Abstract
Background: Cardiac troponin release is related to the cardiomyocyte loss occurring in heart failure (HF). The prognostic role of high-sensitivity cardiac troponin T (hs-cTnT) in several settings of HF is under investigation. The aim of the study is to assess the prognostic role of intrahospital hs-cTnT in patients admitted due to HF. Methods: In this observational, single center, prospective study, patients hospitalized due to HF have been enrolled. Admission, in-hospital peak, and discharge hs-cTnT have been assessed. Patients were followed up for 6 months. Cardiovascular (CV) death, HF hospitalization (HFH), and worsening HF (WHF) (i.e., urgent ambulatory visit/loop diuretics escalation) events have been assessed at 6-month follow up. Results: 253 consecutive patients have been enrolled in the study. The hs-cTnT median values at admission and discharge were 0.031 ng/mL (IQR 0.02–0.078) and 0.031 ng/mL (IQR 0.02–0.077), respectively. The risk of CV death/HFH was higher in patients with admission hs-cTnT values above the median (p = 0.02) and in patients who had an increase in hs-cTnT during hospitalization (p = 0.03). Multivariate Cox regression analysis confirmed that hs-cTnT above the median (OR: 2.06; 95% CI: 1.02–4.1; p = 0.04) and increase in hs-cTnT during hospitalization (OR:1.95; 95%CI: 1.006–3.769; p = 0.04) were predictors of CV death/HFH. In a subgroup analysis of patients with chronic HF, hs-cTnT above the median was associated with increased risk of CV death/HFH (p = 0.03), while in the subgroup of patients with HFmrEF/HFpEF, hs-cTnT above the median was associated with outpatient WHF events (p = 0.03). Conclusions: Inpatient hs-cTnT levels predict CV death/HFH in patients with HF. In particular, in the subgroup of chronic HF patients, hs-cTnT is predictive of CV death/HFH; while in patients with HFmrEF/HFpEF, hs-cTnT predicts WHF events.
1. Introduction
Heart failure (HF) is one of the main causes of morbidity and mortality worldwide []. It is a clinical syndrome caused by the incapacity of the heart to maintain normal systemic perfusion at normal intraventricular filling pressures. Once diagnosed, patients with HF have an average rate of one hospital readmission per year [,] and an estimated mortality rate of 67% within five years [].
HF is characterized by variable periods of symptomatic stability, often interrupted by episodes of decompensated HF despite optimized therapy. The phases of clinical deterioration are increasingly recognized as a distinct phase in the history of HF, termed worsening HF (WHF) []. WHF is a condition of deterioration of clinical signs of HF, despite optimized medical management, requiring escalation of diuretic therapy, hospitalization or urgent ambulatorial visits []. The interesting and challenging aspect of this condition is that the culminating event of WHF is hospitalization, but the progressive worsening develops outside of the hospital, and it is often subclinical, manifesting itself with myocardial biomarkers increase, need for diuretic escalation, as well as symptoms and signs requiring urgent observation by a cardiologist in the outpatient setting. The early identification of patients in need of diuretic dose adjustments and ambulatory urgent visits may be crucial in the management of these patients in order to avoid hospitalization and related adverse events.
Besides echocardiographic parameters, natriuretic peptides (NPs) are fundamental to rule out the clinical condition of HF and to predict short-term mortality in patients hospitalized due to the latter [,]. The association between NPs and poor prognosis has been demonstrated []. High pre-discharge levels of brain natriuretic peptide (BNP) and N-terminal pro B-type natriuretic peptide (NT-proBNP) are associated with a high risk of cardiovascular (CV) death and hospital readmission []. Similar findings have been reported in the OPTIMIZE-HF registry []. In acute HF, congestion is the main factor influencing NP elevation. However, in chronic stable conditions, transmural wall stress is usually the main determinant of NP concentrations. On the other hand, the mechanism behind NP augmentation in HF with preserved ejection fraction (HFpEF) is less clear, since there is a reduction in wall stress due to the generally smaller size of the left ventricular chamber. Comorbidities, such as kidney disease or obesity, may also affect the concentration of NPs and thus the prognostic significance of these biomarkers [].
NPs are sensitive prognostic markers in HF, but it may be important to identify alternative biomarkers for more accurate management and prognostic stratification of HF patients. Recently, the importance of the high-sensitivity cardiac troponin T (hs-cTnT) assay in the diagnosis and prognosis of HF has been demonstrated []. Troponins are part of the skeletal and cardiac myocyte contraction system. Different troponin isoforms are represented in the different muscle types. While troponin C is synthesized in equal manner in skeletal and cardiac myocytes, the troponin T and I isoforms are highly specific []. The latter are expressed especially in cardiac myocytes and are by far the most specific and sensitive indicators for the diagnosis of acute myocardial infarction (AMI) []. Myocyte damage induces troponin release into the circulation. The increase in hs-cTnT levels is directly related to the severity of myocyte damage, making troponins quantitative markers of heart tissue damage []. Molecular events such as cardiomyocyte death and apoptosis also take place during chronic disease, and high hs-cTnT levels are representative of the long-standing cardiac damage occurring in HF. In fact, in patients with dilatative cardiomyopathy, higher hs-cTnT levels were found to be predictive of a deterioration in clinical conditions []. Setsuka et al. [] have shown that higher troponin levels are found in severe HF, with advanced New York Heart Association (NYHA) class, and in patients who developed complications and HF exacerbation. Various studies have investigated the predictive power of troponin levels in HF patients, showing a higher incidence of major CV events in patients with higher troponin levels []. These studies mainly included patients with HF with reduced ejection fraction (HFrEF) and had major CV events as their main endpoints. Evidence regarding the role of cardiac troponins in HF subpopulations is lacking. Furthermore, the prognostic role of cardiac troponins in terms of WHF events (i.e., the need for diuretic escalation or urgent ambulatory visits due to HF) has not been investigated yet.
The aim of the current study was to assess the role of inpatient cardiac hs-cTnT regarding the identification of HF patients at higher risk of adverse events, including CV mortality, HFH and WHF events, with special focus on the different subgroups of HF.
2. Methods
This was an observational, prospective, single center study, enrolling patients with a diagnosis of HF who have been consecutively admitted to the Department of Clinical, Internal, Anesthesiology and Cardiovascular Sciences at Policlinico Umberto I, Sapienza University of Rome. Inclusion criteria were the following: (I) written, signed and dated informed consent; (II) age above 18 years; (III) diagnosis of HF according to the Guidelines []. Exclusion criteria were the following: (I) presence of any condition representing the main cause of hs-cTnT increase beyond HF; (II) planned or history of heart transplantation or ventricular assist device (VAD); (III) end-stage kidney failure and/or dialysis; (IV) any condition limiting life expectancy less than one year; (V) pregnancy or nursing; (VI) non-compliance with the study protocol.
Patients enrolled constituted one study group.
The following parameters were collected: (i) clinical parameters (past medical history, physical examination, electrocardiogram, arterial blood pressure, NYHA class, and pharmacological therapy); (ii) echocardiographic parameters (ventricular chambers size, systolic and diastolic function, and valve disease and severity); (iii) laboratory parameters (hs-cTnT, blood cell count, creatinine, electrolytes, alanine aminotransferase, and aspartate aminotransferase). Specifically, the admission, peak, and discharge values of hs-cTnT were recorded. Moreover, the delta between admission and peak values of hs-cTnT was calculated. An increase in hs-cTnT during an in-hospital stay was defined as a delta of at least 0.014 ng/mL, between the admission and peak hs-cTnT values (representing the upper reference limit of hs-cTnT). The assay Elecsys® (Roche Diagnostics International Ltd., Rotkreuz, Switzerland) for hs-cTnT has been used.
Over a follow-up period of 6 months after the index hospitalization, CV death, HFH, and urgent ambulatory visits/need of loop diuretic escalation were investigated in the outpatient HF clinic.
Specific subgroup analyses according to LVEF values and clinical presentation of HF were performed in order to define the prognostic role of hs-cTnT in terms of CV death, HFH, and urgent ambulatory visits/need of loop diuretic escalation.
Data were collected in a dedicated Excel Database (Version 2405 Build 16.0.17628.20006; 64 bit). The study was conducted according to the Helsinki Declaration. The study protocol was approved by the Ethical Committee of Policlinico Umberto I in Rome (rif.7068, approved on 8 May 2023).
Statistical Analysis
The normal distribution of continuous variables was assessed with the Kolmogorov–Smirnov test. Continuous variables were expressed as mean and standard deviation, whereas median and first and third quartiles were used for non-normally distributed data. Categorical data were described as numbers and percentages. Student’s t-test, the Mann–Whitney test, the χ2 test, and the Fisher exact test were used for comparisons, as needed. The Kaplan–Meier method was used to estimate the cumulative event rates of study outcomes in the overall population, categorized based on admission troponin (above or below the median value of the studied population) and on the basis of the trend in troponin values during the hospitalization (patients with an increase or decrease in troponin values). Kaplan–Meier analysis was used to analyze the differences in clinical outcome rates in subgroups of patients with HFpEF and HF with mildly reduced ejection fraction (HFmrEF), and in patients with chronic HF presentation. The differences in each group were compared using log-rank tests. Univariate and multivariate Cox regression analyses were performed to obtain the odds ratios (ORs) of the associations among hs-cTnT with the endpoints. All the associations among variables and the composite endpoints with a p-value < 0.1 at univariate analysis were included at multivariate analysis. At multivariate analysis, variables potentially associated with the composite outcomes of CV death and HFH have been considered. For all tests, a p-value < 0.05 was considered statistically significant.
The statistical analysis was performed using SPSS version 27.0 for Mac (IBM Software, Inc., Armonk, NY, USA).
3. Results
A total of 253 consecutive patients were enrolled from October 2022 to April 2023 and they were followed-up for a period of 6 months.
The baseline features of the patient population are listed in Table 1. The types of admission and discharge therapies for the total population have been represented in Figure 1. The occurrence of each outcome in the total population has been represented in Figure 2.

Table 1.
Baseline features of the study population at hospital admission.
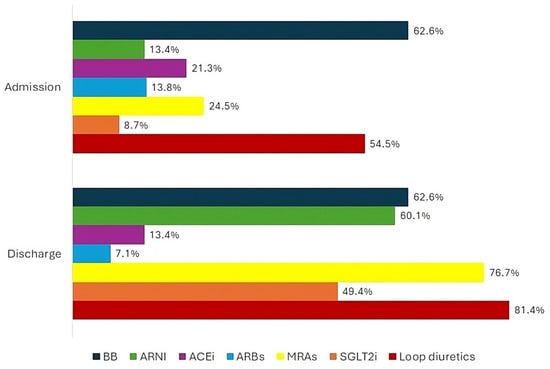
Figure 1.
Percentage of total population on treatment with heart failure disease modifying drugs and loop diuretics at hospital admission and discharge. HF—heart failure; BB—beta blocker; ARNI—angiotensin receptor/neprilysin inhibitor; ACEi—angiotensin-converting enzyme inhibitor; ARBs—angiotensin receptor blockers; MRAs—mineralocorticoid receptor antagonists; SGLT2i—sodium glucose cotransporter 2 inhibitor.
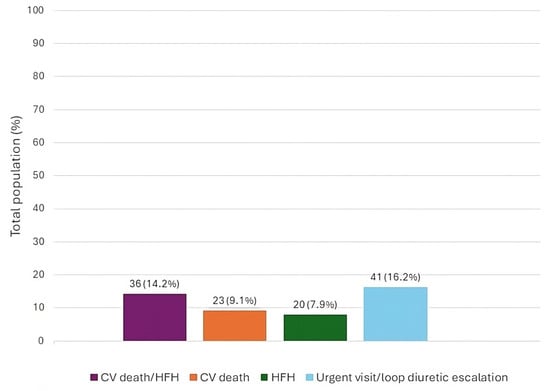
Figure 2.
Total number and percentage rate of adverse events in the total population at 6-month follow-up. CV—cardiovascular; HFH—heart failure hospitalization.
Considering the total population, the composite of CV death and HFH was significantly higher in patients with hs-cTnT levels at admission above the median (23 vs. 13; 19.8% vs. 9.5%; p = 0.02) and in patients with a significant increase in hs-cTnT during hospitalization (20 vs. 16; 20% vs. 10.5%; p = 0.03) (Table 2 and Table 3).

Table 2.
Relationship between high-sensitivity T troponin at admission and the occurrence of each outcome in the total population.

Table 3.
Relationship between high-sensitivity T troponin increase during hospitalization and the occurrence of each outcome in the total population.
Kaplan–Meier survival analysis (Figure 3A,B) demonstrated that patients with admission hs-cTnT levels above the median value and patients with an increase in hs-cTnT during in-hospital stays experienced more commonly the composite outcome CV death and HFH (log-rank p-value = 0.02 and p-value = 0.03, respectively).
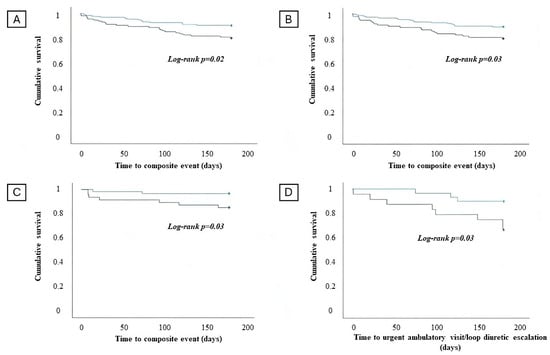
Figure 3.
Survival analysis regarding the occurrence of the composite of cardiovascular (CV) death and heart failure hospitalization (HFH) in patients with an admission high-sensitivity T troponin (hs-cTnT) value below the median (blue line) and admission high-sensitivity T troponin value above the median (green line) in the overall population (A). Survival analysis regarding the occurrence of the composite of CV death and HFH in patients without significant in-hospital hs-cTnT increase (blue line) and with significant hs-cTnT increase (green line) in the overall population (B). Survival analysis regarding the occurrence of the composite of CV death and HFH in patients with an admission hs-cTnT value below the median (blue line) and admission hs-cTnT value above the median (green line) in the chronic HF subgroup (C). Survival analysis regarding the occurrence of worsening HF events and admission hs-cTnT values below the median (blue line) and above the median (green line) in the HFmrEF/HFpEF subgroup (D).
Cox regression analysis showed that an admission hs-cTnT above the median and an in-hospital increase in hs-cTnT represent an independent predictor of the composite of CV death and HFH at 6-month follow-up (Table 4 and Table 5).

Table 4.
Univariate and multivariate analysis regarding the variables considered as predictors of the composite event in the total population. High-sensitivity T troponin above the median at admission represents an independent predictor of cardiovascular death and heart failure hospitalization at 6-month follow-up in patients hospitalized with a diagnosis of heart failure.

Table 5.
Univariate and multivariate analysis regarding the variables considered as predictors of the composite event in the total population. High-sensitivity T troponin increase during hospitalization represents an independent predictor of cardiovascular death and heart failure hospitalization at 6-month follow-up in patients hospitalized with a diagnosis of heart failure.
The subgroup analysis, considering patients with chronic HF, demonstrated that the risk of the composite of CV death and HFH was significantly higher in patients with an admission hs-cTnT above the median value compared to patients with an admission hs-cTnT below the median value (7 vs. 2; 14.9% vs. 3.3%; p = 0.04) (Table 6).

Table 6.
Occurrence of each outcome according to high-sensitivity T troponin at hospital admission in chronic HF subgroup.
Kaplan–Meier survival analysis evidenced that an admission hs-cTnT above the median value in the subgroup of patients with chronic HF is associated with an increased risk of CV death and HFH (log rank p = 0.03) at 6-month follow-up (Figure 3C).
The baseline features of patients according to LVEF values have been reported in Table 7.

Table 7.
Baseline features and discharge therapy of patients according to left ventricular ejection fraction.
Patients with HFmrEF/HFpEF and admission hs-cTnT above the median value had a significantly higher risk of outpatient WHF (i.e., urgent ambulatory visit/loop diuretic escalation) at 6-month follow-up compared to patients with HFmrEF/HFpEF and admission hs-cTnT below the median value (8 vs. 3; 33.3% vs 10%; p = 0.04) (Table 8).

Table 8.
Occurrences of each outcome in the subgroup of patients with HFpEF/HFmrEF according to hs-cTnT values at hospital admission.
Kaplan–Meier survival analysis demonstrated that patients with HFmrEF/HFpEF and an admission hs-cTnT above the median value have a significantly increased risk of experiencing an outpatient WHF event at 6-month follow-up (log rank p = 0.03) (Figure 3D).
4. Discussion
The identification of prognostic and predictive biomarkers is currently one of the biggest challenges for the improvement of HF management. The only validated biomarkers in HF are NPs. Beyond NPs, the most promising biomarkers are hs-cTnT and suppression of tumorigenesis-2 ligand (sST2L), and both have been shown to be independent predictors of mortality in HF []. However, data regarding hs-cTnT as prognostic tool in HF are discordant and often confusing, as well as scarce [].
The results of our study highlighted the role of hs-cTnT as a valid prognostic biomarker in the total population of HF patients. More specifically, our results demonstrated that not only admission hs-cTnT values above the median, but also hs-cTnT increase during hospitalization are independent predictors of the composite of CV death and HFH at 6-month follow-up (OR: 2.06; 95% CI: 1.02–4.1; p = 0.04 and OR:1.95; 95%CI: 1.006–3.769; p = 0.04, respectively).
Previous studies revealed the possible role of troponins as predictive biomarkers of major CV events in HF patients [,,,,,,,,,,]. Latini et al. [] demonstrated that high levels of hs-cTnT were moderately associated with CV death in chronic HF patients, with a risk that was 5% higher when troponin levels above the median were detected. You et al. [] identified that cardiac troponin I (cTnI) was an independent predictor of all-cause mortality in patients with acute decompensated HF. These results were confirmed in different studies including cTnI [,,]. Del Carlo et al. [] demonstrated a higher incidence of 1-year rehospitalization due to HF and mortality in patients with persistent troponin T levels higher than 0.02 ng/dl. Aimo et al. [] conducted a meta-analysis analyzing a global population of 9289 in which it was confirmed that cTnT was an independent predictor of all-cause mortality and CV hospitalizations in patients with chronic HF. In a meta-analysis by Masson et al. [] including 5284 patients, hs-cTnT levels were predictors of cardiovascular events in patients with chronic HF; however, it did not add significant prognostic discrimination. In acute decompensated HF patients, Peacock et al. [] conducted a retrospective analysis on a population of 84872 patients hospitalized due to acute HF decompensation. A higher in-hospital mortality for patients with elevated hs-cTnT levels at admission has been observed []. Furthermore, Pandey et al. [] reported that cardiac troponin elevation in patients with acute decompensated HFpEF was a predictor of adverse in-hospital and post-discharge events. In a recent meta-analysis by Evans et al. [] including 67063 patients, hs-cTnT was associated with incident HF, improving also HF prediction.
These results, including the results of our study, are supported by a physiological explanation. It is known that the blood concentration of cardiac troponins is a consequence of myocardial cell necrosis and that every clinical condition that causes cardiomyocyte damage is also a cause of cardiac troponin blood level elevation [,]. In HF patients, cardiac troponin release may happen as a consequence of chronic ischemia, also in the absence of acute coronary stenosis []. This is due to HF-induced myocardial remodeling and subendocardial ischemia, determined by the excessive myocardial wall stress and cardiomyocyte damage []. Also, increased filling pressures, tachyarrhythmia or bradyarrhythmia, arterial hypotension, anemia, and endothelial dysfunction may be reasons for reduced oxygen supply to cardiomyocytes []. The consequence is the generation of myocardial injury, with an increase in cell permeability, allowing cytosol troponin to be released into the circulation [,].
Also, anemia and iron deficiency are known comorbidities associated with adverse events and worse life quality in patients with HF []. According to the Guidelines and the World Health Organization, anemia is defined by a hemoglobin level < 12 g/dL and <13 g/dL in females and males, respectively []. In our population, hemoglobin represented a predictor of CV death/HFH at univariate analysis for the total population, but it did not reach statistical significance at multivariate analysis.
Most of the mentioned studies are limited to describing an association between troponins and major CV events [], without considering subclinical events in WHF and HF subgroups. Scenarios of WHF without hospitalization, such as escalation of diuretic therapy and/or need for urgent ambulatory visits, are also important concerns in the management of HF patients. This aspect has been highlighted by the consideration that hospitalization can be compared to the “tip of the iceberg” of a complex process of disease-worsening, which occurs outside of the hospital and is often subclinical [,]. It has been demonstrated that outpatient escalation of diuretics therapy increases the risk of 1-year mortality by 75% []. WHF is a transversal condition which involves HF patients regardless of LVEF.
HFrEF is widely studied in the scientific literature, while HFmrEF and HFpEF are entities less studied, but growing evidence demonstrates that their prognoses are similar to HFrEF []. In our study, we found that patients with HFmrEF/HFpEF and an admission hs-cTnT above the median value had a significant higher risk of urgent ambulatory visit/loop diuretic escalation at 6 months compared to HFmrEF/HFpEF patients with hs-cTnT below the median value (p = 0.03). It is known that HFpEF and HFmrEF populations have substantial differences compared to HFrEF patients. HFpEF patients are usually older and have multiple comorbidities with a less frequent history of ischemic heart disease than in HFrEF []. Furthermore, HFpEF has a greater association with extracardiac comorbidities, as well as with the female gender []. Although the mortality is similar in HFpEF and HFrEF, there has been shown to be a higher incidence of hospitalization in HFpEF, which is mainly related to worsening comorbidities []. It has been shown that HFmrEF has more similar outcomes to HFpEF than HFrEF []. The population of HFmrEF, similarly to HFpEF, is composed of older people and has a higher comorbidity burden than the HFrEF population. The prognosis of HFmrEF and HFpEF is mainly influenced by the adverse events related to comorbidities, and the role of hs-cTnT in this patient population may be explained by continuous ventricular pressure overload with consequently subendocardial ischemia [,].
Another important result of our study was that the risk of the composite of CV death and HFH was significantly higher in patients with chronic HF and an admission hs-cTnT above the median value compared to patients with an admission hs-cTnT below the median value (p = 0.03). This finding emphasizes the prognostic significance of hs-cTnT levels at hospital admission in patients with HF. Chronic HF patients with elevated hs-cTnT levels are likely to have underlying cardiac damage or stress, predisposing them to a higher risk of adverse cardiovascular outcomes. The elevated hs-cTnT levels on admission serve as a marker of continuous myocardial injury [] in chronic HF patients. Our results seem to suggest that the long-standing steady myocardial damage in chronic HF may severely impact the prognosis.
The use of biomarkers such as NPs and cardiac troponins is suitable in most hospitals and outpatient services, and their use to manage patients is feasible and standardizable. On the contrary, other biomarkers, albeit interesting, are not always available everywhere. NPs and cardiac troponins reflect two different pathophysiological pathways in HF [,], whose involvement may vary according to the HF subgroup considered. Therefore, the integrated evaluation of the latter may bring relevant information which can be integrated into clinical evaluation in light of better patient management. Importantly, our results highlight the potential role of hs-cTnT quantification, in order to predict adverse events in peculiar HF subpopulations. An emerging and challenging aspect is the possibility of using these biomarkers not only to stratify patients’ prognoses, but also to guide therapy with HF disease-modifying drugs in order to identify patients at higher risk of adverse events and be more aggressive with the up-titration of therapy [,,,]. This aspect has been recently evaluated for NPs, with promising results.
Our study has several limitations. The results should be confirmed on a larger population and larger subgroups of HF patients. Due to the number of patients, multivariate analysis has not been used for subgroups. Other biomarkers have not been included in the study and compared to hs-cTnT in terms of prognostic predictivity. The trend of hs-cTnT has not been evaluated during the follow-up.
5. Conclusions
The assessment of biomarkers in HF represents a crucial aspect of patient management. Our results suggest that in-hospital hs-cTnT levels may predict the composite of CV death/HFH in patients with HF. In particular, in the subgroup of chronic HF patients, hs-cTnT may predict the composite of CV death/HFH, while in HFmrEF/HFpEF subgroup, hs-cTnT may predict out of hospital WHF events. Our results emphasize the importance of the serial assessment of hs-cTnT at admission and during hospitalization to assess the prognosis in HF patients.
Author Contributions
Conceptualization, A.D., P.S., S.P. and F.F.; data curation, A.D., P.S., S.P., M.V.M., A.D.P., R.G., V.M., A.L.F., C.C., S.M.-I., L.T., L.V., S.L.M. and G.D.P.; formal analysis, A.D., P.S., S.P. and M.V.M.; methodology, A.D., P.S., S.P. and M.V.M.; supervision, C.L., G.S., M.M., R.B., F.F. and C.D.V.; validation, C.L., G.S., M.M., R.B., F.F. and C.D.V.; visualization, C.L., G.S., M.M., R.B., F.F. and C.D.V.; writing—original draft, A.D., P.S., S.P., M.V.M., A.D.P., R.G., V.M., A.L.F., C.C., S.M.-I., L.T., L.V., S.L.M. and G.D.P.; writing—review and editing, A.D., P.S., S.P., M.V.M., C.L., G.S., M.M., R.B., F.F. and C.D.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work did not receive external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Policlinico Umberto I of Rome (protocol code 7068, approved on 8 May 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interests.
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee; Maddox, T.M.; Januzzi, J.L., Jr.; Allen, L.A.; Breathett, K.; Butler, J.; Davis, L.L.; Fonarow, G.C.; Ibrahim, N.E.; Lindenfeld, J.; et al. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues about Heart Failure with Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2021, 77, 772–810. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Lyass, A.; Enserro, D.; Larson, M.G.; Ho, J.E.; Kizer, J.R.; Gottdiener, J.S.; Psaty, B.M.; Vasan, R.S. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail. 2018, 6, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.J.; Bauersachs, J.; Brugts, J.J.; Ezekowitz, J.A.; Lam, C.S.P.; Lund, L.H.; Ponikowski, P.; Voors, A.A.; Zannad, F.; Zieroth, S.; et al. Worsening Heart Failure: Nomenclature, Epidemiology, and Future Directions: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2023, 81, 413–424. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, A.; Prosperi, S.; Severino, P.; Myftari, V.; Labbro Francia, A.; Cestiè, C.; Pierucci, N.; Marek-Iannucci, S.; Mariani, M.V.; Germanò, R.; et al. Current Approaches to Worsening Heart Failure: Pathophysiological and Molecular Insights. Int. J. Mol. Sci. 2024, 25, 1574. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Albert, N.M.; Coats, A.J.S.; Anker, S.D.; Bayes-Genis, A.; Butler, J.; Chioncel, O.; Defilippi, C.R.; Drazner, M.H.; Felker, G.M.; et al. Natriuretic Peptides: Role in the Diagnosis and Management of Heart Failure: A Scientific Statement from the Heart Failure Association of the European Society of Cardiology, Heart Failure Society of America and Japanese Heart Failure Society. J. Card. Fail. 2023, 29, 787–804. [Google Scholar] [CrossRef] [PubMed]
- Logeart, D.; Thabut, G.; Jourdain, P.; Chavelas, C.; Beyne, P.; Beauvais, F.; Bouvier, E.; Solal, A.C. Predischarge B-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure. J. Am. Coll. Cardiol. 2004, 43, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Kociol, R.D.; Horton, J.R.; Fonarow, G.C.; Reyes, E.M.; Shaw, L.K.; O’Connor, C.M.; Felker, G.M.; Hernandez, A.F. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: Data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circ. Heart Fail. 2011, 4, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Ezekowitz, J.A.; Alemayehu, W.; Rathwell, S.; Grant, A.D.; Fiuzat, M.; Whellan, D.J.; Ahmad, T.; Adams, K.; Piña, I.L.; Cooper, L.S.; et al. The influence of comorbidities on achieving an N-terminal pro-b-type natriuretic peptide target: A secondary analysis of the GUIDE-IT trial. ESC Heart Fail. 2022, 9, 77–86. [Google Scholar] [CrossRef]
- Del Carlo, C.H.; O’Connor, C.M. Cardiac troponins in congestive heart failure. Am. Heart J. 1999, 138 Pt 1, 646–653. [Google Scholar] [CrossRef]
- Garg, P.; Morris, P.; Fazlanie, A.L.; Vijayan, S.; Dancso, B.; Dastidar, A.G.; Plein, S.; Mueller, C.; Haaf, P. Cardiac biomarkers of acute coronary syndrome: From history to high-sensitivity cardiac troponin. Intern. Emerg. Med. 2017, 12, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Chauin, A. The Main Causes and Mechanisms of Increase in Cardiac Troponin Concentrations Other than Acute Myocardial Infarction (Part 1): Physical Exertion, Inflammatory Heart Disease, Pulmonary Embolism, Renal Failure, Sepsis. Vasc. Health Risk Manag. 2021, 17, 601–617. [Google Scholar] [CrossRef]
- Sandoval, Y.; Apple, F.S.; Mahler, S.A.; Body, R.; Collinson, P.O.; Jaffe, A.S.; International Federation of Clinical Chemistry and Laboratory Medicine Committee on the Clinical Application of Cardiac Biomarkers. High-Sensitivity Cardiac Troponin and the 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guidelines for the Evaluation and Diagnosis of Acute Chest Pain. Circulation 2022, 146, 569–581. [Google Scholar] [CrossRef]
- Sato, Y.; Yamada, T.; Taniguchi, R.; Nagai, K.; Makiyama, T.; Okada, H.; Kataoka, K.; Ito, H.; Matsumori, A.; Sasayama, S.; et al. Persistently increased serum concentrations of cardiac troponin t in patients with idiopathic dilated cardiomyopathy are predictive of adverse outcomes. Circulation 2001, 103, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Setsuta, K.; Seino, Y.; Ogawa, T.; Arao, M.; Miyatake, Y.; Takano, T. Use of cytosolic and myofibril markers in the detection of ongoing myocardial damage in patients with chronic heart failure. Am. J. Med. 2002, 113, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Kociol, R.D.; Pang, P.S.; Gheorghiade, M.; Fonarow, G.C.; O’Connor, C.M.; Felker, G.M. Troponin elevation in heart failure prevalence, mechanisms, and clinical implications. J. Am. Coll. Cardiol. 2010, 56, 1071–1078. [Google Scholar] [CrossRef]
- Castiglione, V.; Aimo, A.; Vergaro, G.; Saccaro, L.; Passino, C.; Emdin, M. Biomarkers for the diagnosis and management of heart failure. Heart Fail. Rev. 2022, 27, 625–643. [Google Scholar] [CrossRef]
- Lazar, D.R.; Lazar, F.L.; Homorodean, C.; Cainap, C.; Focsan, M.; Cainap, S.; Olinic, D.M. High-Sensitivity Troponin: A Review on Characteristics, Assessment, and Clinical Implications. Dis. Markers 2022, 2022, 9713326. [Google Scholar] [CrossRef]
- Latini, R.; Masson, S.; Anand, I.S.; Missov, E.; Carlson, M.; Vago, T.; Angelici, L.; Barlera, S.; Parrinello, G.; Maggioni, A.P.; et al. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation 2007, 116, 1242–1249. [Google Scholar] [CrossRef]
- You, J.J.; Austin, P.C.; Alter, D.A.; Ko, D.T.; Tu, J.V. Relation between cardiac troponin I and mortality in acute decompensated heart failure. Am. Heart J. 2007, 153, 462–470. [Google Scholar] [CrossRef]
- Myhre, P.L.; O’Meara, E.; Claggett, B.L.; de Denus, S.; Jarolim, P.; Anand, I.S.; Beldhuis, I.E.; Fleg, J.L.; Lewis, E.; Pitt, B.; et al. Cardiac Troponin I and Risk of Cardiac Events in Patients with Heart Failure and Preserved Ejection Fraction. Circ. Heart Fail. 2018, 11, e005312. [Google Scholar] [CrossRef] [PubMed]
- Yan, I.; Börschel, C.S.; Neumann, J.T.; Sprünker, N.A.; Makarova, N.; Kontto, J.; Kuulasmaa, K.; Salomaa, V.; Magnussen, C.; Iacoviello, L.; et al. High-Sensitivity Cardiac Troponin I Levels and Prediction of Heart Failure: Results from the BiomarCaRE Consortium. JACC Heart Fail. 2020, 8, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Stelzle, D.; Shah, A.S.V.; Anand, A.; Strachan, F.E.; Chapman, A.R.; Denvir, M.A.; Mills, N.L.; McAllister, D.A. High-sensitivity cardiac troponin I and risk of heart failure in patients with suspected acute coronary syndrome: A cohort study. Eur. Heart J. Qual. Care Clin. Outcomes 2018, 4, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Del Carlo, C.H.; Pereira-Barretto, A.C.; Cassaro-Strunz, C.; Latorre, M.d.R.; Ramires, J.A. Serial measure of cardiac troponin T levels for prediction of clinical events in decompensated heart failure. J. Card. Fail. 2004, 10, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Januzzi, J.L., Jr.; Vergaro, G.; Ripoli, A.; Latini, R.; Masson, S.; Magnoli, M.; Anand, I.S.; Cohn, J.N.; Tavazzi, L.; et al. Prognostic Value of High-Sensitivity Troponin T in Chronic Heart Failure: An Individual Patient Data Meta-Analysis. Circulation 2018, 137, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Masson, S.; Anand, I.; Favero, C.; Barlera, S.; Vago, T.; Bertocchi, F.; Maggioni, A.P.; Tavazzi, L.; Tognoni, G.; Cohn, J.N.; et al. Serial measurement of cardiac troponin T using a highly sensitive assay in patients with chronic heart failure: Data from 2 large randomized clinical trials. Circulation 2012, 125, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Peacock, W.F., 4th; De Marco, T.; Fonarow, G.C.; Diercks, D.; Wynne, J.; Apple, F.S.; Wu, A.H. Cardiac troponin and outcome in acute heart failure. N. Engl. J. Med. 2008, 358, 2117–2126. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Golwala, H.; Sheng, S.; DeVore, A.D.; Hernandez, A.F.; Bhatt, D.L.; Heidenreich, P.A.; Yancy, C.W.; de Lemos, J.A.; Fonarow, G.C. Factors Associated with and Prognostic Implications of Cardiac Troponin Elevation in Decompensated Heart Failure with Preserved Ejection Fraction: Findings from the American Heart Association Get with the Guidelines-Heart Failure Program. JAMA Cardiol. 2017, 2, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.W.; Dobbin, S.J.H.; Pettit, S.J.; Di Angelantonio, E.; Willeit, P. High-Sensitivity Cardiac Troponin and New-Onset Heart Failure: A Systematic Review and Meta-Analysis of 67,063 Patients with 4165 Incident Heart Failure Events. JACC Heart Fail. 2018, 6, 187–197. [Google Scholar] [CrossRef]
- Westermann, D.; Neumann, J.T.; Sörensen, N.A.; Blankenberg, S. High-sensitivity assays for troponin in patients with cardiac disease. Nat. Rev. Cardiol. 2017, 14, 472–483. [Google Scholar] [CrossRef]
- Januzzi, J.L., Jr.; Filippatos, G.; Nieminen, M.; Gheorghiade, M. Troponin elevation in patients with heart failure: On behalf of the third Universal Definition of Myocardial Infarction Global Task Force: Heart Failure Section. Eur. Heart J. 2012, 33, 2265–2271. [Google Scholar] [CrossRef]
- Miller, W.L.; Hartman, K.A.; Burritt, M.F.; Grill, D.E.; Jaffe, A.S. Profiles of serial changes in cardiac troponin T concentrations and outcome in ambulatory patients with chronic heart failure. J. Am. Coll. Cardiol. 2009, 54, 1715–1721. [Google Scholar] [CrossRef]
- Potluri, S.; Ventura, H.O.; Mulumudi, M.; Mehra, M.R. Cardiac troponin levels in heart failure. Cardiol. Rev. 2004, 12, 21–25. [Google Scholar] [CrossRef]
- Meijers, W.C.; Bayes-Genis, A.; Mebazaa, A.; Bauersachs, J.; Cleland, J.G.F.; Coats, A.J.S.; Januzzi, J.L.; Maisel, A.S.; McDonald, K.; Mueller, T.; et al. Circulating heart failure biomarkers beyond natriuretic peptides: Review from the Biomarker Study Group of the Heart Failure Association (HFA), European Society of Cardiology (ESC). Eur. J. Heart Fail. 2021, 23, 1610–1632. [Google Scholar] [CrossRef] [PubMed]
- Gherasim, L. Troponins in Heart Failure—A Perpetual Challenge. Maedica 2019, 14, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Madelaire, C.; Gustafsson, F.; Stevenson, L.W.; Kristensen, S.L.; Køber, L.; Andersen, J.; D’Souza, M.; Biering-Sørensen, T.; Andersson, C.; Torp-Pedersen, C.; et al. One-Year Mortality after Intensification of Outpatient Diuretic Therapy. J. Am. Heart Assoc. 2020, 9, e016010. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Beltrami, M. Are HFpEF and HFmrEF So Different? The Need to Understand Distinct Phenotypes. Front. Cardiovasc. Med. 2021, 8, 676658. [Google Scholar] [CrossRef]
- Li, P.; Zhao, H.; Zhang, J.; Ning, Y.; Tu, Y.; Xu, D.; Zeng, Q. Similarities and Differences between HFmrEF and HFpEF. Front. Cardiovasc. Med. 2021, 8, 678614. [Google Scholar] [CrossRef]
- Simmonds, S.J.; Cuijpers, I.; Heymans, S.; Jones, E.A.V. Cellular and Molecular Differences between HFpEF and HFrEF: A Step Ahead in an Improved Pathological Understanding. Cells 2020, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Streng, K.W.; Nauta, J.F.; Hillege, H.L.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.; Lang, C.C.; Metra, M.; Ng, L.L.; et al. Non-cardiac comorbidities in heart failure with reduced, mid-range and preserved ejection fraction. Int. J. Cardiol. 2018, 271, 132–139. [Google Scholar] [CrossRef]
- Santas, E.; de la Espriella, R.; Palau, P.; Miñana, G.; Amiguet, M.; Sanchis, J.; Lupón, J.; Bayes-Genís, A.; Chorro, F.J.; Villota, J.N. Rehospitalization burden and morbidity risk in patients with heart failure with mid-range ejection fraction. ESC Heart Fail. 2020, 7, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Agdashian, D.; Daniels, L.B. What Is the Clinical Utility of Cardiac Troponins in Heart Failure? Are They Modifiable beyond Their Prognostic Value? Curr. Heart Fail. Rep. 2023, 20, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; D’Amato, A.; Prosperi, S.; Myftari, V.; Canuti, E.S.; Labbro Francia, A.; Cestiè, C.; Maestrini, V.; Lavalle, C.; Badagliacca, R.; et al. Heart Failure Pharmacological Management: Gaps and Current Perspectives. J. Clin. Med. 2023, 12, 1020. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; D’Amato, A.; Prosperi, S.; Dei Cas, A.; Mattioli, A.V.; Cevese, A.; Novo, G.; Prat, M.; Pedrinelli, R.; Raddino, R.; et al. Do the Current Guidelines for Heart Failure Diagnosis and Treatment Fit with Clinical Complexity? J. Clin. Med. 2022, 11, 857. [Google Scholar] [CrossRef] [PubMed]
- Adamo, M.; Pagnesi, M.; Mebazaa, A.; Davison, B.; Edwards, C.; Tomasoni, D.; Arrigo, M.; Barros, M.; Biegus, J.; Celutkiene, J.; et al. NT-proBNP and high intensity care for acute heart failure: The STRONG-HF trial. Eur. Heart J. 2023, 44, 2947–2962. [Google Scholar] [CrossRef]
- Severino, P.; Mancone, M.; D’Amato, A.; Mariani, M.V.; Prosperi, S.; Alunni Fegatelli, D.; Birtolo, L.I.; Angotti, D.; Milanese, A.; Cerrato, E.; et al. Heart failure ‘the cancer of the heart’: The prognostic role of the HLM score. ESC Heart Fail. 2024, 11, 390–399. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).