE, but it does not worsen the prognosis of patients unless complicated with heart failure.
1. Introduction
Heart failure worsens the prognosis of patients with left-sided infective endocarditis (IE) and represents the main surgical indication in these patients [,]. In most patients, heart failure results from severe valvular regurgitation, which affects up to 55% of patients with left-sided IE, according to previous studies [,]. Hypothetically, the presence of severe valvular regurgitation in patients with native left-sided infective endocarditis (NLSIE) is associated with an increased risk of heart failure and the need for surgery; it could be caused by more aggressive micro-organisms and might be associated with a poorer outcome. However, these hypotheses remain unsettled, as there is no study focused on this specific group of patients. Given its high prevalence, it is important to know whether severe valvular regurgitation entails clinical or prognostic specific issues.
The aim of our investigation was to describe the clinical, epidemiological, microbiological, and echocardiographic characteristics of patients with NLSIE with severe valvular regurgitation, to analyze the patient prognosis according to the therapeutic approach, and to determine the prognostic factors of in-hospital mortality.
2. Materials and Methods
2.1. Population
We prospectively recruited all episodes of possible or definite IE diagnosed at three tertiary hospitals between 2005 and 2022 according to diagnostic criteria accepted at each period (modified Duke criteria from 2005 to 2014 and ESC 2015 modified diagnostic criteria from 2015 to 2022). The inclusion criterion for the analysis was NLSIE.
All patients underwent transthoracic (TTE) and transesophageal (TEE) echocardiography performed by imaging experts certified by the Spanish Society of Echocardiography, and the degree of regurgitation was determined according to European Guidelines of Echocardiography present in each period of the study. Quantitative and semiquantitative measures such as Proximal Isovelocity Surface Area (PISA), vena contracta width, pressure half-time, and color Doppler 2D and 3D were used whenever possible [].
Patients were then divided into two groups: patients with severe valvular regurgitation at the time of admission or during hospitalization and patients without severe valvular regurgitation. Patients with severe regurgitation already known before admission were included in the former group.
The main indications for surgery were heart failure, unresponsiveness to medical treatment, septic shock, persistent signs of infection, fungal endocarditis, and recurrent systemic embolism despite adequate antibiotic therapy. The decision to operate on a patient was always made by the IE-Heart Team, composed of cardiologists, heart surgeons, and experts in infectious diseases.
2.2. Variables Studied
We analyzed up to 85 variables concerning epidemiological, clinical, analytical, microbiological, and echocardiographic data at the time of admission and during hospitalization, as well as information about treatment and clinical outcomes. The proportion of missing data was <10% in all analyzed variables.
2.3. Definition of Terms
Heart failure was diagnosed by a clinical cardiologist according to the ESC guidelines criteria []. Acute onset of symptoms was defined by a cut-off of 15 days between the onset of symptoms and hospital admission. Nosocomial IE was defined as IE occurring 72 h or more after admission to the hospital or when the episode was related to a hospitalary procedure performed in the last 8 weeks []. Renal failure was defined as serum creatinine levels greater than 2 mg/dL in patients with previously normal renal function and a creatinine level increase >50% in those with chronic renal insufficiency. Chronic anemia was defined as serum hemoglobin levels lower than 13 g/dL in males and 12 g/dL in females for at least a year. Persistent infection was defined as persistent fever and positive blood cultures after 7 days of adequate antibiotic treatment once other potential causes of fever or the presence of perivalvular complications were excluded []. Patients were assessed in all cases with TTE and TEE at the time of diagnosis, and severe valvular regurgitation was defined according to the European Guidelines of Echocardiography []. Previous disease on the affected valve was defined as any known valvular disease, independently of its severity or condition, prior to admission. The bicuspid aortic valve was included in “congenital valvular disease”, and mitral valve prolapse was involved in “degenerative valvular disease”. Systemic emboli were diagnosed by imaging techniques such as computed tomography and echography. Referred cases were those patients transferred to the tertiary hospital from other healthcare centers where they were initially admitted.
2.4. Statistical Analysis
Continuous variables were reported as the mean ± standard deviation or median [interquartile range, IQR] according to variable distribution (normal or not). Student’s t-test or Mann–Whitney U tests were used for comparison among groups. The normal distribution of continuous variables was verified with the Kolmogorov–Smirnov test and q-q plot. Categorical variables were reported as absolute values and percentages and compared with a Chi-square test or Fisher’s exact test when expected frequencies were less than 5.
Multivariable models by logistic regression with the maximum likelihood backward stepwise method were adjusted to analyze the prognostic factors of in-hospital mortality and heart failure in patients with severe valvular regurgitation.
All prognostic factors with a p value of less than 0.05 in the univariate model were further entered into the multivariate analysis. Age, gender, diabetes mellitus, chronic renal failure, pulmonary hypertension, vegetation, heart failure, septic shock, renal failure, Streptococcus viridans, Staphylococcus aureus, and surgery were included in the model for in-hospital mortality. Age, degenerative valve disease, anemia, atrial fibrillation (AF), mitroaortic severe regurgitation, Streptococcus viridans, Enterococcus spp., coagulase-negative staphylococci, and negative cultures were included in the model for heart failure.
For all adjusted models, the ratio variable/event was controlled to avoid overfitting. Odds ratios (ORs), adjusted for each of the variables included, along with their 95% confidence intervals (95% CI), were calculated. Noncollinearity was checked among the variables. The area under the receiver operating characteristic curve (ROC curve) was used to measure the discriminatory capacity. Calibration was evaluated with the Hosmer–Lemeshow test and with plots.
Kaplan–Meier curves were estimated for 1-year mortality and compared between groups with the log-rank test.
Statistical analysis was performed using R software, version 3.6.1 (R Project for Statistical Computing). No imputation of missing data was performed. All tests were two-sided. Differences were statistically significant when the p value was <0.05.
3. Results
Of the 1756 patients with IE included in our database, 1468 were left-sided, and 874 of those were NLSIE. Of these, 564 (65%) had severe valvular regurgitation: 248 had aortic regurgitation (44%), 201 had mitral regurgitation (35%), and 115 had both mitral and aortic severe regurgitation (21%). The remaining 310 patients (35%) had no, mild, or moderate regurgitation. Table 1 shows the characteristics of patients with and without severe regurgitation (Figure 1).

Table 1.
Descriptive study of patients with left-sided native valve infective endocarditis (LSNVIE).
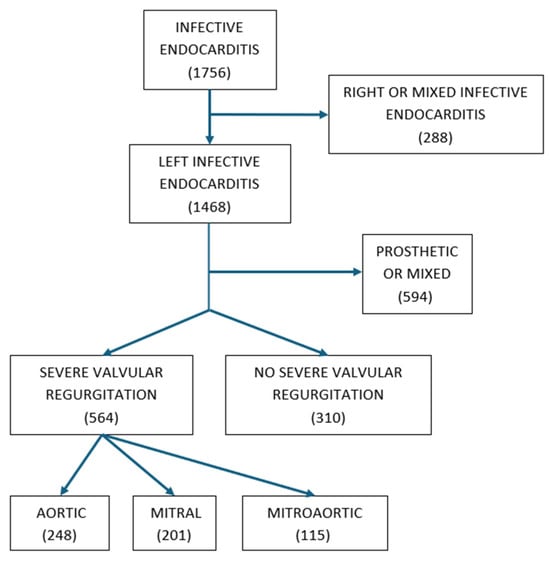
Figure 1.
Eligibility criteria and sample size.
3.1. Characteristics of Patients with and without Severe Regurgitation
Table 1 compares the epidemiologic, clinical, microbiologic, echocardiographic, and prognostic characteristics of patients with and without severe valvular regurgitation.
Of note, patients with severe regurgitation were more frequently referred from other hospitals (73% vs. 55%; p = 0.001). Previous valvular disease of the infected valve in patients with severe regurgitation was less frequent than in patients without severe regurgitation (46% vs. 54.3%; p = 0.027). The most frequent initial manifestation in patients with severe regurgitation was heart failure (44.4% vs. 23.2%; p < 0.001), while other manifestations such as septic shock (20% vs. 14.1%; p = 0.025) and stroke (21% vs. 14.5%; p = 0.014) were more frequent in patients without severe regurgitation.
From a microbiological point of view, positive blood cultures at the time of admission showed no difference (83.3% vs. 83.9%, p = 0.825). Interestingly, 19.2% of patients with severe regurgitation had persistent positive blood cultures (after 72 h of admission) versus 30.3% of patients without severe regurgitation (p = 0.004). Intriguingly, S. aureus was more frequent in patients without severe regurgitation (27.1% vs. 17.2%, p = 0.001) and S. viridans in patients with severe regurgitation (22% vs. 14.2% p = 0.005).
The most common surgical indication among patients with severe regurgitation was heart failure (93.4% vs. 48.1%; p < 0.001). Uncontrolled infection and prevention of emboli were more frequent in patients with no severe regurgitation (34.8% vs. 63.2%; p < 0.001 and 27.4% vs. 40.6%; p = 0.009, respectively). Some patients had more than one surgical indication.
3.2. Mortality in Patients with Severe Valvular Regurgitation
Remarkably, in-hospital mortality was similar among patients with and without severe regurgitation (30.2% vs. 26.5%, p = 0.223), as well as 1-year mortality (Figure 2). However, patients with severe regurgitation who developed heart failure at any point had a higher in-hospital mortality than those who did not (33% vs. 11.4%; p < 0.001), with similar findings in the analysis of 1-year mortality (Figure 2). Table 2 shows the analysis of mortality in patients with severe regurgitation. Independent predictors of mortality in patients with severe regurgitation include heart failure and are shown in Table 3.
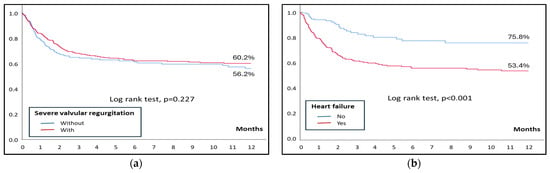
Figure 2.
(a) Kaplan–Meier curves for 1-year mortality in patients with and without severe regurgitation. (b) Kaplan–Meier curves for 1-year mortality in patients with severe regurgitation, with and without heart failure.

Table 2.
Analysis of mortality in patients with severe valvular regurgitation.

Table 3.
Independent factors associated with mortality in patients with severe valvular regurgitation.
3.3. Heart Failure in Patients with Severe Regurgitation
Table 4 compares the characteristics of patients with severe regurgitation to those with and without heart failure.

Table 4.
Comparison between patients with severe valvular regurgitation who developed heart failure and patients who did not develop heart failure.
Patients with severe regurgitation who had heart failure were older (p < 0.001), had more degenerative valvular disease (28.1% vs. 19.1%, p = 0.023), as well as chronic kidney disease (13% vs. 5.6%, p = 0.008), anemia (26.8% vs. 12.9%, p < 0.001), multiple valve involvement (22.9% vs. 15.1%, p = 0.03), and AF (15.5% vs. 5%, p < 0.001).
Patients’ microbiological profile showed a lower frequency of infections by S. viridans (16.7% vs. 33.5%, p < 0.001) and a higher frequency of Enterococcus spp. (15.6% vs. 9.5%, p = 0.005) and coagulase-negative staphylococci (12.2% vs. 6.7%, p = 0.046).
Table 5 shows those factors that were independently associated with heart failure in patients with severe valvular regurgitation. Remarkably, S. viridans infection was associated with not developing heart failure (p = 0.001).

Table 5.
Independent predictors of heart failure in patients with severe valvular regurgitation.
4. Discussion
Scant information is available regarding the impact of severe valvular regurgitation on patients with NLSIE despite it being a common finding among these patients. Our results shed light on the course of these patients, with the largest cohort studied to our knowledge. The main findings were the following: (1) Despite being very frequent, severe valvular regurgitation among patients with NLSIE was not associated with higher in-hospital mortality in our population; (2) Prognosis worsened if patients with severe regurgitation developed heart failure; (3) Predictors of heart failure in patients with NLSIE and severe regurgitation were age, AF, anemia and involvement of both the mitral and aortic valves.
The prevalence of severe regurgitation in our cohort was surprisingly high compared to others [,,], probably due to a selection bias, as all patients with endocarditis and surgical indications from other nearby primary and secondary centers are referred to our hospital. If we exclude all referred patients, the prevalence of severe regurgitation was 55%, resembling other studies’ frequencies. Another baseline difference in our cohort was a higher prevalence of S. viridans in the baseline cohort [,,], which showed a significant correlation with severe regurgitation. As described by previous works [], heart failure was less frequent in patients with S. viridans-related IE as well. The subacute onset of endocarditis caused by this micro-organism may explain why severe regurgitation is better tolerated from a clinical standpoint.
On the other hand, nonsevere regurgitation was surprisingly more frequent in S. aureus-related IE. Its more aggressive course and association with septic shock and stroke could explain alternative mechanisms for heart failure other than valvular regurgitation, such as renal failure, hyperdynamic circulation, or arrhythmias (as later mentioned).
Heart failure has been largely described as a mortality risk factor in previous studies, with some of them reaching 34% mortality, and has been included in predictive models of in-hospital mortality [,,,,]. Recent work focused on patients with IE and surgical indication also proved heart failure to be independently associated not only with all-cause mortality but also with major adverse events, including all-cause death, hospitalizations, and IE relapses []. The main finding of our investigation was that in patients with NLSIE and severe regurgitation from our cohort, it was not the dysfunction of the valve but heart failure that was the main feature related to mortality (11% vs. 33%). We identified several variables related to an increased risk of heart failure in this group of patients, which could help clinicians stratify the severity of the disease when severe regurgitation is present. It could also help in deciding when to refer a patient admitted for NLSIE to a center with surgical facilities.
The presence of AF in patients with IE has previously been described as a mortality risk factor and predictor of heart failure []. Interestingly, severe regurgitation was not more frequent in patients with AF, suggesting an independent mechanism of heart failure. Furthermore, new-onset AF had a poorer outcome than chronic AF []. The loss of atrio-ventricular synchrony, rapid ventricular rate, and the absence of atrial contraction raises filling pressures and pulmonary capillary pressures []. Our study shows that AF strongly increases the risk of heart failure and is associated with greater mortality.
As expected, severe regurgitation of multiple valves was also related to a higher risk of heart failure than single valve involvement []. Aortic regurgitation (AR) results in an increased end-diastolic volume (EDV), which increases the regurgitant flow of an incompetent mitral valve, thus raising capillary wedge pressure and heart failure symptoms. Furthermore, AR and MR both end up in a decreased forward cardiac output (AR initially increases systolic volume but not organ perfusion), resulting in increased activation of the renin–angiotensin–aldosterone system and worsening heart failure []. A previous study already described how bivalvular regurgitation led to more heart failure without increased mortality, which was attributed to a higher surgery rate []. Another study, however, found that patients with bivalvular IE had higher mortality, although early surgery increased survival in this set of patients []. On the other hand, the severe regurgitation of both mitral and aortic valves in a patient with endocarditis is not always caused directly by an infection of both valves. It has already been described how chronic AR can lead to increased LV dilation, which causes an increased sphericity of LV, changing the position and orientation of papillary muscles and interfering with the coaptation of mitral leaflets, leading to an increased MR [].
Another important factor to consider is the time dimension, as the hemodynamic behavior of acute regurgitation differs from chronic regurgitation. Dilation and increased compliance of the left ventricle (LV) derived from chronic severe regurgitation allows the accommodation of excess end-diastolic volume and the maintenance of forward systolic volume. On the contrary, the acute setting of severe regurgitation not only limits LV adaptation to increased volume but, in the case of aortic regurgitation, also results in an increased LV end-diastolic pressure exceeding that of the left atrium, which leads to the premature closure of the mitral valve during diastole, limiting LV filling and further decreasing systolic volume. In acute mitral regurgitation, the left atrium does not have time to adapt to increased volume, and capillary wedge pressure increases drastically [,]. This particularity in the timing of regurgitation suggests that patients without previous significant valvular disease may have a worse hemodynamic tolerance to acute valvular disease and a poorer outcome. However, since valvular disease is also a risk factor for IE, the correlation between those two variables remains a difficult aspect to address.
Nevertheless, early surgery should be targeted to IE patients who develop heart failure or those at a high risk of doing so, irrespective of the number of valves infected.
From a clinical standpoint, our work enhances the importance of simple and readily available clinical markers that help identify patients with NLSIE and severe regurgitation who have worse prognosis because of the high likelihood of developing heart failure and death.
We are aware of several limitations. Although data were prospectively collected, the study is retrospective, and our conclusions are hypothesis-generating at most. An assessment of regurgitation was not evaluated by an independent core laboratory, but it was undertaken in the local imaging units. However, those are busy units with long-recognized expertise in endocarditis, in which guidelines are tightly followed. The inclusion of patients with severe regurgitation already known at admission in the group of patients with severe regurgitation is questionable, given that the hemodynamic behavior of chronic and acute regurgitation is different [,,,]. Nonetheless, few patients had known severe regurgitation (n = 84), and the results did not change if those patients were excluded from the analysis.
5. Conclusions
To summarize, severe valvular regurgitation is very frequent among patients with NLSIE, but it does not worsen the prognosis of patients unless it is complicated with heart failure. We identified several factors associated with the development of heart failure. Future studies should test whether preventive surgery in those patients may improve outcomes.
Author Contributions
Conceptualization, J.L.D. and J.A.S.R.; methodology, M.d.M. and I.G.-S.; validation, J.L.D., I.V. and J.A.S.R.; formal analysis, J.L.D.; investigation, C.O., C.S. and J.B.P.-S.; data curation, A.O., D.P.-G. and P.P.; visualization, P.Z., A.J. and D.G.; writing—original draft preparation, A.L.I.; writing—review and editing, J.L.D. and J.A.S.R.; visualization, G.C.; supervision, J.L.D. and J.A.S.R. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Instituto de Salud Carlos III with grant number PI22/00459.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Committee of Valladolid Este (protocol code CASVE-PI-07-002) on 24 May 2007.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Delgado, V.; Ajmone Marsan, N.; de Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.A.; et al. 2023 ESC Guidelines for the management of endocarditis. Eur. Heart J. 2023, 44, 3948–4042. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Wang, A. Infective Endocarditis. J. Intensive Care Med. 2016, 31, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Bohbot, Y.; Habib, G.; Laroche, C.; Stöhr, E.; Chirouze, C.; Hernandez-Meneses, M.; Melissopoulou, M.; Mutlu, B.; Scheggi, V.; Branco, L.; et al. Characteristics, management, and outcomes of patients with left-sided infective endocarditis complicated by heart failure: A substudy of the ESC-EORP EURO-ENDO (European infective endocarditis) registry. Eur. J. Heart Fail. 2022, 24, 1253–1265. [Google Scholar] [CrossRef]
- Nadji, G.; Rusinaru, D.; Rémadi, J.P.; Jeu, A.; Sorel, C.; Tribouilloy, C. Heart failure in left-sided native valve infective endocarditis: Characteristics, prognosis, and results of surgical treatment. Eur. J. Heart Fail. 2009, 11, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L.; Scientific Document Committee of the European Association of Cardiovascular Imaging. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Peláez Ballesta, A.I.; García Vázquez, E.; Gómez Gómez, J. Infective endocarditis treated in a secondary hospital: Epidemiological, clinical, microbiological characteristics and prognosis, with special reference to patients transferred to a third level hospital. Rev. Esp. Quimioter. Publ. Soc. Esp. Quimioter. 2022, 35, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Cabezón, G.; de Miguel, M.; López, J.; Vilacosta, I.; Pulido, P.; Olmos, C.; Jerónimo, A.; Pérez, J.B.; Lozano, A.; Gómez, I.; et al. Contemporary Clinical Profile of Left-Sided Native Valve Infective Endocarditis: Influence of the Causative Microorganism. J. Clin. Med. 2023, 12, 5441. [Google Scholar] [CrossRef]
- Mistiaen, W.P. What are the main predictors of in-hospital mortality in patients with infective endocarditis: A review. Scand. Cardiovasc. J. SCJ 2018, 52, 58–68. [Google Scholar] [CrossRef]
- Marques, A.; Cruz, I.; Caldeira, D.; Alegria, S.; Gomes, A.C.; Broa, A.L.; João, I.; Pereira, H. Risk Factors for In-Hospital Mortality in Infective Endocarditis. Arq. Bras. Cardiol. 2020, 114, 1–8. [Google Scholar] [CrossRef]
- Sy, R.W.; Chawantanpipat, C.; Richmond, D.R.; Kritharides, L. Development and validation of a time-dependent risk model for predicting mortality in infective endocarditis. Eur. Heart J. 2011, 32, 2016–2026. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, F.; Paradossi, U.; Trimarchi, G.; Benedetti, G.; Marchi, F.; Chiappino, S.; Conti, M.; Di Bella, G.; Murzi, M.; Di Sibio, S.; et al. Clinical Features and Patient Outcomes in Infective Endocarditis with Surgical Indication: A Single-Centre Experience. J. Cardiovasc. Dev. Dis. 2024, 11, 138. [Google Scholar] [CrossRef]
- Ferrera, C.; Vilacosta, I.; Fernández, C.; López, J.; Sarriá, C.; Olmos, C.; Vivas, D.; Sáez, C.; Sánchez-Enrique, C.; Ortiz-Bautista, C.; et al. Usefulness of New-Onset Atrial Fibrillation, as a Strong Predictor of Heart Failure and Death in Patients With Native Left-Sided Infective Endocarditis. Am. J. Cardiol. 2016, 117, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.M.; Desai, R.G.; Krishnan, S. Mitral Regurgitation in Patients With Coexisting Chronic Aortic Regurgitation: An Evidence-Based Narrative Review. J. Cardiothorac. Vasc. Anesth. 2021, 35, 3404–3415. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Lazar, J.M.; Cunha, B.A.; Liao, W.; Minnaganti, V. Multi-valvular endocarditis. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2000, 6, 207–212. [Google Scholar] [CrossRef]
- López, J.; Revilla, A.; Vilacosta, I.; Sevilla, T.; García, H.; Gómez, I.; Pozo, E.; Sarriá, C.; San Román, J.A. Multiple-valve infective endocarditis: Clinical, microbiologic, echocardiographic, and prognostic profile. Medicine 2011, 90, 231–236. [Google Scholar] [CrossRef]
- Bohbot, Y.; Peugnet, F.; Lieu, A.; Carbone, A.; Mouhat, B.; Philip, M.; Gouriet, F.; Arregle, F.; Chevalier, F.; Diouf, M.; et al. Characteristics and Prognosis of Patients With Left-Sided Native Bivalvular Infective Endocarditis. Can. J. Cardiol. 2021, 37, 292–299. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Maheshwari, V.; Barr, B.; Srivastava, M. Acute Valvular Heart Disease. Cardiol. Clin. 2018, 36, 115–127. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Huang, W.C.; Lin, K.L.; Chiou, K.R.; Kuo, F.Y.; Lin, S.K.; Cheng, C.C. Left atrial distensibility and left ventricular filling pressure in acute versus chronic severe mitral regurgitation. Am. J. Cardiol. 2010, 105, 709–715. [Google Scholar] [CrossRef]
- Keane, R.R.; Menon, V.; Cremer, P.C. Acute Heart Valve Emergencies. Cardiol. Clin. 2024, 42, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Akinseye, O.A.; Pathak, A.; Ibebuogu, U.N. Aortic Valve Regurgitation: A Comprehensive Review. Curr. Probl. Cardiol. 2018, 43, 315–334. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).