Abstract
Background. Lower extremity peripheral artery disease (LEPAD) frequently coexists with coronary artery disease (CAD) in patients with multisite vascular disease (MVD). While percutaneous revascularization is well-established for both LEPAD and CAD, limited evidence exists for patients eligible for both procedures. Specifically, the feasibility of concomitant LEPAD and CAD percutaneous revascularization remains unknown. Objectives. To compare the efficacy and safety of concomitant coronary and lower extremity elective percutaneous revascularization. Methods. Between 2012 and 2021, we included 135 patients in an observational, retrospective single-center registry. The population was stratified into two groups: 45 patients (concomitant group) underwent simultaneous coronary and peripheral percutaneous interventions, and 90 patients (deferred group) underwent two separate procedures within one year. The primary efficacy endpoint was major adverse cardiovascular events (MACE) at one year, while the primary safety endpoint was in-hospital contrast-induced nephropathy (CIN). Results. Study groups were well-balanced in baseline characteristics. In terms of coronary features, the concomitant revascularization group more often underwent single-vessel percutaneous coronary intervention (PCI), while the deferred group had multivessel PCI with diffuse coronary disease. No differences were detected in the number of LEPAD lesions between groups. For the primary efficacy endpoint, the incidence of MACE at one year was 37.8% in the concomitant group vs. 34.4% in the deferred group (HR 1.20, 95% CI 0.64–2.10; p = 0.61). No significant differences were found in CIN occurrence between the concomitant and deferred groups (11.1% vs. 8.9%; OR 1.30; 95% CI 0.36–4.21; p = 0.68). Conclusions. Multisite vascular disease patients eligible for CAD and LEPAD percutaneous revascularization exhibited a high cardiovascular risk profile with diffuse multivessel coronary and lower extremity disease. Our study suggests the efficacy and safety of concomitant coronary and lower extremity percutaneous revascularization based on one-year MACE incidence and in-hospital CIN. However, dedicated studies are warranted to confirm the short- and long-term outcomes of the concomitant revascularization strategy.
1. Introduction
Atherosclerotic cardiovascular disease (ASCVD) is a leading cause of major adverse cardiovascular events (MACE) and major adverse limb events (MALE) [1]. Advanced ASCVD affects multiple vascular districts, with prevalence ranging from 25 to 70% for coronary artery disease (CAD) and 14–19% for carotid stenosis in patients affected by lower extremity peripheral artery disease (LEPAD) [1].
Irrespective of limb symptoms, LEPAD patients show an increased risk of overall death, cardiovascular (CV) death, fatal myocardial infarction (MI), and stroke [2]. The risk of ischemic events is higher in patients with polyvascular disease compared to those without [3]. Data from the VOYAGER-PAD trial confirmed the higher risk of MACE plus MALE in patients with CAD compared to those without in a primary PAD population underwent lower extremity revascularization (LER) [4].
Despite the known prognostic value of concomitant CAD and LEPAD, established recommendations for coronary disease screening in lower extremity disease patients suitable for endovascular revascularization, or vice versa, are lacking. This gap in evidence hinders the clarification of the risk-benefit balance between concomitant and deferred percutaneous revascularization for elective patients eligible for CAD and LEPAD interventions.
The aim of our study was to assess the safety and effectiveness of concomitant vs. deferred CAD and LEPAD percutaneous revascularization in a cohort of LEPAD patients with multisite vascular disease.
2. Methods
2.1. Patient Population and Data Collection
Consecutive patients with concomitant CAD and LEPAD undergoing elective coronary and lower extremity arterial percutaneous revascularization between 2012 and 2021 at our institution were included in the study. Patients requiring urgent/primary percutaneous coronary intervention (PCI) or urgent percutaneous transluminal angioplasty (PTA) were excluded. A full list of inclusion and exclusion criteria is listed in Table 1. Clinical, procedural, and follow-up data were anonymously entered in a web-based database (https://www.redcap.unina.it/redcap/, 1 March 2022). The present study has been approved by the local ethic committee, and all study-related procedures were carried out in accordance with the Declaration of Helsinki. Written informed consent was obtained for all patients included in the study.

Table 1.
Study inclusion and exclusion criteria.
2.2. Indication to Procedure
According to current American College of Cardiology/American Heart Association (ACC/AHA) and European Society of Cardiology (ESC) guidelines, LEPAD was defined as the finding of a value ≤ 0.9 of ankle-brachial index (ABI) [5]. In patients with a high clinical suspicion of LEPAD despite an ABI > 0.9, the first line imaging method to confirm disease was duplex ultrasound. Moreover, patients with life-limiting symptoms despite optimized pharmacological therapy (defined at least as an IIB class according to Fontaine classification) and/or with a hemodynamically significant lesion of aorto-iliac, femoral-popliteal, or below the knee district were considered suitable for percutaneous revascularization and included in the study [1,5].
Significant CAD was defined as the finding of an ischemic coronary lesion documented by an abnormal stress-electrocardiogram or stress-echocardiography, an ischemic area > 10% of the left ventricle documented by myocardial single photon emission computed tomography (SPECT) or positron emission tomography (PET), a significant stenosis of at least one vessel detected by coronary computed tomography, a coronary stenosis > 70% in a non-left main vessel and >50% in the left main without a non-invasive or invasive ischemic probe, an intermediate stenosis in a non-left main vessel without a non-invasive ischemia probe but with a fractional flow reserve (FFR) value ≤ 0.8 [6,7]. The choice of concomitant vs. deferred coronary and LEPAD percutaneous was left to operator discretion.
The institution where the study was performed includes a 24/7 service of cardiac and vascular surgery. The catheterization laboratory includes 3 cardiologists with high and recognized experience in coronary and peripheral revascularization.
2.3. Clinical Follow-Up and Study Endpoints
After hospital discharge, follow-up was performed at 30-day intervals and after 1 year through clinical visits, telephone contacts, electronic medical records, or by formal inquiries to primary care physicians. All adverse events were systematically recorded using a web-based reporting system and classified according to the following definitions.
The primary efficacy endpoint of the study was the incidence of MACE, defined as the composite of spontaneous myocardial infarction, death for all causes, any percutaneous transluminal angioplasty, any percutaneous coronary intervention, any amputation for vascular causes, or ischemic stroke at 30-day follow-up and up to 1 year after the last intervention. Secondary endpoints included in-hospital vascular access complications, periprocedural MI, and periprocedural myocardial injury. The primary safety endpoint was in-hospital contrast-induced nephropathy (CIN). Major adverse limb events (MALE) were defined as any percutaneous transluminal angioplasty or any amputation for vascular causes. The incidence of vascular complications was assessed by duplex ultrasound during hospitalization, while the laboratory data were collected at baseline and after 24–48 h from the procedures (i.e., hemoglobin, Hs-TnI, CK-MB, and creatinine). Vascular access complications were defined as arterial injury (i.e., perforation, rupture, dissection, arteriovenous fistula, pseudoaneurysm, hematoma, retroperitoneal hematoma, infection) or compartment syndrome resulting in death, Bleeding Academic Research Consortium (BARC) type ≥ 3a bleeding [8,9].
Periprocedural, spontaneous MI, and myocardial injury were assessed according to the fourth universal definition (UDMI) based on rise/fall of cardiac troponin I (cTnI) values above the 99th percentile Upper Reference Limit (URL) with or without (as myocardial injury) at least one between symptoms of myocardial ischaemia, new ischaemic ECG changes, development of pathological Q waves, imaging evidence of new loss of viable myocardium, or new regional wall motion abnormality in a pattern consistent with an ischaemic aetiology or identification of a coronary thrombus by angiography or autopsy [10].
Chronic kidney disease (CKD) was considered as an estimated glomerulation filtration rate (eGFR) < 60 mL/min/1.73 m2 assessed by the Modification of Diet in Renal Disease (MDRD) equation, and CIN was defined as an increase in serum creatinine by ≥0.3 mg/dL (≥26.5 μmol/L) within 48 h [11].
Stroke was defined as an episode of neurological dysfunction caused by focal cerebral, spinal, or retinal infarction [12]. Any PTA and any PCI were both considered as peripheral or coronary revascularization within 1 year from the index one of the same or another lesion. Amputation due to a vascular etiology was defined as above (major) or below (minor) the ankle for non-traumatic causes and due to a vascular cause, including worsening perfusion of the limb as the primary indication, with the exclusion of infectious causes [13].
2.4. Statistical Analysis
Continuous data distribution was assessed with the Kolmogorov–Smirnov test. Normally distributed variables are expressed as mean ± standard deviation, while non-normally distributed ones are expressed as median and interquartile range. Categorical variables are expressed as numbers and percentages. Continuous normally distributed variables were compared by using the unpaired t test, and differences between non-normally distributed variables were assessed with the Mann–Whitney U test. Categorical variables were assessed using the Fisher’s exact test. A p value of <0.05 was considered to indicate a statistically significant difference for all the analyses performed. A logistic regression model was used to test for between-group differences with adjustment for clinical outcomes (cardiac arrest, fourth UDMI Periprocedural MI, fourth UDMI myocardial injury, contrast-induced nephropathy) at the baseline. The estimates from the model are expressed in terms of odds ratios (OR).
In addition, a Kaplan–Meier analysis was performed to determine survival cumulative probabilities for time-to-event outcomes, including six MACE: death for all causes, any amputation for vascular causes, spontaneous MI, any PTA, any PCI, or ischemic stroke. Cox proportional hazard models were used to investigate the association of concomitant PTA at the time of PCI with risk of clinical outcomes and were estimated using the HRs and their 95% confidence intervals (CI). Time to response was displayed by a Kaplan–Meier plot. Since the retrospective nature of this proof-of-concept study, no formal assessment for sample size was performed. All data analyses were performed using the R version 4.1.3 statistical software.
3. Results
3.1. Baseline Characteristics
Among 151 screened patients with CAD and LEPAD, 16 patients were excluded due to urgent PCI (n = 9), absence of complete laboratory assessment (n = 6), and deferred CAD and LEPAD revascularization for more than 1 year (n = 1). The overall cohort of 135 patients was divided into two groups according to revascularization timing: 45 patients (33.3%) underwent PCI and PTA in the same procedure (concomitant group), whereas 90 patients (66.6%) underwent PCI and PTA in two different procedures (deferred group). Concomitant or deferred CAD and LEPAD percutaneous revascularization strategy was performed according to operator discretion. Study flowchart is represented in Figure 1.
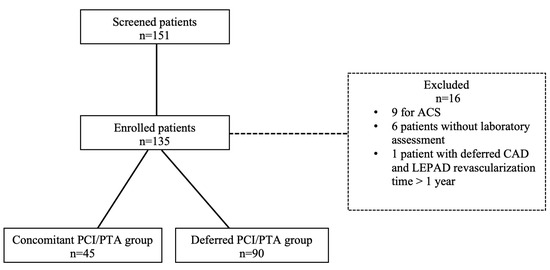
Figure 1.
Study flowchart. ACS: acute coronary syndrome; CAD: coronary artery disease; LEPAD: lower extremity peripheral artery disease; PCI: percutaneous coronary intervention; PTA: percutaneous transluminal angioplasty.
Baseline characteristics of the study population are reported in Table 2. The two groups showed similar baseline characteristics, including age, sex, and body mass index. Except for the familiar history of CAD being more frequent in the concomitant group (17.8% vs. 35.6%, p = 0.04), no differences were found in the baseline CV risk profile among study groups. Patients with histories of diabetes mellitus resulted in 66.7% and 54.4% in the concomitant and deferred groups, respectively (p = 0.2). Moreover, hypertension (88.9% and 85.6%, p = 0.79) and hypercholesterolemia (82.2% and 81.1%, p = 1.0) showed similar rates in the concomitant and deferred groups respectively. Even previous MI showed no significant difference considering concomitant vs. deferred group (24.4% vs. 30.0%, p = 0.55) while history of CKD was detected in 33.3% and 27.8% among concomitant and deferred groups respectively (p = 0.55). Previous history of bleeding (according to thrombolysis in myocardial infarction classification) [14] was similar between groups irrespective of major (2.2% vs. 4.4%, p = 0.66) or minor (4.4% vs. 1.1%, p = 0.26) bleeding events comparing concomitant vs. deferred groups, respectively. Finally, no difference was found among baseline medications considering ASCVD drugs as antithrombotic, hypertension medications, and lipid-lowering therapy (Table 3). Insulin therapy was more likely prescribed among the concomitant group compared to the deferred group (44.4% vs. 24.4%, p = 0.03), while proton pump inhibitor prescriptions were more frequent in the deferred group (51.1% vs. 76.7%, p = 0.003).

Table 2.
Demographic baseline characteristics of the study population.

Table 3.
Medications at baseline.
3.2. Coronary and Peripheral Periprocedural Features
Patients in the deferred group presented with complex CAD considering total lesions, the number of diseased vessels, as well as bifurcation lesions. The overall average number of coronary lesions treated was 1.35 ± 0.7, with a higher number of lesions treated in the deferred group (1.5 ± 0.8 vs. 1.1 ± 0.3, p < 0.001), where multivessel coronary disease (75.6% vs. 57.8%, p = 0.05) and type B2/C lesions according to the ACC/AHA classification were more frequent. In the concomitant group, one lesion was more frequently treated when compared to deferred (88.9% vs. 64.4%, p = 0.002), with PCI localized in 95.6% of cases in only one vessel. The treatment of bifurcation lesions was predominant in the deferred group (25.6% vs. 6.7%, p = 0.01) as well as length of stents (Table 4). None of the patients in the concomitant group underwent treatment of coronary total occlusion (CTO). During coronary intervention, the femoral artery was the most used vascular access in the concomitant group, while in the deferred group the radial access was mostly prevalent (Table 4). Among peripheral angiographic findings, the number in average of lesions treated was 2 ± 0.8 overall with a similar distribution into two groups. Lesions were mostly localized in the femoral-popliteal site (68.9% of the patients). The prevalence of peripheral CTO was more frequent in the deferred group (35.6% vs. 8.9%, p = 0.001). Also, regarding peripheral angioplasty, the lesions treated in the deferred group were significantly longer, more stenotic, and required more often a stent implantation (Table 5).

Table 4.
Coronary angiography findings.

Table 5.
Peripheral angiography findings.
The contrast amount was higher in the concomitant group (293.2 ± 100.3 mL vs. 211 ± 92.6 mL, p = 0.01). Regarding procedural medications, patients underwent concomitant revascularization and received a higher dose of intravenous unfractioned heparin, as well as more often oral loading doses of P2Y12 inhibitors and intravenous doses of aspirin (Table 4).
3.3. Laboratory Assessment
Cardiac troponin I (cTnI), creatine-kinase binding protein (CK-MB), creatinine, and complete hematological evaluation were systematically collected before the index procedure and during the hospitalization. No significant difference was found in myocardial injury incidence between groups. Also, baseline creatinine value (1.42 ± 1.36 mg/dL vs. 1.42 ± 1.50 in concomitant and deferred groups, respectively; p = 0.98) and hemoglobin value (12.66 ± 1.72 g/dL vs. 13.05 ± 1.86 g/dL in concomitant and deferred groups, respectively; p = 0.23) were comparable among study groups.
After 24 h from PCI, the cTnI peak increased in the deferred group (4655.23 ± 26,074.78 ng/L) vs. the concomitant group (568.91 ± 971.64 ng/L, p = 0.0005). CK-MB peak resulted in lower concomitant (4.47 ± 4.09 UI/L) vs. deferred group (11.81 ± 37.06 UI/L) without statistical significance (p = 0.07). Finally, hemoglobin and creatinine assessments found no difference among groups (Table 6).

Table 6.
Laboratory findings.
3.4. Clinical Outcomes
In-hospital clinical outcomes are presented in Table 7. Periprocedural MI occurred more frequently in the deferred group (4.4% vs. 18.9%, p = 0.04 in the concomitant and deferred groups, respectively). The occurrence of myocardial injury (77.8% vs. 62.0%, p = 0.07) and CIN (11.1% vs. 8.9%, p = 0.68) were similar between two study groups. Despite the more frequent adoption of the femoral artery approach in the concomitant group, no significant differences were found in vascular access complications (p = 0.21). Considering in hospital CIN, no significant differences were found among the concomitant (11.1%) vs. deferred (8.9%) group, OR 1.3; 95% CI 0.36–4.21.

Table 7.
In hospital outcomes.
At 1 year follow-up, no differences were detected on the primary efficacy endpoint of MACE among the two groups (37.8% and 34.4%, HR 1.20, 95% CI 0.64–2.10, in the concomitant and deferred groups, respectively) (Table 8, Figure 2). Moreover, 1-year MALE occurrence was similar between study groups (24.4% vs. 27.8% in concomitant and deferred groups, respectively), p = 0.77.

Table 8.
Follow up after 1 month and 1 year.
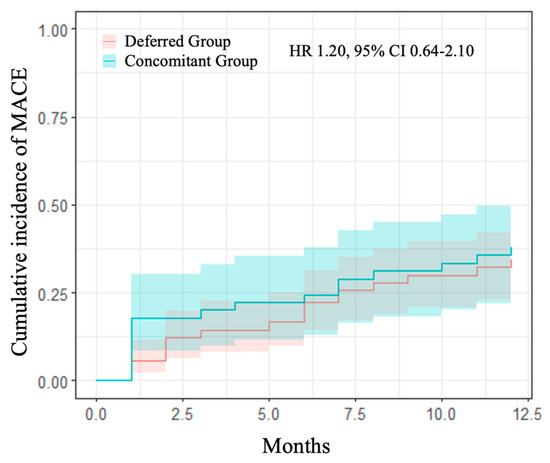
Figure 2.
1-year cumulative incidence of MACE comparing the study groups. Kaplan–Meier curves for primary efficacy endpoint (MACE—a composite of spontaneous MI, death for all causes, any PTA, any PCI, any amputation for vascular causes, and ischemic stroke). In blue, concomitant group vs. separate group in red with relative confidence intervals. HR: hazards ratio; CI: confidence interval.
Even the incidence of the single adverse events after one month did not show any significant difference. Considering concomitant vs. deferred group, all-causes of death occurred in 0% vs. 1.1% (p = 1), spontaneous MI in 4.4% vs. 1.1% (p = 0.25), any-PTA in 4.4% vs. 5.6% (p = 0.79) with no cases of ischemic stroke. Patients in the concomitant group had more frequently amputation adverse at 1 month follow-up (6.7%) compared to the deferred group (0%), p = 0.03, as well as any PCI in the concomitant (8.9%) vs. the deferred group (0%), p = 0.01 (Table 8).
After 1-year of follow-up, no differences among two study groups were found in all-causes death (6.7% in concomitant and 2.2% in deferred, p = 0.21), MI (4.4% vs. 3.3%, p = 0.74), any-PTA (20.0% vs. 27.8%, p = 0.31) and any PCI (8.9% vs. 7.8%, p = 0.79) without any IS. Even at 1-year follow-up, amputation rates increased in the concomitant vs. deferred group (6.7% vs. 0%, p = 0.03) (Table 8).
4. Discussion
This study is focused on the evaluation of the safety and efficacy of concomitant versus deferred CAD and PAD revascularization in patients affected by MVD, representing a high CV risk population for hard clinical endpoint [15].
Among PAD patients, prior evidence confirmed the risk of MACE, with 20% of patients with intermittent claudication experiencing MI or stroke at 5 years follow-up, resulting in a related mortality of 10–15% [14]. Conversely, CAD patients with concomitant LEPAD exhibited a 2-fold increased rate of all-cause death and MACE in the PEGASUS TIMI-54 trial [16]. Our population’s baseline risk profile aligns with other clinical trials enrolling similar risk-profiles [9,13].
Managing percutaneous revascularization timing among CAD and LEPAD patients poses a challenge. To date, only one study investigated the timing of coronary and peripheral revascularization within a 12 month frame, showing no differences in the incidence of MACE, mortality, and CIN [17]. Our study demonstrates the safety and efficacy of concomitant CAD and LEPAD percutaneous revascularization among a very high CV risk population. Considering the primary efficacy endpoint, 1 year rates of MACE were similar between study groups, suggesting that a strategy of concomitant revascularization is effective and safe, particularly for patients with non-complex CAD. The prognostic value of MACE among these patients was assessed by the TRA 2°P-TIMI 50 trial, revealing an almost twofold higher rate of MACE at 3 years in patients with MVD compared to those with only peripheral disease (12.8% vs. 7.6%) [18]. In our study, even other 1-year efficacy endpoints were comparable in both groups, but we observed a higher rate of amputation and any PCI in the concomitant group. We may speculate that concomitant revascularization enhances patient compliance due to a single hospitalization and procedure, thereby reducing inherent costs. Some concerns may arise regarding the widespread use of femoral access. Given the complexity of concomitant coronary and lower extremity revascularization, in some cases operators preferred femoral access considering complex procedures.
Despite strong evidence supporting radial artery access as the preferred route for PCI [19], our study did not find a significant difference between the two approaches. This might be attributed to the local expertise in peripheral and structural intervention. The rate of complications associated with femoral access, detected through ultrasound, was as high as 8.9%, with no statistical variances between the two-study group, consistent with other reports [20].
Considering the characteristics of the coronary lesions, we observed higher complexity, total lesion count, number of diseased vessels, and bifurcation lesions in the deferred group, with no left main lesions treated in the concomitant group. This observation may be a consequence of operators’ choice to manage peripheral and coronary revascularization separately upon encountering complex CAD. Similarly, in the deferred group, we found a higher complexity of lower extremity arterial disease (longer lesions, higher degree of stenosis, presence of CTO). Contrast-induced nephropathy is a common complication after percutaneous procedures, correlated with the amount of contrast medium used and pre-existing CKD as the strongest predictor [21]. Previous studies confirmed CIN impact on renal function and on long-term prognosis, particularly when kidney injury is persistent [22]. Our center employs a preventive protocol, including hydration with a saline solution of NaCl 0.9% at 1 mL/kg/h starting 12 h before the procedure and continuing until 12 h after, with an adjustment rate according to the patient’s ejection fraction, filling pressures, and volemic state [20]. Despite the mean amount of contrast medium being higher in the concomitant group, CIN occurrence was comparable between the concomitant and deferred groups, emphasizing the importance of adopting the preventive protocol. Another adverse event affecting the prognosis of patients undergoing PCI for stable CAD is periprocedural MI [23]. In our study, we found an overall incidence of periprocedural MI of 14.1%, driven by the deferred group (18.9%) and aligned with previously reported data [23,24]. Moreover, in these registries, patients affected by periprocedural MI had a higher complex degree of coronary disease [25]. Accordingly, in our study, periprocedural MI incidence was significantly higher in the deferred group, which exhibited more complex CAD with multivessel complex lesions leading to periprocedural MI.
In summary, the right revascularization timing between coronary and lower extremity peripheral artery disease is still debated. There is a lack of data in this field since the challenge to design a dedicated randomized trial on clinical outcomes comparing concomitant vs. deferred coronary and lower extremity revascularization. The most important barrier may be the systematic selection of patients suitable for elective revascularization procedures, even considering the different approach (i.e., percutaneous, surgical, or hybrid). Moreover, compared to coronary, LEPAD percutaneous revascularization still shows wide variability in terms of operators and lesions management.
5. Limitations
Our study has several limitations. First, it is an observational single-center retrospective study based on a small sample size with no formal assessment performed. The study reference period is as long as 9 years, a range of time with extensive changes in catheterization laboratory equipment, indications for revascularization, and medical management. Finally, management of CAD/LEPAD revascularization in either concomitant or deferred procedures was according to operators’ choice and not based on systematic stratification.
6. Conclusions
Concomitant CAD and PAD percutaneous revascularization results are effective and safe regarding the occurrence of MACE at 1 year follow-up and in hospital CIN in a very high risk population with multisite vascular disease. Dedicated studies are warranted to confirm short and long-term outcomes of concomitant CAD and LEPAD revascularization.
Author Contributions
Conceptualization, M.E.C. and N.V.; Methodology, G.G., E.S. and R.P.; Formal Analysis, M.C.; Investigation, A.L. and M.A.; Data Curation, F.S., S.E. and L.B.; Writing—Original Draft Preparation, M.E.C. and N.V.; Writing—Review and Editing, R.P.; Supervision, G.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of University of Naples Federico II (protocol code 95/21 and date of approval 23 April 2021) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available upon reasonable request to corresponding author.
Conflicts of Interest
Piccolo reports personal fees from Abiomed, Biotronik, Chiesi, and Medtronic outside the submitted work. Esposito reports personal fees from Abbott Vascular, Amgen, Edwards Lifesciences, and Sanofi outside the submitted work and research grants to the institution from Alvimedica, Boston Scientific, and Medtronic. The other authors have no conflicts of interest to declare.
Abbreviations
| ACC | American College of Cardiology |
| AHA | American Heart Association |
| ASCVD | atherosclerotic cardiovascular disease |
| CAD | coronary artery disease |
| CKD | chronic kidney disease |
| CI | confidence intervals |
| CIN | contrast induced nephropathy |
| CTO | chronic total occlusion |
| CV | cardiovascular |
| DM | diabetes mellitus |
| eGFR | estimated glomerulation filtration rate |
| ESC | European Society of Cardiology |
| LEPAD | lower extremity peripheral artery disease |
| LER | lower extremity revascularization |
| MACE | major adverse cardiovascular events |
| MALE | major adverse limb events |
| MI | myocardial infarction |
| MVD | multisite vascular disease |
| PCI | percutaneous coronary intervention |
| PTA | percutaneous transluminal angioplasty |
| UFH | unfractionated heparin |
References
- Aboyans, V.; Ricco, J.B.; Bartelink, M.-L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.-P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [PubMed]
- Aday, A.W.; Matsushita, K. Epidemiology of Peripheral Artery Disease and Polyvascular Disease. Circ. Res. 2021, 128, 1818–1832. [Google Scholar] [CrossRef] [PubMed]
- Canonico, M.E.; Piccolo, R.; Avvedimento, M.; Leone, A.; Esposito, S.; Franzone, A.; Giugliano, G.; Gargiulo, G.; Hess, C.N.; Berkowitz, S.D.; et al. Antithrombotic Therapy in Peripheral Artery Disease: Current Evidence and Future Directions. J. Cardiovasc. Dev. Dis. 2023, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.T.; Canonico, M.E.; Anand, S.S.; Patel, M.R.; Debus, E.S.; Nehler, M.R.; Hess, C.N.; Hsia, J.; Capell, W.H.; Muehlhofer, E.; et al. Low-Dose Rivaroxaban Plus Aspirin in Patients with Peripheral Artery Disease Undergoing Lower Extremity Revascularization with and without Concomitant Coronary Artery Disease: Insights from VOYAGER PAD. Circulation 2024, 149, 1536–1539. [Google Scholar] [CrossRef] [PubMed]
- Gornik, H.L.; Aronow, H.D.; Goodney, P.P.; Arya, S.; Brewster, L.P.; Byrd, L.; Chandra, V.; Drachman, D.E.; Eaves, J.M.; Ehrman, J.K.; et al. 2024 ACC/AHA/AACVPR/APMA/ABC/SCAI/SVM/SVN/SVS/SIR/VESS Guideline for the Management of Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1313–e1410. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477, Erratum in Eur. Heart J. 2020, 41, 4242. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e18–e114, Erratum in Circulation 2022, 145, e772. [Google Scholar] [CrossRef] [PubMed]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef] [PubMed]
- Farber, A.; Menard, M.T.; Conte, M.S.; Kaufman, J.A.; Powell, R.J.; Choudhry, N.K.; Hamza, T.H.; Assmann, S.F.; Creager, M.A.; Cziraky, M.J.; et al. Surgery or Endovascular Therapy for Chronic Limb-Threatening Ischemia. N. Engl. J. Med. 2022, 387, 2305–2316. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Ilardi, F.; Gargiulo, G.; Paolillo, R.; Ferrone, M.; Cimino, S.; Giugliano, G.; Schiattarella, G.G.; Verde, N.; Stabile, E.; Perrino, C.; et al. Impact of chronic kidney disease on platelet aggregation in patients with acute coronary syndrome. J. Cardiovasc. Med. 2020, 21, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Scudiero, F.; Canonico, M.E.; Sanna, G.D.; Dossi, F.; Silverio, A.; Galasso, G.; Esposito, G.; Porto, I. Dual Antiplatelet Therapy with 3rd Generation P2Y12 Inhibitors in STEMI Patients: Impact of Body Mass Index on Loading Dose-Response. Cardiovasc. Drugs Ther. 2023, 37, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Bauersachs, R.M.; Anand, S.S.; Debus, E.S.; Nehler, M.R.; Patel, M.R.; Fanelli, F.; Capell, W.H.; Diao, L.; Jaeger, N.; et al. Rivaroxaban in Peripheral Artery Disease after Revascularization. N. Engl. J. Med. 2020, 382, 1994–2004. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.I.; Byrne, J.; Clagett, G.P.; Farkouh, M.E.; Porter, J.M.; Sackett, D.L.; Strandness, D.E., Jr.; Taylor, L.M. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: A critical review. Circulation 1996, 94, 3026–3049. [Google Scholar] [CrossRef]
- King, R.W.; Canonico, M.E.; Bonaca, M.P.; Hess, C.N. Management of Peripheral Arterial Disease: Lifestyle Modifications and Medical Therapies. J. SCAI 2022, 1, 100513. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Bhatt, D.L.; Storey, R.F.; Steg, P.G.; Cohen, M.; Kuder, J.; Goodrich, E.; Nicolau, J.C.; Parkhomenko, A.; López-Sendón, J.; et al. Ticagrelor for Prevention of Ischemic Events After Myocardial Infarction in Patients with Peripheral Artery Disease. J. Am. Coll. Cardiol. 2016, 67, 2719–2728. [Google Scholar] [CrossRef] [PubMed]
- Koren, O.; Abu Rajab Saaida, R.; Rozner, E.; Turgeman, Y. Outcomes and safety of concurrent coronary and peripheral catheterization (REVascularization in concomitant PERIpheral artery disease and coronary artery disease REV-PERICAD Study). Catheter. Cardiovasc. Interv. 2020, 96, E317–E323. [Google Scholar] [CrossRef] [PubMed]
- Qamar, A.; Morrow, D.A.; Creager, M.A.; Scirica, B.M.; Olin, J.W.; Beckman, J.A.; Murphy, S.A.; Bonaca, M.P. Effect of vorapaxar on cardiovascular and limb outcomes in patients with peripheral artery disease with and without coronary artery disease: Analysis from the TRA 2°P-TIMI 50 trial. Vasc. Med. 2020, 25, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Chiarito, M.; Cao, D.; Nicolas, J.; Roumeliotis, A.; Power, D.; Chandiramani, R.; Sartori, S.; Camaj, A.; Goel, R.; Claessen, B.E.; et al. Radial versus femoral access for coronary interventions: An updated systematic review and meta-analysis of randomized trials. Catheter. Cardiovasc. Interv. 2021, 97, 1387–1396. [Google Scholar] [CrossRef]
- Hetrodt, J.; Engelbertz, C.; Gebauer, K.; Stella, J.; Meyborg, M.; Freisinger, E.; Reinecke, H.; Malyar, N. Access Site Related Vascular Complications following Percutaneous Cardiovascular Procedures. J. Cardiovasc. Dev. Dis. 2021, 8, 136. [Google Scholar] [CrossRef]
- Mehran, R.; Dangas, G.D.; Weisbord, S.D. Contrast-Associated Acute Kidney Injury. N. Engl. J. Med. 2019, 380, 2146–2155. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Morimoto, T.; Nakagawa, Y.; Furukawa, Y.; Ono, K.; Kato, T.; Kadota, K.; Ando, K.; Ishii, M.; Masunaga, N.; et al. Impact of Transient or Persistent Contrast-induced Nephropathy on Long-term Mortality After Elective Percutaneous Coronary Intervention. Am. J. Cardiol. 2017, 120, 2146–2153. [Google Scholar] [CrossRef] [PubMed]
- Ueki, Y.; Otsuka, T.; Bär, S.; Koskinas, K.C.; Heg, D.; Häner, J.; Siontis, G.C.M.; Praz, F.; Hunziker, L.; Lanz, J.; et al. Frequency and Outcomes of Periprocedural MI in Patients With Chronic Coronary Syndromes Undergoing PCI. J. Am. Coll. Cardiol. 2022, 79, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, R.; Leone, A.; Avvedimento, M.; Simonetti, F.; Ippolito, D.; Angellotti, D.; Verde, N.; Manzi, L.; Cirillo, P.; Serafino, L.D.; et al. Impact of biomarker type on periprocedural myocardial infarction in patients undergoing elective PCI. Eur. Heart J. Qual. Care Clin. Outcomes 2023, 9, 680–690. [Google Scholar] [CrossRef]
- Piccolo, R.; Leone, A.; Simonetti, F.; Avvedimento, M.; Angellotti, D.; Manzi, L.; Verde, N.; Spaccarotella, C.A.M.; Di Serafino, L.; Cirillo, P.; et al. Periprocedural myocardial infarction in patients undergoing complex versus noncomplex percutaneous coronary intervention. Catheter. Cardiovasc. Interv. 2023, 102, 212–220. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).