Therapeutic Effectiveness of a Novel Cranial Remolding Helmet (baby band2) for Positional Plagiocephaly: A Multicenter Clinical Observational Study
Abstract
1. Introduction
- Baby band2 (Medical Device Approval No.: 30400BZX00252000, Figure 1) is a cranial remolding helmet developed by Berry Inc. and introduced in November 2022. This device is designed to optimize therapeutic efficacy, patient comfort, and clinical practicality simultaneously. The key features of baby band2 are as follows.
- Customized Manufacturing: The helmet utilizes 3D printing technology for the production of individually tailored helmets optimized for each patient’s specific cranial shapes, ensuring precise treatment for various degrees of positional plagiocephaly.
- A Single-Device Treatment: The helmet’s external structure is pre-formed to accommodate the anticipated final corrected cranial shape, enabling the completion of treatment with a single device potentially reducing overall treatment duration and cost.
- Efficient Growth Promotion Mechanism: A proprietary internal cushioning system temporarily inhibits growth along the longitudinal axis, thereby promoting more effective growth in the flattened area.
- Adjustment System Without Specialized Technicians: The helmet incorporates a system for adjusting the flattened area with supplementary cushioning to accommodate cranial growth, thus minimizing helmet displacement. The automated placement of additional cushioning, guided by 3D data analysis, eliminates the requirement for specialized cranial orthotic specialists.
- Cloud-Based Data Management System: Three-dimensional cranial data are stored and managed in a cloud-based system, accessible to both clinicians and patients. This data visualization capability facilitates multi-institutional clinical management and enhances the safety and reliability of patient care.
- Customizable Aesthetics: The helmet offers various color and pattern options for its external shell, facilitating personalization. This feature is designed to enhance patient engagement and potentially improve treatment adherence through increased personal connection to the device.
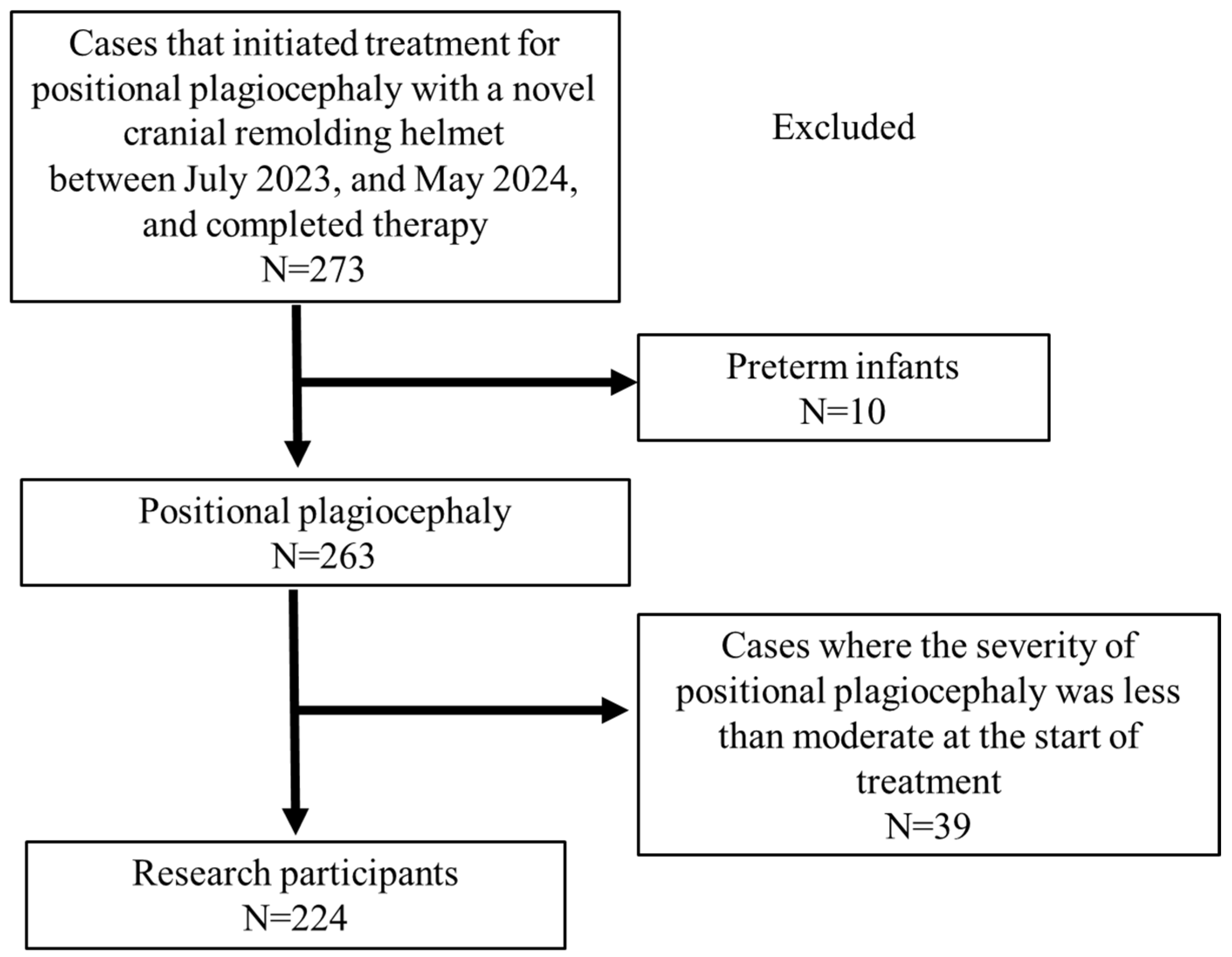
2. Subjects and Methods
2.1. 3D Cranial Shape Parameters
2.2. Investigation Items
- Study 1: The effectiveness of a novel cranial remolding helmet (baby band2) was evaluated by examining changes in both CVAI and the severity of plagiocephaly before and after helmet therapy.
- Study 2: CVAI improvement (ΔCVAI: CVAI before therapy − CVAI after therapy) was compared between the attending physicians.
- Study 3: The effectiveness of helmet therapy was compared between the infants who started therapy at less than the age of 7 months and those who started therapy at the age of 7 months or later.
- Study 4: We examined whether gender affects CVAI improvement.
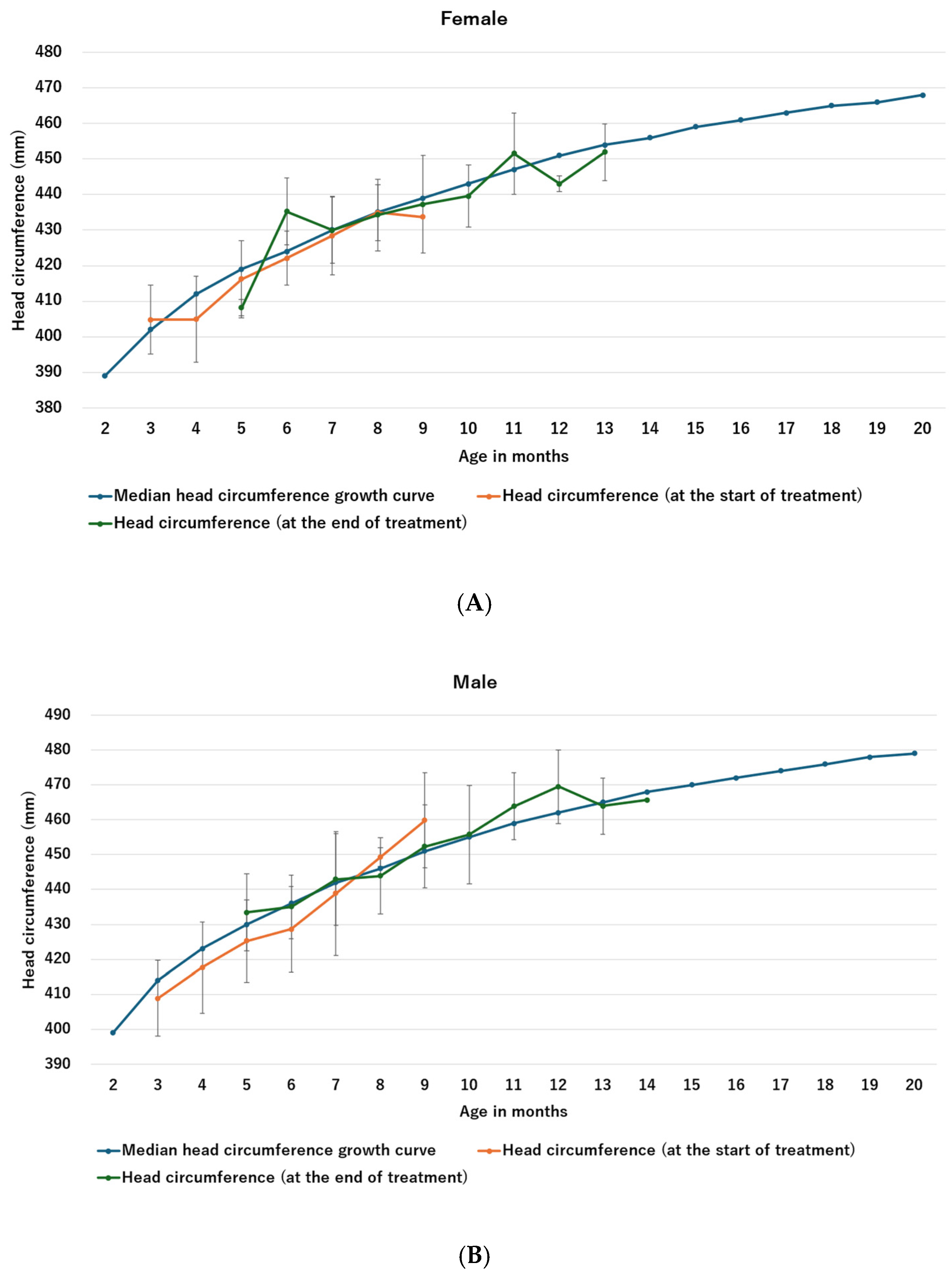
- Study 5: To determine whether helmet therapy affects head circumference by impairing growth, mean head circumference at the beginning and end of helmet therapy was compared with previously reported head circumference growth curves [26].
- Study 6: The frequency of head dermatitis was investigated as a side effect of helmet therapy.
- Study 7: The clinical factors affecting ΔCVAI were examined.
2.3. Statistical Analysis Methods
3. Results
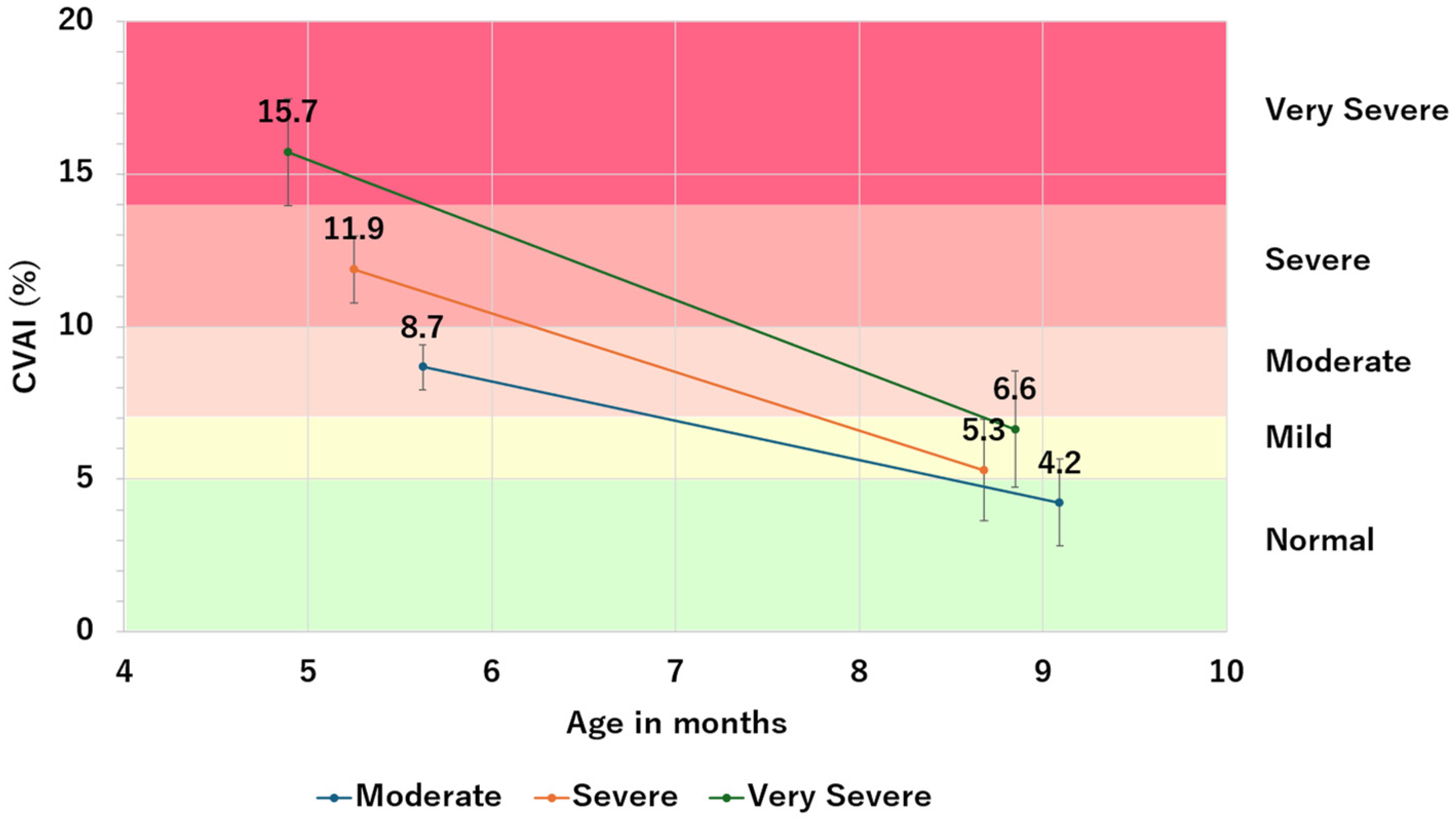
3.1. Study 1.1: Changes in CVAI before and after Helmet Therapy
Study 1.2: Change in Severity of Plagiocephaly before and after the Helmet Therapy
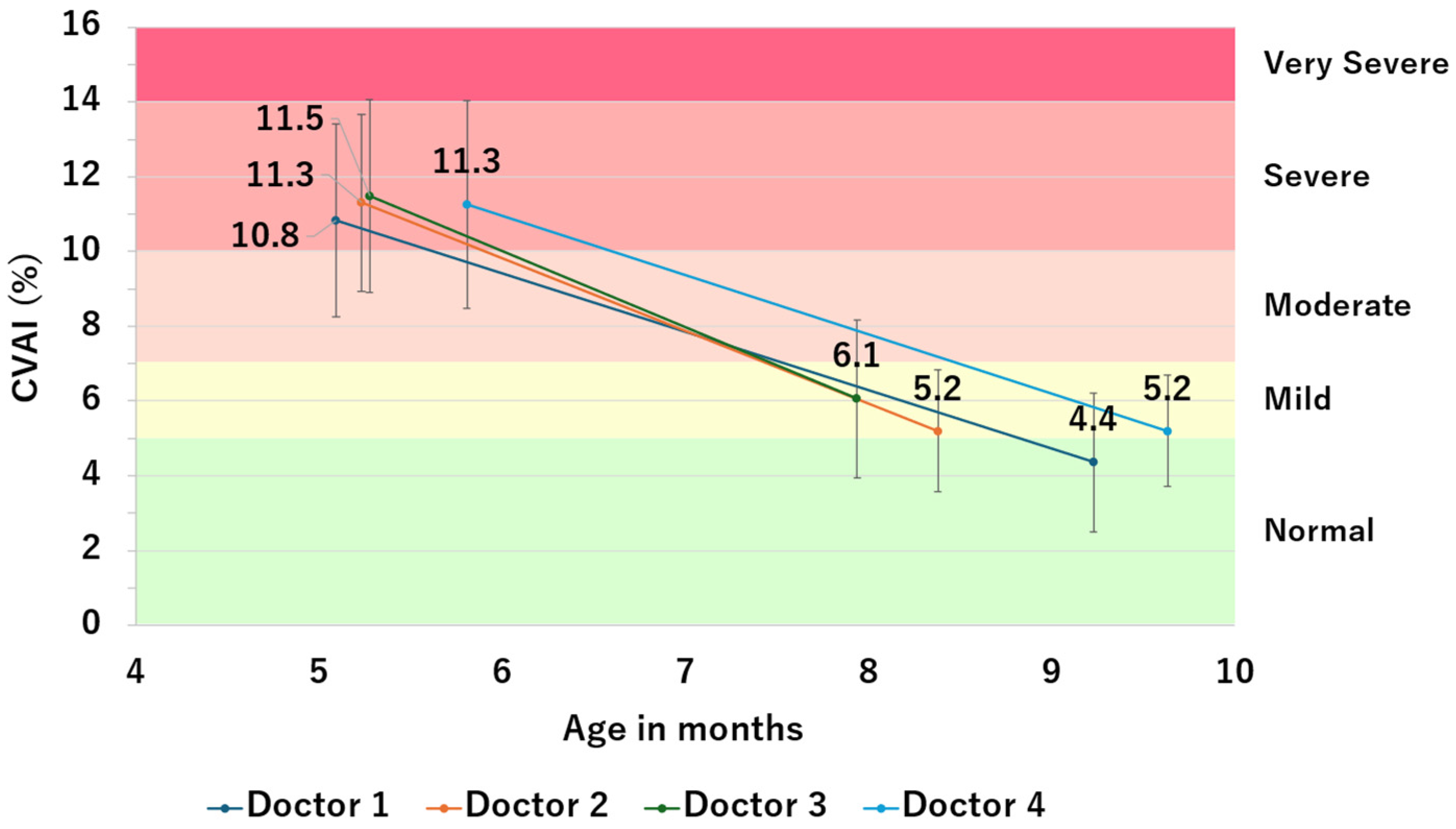
3.2. Study 2: Comparison of CVAI Improvement Values between Attending Physicians
3.3. Study 3: Comparison of Helmet Therapy Efficacy by Age at Start of Therapy (<7 Months vs. ≥7 Months)
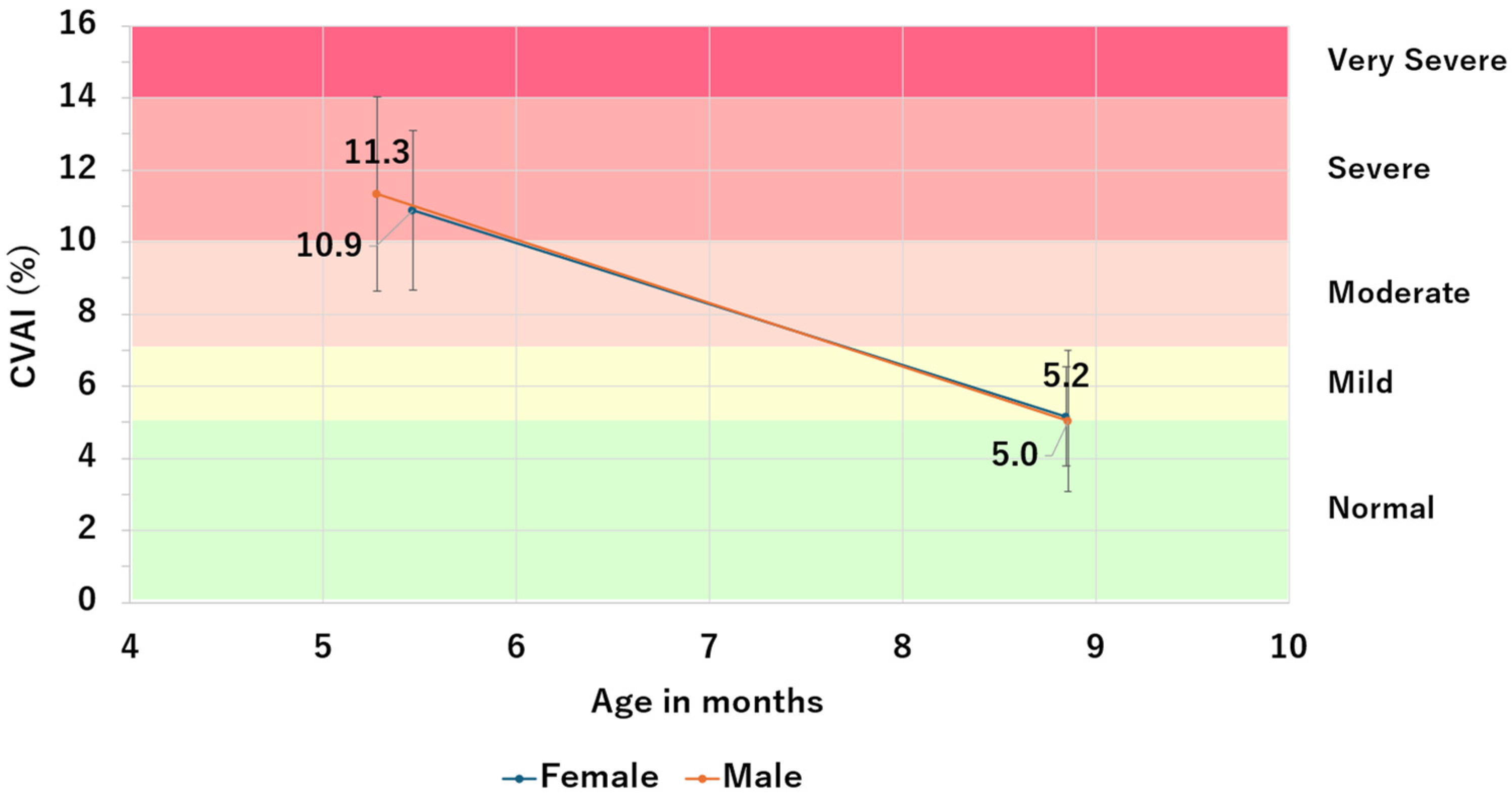
3.4. Study 4: Impact of Sex on CVAI Improvement
3.5. Study 5: Effects of Helmet Therapy on Head Circumference Stunting
3.6. Study 6: Frequency of Dermatitis Due to Helmet Therapy
3.7. Study 7: Examination of Clinical Factors Affecting ΔCVAI
4. Discussion
4.1. Improvement of CVAI
4.2. Treatment Period
4.3. Starting Age of Therapy
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Argenta, L.C.; David, L.R.; Wilson, J.A.; Bell, W.O. An Increase in Infant Cranial Deformity with Supine Sleeping Position. J. Craniofac. Surg. 1996, 7, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.A.; Mitchell, L.E.; Craven, K.P.; Marsh, J.L. Observations on a Recent Increase in Plagiocephaly without Synostosis. Pediatrics 1996, 97, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Branch, L.G.; Kesty, K.; Krebs, E.; Wright, L.; Leger, S.; David, L.R. Deformational plagiocephaly and craniosynostosis: Trends in diagnosis and treatment after the “back to sleep” campaign. J. Craniofac. Surg. 2015, 26, 147–150. [Google Scholar] [CrossRef]
- Noto, T.; Nagano, N.; Kato, R.; Hashimoto, S.; Saito, K.; Miyabayashi, H.; Sasano, M.; Sumi, K.; Yoshino, A.; Morioka, I. Natural-Course Evaluation of Infants with Positional Severe Plagiocephaly Using a Three-Dimensional Scanner in Japan: Comparison with Those Who Received Cranial Helmet Therapy. J. Clin. Med. 2021, 10, 3531. [Google Scholar] [CrossRef] [PubMed]
- Roider, L.; Ungerer, G.; Shock, L.; Aldridge, K.; Al-Samarraie, M.; Tanaka, T.; Muzaffar, A. Increased Incidence of Ophthamologic Findings in Children With Concurrent Isolated Nonsyndromic Metopic Suture Abnormalities and Deformational Cranial Vault Asymmetry. Cleft Palate-Craniofac. J. 2021, 58, 497–504. [Google Scholar] [CrossRef]
- Purzycki, A.; Thompson, E.; Argenta, L.; David, L. Incidence of otitis media in children with deformational plagiocephaly. J. Craniofac. Surg. 2009, 20, 1407–1411. [Google Scholar] [CrossRef]
- Orlando, M.P.; Bonanno, M.A.; Russo, F.Y.; Ralli, M.; Turchetta, R.; Passali, F.M.; Minni, A.; Greco, A.; De Vincentiis, M.; Tattoli, M. Correlation between otitis media with effusion and cranial deformation in children. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 55–59. [Google Scholar]
- Martiniuk, A.L.; Vujovich-Dunn, C.; Park, M.; Yu, W.; Lucas, B.R. Plagiocephaly and Developmental Delay: A Systematic Review. J. Dev. Behav. Pediatr. 2017, 38, 67–78. [Google Scholar] [CrossRef]
- Collett, B.R.; Gray, K.E.; Starr, J.R.; Heike, C.L.; Cunningham, M.L.; Speltz, M.L. Development at age 36 months in children with deformational plagiocephaly. Pediatrics 2013, 131, e109–e115. [Google Scholar] [CrossRef]
- Miller, R.I.; Clarren, S.K. Long-term developmental outcomes in patients with deformational plagiocephaly. Pediatrics 2000, 105, e26. [Google Scholar] [CrossRef]
- Kim, D.H.; Kwon, D.R. Neurodevelopmental delay according to severity of deformational plagiocephaly in children. Medicine 2020, 99, e21194. [Google Scholar] [CrossRef] [PubMed]
- González-Santos, J.; González-Bernal, J.J.; De-la-Fuente Anuncibay, R.; Soto-Cámara, R.; Cubo, E.; Aguilar-Parra, J.M.; Trigueros, R.; López-Liria, R. Infant Cranial Deformity: Cranial Helmet Therapy or Physiotherapy? Int. J. Environ. Res. Public Health 2020, 17, 2612. [Google Scholar] [CrossRef] [PubMed]
- González-Santos, J.; González-Bernal, J.J.; De-la-Fuente Anuncibay, R.; Aguilar-Parra, J.M.; Trigueros, R.; Soto-Cámara, R.; López-Liria, R. A Prospective Study of Cranial Deformity and Delayed Development in Children. Sustainability 2020, 12, 1949. [Google Scholar] [CrossRef]
- Kluba, S.; Roßkopf, F.; Kraut, W.; Peters, J.P.; Calgeer, B.; Reinert, S.; Krimmel, M. Malocclusion in the primary dentition in children with and without deformational plagiocephaly. Clin. Oral Investig. 2016, 20, 2395–2401. [Google Scholar] [CrossRef] [PubMed]
- Rauten, A.M.; Maglaviceanu, C.; Popescu, M.R.; Martu, I.; Popescu, D.; Surlin, P.; Suciu, M.; Bogdan, M. Correlations between craniofacial morphology and dento-maxillary anomalies in a population of children in the South west region of Romania. Curr. Health Sci. J. 2014, 40, 200–204. [Google Scholar]
- Aihara, Y.; Komatsu, K.; Dairoku, H.; Kubo, O.; Hori, T.; Okada, Y. Cranial Molding Helmet Therapy and Establishment of Practical Criteria for Management in Asian Infant Positional Head Deformity. Childs Nerv. Syst. 2014, 30, 1499–1509. [Google Scholar] [CrossRef]
- Takamatsu, A.; Hikosaka, M.; Kaneko, T.; Mikami, M.; Kaneko, A. Evaluation of the Molding Helmet Therapy for Japanese Infants with Deformational Plagiocephaly. JMA J. 2021, 4, 50–60. [Google Scholar]
- Kajita, H.; Tanaka, I.; Komuro, H.; Nishimaki, S.; Kusakawa, I.; Sakamoto, K. Efficacy of Cranial Orthosis for Plagiocephaly Based on 2D and 3D Evaluation. Arch. Plast. Surg. 2024, 51, 169–181. [Google Scholar] [CrossRef]
- Plank, L.H.; Giavedoni, B.; Lombardo, J.R.; Geil, M.D.; Reisner, A. Comparison of infant head shape changes in deformational plagiocephaly following treatment with a cranial remolding orthosis using a noninvasive laser shape digitizer. J. Craniofac. Surg. 2006, 17, 1084–1091. [Google Scholar] [CrossRef]
- Ifflaender, S.; Rüdiger, M.; Koch, A.; Burkhardt, W. Three-dimensional digital capture of head size in neonates—A method evaluation. PLoS ONE 2013, 8, e61274. [Google Scholar] [CrossRef]
- Loveday, B.P.; de Chalain, T.B. Active counterpositioning or orthotic device to treat positional plagiocephaly? J. Craniofac. Surg. 2001, 12, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Miyabayashi, H.; Saito, K.; Kato, R.; Noto, T.; Nagano, N.; Morioka, I. Denominator of Cranial Vault Asymmetry Index: Choosing Between Longer and Shorter Diagonal Lengths. J. Craniofac. Surg. 2023, 4, e369–e372. [Google Scholar] [CrossRef]
- Kunz, F.; Schweitzer, T.; Große, S.; Waßmuth, N.; Stellzig-Eisenhauer, A.; Böhm, H.; Meyer-Marcotty, P.; Linz, C. Head orthosis therapy in positional plagiocephaly: Longitudinal 3D-investigation of long-term outcomes, compared with untreated infants and with a control group. Eur. J. Orthod. 2019, 41, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Holowka, M.A.; Reisner, A.; Giavedoni, B.; Lombardo, J.R.; Coulter, C. Plagiocephaly Severity Scale to Aid in Clinical Treatment Recommendations. J. Craniofac. Surg. 2017, 28, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Argenta, L.; David, L.; Thompson, J. Clinical classification of positional plagiocephaly. J. Craniofac. Surg. 2004, 15, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Takimoto, H.; Yokoyama, T. Results of the 2010 Infant and Toddler Physical Growth Survey in Japan. J. Child Health 2012, 71, 671–680. (In Japanese) [Google Scholar]
- Couture, D.E.; Crantford, J.C.; Somasundaram, A.; Sanger, C.; Argenta, A.E.; David, L.R. Efficacy of passive helmet therapy for deformational plagiocephaly: Report of 1050 cases. Neurosurg. Focus 2013, 35, E4. [Google Scholar] [CrossRef]
- Hallac, R.R.; Ajiwe, T.; Effendi, M.; Seaward, J.R.; Kane, A.A. Molding Helmet Therapy for Deformational Brachycephaly. J. Craniofac. Surg. 2019, 30, 1756–1759. [Google Scholar] [CrossRef]
- Goh, J.L.; Bauer, D.F.; Durham, S.R.; Stotland, M.A. Orthotic (helmet) therapy in the treatment of plagiocephaly. Neurosurg. Focus 2013, 35, E2. [Google Scholar] [CrossRef]
- Lin, R.S.; Stevens, P.M.; Wininger, M.; Castiglione, C.L. Orthotic Management of Deformational Plagiocephaly: Consensus Clinical Standards of Care. Cleft Palate-Craniofac. J. 2016, 53, 394–403. [Google Scholar] [CrossRef]
- Di Chiara, A.; La Rosa, E.; Ramieri, V.; Vellone, V.; Cascone, P. Treatment of Deformational Plagiocephaly with Physiotherapy. J. Craniofac. Surg. 2019, 30, 2008–2013. [Google Scholar] [CrossRef] [PubMed]


| Moderate | Severe | Very Severe | Total | |
|---|---|---|---|---|
| Hospital 1 | 23 | 31 | 4 | 58 |
| Hospital 2 | 27 | 46 | 12 | 85 |
| Hospital 3 | 10 | 12 | 4 | 26 |
| Hospital 4 | 23 | 23 | 9 | 55 |
| Total | 83 | 112 | 29 | 224 |
| Severity | Number of Patients | Pre-Therapy CVAI | Post-Therapy CVAI | ΔCVAI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Variance | Mean | SD | Variance | Mean | SD | Variance | ||
| Moderate | 83 | 8.7 | 0.7 | 0.5 | 4.2 | 1.4 | 1.9 | −4.4 | 1.4 | 1.9 |
| Severe | 112 | 11.9 | 1.1 | 1.2 | 5.3 | 1.6 | 3.2 | −6.6 | 1.8 | 3.2 |
| Very Severe | 29 | 15.7 | 1.8 | 3.1 | 6.6 | 1.9 | 5.4 | −9.1 | 2.3 | 5.4 |
| Total | 224 | 11.2 | 2.5 | 6.5 | 5.1 | 1.8 | 5.3 | −6.1 | 2.3 | 5.3 |
| Severity | Number of Patients | Age at Therapy Initiation | Age at Therapy Completion | Therapy Duration (Months) | p-Value |
|---|---|---|---|---|---|
| Moderate | 83 | 5.6 ± 1.4 | 9.1 ± 1.9 | 3.5 ± 1.3 | 0.028 |
| Severe | 112 | 5.3 ± 1.2 | 8.7 ± 1.8 | 3.4 ± 1.0 | |
| Very Severe | 29 | 4.9 ± 0.8 | 8.8 ± 1.4 | 4.0 ± 0.9 | |
| Total | 224 | 5.3 ± 1.3 | 8.9 ± 1.8 | 3.5 ± 1.1 |
| After Therapy | |||||||
|---|---|---|---|---|---|---|---|
| Normal | Mild | Moderate | Severe | Very Severe | Total | ||
| Before Therapy | Moderate | 55 | 28 | 0 | 0 | 0 | 83 |
| Severe | 48 | 49 | 15 | 0 | 0 | 112 | |
| Very Severe | 6 | 11 | 11 | 1 | 0 | 29 | |
| Total | 109 | 88 | 26 | 1 | 0 | 224 | |
| Number of Patients | Age at Therapy Initiation | Age at Therapy Completion | Therapy Duration (Months) | p-Value | |
|---|---|---|---|---|---|
| Doctor 1 | 58 | 5.1 ± 1.1 | 9.2 ± 1.9 | 4.1 ± 1.4 | <0.001 |
| Doctor 2 | 85 | 5.2 ± 1.2 | 8.4 ± 1.6 | 3.1 ± 0.8 | |
| Doctor 3 | 26 | 5.3 ± 1.3 | 7.9 ± 1.6 | 2.7 ± 0.9 | |
| Doctor 4 | 55 | 5.8 ± 1.3 | 9.6 ± 1.6 | 3.8 ± 0.9 | |
| Total | 224 | 5.3 ± 1.3 | 8.9 ± 1.8 | 3.5 ± 1.1 |
| Number of Patients | Pre-Therapy CVAI | Post-Therapy CVAI | ΔCVAI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Variance | Mean | SD | Variance | Mean | SD | Variance | ||
| Doctor 1 | 58 | 10.8 | 2.6 | 6.7 | 4.4 | 1.8 | 7.4 | −6.5 | 2.7 | 7.4 |
| Doctor 2 | 85 | 11.3 | 2.4 | 5.6 | 5.2 | 1.6 | 4.5 | −6.1 | 2.1 | 4.5 |
| Doctor 3 | 26 | 11.5 | 2.6 | 6.6 | 6.1 | 2.1 | 3.5 | −5.4 | 1.9 | 3.5 |
| Doctor 4 | 55 | 11.3 | 2.8 | 7.7 | 5.2 | 1.5 | 5.0 | −6.1 | 2.2 | 5.0 |
| Total | 224 | 11.2 | 2.5 | 6.5 | 5.1 | 1.8 | 5.3 | −6.1 | 2.3 | 5.3 |
| Age at Therapy Initiation | Number of Patients | Pre-Therapy CVAI | Post-Therapy CVAI | ΔCVAI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Variance | Mean | SD | Variance | Mean | SD | Variance | ||
| Under 7 months | 202 | 11.3 | 2.6 | 6.7 | 5.0 | 1.8 | 5.2 | −6.3 | 2.3 | 5.2 |
| 7 months or older | 22 | 10.2 | 1.8 | 3.1 | 5.8 | 1.2 | 2.6 | −4.4 | 1.6 | 2.6 |
| Total | 224 | 11.2 | 2.5 | 6.5 | 5.1 | 1.8 | 5.3 | −6.1 | 2.3 | 5.3 |
| Age at Therapy Initiation | Number of Patients | Age at Therapy Initiation | Age at Therapy Completion | Therapy Duration (Months) | p-Value |
|---|---|---|---|---|---|
| Under 7 months | 202 | 5.0 ± 0.9 | 8.5 ± 1.5 | 3.5 ± 1.2 | 0.043 |
| 7 months or older | 22 | 8.1 ± 0.9 | 12.0 ± 1.2 | 3.9 ± 0.9 | |
| Total | 224 | 5.3 ± 1.3 | 8.9 ± 1.8 | 3.5 ± 1.1 |
| Sex | Number of patients | Pre-Therapy CVAI | Post-Therapy CVAI | ΔCVAI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Variance | Mean | SD | Variance | Mean | SD | Variance | ||
| Female | 76 | 10.9 | 2.2 | 4.9 | 5.2 | 1.4 | 4.1 | −5.7 | 2.0 | 4.1 |
| Male | 148 | 11.3 | 2.7 | 7.3 | 5.0 | 2.0 | 5.8 | −6.3 | 2.4 | 5.8 |
| Total | 224 | 11.2 | 2.5 | 6.5 | 5.1 | 1.8 | 5.3 | −6.1 | 2.3 | 5.3 |
| Sex | Number of Patients | Age at Therapy Initiation | Age at Therapy Completion | Therapy Duration (Months) | p-Value |
|---|---|---|---|---|---|
| Female | 76 | 5.5 ± 1.2 | 8.9 ± 1.7 | 3.4 ± 1.1 | 0.343 |
| Male | 148 | 5.3 ± 1.3 | 8.9 ± 1.8 | 3.6 ± 1.2 | |
| Total | 224 | 5.3 ± 1.3 | 8.9 ± 1.8 | 3.5 ± 1.1 |
| Factor | Logarithmic Worth (95% Confidence Interval) | p-Value |
|---|---|---|
| Age at treatment initiation | 8.117 (0.48–0.94) | <0.001 |
| Sex | 0.743 (−0.50–0.10) | 0.181 |
| Treatment duration | 0.661 (−0.45–0.10) | 0.218 |
| Doctor | 0.270 (−0.70–0.37) | 0.537 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagano, N.; Kato, R.; Noto, T.; Hijikata, M.; Okahashi, A.; Nakanomori, A.; Miyabayashi, H.; Yoshikawa, K.; Ichiwata, N.; Saito, H.; et al. Therapeutic Effectiveness of a Novel Cranial Remolding Helmet (baby band2) for Positional Plagiocephaly: A Multicenter Clinical Observational Study. J. Clin. Med. 2024, 13, 5952. https://doi.org/10.3390/jcm13195952
Nagano N, Kato R, Noto T, Hijikata M, Okahashi A, Nakanomori A, Miyabayashi H, Yoshikawa K, Ichiwata N, Saito H, et al. Therapeutic Effectiveness of a Novel Cranial Remolding Helmet (baby band2) for Positional Plagiocephaly: A Multicenter Clinical Observational Study. Journal of Clinical Medicine. 2024; 13(19):5952. https://doi.org/10.3390/jcm13195952
Chicago/Turabian StyleNagano, Nobuhiko, Risa Kato, Takanori Noto, Midori Hijikata, Aya Okahashi, Aya Nakanomori, Hiroshi Miyabayashi, Kayo Yoshikawa, Nobutaka Ichiwata, Hiroshi Saito, and et al. 2024. "Therapeutic Effectiveness of a Novel Cranial Remolding Helmet (baby band2) for Positional Plagiocephaly: A Multicenter Clinical Observational Study" Journal of Clinical Medicine 13, no. 19: 5952. https://doi.org/10.3390/jcm13195952
APA StyleNagano, N., Kato, R., Noto, T., Hijikata, M., Okahashi, A., Nakanomori, A., Miyabayashi, H., Yoshikawa, K., Ichiwata, N., Saito, H., Sasano, M., Sumi, K., & Morioka, I. (2024). Therapeutic Effectiveness of a Novel Cranial Remolding Helmet (baby band2) for Positional Plagiocephaly: A Multicenter Clinical Observational Study. Journal of Clinical Medicine, 13(19), 5952. https://doi.org/10.3390/jcm13195952







