Abstract
Background: The coronary artery calcium score and left atrial volume have been shown to predict the incidence of acute myocardial infarction and death from cardiovascular disease in patients undergoing peritoneal dialysis. However, the association between these factors has not been well-established. Methods: This cross-sectional, prospective, single-center study was conducted on patients undergoing outpatient peritoneal dialysis, who were followed up at a university hospital between March 2018 and August 2019. The coronary artery calcium score was calculated based on cardiovascular computed tomography findings. The score was “positive” when it was ≥100 Agatston and “negative” when it was <100 Agatston. The left atrial volume was obtained using the biplane disc method at the end of the left ventricular systole, and then it was indexed to the body surface. Results: Forty-four patients were evaluated. They had an age [mean (range)] of 56 (43–65) years and had been on dialysis therapy for 11.7 (6.8–25.4) months. Univariate analysis revealed a relationship between the coronary artery calcium score and left atrial volume index and the following variables: age, diabetes, overhydration, pulse wave velocity, E/A ratio, and left ventricular mass index. In multivariate logistic regression analysis, only the left atrial volume index was independently associated with a positive coronary artery calcium score. Conclusions: The left atrial volume index was associated with a positive coronary artery calcium score in patients on peritoneal dialysis, regardless of other factors. It may be a useful risk marker for coronary artery disease in this population.
1. Introduction
Chronic kidney disease (CKD) increases vascular calcification because of the accelerated progression of atherosclerosis and changes in calcium homeostasis [1]. Furthermore, clinical factors such as advanced age, vitamin D therapy, dialysis time, cellular and inflammatory processes, and active osteogenesis in the vascular bed have an important role in this process [2,3,4,5].
Coronary artery disease (CAD) is the major cause of morbidity and mortality in patients with end-stage CKD. These patients are generally asymptomatic until acute myocardial infarction or sudden cardiac death occurs [6]. Coronary calcification, assessed via the coronary artery calcium (CAC) score, which is measured with multidetector computed tomography (CT), is a marker of the atherosclerotic plaque burden, and is useful for predicting the incidence of acute myocardial infarction and death from cardiovascular disease.
In median-aged men without CKD, the diameter of the left atrium has been correlated with the severity of CAD and cardiovascular mortality, with the adjusted risk being 1.2 times for each 5 mm increase in the diameter of the left atrium [7]. Three-vessel CAD is more common than single-vessel CAD in patients with a large left atrium [8]. In patients with dialysis CKD, the left atrial volume (LAV), measured via transthoracic Doppler echocardiography, is independently associated with the presence of silent ischemia [9]. In 2007, researchers in one study [10] evaluated 249 patients on dialysis therapy, of whom 50 patients were on peritoneal dialysis (PD), and demonstrated that a progressive increase in the LAV was a predictor of cardiovascular events in this population.
In the general population, LAV is associated with an elevated CAC score [11], which is a useful marker of advanced CAD [8]. This fact may be mediated by direct ischemia or infarction of the atrium, or by pressure and volume overload secondary to ischemia, and infarction of the left ventricle. Some studies [12,13] have demonstrated that remodeling of the left atrium in patients with stable CAD is predictive of cardiovascular events. In 2017, a study [14] that evaluated patients undergoing hemodialysis revealed an association between the LAV and CAC.
In this context, little evidence exists in the literature regarding the correlation between the LAV and the CAC score in patients with CKD undergoing dialysis, especially the peritoneal modality, and Doppler echocardiography is more accessible than computed tomography in most dialysis services. Thus, the aim of this study was to evaluate this association so that the left atrial volume can emerge as a risk marker for CAD in this population.
2. Methods
2.1. Study Design
This cross-sectional study included patients with CKD who underwent outpatient PD between March 2018 and August 2019 at the Botucatu Medical School University Hospital, Sao Paulo State University, UNESP; Botucatu, Brazil.
Patients with prevalent PD aged between 18 years and 75 years were included in the study. Individuals who had active or recent infections (<7 days), autoimmune disease, malignant neoplasm, unstable heart disease (e.g., acute coronary syndrome, decompensated heart failure, unstable arrhythmias), previous coronary artery disease, severe left ventricular systolic dysfunction, pericardial effusion, significant mitral or aortic valve disease, primary hyperparathyroidism, calcium supplementation, or who used calcium-based phosphorus binders were not included in this study.
Demographic and clinical data were recorded. The following complementary examinations were conducted with a maximum interval of 2 weeks: biochemical examinations, assessment of nutritional status via anthropometry and bioimpedance bioelectrical impedance analysis (BIA), calculation of residual renal function and dialysis adequacy, Doppler echocardiography, carotid ultrasound, pulse wave velocity (PWV), ankle–brachial index, 24-h ambulatory blood pressure monitoring, and CAC score.
2.2. Coronary Artery Calcium Score
CAC scores were calculated using cardiovascular computed tomography (multislice 64-channel Optima scanner; GE Medical Systems, Waukesha, WI, USA). Calcification was defined as a hyperattenuating lesion above a threshold of 130 Hounsfield units (HU) in an area of two or more adjacent pixels observed in the coronary path. The product of the total calcium area and a factor derived from the maximum attenuation (i.e., maximal computed tomographic number) was the calcium score, as described by Agatston et al. [4]; the unit bears his name. The sensitivity and specificity reported in detecting this score are 98.7% and 100%, respectively [15]. The images, including their quality and precision, were analyzed by a single examiner who specialized in cardiovascular tomography and was “blind” to the clinical and laboratory information and echocardiography of the patient. The CAC score was “positive” when it was ≥100 Agatston and “negative” when it was <100 Agatston.
2.3. Doppler Echocardiographic Evaluation
The Doppler echocardiogram examinations were conducted by a single expert examiner in the field (who was “blind” to the patient‘s clinical, laboratory, and tomographic information) by using an ultrasound scanner (model HD 15; Philips, Amsterdam, The Netherlands) equipped with a 2.0–5.0 MHz multifrequency ultrasonic transducer and image registration system. Images were obtained and analyzed based on the recommendations of the American Society of Echocardiography [16].
Left ventricular mass (LVM) was calculated using the Devereux [17] formula, and it was indexed to the body surface. The relative thickness of the ventricular wall (2 × posterior wall diastolic thickness/left ventricular [LV] diastolic diameter) was measured to analyze LV geometry based on the description by Ganau et al. [18].
The E and A waves (maximum velocity of rapid and late ventricular filling during atrial contraction, respectively) were obtained via Doppler spectral recording of the transmitral diastolic flow. The measurement of e′ was obtained using spectral tissue Doppler recording of the movement of the middle portion of the mitral annulus. Based on the flow profile and movement of the mitral annulus, the E/A and E/e′ ratios were calculated as markers of LV diastolic function. The LAV was obtained using the biplane disc method, at the end of LV systole. For the analysis, this measurement was indexed to the body surface [19].
2.4. Statistical Analysis
A sample size of 47 patients was calculated based on the method described by Baloglu et al. [14], with a correlation coefficient of 0.4, a beta error of 0.2, and an alpha error of 0.05. The included patients were divided into two groups, based on their CAC score, as follows: they were put in the positive group if the score was ≥100 Agatston and the negative group if the score was <100 Agatston. The association between the CAC score and the LAV was evaluated, along with the presence of other CAC markers.
Statistical analyses were conducted using SPSS (version 23.0; SPSS Inc., Chicago, IL, USA). Data were expressed as the frequency, the mean ± standard deviation, or the median (interquartile range), as appropriate. Statistical comparisons between study groups (i.e., between positive and negative calcium scores) were conducted by using the Student’s t-test for continuous variables with a normal distribution, the Mann–Whitney test for variables with a non-normal distribution, and the chi-square test for categorical variables. Associations were assessed between the study variables via univariate and multivariate logistic regression models. The positive and negative predictive values, sensitivity, specificity, and accuracy between the LAV and CAC scores were analyzed using the receiver operating characteristic (ROC) curve, with calculation of the area under the ROC curve (AUC). The significance level was set at p < 0.05.
2.5. Ethical Aspects
This study was approved by the Research Ethics Committee of Botucatu Medical School, São Paulo State University, UNESP (approval no. CAAE 80051517.1.0000.5411). All individuals included in the study signed an informed consent form. All clinical investigation has been conducted according to the principles expressed in the Declaration of Helsinki.
3. Results
Of the 76 patients who underwent PD during the inclusion period, 61 patients were eligible. Of these, seven patients refused to participate in the study. Fifty-four patients were included; however, four patients withdrew their consent during the study, two patients were excluded because of previous myocardial revascularization, three patients withdrew because of significant aortic valve disease, and one patient withdrew because of an ejection fraction <30% on Doppler echocardiography. Therefore, 44 patients were included in the analysis (Figure 1).
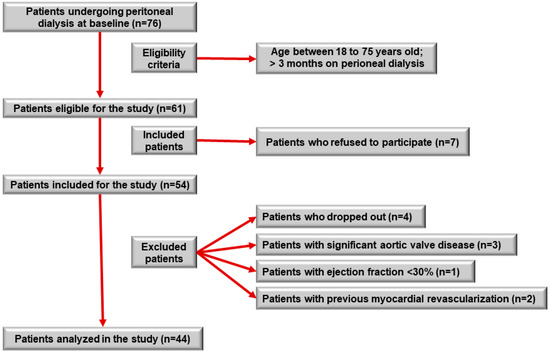
Figure 1.
Study participant selection flowchart.
The median age of the patients was 56 years; most patients were male, white, and had low educational levels (i.e., ≤9 years). Most patients were hypertensive and dyslipidemic, with nearly one-half of them having diabetes. Of the patients investigated, 38.6% (17) of patients tested positive for CAC. Compared to patients who tested negative for CAC, patients who tested positive were older (52 years vs. 60 years, p = 0.017) and had a higher incidence of diabetes (22.2% vs. 70.6%, p = 0.001) (Table 1).

Table 1.
Demographic and clinical characteristics of the study participants.
Nearly one-third (31.8%) of all patients had hypertension as the underlying disease of CKD, followed by diabetes (27.3%). The median duration of dialysis therapy was 11.7 months, and the most frequent treatment modality was nocturnal intermittent peritoneal dialysis (NIPD) (54.5%). The groups testing positive and negative for CAC were similar in regard to these variables (Table 2).

Table 2.
Dialysis data of the study participants.
In the BIA evaluation, compared to patients who were negative for CAC, patients who were positive for CAC presented with lower mean values for the phase angle (6.5° ± 0.8° vs. 5.5° ± 0.8°, p ≤ 0.001), reactance (60.4 ± 10.9Xc vs. 48.6 ± 8.7Xc, p ≤ 0.001), and intracellular water (54.5% ± 3.3% vs. 51.1% ± 3.2%, p = 0.002); as well as a higher median extracellular water value (45.2% vs. 48.8%, p = 0.002); and a higher mean hyperhydration (OH) index value (0.0 ± 1.4l vs. 1.3 ± 1.2l, p = 0.002). The other analyzed variables were similar between the groups (Table 3).

Table 3.
Bioimpedance variables among the study participants.
Analysis of the laboratory tests revealed that the positive CAC group, compared to the negative CAC group, had a lower HDL value (32.0 mg/dL vs. 39.5 mg/dL, p = 0.01), a lower uric acid value (5.6 ± 1.0 mg/dL vs. 6.5 ± 1.5 mg/dL, p = 0.038), a higher glycated hemoglobin value (6.2% vs. 5.1%, p = 0.010), and a higher alkaline phosphatase value (76.0 U/L vs. 70.5 U/L, p = 0.049) (Table 4).

Table 4.
Laboratory test results of the study participants.
Analysis of the subclinical markers of atherosclerosis revealed that the positive and negative CAC groups were significantly different for femoral PWV (11.4 m/s vs. 8.9 m/s, p = 0.025), intima-media thickness of the right carotid artery (0.7 mm vs. 0.6 mm, p = 0.037) and left carotid artery (0.7 mm vs. 0.7 mm, p = 0.034), and the absence of atherosclerotic plaque in the left carotid artery (46.2% vs. 71.4%, p = 0.005) (Table 5).

Table 5.
Subclinical markers of atherosclerosis among the study participants.
When comparing the positive CAC and negative CAC groups in relation to ambulatory blood pressure monitoring data, a significant difference was observed only in the nocturnal decrease in systolic pressure (0.4% ± 6.4% vs. 6.0% ± 8.3%, p = 0.031) (Table 6).

Table 6.
Ambulatory blood pressure monitoring data of the study participants.
Analysis of the echocardiographic morphological variables revealed that, compared to the negative CAC group, the positive CAC group had greater values for the thickness of the posterior wall, interventricular septum, and relative wall; as well as the left ventricular mass index (LVMI); the anteroposterior diameter of the left atrium; and the basal diameter of the right ventricle. Among the variables used to evaluate systolic function, only the tricuspid annular plane systolic excursion differed between the groups with mean values, being lower for patients testing positive for CAC than for patients testing negative for CAC (22.6 ± 1.8 mm vs. 24.6 ± 2.0 mm, p = 0.002). With regard to diastolic function variables, patients testing positive for CAC, compared to patients testing negative for CAC, had a higher median left atrial volume index (LAVi), based on body surface area (36.1 mL/m2 vs. 28.7 mL/m2, p < 0.001), with lower values for the E wave, A wave, E/A ratio, and e′ septal, e′ lateral, e′ medium, and e′ t waves (Table 7).

Table 7.
Doppler echocardiographic variables of the study participants.
Multiple logistic regression was conducted with the predictor variables for positive CAC in the univariate analysis (i.e., age, diabetes, OH index, femoral PWV, LVMI, and E/A ratio), including LAVi. In this evaluation, only the LAVi remained an independent predictor of positive CAC among the patients studied (Table 8).

Table 8.
Multivariate logistic regression with variables predictive of CAC positivity among the study patients.
In the analysis of the ROC curve for diagnosing positive CAC, based on the LAVi, the best cutoff point was ≥34.4 mL/m2. This value had a sensitivity of 76.5% and a specificity of 96.3%, with a positive predictive value of 92.8% and negative predictive value of 86.6% (Figure 2).
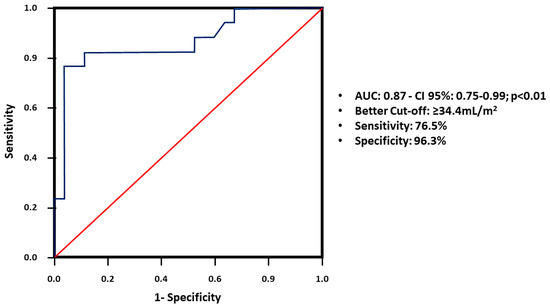
Figure 2.
ROC curve for diagnosing positive CAC, based on the LAVi. Abbreviations: ROC, receiver operating characteristic; CAC, coronary artery calcium; LAVi, left atrial volume index; AUC, area under the ROC curve.
4. Discussion
The aim of this study was to evaluate this association so that the left atrial volume can emerge as a risk marker for CAD in this population. This study demonstrated an independent association between the LAVi and CAC in patients with PD, even after adjusting for the following variables: age, diabetes, OH index, femoral PWV, LVMI, and E/A ratio.
In the general population, several clinical studies have demonstrated that the size of the left atrium, measured with Doppler echocardiography, is correlated with coronary atherosclerosis [8,14]. In one study, carried out with 100 patients with CAD who underwent coronary cineangiography and Doppler echocardiography, the authors observed that three-vessel coronary disease was much more frequent among individuals who had larger left atria [8]. Laukkanen et al. [7] found a direct and linear relationship between the diameter of the left atrium and the risk of cardiovascular death. They observed that, for each 5 mm increase in diameter of the left atrium, the adjusted risk for cardiovascular death increased by 1.23 times, and that, when the size of the left atrium was greater than 43 mm, patients had a 2.34 times greater risk of cardiovascular death than patients with a normal-sized atrium.
A study by Pan et al. [11], who evaluated 166 patients from the general population who underwent computed tomography of the heart, demonstrated that individuals with higher CAC values (>400 Agatston) had a larger diameter of the left atrium [11]. Baloglu et al. [14], on evaluating 32 patients on hemodialysis, recently found a significant relationship between the size of the left atrium and CAC. The present study demonstrated the same association in patients on PD.
Several pathophysiological links exist between LAV and CAD [20]. First, oxidative stress and endothelial dysfunction [21], which are more pronounced in patients with dialysis kidney disease, are associated with atherosclerosis and can predispose patients to arterial stiffness, thereby causing LV diastolic dysfunction and consequent left atrium (LA) enlargement. Second, the LA is strongly associated with left ventricular hypertrophy [19], a common condition in patients with renal failure, and this fact is associated with abnormal myocardial perfusion [22]. Yameogo et al. [23] found that myocardial ischemia is significantly more frequent in patients with a dilated LA, with increased filling pressure and left ventricular mass. Third, overhydration, which can be measured with the OH index in patients on PD, results in pathological mechanical stimuli in the vascular endothelium and smooth muscle cells, with elevated superoxide production and reduced nitric oxide bioavailability, thereby predisposing patients to atherosclerosis and vascular calcification [10]. Extracellular volume overload also directly contributes to the increase in the LA size.
In this study, when the LAVi was greater than 34.4 mL/m2, predicting CAC positivity was possible in 92.8% of cases with a sensitivity of 76.5%, specificity of 96.3%, and accuracy of 88.6%. The LAVi showed a correlation with CAC (>100 Agatston), regardless of diastolic dysfunction, left ventricular mass, arterial stiffness, and fluid overload. This finding indicated that other mechanisms, which could be the subject of future studies, may be involved in this relationship. Therefore, the LAVi can be useful in clinical practice as a marker of CAD risk in patients with PD, regardless of the known traditional risk factors. For example, in patients with few or no traditional risk factors for CAD, but with an enlarged left atrium, we should have more intensive control of comorbidities and greater suspicion regarding the diagnosis of CAD, considering that they have a high risk of significant coronary calcification.
The strengths of this study include the large number of variables analyzed and its prospective design. However, this study had limitations such as the small sample size, although the number of patients was sufficient to identify statistically significant associations. Another limitation is that the study was conducted at a single center; in the future, we intend to subsequently expand our sample size and the external validity of the study in a multicenter study. Furthermore, the cross-sectional observational study design did not allow the establishment of a causal relationship between the LAVi and CAC variables.
These results confirmed the hypothesis that the LAVi, measured with Doppler echocardiography, was associated with a positive CAC score, measured via computed tomography among patients with chronic kidney disease undergoing PD. Therefore, considering that Doppler echocardiography is a more accessible and noninvasive test performed routinely for patients on PD in many services, using the LAVi as a marker of risk for coronary artery disease is feasible for this population.
Author Contributions
F.M.R.: conceptualization; methodology; formal analysis; investigation; study design; data collection; data analysis and interpretation; writing—original draft. E.B.F.: literature search; data collection; data analysis and interpretation; writing—original draft. N.S.d.C.R.: literature search; data analysis and interpretation; writing—original draft. F.L.C.: literature search; data collection; data analysis and interpretation; writing—original draft. P.N.M.: literature search; data collection; data analysis and interpretation; writing—original draft. A.D.C.V.M.: literature search; data collection; data analysis and interpretation; writing—original draft. J.C.H.: literature search; data analysis and interpretation; writing—original draft. R.B.: literature search; data analysis and interpretation; writing—review and editing. V.B.B.: literature search; data analysis and interpretation; writing—review and editing. P.B.: literature search; data analysis and interpretation; writing—review and editing. L.C.M.: conceptualization; methodology; formal analysis; study design; data analysis and interpretation; writing—review and editing. S.G.Z.B.: conceptualization; methodology; formal analysis; study design; data analysis and interpretation; supervision; funding acquisition; writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CNPq (Proc. n. 311272/2022-3) and FAPESP (Proc. n. 2019/05912-4).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Research Ethics Committee of Botucatu Medical School, São Paulo State University – UNESP (approval n° CAAE 80051517.1.0000.5411, 7 December 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: http://hdl.handle.net/11449/215612 (accessed on 24 August 2024).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kurabayashi, M. Bone and calcium update; diagnosis and therapy of bone metabolism disease update. Calcification of atherosclerotic plaques: Mechanism and clinical significance. Clin. Calcium. 2011, 21, 43–50. [Google Scholar]
- Dellegrottaglie, S.; Sanz, J.; Rajagopalan, S. molecular determinants of vascular calcification: A bench to bedside view. Curr. Mol. Med. 2006, 6, 515–524. [Google Scholar] [CrossRef]
- Haydar, A.A.; Hujairi, N.M.A.; Covic, A.A.; Pereira, D.; Rubens, M.; Goldsmith, D.J.A. Coronary artery calcification is related to coronary atherosclerosis in chronic renal disease patients: A study comparing EBCT-generated coronary artery calcium scores and coronary angiography. Nephrol. Dial. Transplant. 2004, 19, 2307–2312. [Google Scholar] [CrossRef][Green Version]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Turkmen, K.; Kayikcioglu, H.; Ozbek, O.; Solak, Y.; Kayrak, M.; Samur, C.; Anil, M.; Tonbul, H.Z. The relationship between epicardial adipose tissue and malnutrition, inflammation, atherosclerosis/calcification syndrome in ESRD patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 1920–1925. [Google Scholar] [CrossRef]
- Kim, J.-K.; Kim, S.G.; Kim, H.J.; Song, Y.R. Cardiac risk assessment by gated single-photon emission computed tomography in asymptomatic end-stage renal disease patients at the start of dialysis. J. Nucl. Cardiol. 2012, 19, 438–447. [Google Scholar] [CrossRef]
- Laukkanen, J.A.; Kurl, S.; Eränen, J.; Huttunen, M.; Salonen, J.T. Left atrium size and the risk of cardiovascular death in middle-aged men. Arch. Intern. Med. 2005, 165, 1788–1793. [Google Scholar] [CrossRef]
- Hamby, R.I.; Zeldis, S.M.; Hoffman, I.; Sarli, P. Left atrial size and left ventricular function in coronary artery disease: An echocardiographic-angiographic correlative study. Catheter. Cardiovasc. Diagn. 1982, 8, 173–183. [Google Scholar] [CrossRef]
- Choi, M.J.; Kim, J.-K.; Kim, S.G.; Yoon, J.W.; Koo, J.R.; Kim, H.J.; Song, Y.R. Left atrial volume index is a predictor of silent myocardial ischemia in high-risk patients with end-stage renal disease. Int. J. Cardiovasc. Imaging 2013, 29, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, C.J.; Jhee, J.H.; Yun, H.-R.; Kim, H.; Jung, S.; Kee, Y.K.; Yoon, C.; Park, J.T.; Kim, H.C.; et al. Extracellular fluid excess is significantly associated with coronary artery calcification in patients with chronic kidney disease. J. Am. Heart Assoc. 2018, 7, e008935. [Google Scholar] [CrossRef] [PubMed]
- Pan, N.H.; Tsao, H.M.; Chang, N.C.; Lee, C.M.; Chen, Y.J.; Chen, S.A. Dilated left atrium and pulmonary veins in patients with calcified coronary artery: A potential contributor to the genesis of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2009, 20, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; Welles, C.C.; Azarbal, F.; Whooley, M.A.; Schiller, N.B.; Turakhia, M.P. Relation of left atrial dysfunction to ischemic stroke in patients with coronary heart disease (from the Heart and Soul Study). Am. J. Cardiol. 2014, 113, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Welles, C.C.; Ku, I.A.; Kwan, D.M.; Whooley, M.A.; Schiller, N.B.; Turakhia, M.P. Left atrial function predicts heart failure hospitalization in subjects with preserved ejection fraction and coronary heart disease: Longitudinal data from the heart and soul study. J. Am. Coll. Cardiol. 2012, 59, 673–680. [Google Scholar] [CrossRef]
- Baloglu, I.; Turkmen, K.; Tonbul, H.Z.; Selcuk, N.Y. The relationship between coronary artery calcium scores and left atrium size in hemodialysis patients. Int. Urol. Nephrol. 2017, 49, 1661–1666. [Google Scholar] [CrossRef]
- Horiguchi, J.; Yamamoto, H.; Akiyama, Y.; Marukawa, K.; Hirai, N.; Ito, K. Coronary artery calcium scoring using 16-MDCT and a retrospective ECG-gating reconstruction algorithm. AJR Am. J. Roentgenol. 2004, 183, 103–108. [Google Scholar] [CrossRef]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: Recommendations from the American society of echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef]
- de Simone, G.; Devereux, R.B.; Daniels, S.R.; Koren, M.J.; Meyer, R.A.; Laragh, J.H. Effect of growth on variability of left ventricular mass: Assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J. Am. Coll. Cardiol. 1995, 25, 1056–1062. [Google Scholar] [CrossRef]
- Ganau, A.; Devereux, R.B.; Pickering, T.G.; Roman, M.J.; Schnall, P.L.; Santucci, S.; Spitzer, M.C.; Laragh, J.H. Relation of left ventricular hemodynamic load and contractile performance to left ventricular mass in hypertension. Circulation 1990, 81, 25–36. [Google Scholar] [CrossRef]
- Lester, S.J.; Ryan, E.W.; Schiller, N.B.; Foster, E. Best method in clinical practice and in research studies to determine left atrial size. Am. J. Cardiol. 1999, 84, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Møller, J.E.; Hillis, G.S.; Oh, J.K.; Seward, J.B.; Reeder, G.S.; Wright, R.S.; Park, S.W.; Bailey, K.R.; Pellikka, P.A. Left Atrial volume: A powerful predictor of survival after acute myocardial infarction. Circulation 2003, 107, 2207–2212. [Google Scholar] [CrossRef]
- Zapolski, T.; Wysokiński, A.; Książek, A.; Jaroszyński, A. Aortic stiffness and left atrial volume index in patients on continuous ambulatory peritoneal dialysis: Role of endothelial dysfunction. Int. J. Cardiol. 2013, 162, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.K.; Henriksen, J.E.; Dahl, J.; Johansen, A.; Gerke, O.; Vach, W.; Haghfelt, T.; Beck-Nielsen, H.; Høilund-Carlsen, P.F.; Møller, J.E. Left ventricular diastolic function in type 2 diabetes mellitus: Prevalence and association with myocardial and vascular disease. Circ. Cardiovasc. Imaging 2010, 3, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Yaméogo, N.V.; Mbaye, A.; Diack, B.; Ndour, M.; Kane, M.; Diagne-Sow, D.; Diallo, A.; Diop, S.N.; Kane, A. Study of echocardiographic parameters of type 2 black African diabetics at high cardiovascular risk. A cross-sectional study of 79 cases in Senegal. Ann. Cardiol. Angeiol. 2013, 62, 3–7. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).