Abstract
Nail apparatus melanoma (NAM) is a rare type of cutaneous melanoma that belongs to the acral melanoma subtype. NAM is managed principally in accordance with the general treatment for cutaneous melanoma, but there is scarce evidence in support of this in the literature. Acral melanoma is genetically different from non-acral cutaneous melanoma, while recently accumulated data suggest that NAM also has a different genetic background from acral melanoma. In this review, we focus on recent advances in the management of NAM. Localized NAM should be surgically removed; amputation of the digit and digit-preserving surgery have been reported. Sentinel lymph node biopsy can be considered for invasive NAM for the purpose of accurate staging. However, it is yet to be clarified whether patients with metastatic sentinel lymph nodes can be safely spared completion lymph node dissection. Similar to cutaneous melanoma, immune checkpoint inhibitors and BRAF/MEK inhibitors are used as the first-line treatment for metastatic NAM, but data on the efficacy of these therapies remain scarce. The therapeutic effects of immune checkpoint inhibitors could be lower for NAM than for cutaneous melanoma. This review highlights the urgent need to accumulate data to better define the optimal management of this rare melanoma.
1. Introduction
Nail apparatus melanoma (NAM) is a distinct type of cutaneous melanoma occurring in the nail apparatus of the hand and foot, which belongs to the acral melanoma subgroup. Although acral melanoma has similar incidences across different ethnic groups [1,2], it accounts for a higher proportion of melanoma cases in darker-skinned individuals since non-acral melanomas are less common in people of color [3,4]. Complete removal of the tumor at an early stage is curative, while invasive NAM increases the risk of lymph node or distant metastasis. Early detection and prompt therapeutic intervention are therefore important. However, there are several mimics of NAM and the diagnosis may sometimes be delayed. Systemic therapies are required for unresectable or metastatic NAM. In this review article, we present an overview of recent advances in the treatment of NAM and discuss the management of this condition.
2. Materials and Methods
We have conducted a narrative review by browsing the PubMed databases with the keywords “acral”, “nail” and “melanoma”. We have selected original articles, case reports, and review articles written in English. Several reports were also adopted from the References in the selected articles.
3. Epidemiology
NAM, comprising 0.7–3.5% of all cutaneous melanomas [5], accounts for 0.18–2.8% of melanomas in Europeans, 10–23% in Asians, and 25% in African–Americans [6]. NAM typically occurs in older people (mean age at diagnosis of 60–70 years old) [6], but on very rare occasions, pediatric cases have been reported [7,8]. The most commonly affected anatomical locations are the thumbnail and big toenail [9,10]. Common risk factors for cutaneous melanoma, such as fair skin, sun exposure, and a family history of melanoma, do not apply to NAM [11,12]. Instead, trauma, chronic inflammation, and mechanical stress, which are thought to contribute to acral melanoma development [13,14,15,16], may also play some roles in NAM development [17]. A recent report comparing NAM with non-NAM acral melanoma (54 NAMs and 78 non-NAM acral melanomas) reported that patients with NAM were younger at diagnosis, had a higher prevalence of primary melanoma on the hand, and had more frequent reports of previous trauma at the tumor site [18]. Like other types of melanomas, most NAM cases occur in the epidermis (nail bed epithelium) as an in situ tumor. Regarding the prognostic factors, a large cohort study (2050 acral melanomas) revealed that acral melanoma shares the same prognostic factors (age, ulceration, tumor thickness, and tumor spread) as other types of melanomas [19], and NAM presumably has similar prognostic factors [20,21]. A large retrospective study in China (1157 acral melanomas, including 270 NAMs) reported that NAM and palmar acral melanoma had a better prognosis than plantar acral melanoma [21].
4. Diagnosis
4.1. Clinical Findings
NAM at an early stage generally presents longitudinal melanonychia, which is irregular in width and color. In contrast, benign melanocytic nevus, which is a major differential diagnosis, is characterized by brown or black pigmented streaks extending from the proximal nail fold to the distal end of the nail plate. Typical melanocytic nevus is regular in color with a stable width throughout the nail. Extended pigmentation to the surrounding skin (Hutchinson’s sign), widening of melanonychia toward the proximal nail, destruction of the nail plate, bleeding, localization to one nail, and rapid enlargement and darkening during adulthood are all signs suggestive of NAM. However, information beyond the clinical appearance, such as age, history, and involvement of other nails, is important and should be considered when deciding on the appropriate treatment [9,22,23,24,25,26]. Representative images of NAMs are shown in Figure 1.
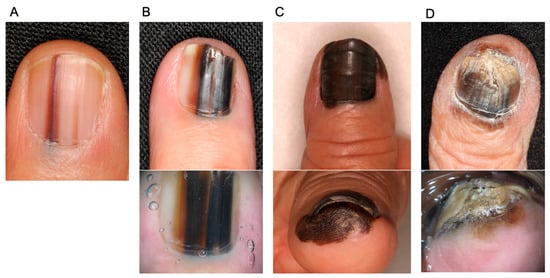
Figure 1.
Representative images of nail apparatus melanoma and dermoscopy (lower images). (A): An early lesion of nail apparatus melanoma. Both of the streaks taper toward the distal end of the nail. (B): An invasive nail apparatus melanoma. The color is highly irregular. (C): An invasive nail apparatus melanoma. The entire thumbnail is deeply pigmented, accompanied by Hutchinson’s sign. (D): An invasive toenail apparatus melanoma. Hutchinson’s sign and nail destruction are evident.
Like melanomas at other sites, dermoscopy improves the diagnostic accuracy of NAM [22,27,28]. Pigmentation of NAM typically involves more than two-thirds of the nail plate, while that of benign melanonychia shows an involvement of less than one-third. Irregularly pigmented streaks, Hutchinson’s and micro-Hutchinson’s signs, and nail destruction are other dermoscopic clues of NAM. Notably, these clues are more common in benign melanonychia of children than that of adults [22,29]. Congenital nevi of the nail matrix may mimic melanoma, but NAM in prepubescent children is exceedingly rare. Therefore, a nail matrix biopsy in children is considered only in exceptional cases [22], since it may permanently deform the nail. A close follow-up is a judicious approach for clinically ambiguous cases.
Clinical differential diagnoses of NAM include a wide range of benign and malignant conditions, namely, subungual hemorrhage, fungal melanonychia, onychopapilloma, onychomatricoma, Bowen’s disease/squamous cell carcinoma in the nail apparatus, and benign melanocytic macules [Figure 2] [30,31,32]. A definitive diagnosis is made by histopathological analysis. For biopsy, the nail matrix area should be sampled since NAM typically arises from melanocytes in the nail matrix [23]. It is generally easy to distinguish NAM from the above-mentioned differential diagnoses, with the exception of melanocytic nevus, since these conditions have distinct histopathological features (e.g., atypical keratinocyte proliferation in Bowen’s disease). The differentiation between NAM and benign melanocytic macules is discussed in Section 4.3. Histopathological findings section.
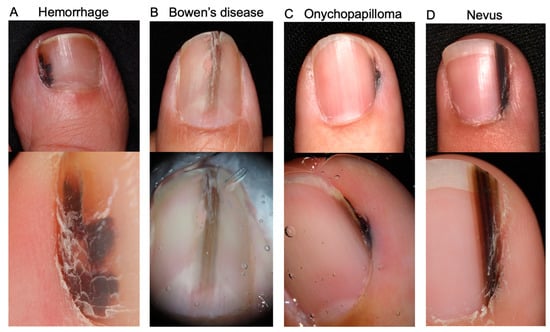
Figure 2.
Representative images of differential diagnoses of nail apparatus melanoma together with their dermoscopy images (lower images). (A): Subungual hemorrhage. Reddish black macules are evident in dermoscopy. (B): Subungual Bowen’s disease. (A) A slightly elevated brown longitudinal streak is observed in the middle of the nail bed. Nail deformity is evident. (C): Onychopapilloma. An irregular melanonychia in the lateral nail fold. (D): Melanocytic nevus. The melanonychia consists of regularly arranged brown to black streaks. No nail deformity or Hutchinson’s sign is observed.
4.2. Treatment of Obtained Samples
Particular attention should be paid to the fixation and decalcification of the obtained tissue so as to preserve the tissue quality at a level suitable for genetic analysis (BRAF mutations, etc.). For example, a fresh surgical tissue sample should be quickly immersed in formalin (ideally within 30 min) but not immersed for a long time (6–72 h).
Specific decalcifying agents should be selected for preserving tumor DNA/RNA. For amputated finger samples, bone samples can be separated before (or after) formalin fixation to minimize the decalcification process, although caution should be taken so as not to damage the tumor in order to accurately measure the tumor thickness.
The use of tissue softeners may be an aid in sectioning nail tissue [33,34].
4.3. Histopathological Findings
Histopathological diagnostic criteria of NAM are similar to those of other types of melanomas. The diagnosis is based on multiple criteria, including architectural and cytological features. Architectural features suggestive of melanoma include asymmetry, poor circumscription, consumption of the epidermis, haphazard interval, and irregular arrangement. Cytological features include nuclear pleomorphism, nuclear variability, and atypical mitoses. Histopathologically, most NAM cases are of the acral lentiginous type [35], which is characterized by the proliferation of atypical melanocytes along the basal layer of the epidermis [36]. At a very early stage, there may be only scattered lentiginous, atypical melanocytes. Lymphocytic infiltrates, pagetoid scatter, and cytological atypia with mitoses then become evident with time [36].
Compared with NAM, nevus in the nail apparatus is relatively small and circumscribed. Although nevus in the nail matrix belongs to the category of “special-site” nevi that may show atypical histopathological features, pagetoid scatter and bridging between rete ridges tend to be sparse. The diagnosis of NAM at an early stage is sometimes challenging since the histopathological changes are subtle [23]. To identify melanocytes and melanoma cells in the nail matrix, immunochemical staining using Melan-A, HMB45, S-100, SOX-10, and MITF is useful [22].
To date, no definitive marker that can distinguish early melanoma from benign melanocytic lesions has been established. A recent study found that p16, HMB45, and Ki-67/Melan-A staining did not distinguish benign activated melanocytes from nail apparatus melanoma in situ [37]. Recently, PRAME, a cancer testis antigen, has attracted attention as a useful marker in the differential diagnosis between benign and malignant melanocytic lesions [38,39,40]. An immunohistochemical study investigating 22 NAMs, 20 non-NAM acral melanomas, and 14 benign acral nevi reported that 73% of NAMs, 95% of non-NAM acral melanomas were positive for PRAME at least in part, whereas only one (7%) acral nevus exhibited PRAME expression [38]. In a study on 127 acral melanocytic lesions (including 20 nail melanocytic lesions), a PRAME expression score calculated by percentage positivity and expression intensity demonstrated good sensitivity and specificity in the diagnosis of acral melanocytic lesions [39]. However, the authors also identified a subset of challenging cases such as acral Spitz nevi, in situ melanomas, and small, thin, invasive melanomas [39]. In contrast, another study comparing 14 benign subungual melanocytic proliferations and 13 in situ NAMs showed that PRAME nuclear immunostaining (cutoff of 10%) exhibited good overall discrimination between benign melanocytic proliferation and NAM in situ [40]. Collectively, although not perfect, PRAME will aid for the diagnosis of NAM under the appropriate cutoff or scoring. Fluorescence in situ hybridization (FISH) can provide additional information [41,42,43], but it may not be routinely available.
4.4. Genetic Findings
Cutaneous melanoma is a tumor rich in genetic alterations [44]. Most such cases have coding and noncoding mutations primarily driven by exposure to ultraviolet (UV) radiation, whereas acral and mucosal melanomas present with fewer mutations of the UV signature [45,46]. Interestingly, melanomas at acral sites show distinct patterns based on the site and relative sun exposure, and accumulating evidence suggests that NAM is distinct from acral melanoma [19,47,48,49]. For instance, a recent study showed evidence of a UV signature in 85.7% (6/7) of NAMs on the fingernails and 33.3% (5/15) of them on the toenails [49]. Mutations found in melanomas on the dorsal hand and foot were reported to be similar to those in melanomas at other sites of intermittently sun-damaged skin, whereas subungual and interdigital melanomas (n = 13) exhibited diverse mutations in PIK3CA, STK11, EGFR, FGFR3, and PTPN11 [48]. Copy number aberrations were common (8/12, 67%), particularly in CDK4 and CCND1 [48]. One study comparing NAM (n = 54) and non-NAM acral melanoma (n = 78) revealed that, among common melanoma driver genes, mutations in KIT and KRAS were predominantly found in NAM, whereas those in BRAF and NRAS occurred almost exclusively in acral melanoma [18]. Regarding the cell cycle pathway, CDK4/CCND1 amplifications were more common in NAM and CDKN2A/B loss occurred mostly in acral melanoma [18].
To summarize the reported cases, observed alterations of major genes are as follows: BRAF, 10.2% (20/196), range 0–42.9%; NRAS, 12.3% (20/162), range 0–30.7%; KIT, 18.7% (36/193), range 0–50.0%; and NF1, 23.1% (18/78), range 0–50.0% [18,44,46,47,48,49,50,51,52,53,54,55,56,57]. Note that these data on genetic alterations are highly heterogeneous, with different sample sizes, different detection methods, etc.
Common genetic mutations observed in NAM, acral melanoma, and cutaneous melanoma are summarized in Table 1.

Table 1.
Comparison of common mutations among melanoma subtypes.
5. Treatment
Surgical eradication is the mainstay in the treatment for melanoma, including NAM. Postoperative adjuvant systemic therapy can be considered for selected patients at a high risk of metastasis. For metastatic NAM, immune checkpoint inhibitors and BRAF-targeted therapy are first-line treatments. Anti-CTLA4 monotherapy or cytotoxic chemotherapy such as dacarbazine have no more been used as first-line. Here, we review the current status of the treatment for NAM.
5.1. Surgical Management
5.1.1. Surgery for Primary Tumor
Complete surgical removal of the tumor is the treatment of choice for NAM. However, surgical procedures are sometimes challenging because of the anatomical complexity of the nail unit [23]. Amputation has been performed to achieve tumor eradication since thick NAM sometimes invades the underlying distal phalanx. For cases with no sign of bone invasion, non-amputative digit-preserving surgery has been utilized for in situ or thin NAM [58,59,60,61,62,63]. This technique preserved limb function and improved cosmetic outcomes without decreasing the survival rate in several retrospective studies [58,59,60,61,62,63]. To confirm the safety and usefulness of digit-preserving surgery, a prospective clinical trial is now underway in Japan (JCOG1602, J-NAIL; UMIN000029997). In this trial, eligible patients have invasive NAM with no sign of bone invasion in X-ray radiography. NAM including the periosteum of the distal phalanx is excised, and the defect is closed by skin grafting after confirming clear margins histopathologically.
Lateral surgical margins may be set in accordance with the current guidelines for cutaneous melanoma [64,65]. For instance, the latest National Comprehensive Cancer Network (NCCN) guidelines recommend surgical margins according to the Breslow thickness of the primary melanoma: 5 mm for in situ melanoma, 10 mm for T1 melanoma, 10–20 mm for T2 melanoma, and 20 mm for T3 and T4 melanoma [64]. However, acral melanoma, including NAM, may show a relatively small nodule surrounded by a widely spreading in situ macule. Extensive excision may require amputation at more proximal sites. Narrow-margin excisions have sometimes been attempted at our institute, which yielded no statistically significant difference in survival on multivariate analyses between narrow-margin excision and NCCN-recommended-margin excision, but these results should be confirmed via the prospective collection of data [66].
5.1.2. Sentinel Lymph Node Biopsy
Sentinel lymph node (SLN) biopsy is a standard procedure for staging patients with NAM who have no clinical metastasis [57,67,68,69,70,71,72,73]. Although reported case series involve relatively small sample sizes, a tumor-positive sentinel lymph node is uncommon in thin melanoma. The indication of SLN biopsy specific for NAM is lacking, so we perform SLN biopsy for patients with NAM of ≥T1b thickness in accordance with current guidelines for melanoma. Another group also recommended SLN biopsy to determine the stage and predict the prognosis [57].
5.1.3. Completion Lymph Node Dissection
Based on the two hallmark trials on completion lymph node dissection for SLN-positive patients, MSLT-II and DeCOG-SLT [74,75], close node observation using ultrasound sonography has become the standard management rather than simultaneous completion lymph node dissection. However, only a few patients with NAM may have been included in these trials, and the results should be interpreted with caution. Decision-making on the treatment may need to be made on a case-by-case basis.
For patients with lymphadenopathy in regional nodes, completion lymph node dissection is recommended [64,65]. Elective lymph node dissection beyond the regional nodes may not be necessary [65].
5.2. Systemic Adjuvant Therapy for Resected NAM
Adjuvant therapy is offered to patients without evidence of macroscopic metastasis but at a high risk of having microscopic metastasis [64,76]. Several clinical trials have been performed in the adjuvant setting of cutaneous melanoma [77,78,79,80], and adjuvant therapy with nivolumab, pembrolizumab, or dabrafenib/trametinib is commonly used for resected stage IIB/C [79], stage III [78,80,81], and stage IV [81] cutaneous melanoma. However, data on the efficacy of these systemic adjuvant therapies for acral melanoma and NAM are still limited. A retrospective cohort study in China investigating 136 patients with stage III acral melanoma compared the outcome among patients treated with adjuvant anti-PD-1 inhibitor (n = 84), adjuvant interferon (n = 18) and those without adjuvant therapy (n = 34) [82]. They found a lower hazard ratio (0.64; 95% confidence interval, 0.40–1.02) of relapse-free survival in the adjuvant anti-PD-1 inhibitor group than the interferon/observation group, although the difference did not reach statistical significance [82]. Another retrospective study on 90 Chinese patients with stage III cutaneous (n = 54) and acral (n = 36) melanoma comparing adjuvant anti-PD-1 inhibitor monotherapy and high-dose interferon α2b found that adjuvant anti-PD-1 treatment yielded significantly better recurrence-free survival (hazard ratio, 0.402; 95% confidence interval, 0.183–0.886) and distant metastasis-free survival (hazard ratio, 0.324; 95% confidence interval, 0.122–0.861) in patients with cutaneous melanoma, but a significant difference was not observed in those with acral melanoma [83]. Furthermore, a multicenter study of 78 Japanese patients with melanoma (including 31 acral melanomas) retrospectively analyzed the efficacy and safety profile of adjuvant anti-PD-1 monotherapy and reported that the acral type had a significantly lower 12-month relapse-free survival than other cutaneous types (p = 0.029). The acral type was an independent worse prognostic factor on multivariate analysis (p = 0.015) [84]. A single-center retrospective study in Japan investigated adjuvant nivolumab (n = 5) and other treatments (n = 22; 12 patients with interferon β, 4 with chemotherapy, 6 with no treatment) and did not find a superior disease-free survival in the adjuvant nivolumab group over the non-nivolumab group [85]. These results suggest that the adjuvant anti-PD-1 inhibitor monotherapy exert an anti-tumor effect for acral melanoma, but may be less effective than for other types of cutaneous melanoma. Data on the efficacy of adjuvant therapy for NAM are currently unavailable.
5.3. Systemic Therapy for Metastatic NAM
Recent advances in immune checkpoint inhibitors and targeted therapy have dramatically changed the management of melanoma and improved patient survival [86,87,88,89,90]. However, it is unclear whether the results of these clinical trials can be applied to patients with NAM, since only a very small number of NAM cases (or none at all) may have been included in these trials (exact data are not available). Although there is a lack of firm evidence of the efficacy of immune checkpoint inhibitors and targeted therapy for NAM, an increasing number of studies on acral melanoma and NAM have recently been reported mainly from Asia, where acral melanomas constitute about half of cutaneous melanoma cases [70,91]. Here, we review these studies and discuss systemic therapy for NAM.
5.3.1. Immune Checkpoint Inhibitors
For unresectable BRAF wild-type melanoma, immune checkpoint inhibitors, including anti-PD-1 monotherapy (nivolumab or pembrolizumab) or combination therapy (nivolumab/ipilimumab or nivolumab/relatlimab) are recommended as first-line treatments [64]. Since most NAM cases have wild-type BRAF, unresectable cases are principally treated with immune checkpoint inhibitors [52,92,93,94]. Cutaneous melanomas are rich in tumor mutations [45], which is a biomarker for the sensitivity to immune checkpoint inhibitors [95]. However, acral melanoma and NAM have a much lower tumor mutation burden [46], so they are likely to be refractory to immune checkpoint inhibitors [96,97,98]. Indeed, a retrospective study (256 cutaneous, 50 acral, 38 mucosal, and 52 unknown primary melanomas) reported that a complete response to an anti-PD-1 agent was uncommon in acral melanoma (12.0%) compared with the rate in cutaneous melanoma (30.9%) [99]. Other studies focusing on immune checkpoint inhibitors for acral melanoma identified low objective response rates [100,101,102,103,104,105,106,107], although these studies involved relatively small sample sizes of acral melanoma (6–39 patients), and the numbers of NAM cases included are not available. Furthermore, immune checkpoint inhibitors could be less effective for NAM than for acral melanoma. One of the largest retrospective studies from Japan (70 NAMs and 123 non-NAM acral melanomas) reported low objective response rate of anti-PD-1 monotherapy: 21.1% in patients with acral melanoma and 8.6% in patients with NAM [108]. The median overall survival in patients with NAM was significantly worse than for those with non-NAM acral melanoma (12.8 vs. 22.3 months; p = 0.03). Another large study with 547 melanomas (including acral melanomas) treated with ipilimumab, however, showed no statistically significant difference of overall survival among melanoma subtypes [109]. A single-arm, open-label, phase II study (CheckMate 172) examined the efficacy and safety profile of nivolumab after the failure of ipilimumab [110]. This study involved a total of 1008 patients, 55 of whom had acral melanoma, and patients with acral melanoma had survival outcomes similar to those of patients with non-acral cutaneous melanoma. There were no meaningful differences in the incidence of grade ≥ 3, treatment-related select adverse events among melanoma subtypes [110]. Taking these findings together, although conflicting results about the efficacy of immune checkpoint inhibitors for acral melanoma and NAM have been reported, these subtypes are potentially refractory to immune checkpoint inhibitors.
In terms of comparisons among immune checkpoint inhibitors for acral melanoma, a systematic review reported the better outcome of the combination therapy of anti-CTLA4 and anti-PD-1 agents (objective response rate: 42.9%) compared with that of each monotherapy [111]. Anti-PD-1 monotherapy showed better objective response rate and progression-free survival than anti-CTLA4 monotherapy (objective response rate 14%–42.9%, progression-free survival 3.2–9.2 months vs. objective response rate 11.4%–25%, progression-free survival 2.1–6.7 months). A recent study involving single-cell RNA sequencing suggested VISTA, ADORA2, TIGIT, and TIM-3 as potential novel immune checkpoints with research value for acral melanoma [112,113].
5.3.2. Targeted Therapy
The latest NCCN guidelines recommend BRAF/MEK inhibitor combination therapy (dabrafenib/trametinib, encorafenib/binimetinib, and vemurafenib/cobimetinib) as one of the first-line treatments for BRAF-mutated cutaneous melanoma [64]. As already mentioned, BRAF mutations are uncommon in acral melanoma (about 20%) and NAM (about 10%), and BRAF/MEK inhibitor therapy is suitable for only a subset of patients [23,52,92,93,94,114]. Evidence on the efficacy of BRAF/MEK inhibitors for acral melanoma, especially NAM, is accordingly limited. A recent multicenter retrospective study in Japan examined the outcome of BRAF/MEK inhibitors. The objective response rate in acral/mucosal melanoma was relatively high, with no statistically significant difference between acral/mucosal (n = 14) and non-acral cutaneous (n = 85) melanoma (64.3% vs. 76.5%) [115]. A retrospective study from South Korea reported similar results (objective response rate of 78.9% in 10 acral/mucosal melanomas) [116]. Furthermore, a single-arm phase II trial in China (12 acral, 41 non-acral cutaneous, and 7 unknown primary melanomas) reported the efficacy of dabrafenib/trametinib combination therapy; the 3-year overall survival was 28.8% in the overall population and 35.7% in acral melanoma patients [117]. These results may imply that BRAF/MEK inhibitors can exert efficacy for acral melanoma (including NAM) comparable to that for cutaneous melanoma. Other case series may also support this notion [108,109,110,111,112,113,114,115,116,117,118,119,120,121,122]. However, the long-term efficacy of targeted therapies remains unclear.
Overall, therapy for unresectable NAM is still challenging, even after the clinical application of targeted therapy and immune checkpoint inhibitors, due to the infrequency of BRAF mutations and resistance to immunotherapy. Other targeted therapies, including dasatinib, imatinib mesylate, nilotinib, sunitinib, and CDK4/6 inhibitor, have also been applied to acral melanoma [2].
5.3.3. New Classes of Therapeutic Antibodies
Besides naked therapeutic antibodies (anti-PD-1 antibody, etc.), several new classes of antibody drugs, which have been modified to enhance their therapeutic value, have been utilized for solid cancer therapy. These new classes include bispecific antibodies, chimeric antigen receptor T-cell (CAR-T) therapies, and antibody-drug conjugates (ADCs). In this section, we summarize the current knowledge of these novel therapies for melanoma treatment.
In 2021, the results of a randomized controlled study of tebentafusp for the treatment of metastatic uveal melanoma were published [123]. Tebentafusp is a bispecific protein consisting of an affinity-enhanced T-cell receptor fused to an anti-CD3 effector and can redirect T cells to target glycoprotein 100-positive cells (melanoma cells and activated melanocytes). In this trial, a total of 378 patients were randomly assigned to either the tebentafusp group (252 patients) or the control group (126 patients). The control group received the investigator’s choice of therapy with single-agent pembrolizumab, ipilimumab, or dacarbazine. Treatment with tebentafusp resulted in longer overall survival than the control therapy; overall survival at 1 year was 73% in the tebentafusp group and 59% in the control group (hazard ratio for death, 0.51; 95% confidence interval, 0.37 to 0.71; p < 0.001) and progression-free survival was 31% in the tebentafusp group and 19% in the control group at 6 months (hazard ratio for disease progression or death, 0.73; 95% confidence interval, 0.58 to 0.94; p = 0.01). Treatment-related adverse events were generally tolerable [123]. Based on these results, tebentafusp has been approved by the FDA for the treatment of metastatic uveal melanoma. A clinical trial for its use in metastatic cutaneous melanoma is also now ongoing (NCT02535078). Although limited to patients with HLA-A*02:01, tebentafusp is a potential therapy for metastatic melanoma, including NAM.
CAR-T therapies were first applied to hematological malignancies and have been vigorously expanded to solid tumors [124,125]. T-cell receptors of CAR-T cells are artificially engineered to redirect them to a specific antigen. CAR-T cells kill tumor cells by recognizing target antigens on their surface in a non-MHC-restricted manner [125]. Many preclinical and clinical trials of CAR-T therapy with various potential targets in melanoma have been carried out, with targets including VEGFR2, GD2, cMet, hCD70, gp100, NY-ESO-1, CD20, IL13R-α2, B7H3, and CD19 [123]. All of these trials involved phase I or II non-randomized or single-arm trials, so further investigation is needed [125].
ADCs are another emerging class of therapeutics consisting of a monoclonal antibody linked to a cytotoxic agent through a linker. Upon binding with the cell surface antigen, ADCs are internalized by tumor cells and processed by the endolysosomal system, the linker then being cleaved. The cytotoxic drug is released into the cytoplasm and induces apoptosis of the cell via its cytotoxicity. ADCs have historically been used for hematological malignancies (e.g., ADCs targeting CD30, CD79b, CD33, CD22, CD19, and CD38) but have recently been rapidly expanded to solid tumors [126,127]. FDA-approved ADCs for solid tumors include trastuzumab emtansine and trastuzumab deruxtecan (anti-HER2 ADCs) for breast cancer, enfortumab vedotin (anti-NECTIN-4 ADC) for urothelial cancer, sacituzumab govitecan (anti-TROP-2 ADC) for breast cancer, and tisotumab vedotin (anti-tissue factor ADC) for cervical cancer. Interestingly, NECTIN-4 and TROP-2 are highly expressed in normal skin and its appendages, as well as in various skin cancers [128,129,130,131,132,133,134,135], and anti-NECTIN-4 ADC might be a candidate for unresectable skin cancers, including NAM. Several melanoma-specific ADCs are currently undergoing preclinical and clinical trials. Reported target antigens are gpNMB, PMEL17, HER3, endothelin B receptor, c-KIT, and anexelekto [126,136]. Although no NAM-specific ADCs have been reported, these antigens are likely to be expressed in NAM.
6. Conclusions
This paper has focused on the current management and future perspectives of NAM. Although the diagnosis of NAM at an early stage is sometimes challenging, the diagnosis of advanced NAM is well-established histopathologically. Immunohistochemistry and FISH may provide additional clues to discriminate NAM from benign melanocytic lesions. Dermoscopy can benefit the correct diagnosis of NAM, although standardized treatment guidelines for NAM are lacking. At present, it may be most appropriate to administer treatments in accordance with current guidelines for cutaneous melanoma. A current treatment algorithm for NAM in our institute is given in Figure 3. However, this algorithm should be updated whenever firm evidence comes out, or novel agents are available. Prospective data on the safety profile of digit-preserving surgery will soon become available. Evidence on the efficacy of systemic therapy for unresectable NAM is scarce, but immune checkpoint inhibitors and BRAF/MEK inhibitors have superseded conventional cytotoxic chemotherapy. A lot of novel agents have vigorously been tried for melanoma treatment. Recent genetic analyses have suggested that NAM may differ from cutaneous melanoma and even from acral melanoma. The accumulation of data to better define the optimal management of this uncommon type of melanoma is highly desirable.
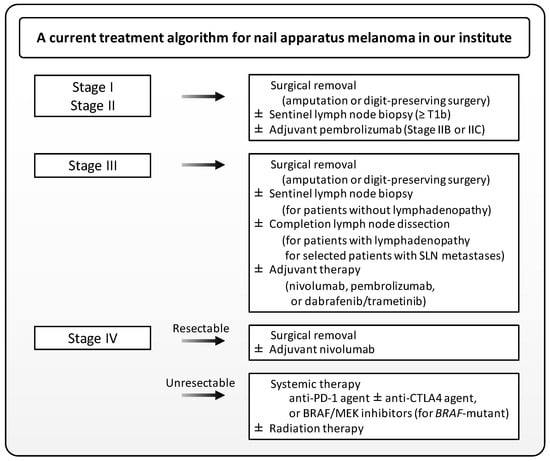
Figure 3.
A treatment algorithm for nail apparatus melanoma in our institute. Note that not all FDA-approved drugs are available in Japan. SLN, sentinel lymph node.
Author Contributions
Conceptualization, T.I.; methodology, T.I., H.H. and Y.K.-I.; validation, T.I.; formal analysis, T.I. and Y.K.-I.; investigation, T.I., H.H. and Y.K.-I.; resources, T.I.; data curation, T.I.; writing—original draft preparation, T.I.; writing—review and editing, H.H., Y.K.-I., Y.T. and T.N.; visualization, T.I.; supervision, project administration, T.I. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI, grant number JP 22K15543.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We wish to thank our patients, as well as all members of our laboratory, for their helpful advice.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Falotico, J.M.; Lipner, S.R. The pharmacotherapeutic management of nail unit and acral melanomas. Expert Opin. Pharmacother. 2022, 23, 1273–1289. [Google Scholar] [CrossRef] [PubMed]
- Kibbi, N.; Kluger, H.; Choi, J.N. Melanoma: Clinical presentations. Melanoma 2015, 167, 107–129. [Google Scholar] [CrossRef]
- Bradford, P.T.; Goldstein, A.M.; McMaster, M.L.; Tucker, M.A. Acral lentiginous melanoma: Incidence and survival patterns in the United States, 1986–2005. Arch. Dermatol. 2009, 145, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.A.; Adamson, A.S.; Halpern, A.C. Melanoma and racial health disparities in black individuals-facts, fallacies, and fixes. JAMA Dermatol. 2021, 157, 1031–1032. [Google Scholar] [CrossRef]
- De Giorgi, V.; Saggini, A.; Grazzini, M.; Gori, A.; Rossari, S.; Scarfì, F.; Verdelli, A.; Chimenti, S.; Lotti, T.; Massi, D. Specific challenges in the management of subungual melanoma. Expert Rev. Anticancer. Ther. 2011, 11, 749–761. [Google Scholar] [CrossRef]
- Nevares-Pomales, O.W.; Sarriera-Lazaro, C.J.; Barrera-Llaurador, J.; Santiago-Vazquez, M.; Lugo-Fagundo, N.; Sanchez, J.E.; Sanchez, J.L. Pigmented lesions of the nail unit. Am. J. Dermatopathol. 2018, 40, 793–804. [Google Scholar] [CrossRef]
- Tosti, A.; Piraccini, B.M.; Cagalli, A.; Haneke, E. In situ melanoma of the nail unit in children: Report of two cases in fair-skinned caucasian children. Pediatr. Dermatol. 2011, 29, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Tosti, A.; Di Chiacchio, N.; Hirata, S.H.; Misciali, C.; Michalany, N.; Domiguez, J.; Toussaint, S. Nail melanoma in children: Differential diagnosis and management. Dermatol. Surg. 2008, 34, 974–978. [Google Scholar] [CrossRef]
- Levit, E.K.; Kagen, M.H.; Scher, R.K.; Grossman, M.; Altman, E. The ABC rule for clinical detection of subungual melanoma. J. Am. Acad. Dermatol. 2000, 42, 269–274. [Google Scholar] [CrossRef]
- Jellinek, N.J.; Lipner, S.R. Longitudinal erythronychia: Retrospective single-center study evaluating differential diagnosis and the likelihood of malignancy. Dermatol. Surg. 2016, 42, 310–319. [Google Scholar] [CrossRef]
- Phan, A.; Touzet, S.; Dalle, S.; Ronger-Savlé, S.; Balme, B.; Thomas, L. Acral lentiginous melanoma: A clinicoprognostic study of 126 cases. Br. J. Dermatol. 2006, 155, 561–569. [Google Scholar] [CrossRef]
- Kuchelmeister, C.; Schaumburg-Lever, G.; Garbe, C. Acral cutaneous melanoma in caucasians: Clinical features, histopathology and prognosis in 112 patients. Br. J. Dermatol. 2000, 143, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Nagore, E.; Pereda, C.; Botella-Estrada, R.; Requena, C.; Guillén, C. Acral lentiginous melanoma presents distinct clinical profile with high cancer susceptibility. Cancer Causes Control 2008, 20, 115–119. [Google Scholar] [CrossRef]
- Jung, H.J.; Kweon, S.S.; Lee, J.B.; Lee, S.C.; Yun, S.J. A clinicopathologic analysis of 177 acral melanomas in Koreans: Relevance of spreading pattern and physical stress. JAMA Dermatol. 2013, 149, 1281–1288. [Google Scholar]
- Minagawa, A.; Omodaka, T.; Okuyama, R. Melanomas and mechanical stress points on the plantar surface of the foot. N. Engl. J. Med. 2016, 374, 2404–2406. [Google Scholar] [CrossRef]
- Sheen, Y.-S.; Liao, Y.-H.; Lin, M.-H.; Chen, J.-S.; Liau, J.-Y.; Tseng, Y.-J.; Lee, C.-H.; Chang, Y.-L.; Chu, C.-Y. A clinicopathological analysis of 153 acral melanomas and the relevance of mechanical stress. Sci. Rep. 2017, 7, 1–6. [Google Scholar] [CrossRef]
- Choi, M.E.; Cho, H.; Won, C.H.; Chang, S.E.; Lee, M.W.; Lee, W.J. Clinicopathologic characteristics of trauma-related nail apparatus melanoma: A comparative study according to the presence of trauma prior to melanoma development. Dermatology 2022, 239, 165–173. [Google Scholar] [CrossRef]
- Holman, B.N.; Van Gulick, R.J.; Amato, C.M.; Macbeth, M.L.; Davies, K.D.; Aisner, D.L.; Robinson, W.A.; Couts, K.L. Clinical and molecular features of subungual melanomas are site-specific and distinct from acral melanomas. Melanoma Res. 2020, 30, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, Y.; Keim, U.; Gesierich, A.; Schuler, G.; Fiedler, E.; Tüting, T.; Ulrich, C.; Wollina, U.; Hassel, J.; Gutzmer, R.; et al. Acral lentiginous melanoma: A skin cancer with unfavourable prognostic features. A study of the German central malignant melanoma registry (CMMR) in 2050 patients. Br. J. Dermatol. 2017, 178, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Kim, H.; Kwon, S.T.; Jo, S.J.; Mun, J.-H.; Lee, C.; Kwak, Y.; Kim, B.J. Tumor invasion in the hyponychium is associated with distant metastasis and poor prognosis in subungual melanoma: A histologic landscape of 44 cases. J. Am. Acad. Dermatol. 2021, 86, 1027–1034. [Google Scholar] [CrossRef]
- Wei, X.; Wu, D.; Li, H.; Zhang, R.; Chen, Y.; Yao, H.; Chi, Z.; Sheng, X.; Cui, C.; Bai, X.; et al. The clinicopathological and survival profiles comparison across primary sites in acral melanoma. Ann. Surg. Oncol. 2020, 27, 3478–3485. [Google Scholar] [CrossRef]
- Weber, P.; Tschandl, P.; Sinz, C.; Kittler, H. Dermatoscopy of neoplastic skin lesions: Recent advances, updates, and revisions. Curr. Treat. Options Oncol. 2018, 19, 1–17. [Google Scholar] [CrossRef]
- Darmawan, C.C.; Ohn, J.; Mun, J.; Kim, S.; Lim, Y.; Jo, S.J.; Kim, Y.; Kim, B.; Seong, M.; Lee, C.; et al. Diagnosis and treatment of nail melanoma: A review of the clinicopathologic, dermoscopic, and genetic characteristics. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 651–660. [Google Scholar] [CrossRef]
- Mannava, K.A.; Mannava, S.; Koman, L.A.; Robinson-Bostom, L.; Jellinek, N. Longitudinal melanonychia: Detection and management of nail melanoma. Hand Surg. 2013, 18, 133–139. [Google Scholar] [CrossRef]
- Barros, A.M.; Duarte, A.F.; Haneke, E.; Correia, O.; Ventura, F. Nail melanoma in situ: Clinical, dermoscopic, pathologic clues, and steps for minimally invasive treatment. Dermatol. Surg. 2015, 41, 59–68. [Google Scholar] [CrossRef]
- Goettmann, S.; Moulonguet, I.; Zaraa, I. In situ nail unit melanoma: Epidemiological and clinic-pathologic features with conservative treatment and long-term follow-up. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 2300–2306. [Google Scholar] [CrossRef]
- Thomas, L.; Dalle, S. Dermoscopy provides useful information for the management of melanonychia striata. Dermatol. Ther. 2007, 20, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Benati, E.; Ribero, S.; Longo, C.; Piana, S.; Puig, S.; Carrera, C.; Cicero, F.; Kittler, H.; Deinlein, T.; Zalaudek, I.; et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: An international dermoscopy society study. J. Eur. Acad. Dermatol. Venereol. 2016, 31, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Ohn, J.; Choe, Y.S.; Mun, J.-H. Dermoscopic features of nail matrix nevus (NMN) in adults and children: A comparative analysis. J. Am. Acad. Dermatol. 2016, 75, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Uchi, H.; Yamada, Y.; Oda, Y.; Furue, M. Onychopapilloma manifesting longitudinal melanonychia: A mimic of subungual malignancy. J. Dermatol. 2015, 42, 1199–1201. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Ito, T.; Yamada, Y.; Oda, Y.; Furue, M. Onychopapilloma presenting as longitudinal melanonychia: A case report and literature review. Australas. J. Dermatol. 2021, 62, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Peruilh-Bagolini, L.; Dossi, M.T.; Wortsman, X.; Montero, T. Pigmented onychomatricoma: A clinical simulator that could not mislead ultrasound. Acta Bio-Med. Atenei Parm. 2021, 92, e2021158–e20211582021. [Google Scholar] [CrossRef]
- Orchard, G.E.; Torres, J.; Sounthararajah, R. Use of softening agents to improve the production of formalin-fixed, paraffin-embedded sections of nail tissue: An assessment. Br. J. Biomed. Sci. 2008, 65, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Orchard, G.E.; Torres, J.; Poirier, A.; Sounthararajah, R.; Webster, J.; Notini, L.; Hacker, L.; Ismail, F.; Nwokie, T.; Humphrey, P.; et al. Investigation into a new softening agent for use on formalin-fixed, paraffin wax-embedded tissue. Br. J. Biomed. Sci. 2009, 66, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.B.; Moncrieff, M.; Thompson, J.F.; McCarthy, S.W.; Shaw, H.M.; Quinn, M.J.; Li, L.X.; Crotty, K.A.; Stretch, J.R.; Scolyer, R.A. Subungual melanoma: A study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am. J. Surg. Pathol. 2007, 31, 1902–1912. [Google Scholar]
- Yun, S.J.; Bastian, B.C.; Duncan, L.M.; Haneke, E.; Uhara, H. Acral melanoma. In WHO Classification of Skin Tumours, 4th ed.; Elder, D.E., Massi, D., Scolyer, R.A., Willemze, R., Eds.; IARC Press: Lyon, France, 2018; pp. 116–118. [Google Scholar]
- Chu, A.; André, J.; Rich, P.; Leachman, S.; Thompson, C.T. Immunohistochemical characterization of benign activation of junctional melanocytes and melanoma in situ of the nail unit. J. Cutan. Pathol. 2019, 46, 479–483. [Google Scholar] [CrossRef]
- Rothrock, A.T.; Torres-Cabala, C.A.; Milton, D.R.; Cho, W.C.; Nagarajan, P.; Vanderbeck, K.; Curry, J.L.; Ivan, D.; Prieto, V.G.; Aung, P.P. Diagnostic utility of PRAME expression by immunohistochemistry in subungual and non-subungual acral melanocytic lesions. J. Cutan. Pathol. 2022, 49, 859–867. [Google Scholar] [CrossRef]
- Santandrea, G.; Valli, R.; Zanetti, E.; Ragazzi, M.; Pampena, R.; Longo, C.; Lai, M.; Piana, S.; Cesinaro, A.M. Comparative analysis of PRAME expression in 127 acral and nail melanocytic lesions. Am. J. Surg. Pathol. 2022, 46, 579–590. [Google Scholar] [CrossRef]
- Kim, Y.J.; Jung, C.J.; Na, H.; Lee, W.J.; Chang, S.E.; Lee, M.W.; Park, C.-S.; Lim, Y.; Won, C.H. Cyclin D1 and PRAME expression in distinguishing melanoma in situ from benign melanocytic proliferation of the nail unit. Diagn. Pathol. 2022, 17, 1–10. [Google Scholar] [CrossRef]
- Ren, M.; Ren, J.; Cai, X.; Shen, X.X.; Kong, J.C.; Dai, B.; Kong, Y.Y. Clinicopathological, Immunohistochemical and fluorescence in-situ hybridisation features of early subungual melanoma: An analysis of 65 cases. Histopathology 2021, 78, 717–726. [Google Scholar]
- Romano, R.C.; Shon, W.; Sukov, W.R. Malignant melanoma of the nail apparatus: A fluorescence in situ hybridization analysis of 7 cases. Int. J. Surg. Pathol. 2016, 24, 512–518. [Google Scholar]
- Shojiguchi, N.; Takai, S.; Arai, E. Hyperplastic melanocytes with chromosomal aberrations in surrounding skin of subungual melanoma: Fluorescence in situ hybridisation analysis using whole-slide digital imaging. Histopathology 2022, 81, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Curtin, J.A.; Fridlyand, J.; Kageshita, T.; Patel, H.N.; Busam, K.J.; Kutzner, H.; Cho, K.-H.; Aiba, S.; Bröcker, E.-B.; LeBoit, P.E.; et al. Distinct sets of genetic alterations in melanoma. N. Engl. J. Med. 2005, 353, 2135–2147. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Hayward, N.K.; Wilmott, J.S.; Waddell, N.; Johansson, P.A.; Field, M.A.; Nones, K.; Patch, A.-M.; Kakavand, H.; Alexandrov, L.B.; Burke, H.; et al. Whole-genome landscapes of major melanoma subtypes. Nature 2017, 545, 175–180. [Google Scholar] [CrossRef]
- Lee, M.; Yoon, J.; Chung, Y.-J.; Lee, S.Y.; Choi, J.Y.; Shin, O.R.; Park, H.Y.; Bahk, W.-J.; Yu, D.S.; Lee, Y.B. Whole-exome sequencing reveals differences between nail apparatus melanoma and acral melanoma. J. Am. Acad. Dermatol. 2018, 79, 559–561.e1. [Google Scholar] [CrossRef] [PubMed]
- Haugh, A.M.; Zhang, B.; Quan, V.L.; Garfield, E.M.; Bubley, J.A.; Kudalkar, E.; Verzi, A.E.; Walton, K.; VandenBoom, T.; Merkel, E.A.; et al. Distinct patterns of acral melanoma based on site and relative sun exposure. J. Investig. Dermatol. 2018, 138, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Newell, F.; Wilmott, J.S.; Johansson, P.A.; Nones, K.; Addala, V.; Mukhopadhyay, P.; Broit, N.; Amato, C.M.; Van Gulick, R.; Kazakoff, S.H.; et al. Whole-genome sequencing of acral melanoma reveals genomic complexity and diversity. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Dai, B.; Cai, X.; Kong, Y.-Y.; Yang, F.; Shen, X.-X.; Wang, L.-W.; Kong, J.-C. Analysis of KIT expression and gene mutation in human acral melanoma: With a comparison between primary tumors and corresponding metastases/recurrences. Hum. Pathol. 2013, 44, 1472–1478. [Google Scholar] [CrossRef]
- Dika, E.; Altimari, A.; Patrizi, A.; Gruppioni, E.; Fiorentino, M.; Piraccini, B.M.; Misciali, C.; Barisani, A.; Fanti, P.A. KIT, NRAS, and BRAF mutations in nail apparatus melanoma. Pigment. Cell Melanoma Res. 2013, 26, 758–760. [Google Scholar] [CrossRef]
- Sakaizawa, K.; Ashida, A.; Uchiyama, A.; Ito, T.; Fujisawa, Y.; Ogata, D.; Matsushita, S.; Fujii, K.; Fukushima, S.; Shibayama, Y.; et al. Clinical characteristics associated with BRAF, NRAS and KIT mutations in Japanese melanoma patients. J. Dermatol. Sci. 2015, 80, 33–37. [Google Scholar] [CrossRef]
- Moon, K.R.; Choi, Y.D.; Kim, J.M.; Jin, S.; Shin, M.-H.; Shim, H.-J.; Lee, J.-B.; Yun, S.J. Genetic alterations in primary acral melanoma and acral melanocytic nevus in Korea: Common mutated genes show distinct cytomorphological features. J. Investig. Dermatol. 2018, 138, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Sheen, Y.-S.; Tan, K.-T.; Tse, K.-P.; Liao, Y.-H.; Lin, M.-H.; Chen, J.-S.; Liau, J.-Y.; Tseng, Y.-J.; Lee, C.-H.; Hong, C.-B.; et al. Genetic alterations in primary melanoma in Taiwan. Br. J. Dermatol. 2020, 182, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Yoon, D.; Lee, D.-Y. Novel mutations identified by whole-exome sequencing in acral melanoma. J. Am. Acad. Dermatol. 2020, 83, 1792–1794. [Google Scholar] [CrossRef] [PubMed]
- Borkowska, A.; Szumera-Ciećkiewicz, A.; Spałek, M.; Teterycz, P.; Czarnecka, A.; Kowalik, A.; Rutkowski, P. Mutation profile of primary subungual melanomas in Caucasians. Oncotarget 2020, 11, 2404–2413. [Google Scholar] [CrossRef]
- Reilly, D.; Aksakal, G.; Gilmour, R.; Gyorki, D.; Chauhan, A.; Webb, A.; Henderson, M. Subungual melanoma: Management in the modern era. J. Plast. Reconstr. Aesthetic Surg. 2017, 70, 1746–1752. [Google Scholar] [CrossRef]
- Cochran, A.M.; Buchanan, P.J.; Bueno, R.A., Jr.; Neumeister, M.W. Subungual melanoma: A review of current treatment. Plast. Reconstr. Surg. 2014, 134, 259–273. [Google Scholar]
- Sureda, N.; Phan, A.; Poulalhon, N.; Balme, B.; Dalle, S.; Thomas, L. Conservative surgical management of subungual (matrix derived) melanoma: Report of seven cases and literature review. Br. J. Dermatol. 2011, 165, 852–858. [Google Scholar] [CrossRef]
- Chow, W.; Bhat, W.; Magdub, S.; Orlando, A. In situ subungual melanoma: Digit salvaging clearance. J. Plast. Reconstr. Aesthetic Surg. 2013, 66, 274–276. [Google Scholar] [CrossRef]
- Cohen, T.; Busam, K.J.; Patel, A.; Brady, M.S. Subungual melanoma: Management considerations. Am. J. Surg. 2008, 195, 244–248. [Google Scholar] [CrossRef]
- Zhang, J.; Yun, S.J.; McMurray, S.L.; Miller, C.J. Management of nail unit melanoma. Dermatol. Clin. 2021, 39, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Ogata, D.; Uhara, H.; Tsutsumida, A.; Yamazaki, N.; Mochida, K.; Amano, M.; Yoshikawa, S.; Kiyohara, Y.; Tsuchida, T. Nail apparatus melanoma in a Japanese population: A comparative study of surgical procedures and prognoses in a large series of 151 cases. Eur. J. Dermatol. 2017, 27, 620–626. [Google Scholar] [CrossRef]
- NCCN Guidelines Version 3.2020 Cutaneous Melanoma. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf (accessed on 16 June 2020).
- Nakamura, Y.; Asai, J.; Igaki, H.; Inozume, T.; Namikawa, K.; Hayashi, A.; Fukushima, S.; Fujimura, T.; Ito, T.; Imafuku, K.; et al. Japanese dermatological association guidelines: Outlines of guidelines for cutaneous melanoma 2019. J. Dermatol. 2019, 47, 89–103. [Google Scholar] [CrossRef]
- Ito, T.; Kaku-Ito, Y.; Wada-Ohno, M.; Furue, M. Narrow-margin excision for invasive acral melanoma: Is it acceptable? J. Clin. Med. 2020, 9, 2266. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Wada, M.; Nagae, K.; Nakano-Nakamura, M.; Nakahara, T.; Hagihara, A.; Furue, M.; Uchi, H. Acral lentiginous melanoma: Who benefits from sentinel lymph node biopsy? J. Am. Acad. Dermatol. 2015, 72, 71–77. [Google Scholar] [CrossRef] [PubMed]
- LaRocca, C.J.; Lai, L.; Nelson, R.A.; Modi, B.; Crawford, B. Subungual melanoma: A single institution experience. Med. Sci. 2021, 9, 57. [Google Scholar] [CrossRef]
- Ito, T.; Moroi, Y.; Oba, J.; Nakahara, T.; Takeuchi, S.; Uchi, H.; Takahara, M.; Masuda, T.; Furue, M. The prognostic value of a reverse transcriptase-PCR assay of sentinel lymph node biopsy for patients with cutaneous melanoma: A single-center analysis in Japan. Melanoma Res. 2012, 22, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Ito, T.; Tsuji, G.; Nakahara, T.; Hagihara, A.; Furue, M.; Uchi, H. Acral lentiginous melanoma versus other melanoma: A single-center analysis in Japan. J. Dermatol. 2017, 44, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yeh, J.; Shen, S.; Lo, Y.; Kuo, T.; Chang, J.W.C. Regressed subungual melanoma simulating cellular blue nevus: Managed with sentinel lymph node biopsy. Dermatol. Surg. 2006, 32, 577–581. [Google Scholar] [CrossRef]
- Chakera, A.H.; Quinn, M.J.; Lo, S.; Drummond, M.; Haydu, L.E.; Bond, J.S.; Stretch, J.R.; Saw, R.P.M.; Lee, K.J.; McCarthy, W.H.; et al. Subungual melanoma of the hand. Ann. Surg. Oncol. 2018, 26, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Wada, M.; Nagae, K.; Nakano-Nakamura, M.; Nakahara, T.; Hagihara, A.; Furue, M.; Uchi, H. Triple-marker PCR assay of sentinel lymph node as a prognostic factor in melanoma. J. Eur. Acad. Dermatol. Venereol. 2014, 29, 912–918. [Google Scholar] [CrossRef]
- Faries, M.B.; Thompson, J.F.; Cochran, A.J.; Andtbacka, R.H.; Mozzillo, N.; Zager, J.S.; Jahkola, T.; Bowles, T.L.; Testori, A.; Beitsch, P.D.; et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N. Engl. J. Med. 2017, 376, 2211–2222. [Google Scholar] [CrossRef]
- Leiter, U.; Stadler, R.; Mauch, C.; Hohenberger, W.; Brockmeyer, N.H.; Berking, C.; Sunderkötter, C.; Kaatz, M.; Schatton, K.; Lehmann, P.; et al. German dermatologic cooperative oncology group (2019). Final analysis of DeCOG-SLT trial: No survival benefit for complete lymph node dissection in patients with melanoma with positive sentinel node. J. Clin. Oncol. 2019, 37, 3000–3008. [Google Scholar] [CrossRef] [PubMed]
- Wada-Ohno, M.; Ito, T.; Furue, M. Adjuvant therapy for melanoma. Curr. Treat. Options Oncol. 2019, 20, 63. [Google Scholar] [CrossRef]
- Weber, J.; Mandalà, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef]
- Long, G.V.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Chiarion-Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; Haydon, A.; et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N. Engl. J. Med. 2017, 377, 1813–1823. [Google Scholar] [CrossRef]
- Luke, P.R.J.J.; Queirolo, P.; Del Vecchio, M.; Mackiewicz, J.; Chiarion Sileni, V.; de la Cruz Merino, L.; Khattak, M.A.; Shadendorf, D.; Long, G.V.; Ascierto, P.A.; et al. LBA3 PR-pembrolizumab versus placebo after complete resection of high-risk stage II melanoma: Efficacy and safety results from the KEYNOTE-716 double-blind phase III trial. Ann. Oncol. 2021, 32, S1283e346. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Blank, C.U.; Mandalà, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, M.; Carlino, M.S.; et al. Pembrolizumab versus placebo after complete resection of high-risk stage III melanoma: New recurrence-free survival results from the EORTC 1325-MG/Keynote 054 double-blinded phase III trial at three-year median follow-up. J. Clin. Oncol. 2020, 38, 10000. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Del Vecchio, M.; Mandalá, M.; Gogas, H.; Arance, A.M.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.J.; Chiarion-Sileni, V.; et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xu, Y.; Sun, W.; Yan, W.; Wang, C.; Hu, T.; Zhang, X.; Luo, Z.; Liu, X.; Chen, Y. Adjuvant Anti-PD-1 Immunotherapy versus conventional therapy for stage III melanoma: A real-world retrospective cohort study. Pharmaceuticals 2022, 16, 41. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Liu, X.; Wen, X.; Li, D.; Ding, Y.; Jiang, H.; Huang, F.; Zhang, X. Adjuvant PD-1 inhibitor versus high-dose interferon α-2b for Chinese patients with cutaneous and acral melanoma: A retrospective cohort analysis. J. Clin. Oncol. 2021, 39, e21516–e215162021. [Google Scholar] [CrossRef]
- Muto, Y.; Kambayashi, Y.; Kato, H.; Fukushima, S.; Ito, T.; Maekawa, T.; Fujisawa, Y.; Yoshino, K.; Uchi, H.; Matsushita, S.; et al. Adjuvant anti-PD-1 antibody therapy for advanced melanoma: A multicentre study of 78 japanese cases. Acta Derm.-Venereol. 2022, 102, adv00756. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Yanagi, T.; Miyamoto, K.; Tokuchi, K.; Kitamura, S.; Ujiie, H. Adjuvant nivolumab therapy may not improve disease-free survival in resected acral lentiginous melanoma patients: A retrospective case series. Dermatol. Ther. 2022, 35, e15817. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Ito, T.; Wada, N.; Wada, M.; Kadono, T.; Uchi, H. Melanoma and immune checkpoint inhibitors. Curr. Oncol. Rep. 2018, 20, 29. [Google Scholar] [CrossRef]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.; Weber, J.S.; et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann. Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion Sileni, V.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef]
- Dummer, R.; Flaherty, K.T.; Robert, C.; Arance, A.; de Groot, J.W.B.; Garbe, C.; Gogas, H.J.; Gutzmer, R.; Krajsová, I.; Liszkay, G.; et al. COLUMBUS 5-year update: A randomized, open-label, phase III trial of encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF V600-mutant melanoma. J. Clin. Oncol. 2022, 40, 4178–4188. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Fujisawa, Y. Diagnosis and management of acral lentiginous melanoma. Curr. Treat. Options Oncol. 2018, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Yeh, I.; Jorgenson, E.; Shen, L.; Xu, M.; North, J.P.; Shain, A.H.; Reuss, D.; Wu, H.; Robinson, W.A.; Olshen, A.; et al. Targeted genomic profiling of acral melanoma. Gynecol. Oncol. 2019, 111, 1068–1077. [Google Scholar] [CrossRef]
- Broit, N.; Johansson, P.A.; Rodgers, C.B.; Walpole, S.T.; Hayward, N.K.; Pritchard, A.L. Systematic review and meta-analysis of genomic alterations in acral melanoma. Pigment. Cell Melanoma Res. 2022, 35, 369–386. [Google Scholar] [CrossRef]
- Zebary, A.; Omholt, K.; Vassilaki, I.; Höiom, V.; Lindén, D.; Viberg, L.; Kanter-Lewensohn, L.; Johansson, C.H.; Hansson, J. KIT, NRAS, BRAF and PTEN mutations in a sample of Swedish patients with acral lentiginous melanoma. J. Dermatol. Sci. 2013, 72, 284–289. [Google Scholar] [CrossRef]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor mutational burden and response rate to PD-1 inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef]
- Cho, K.K.; Cust, A.E.; Foo, Y.M.; Long, G.V.; Menzies, A.M.; Eslick, G.D. Metastatic acral melanoma treatment outcomes: A systematic review and meta-analysis. Melanoma Res. 2021, 31, 482–486. [Google Scholar] [CrossRef]
- Fujimura, T.; Muto, Y.; Asano, Y. Immunotherapy for melanoma: The Significance of immune checkpoint inhibitors for the treatment of advanced melanoma. Int. J. Mol. Sci. 2022, 23, 15720. [Google Scholar] [CrossRef]
- Mao, L.; Qi, Z.; Zhang, L.; Guo, J.; Si, L. Immunotherapy in acral and mucosal melanoma: Current status and future directions. Front. Immunol. 2021, 12, 680407. [Google Scholar] [CrossRef]
- Warner, A.B.; Palmer, J.S.; Shoushtari, A.N.; Goldman, D.A.; Panageas, K.S.; Hayes, S.A.; Bajwa, R.; Momtaz, P.; Callahan, M.K.; Wolchok, J.D.; et al. Long-term outcomes and responses to retreatment in patients with melanoma treated with PD-1 blockade. J. Clin. Oncol. 2020, 38, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Ogata, D.; Haydu, L.E.; Glitza, I.C.; Patel, S.P.; Tawbi, H.A.; McQuade, J.L.; Diab, A.; Ekmekcioglu, S.; Wong, M.K.; Davies, M.A.; et al. The efficacy of anti-programmed cell death protein 1 therapy among patients with metastatic acral and metastatic mucosal melanoma. Cancer Med. 2021, 10, 2293–2299. [Google Scholar] [CrossRef] [PubMed]
- Shoushtari, A.N.; Munhoz, R.R.; Kuk, D.; Ott, P.A.; Johnson, D.B.; Tsai, K.K.; Rapisuwon, S.; Eroglu, Z.; Sullivan, R.J.; Luke, J.J.; et al. The efficacy of anti-PD-1 agents in acral and mucosal melanoma. Cancer 2016, 122, 3354–3362. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Yoshino, K.; Nagai, K.; Oaku, S.; Kato, M.; Hiura, A.; Hata, H. Efficacy of nivolumab monotherapy against acral lentiginous melanoma and mucosal melanoma in Asian patients. Br. J. Dermatol. 2018, 180, 1230–1231. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Namikawa, K.; Ogata, D.; Jinnai, S.; Nakano, E.; Yamazaki, N. Updated analysis of nivolumab and ipilimumab combination therapy in Japanese patients with advanced melanoma. J. Dermatol. 2022. [Google Scholar] [CrossRef]
- Namikawa, K.; Kiyohara, Y.; Takenouchi, T.; Uhara, H.; Uchi, H.; Yoshikawa, S.; Takatsuka, S.; Koga, H.; Wada, N.; Minami, H.; et al. Final analysis of a phase II study of nivolumab in combination with ipilimumab for unresectable chemotherapy—Naive advanced melanoma. J. Dermatol. 2020, 47, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Namikawa, K.; Ogata, D.; Nakano, E.; Jinnai, S.; Nakama, K.; Tsutsui, K.; Muto, Y.; Mizuta, H.; Yamazaki, N. Real-world efficacy and safety data of nivolumab and ipilimumab combination therapy in Japanese patients with advanced melanoma. J. Dermatol. 2020, 47, 1267–1275. [Google Scholar] [CrossRef]
- Si, L.; Zhang, X.; Shu, Y.; Pan, H.; Wu, D.; Liu, J.; Lou, F.; Mao, L.; Wang, X.; Wen, X.; et al. A phase Ib study of pembrolizumab as second-line therapy for Chinese patients with advanced or metastatic melanoma (KEYNOTE-151). Transl. Oncol. 2019, 12, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Ding, Y.; Li, J.; Zhao, J.; Peng, R.; Li, D.; Zhu, B.; Wang, Y.; Zhang, X.; Zhang, X. The experience of immune checkpoint inhibitors in Chinese patients with metastatic melanoma: A retrospective case series. Cancer Immunol. Immunother. 2017, 66, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Namikawa, K.; Yoshino, K.; Yoshikawa, S.; Uchi, H.; Goto, K.; Nakamura, Y.; Fukushima, S.; Kiniwa, Y.; Takenouchi, T.; et al. Anti-PD1 checkpoint inhibitor therapy in acral melanoma: A multicenter study of 193 Japanese patients. Ann. Oncol. 2020, 31, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, N.; Kiyohara, Y.; Uhara, H.; Tsuchida, T.; Maruyama, K.; Shakunaga, N.; Itakura, E.; Komoto, A. Real-world safety and efficacy data of ipilimumab in Japanese radically unresectable malignant melanoma patients: A postmarketing surveillance. J. Dermatol. 2020, 47, 834–848. [Google Scholar] [CrossRef]
- Nathan, P.; Ascierto, P.A.; Haanen, J.; Espinosa, E.; Demidov, L.; Garbe, C.; Guida, M.; Lorigan, P.; Chiarion-Sileni, V.; Gogas, H.; et al. Safety and efficacy of nivolumab in patients with rare melanoma subtypes who progressed on or after ipilimumab treatment: A single-arm, open-label, phase II study (CheckMate 172). Eur. J. Cancer 2019, 119, 168–178. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, J.; Zhang, H.; Wang, Y.; Zhang, S. Immune checkpoint inhibitors in advanced acral melanoma: A systematic review. Front. Oncol. 2020, 10, 602705. [Google Scholar] [CrossRef]
- Li, J.; Smalley, I.; Chen, Z.; Wu, J.-Y.; Phadke, M.S.; Teer, J.K.; Nguyen, T.; Karreth, F.A.; Koomen, J.M.; Sarnaik, A.A.; et al. Single-cell characterization of the cellular landscape of acral melanoma identifies novel targets for immunotherapy. Clin. Cancer Res. 2022, 28, 2131–2146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lan, S.; Wu, D. Advanced acral melanoma therapies: Current status and future directions. Curr. Treat. Options Oncol. 2022, 23, 1405–1427. [Google Scholar] [CrossRef] [PubMed]
- Zaremba, A.; Murali, R.; Jansen, P.; Möller, I.; Sucker, A.; Paschen, A.; Zimmer, L.; Livingstone, E.; Brinker, T.J.; Hadaschik, E.; et al. Clinical and genetic analysis of melanomas arising in acral sites. Eur. J. Cancer 2019, 119, 66–76. [Google Scholar] [CrossRef]
- Fujisawa, Y.; Ito, T.; Kato, H.; Irie, H.; Kaji, T.; Maekawa, T.; Asai, J.; Yamamoto, Y.; Fujimura, T.; Nakai, Y.; et al. Outcome of combination therapy using BRAF and MEK inhibitors among Asian patients with advanced melanoma: An analysis of 112 cases. Eur. J. Cancer 2021, 145, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Lee, S.; Kim, K.; Heo, M.H.; Lee, H.; Cho, J.; Kim, N.K.; Park, W.; Lee, S.J.; Kim, J.H.; et al. Efficacy of BRAF inhibitors in Asian metastatic melanoma patients: Potential implications of genomic sequencing in BRAF-mutated melanoma. Transl. Oncol. 2016, 9, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Ding, Y.; Bai, X.; Sheng, X.; Dai, J.; Chi, Z.; Cui, C.; Kong, Y.; Fan, Y.; Xu, Y.; et al. Overall survival of patients with unresectable or metastatic BRAF V600-mutant acral/cutaneous melanoma administered dabrafenib plus trametinib: Long-term follow-up of a multicenter, single-arm phase IIa trial. Front. Oncol. 2021, 11, 720044. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Namikawa, K.; Nakano, E.; Yamazaki, N. Real-world efficacy and safety data for dabrafenib and trametinib combination therapy in Japanese patients with BRAF V600 mutation-positive advanced melanoma. J. Dermatol. 2019, 47, 257–264. [Google Scholar] [CrossRef]
- Si, L.; Zhang, X.; Shin, S.J.; Fan, Y.; Lin, C.-C.; Kim, T.M.; Dechaphunkul, A.; Maneechavakajorn, J.; Wong, C.S.; Ilankumaran, P.; et al. Open-label, phase IIa study of dabrafenib plus trametinib in East Asian patients with advanced BRAF V600-mutant cutaneous melanoma. Eur. J. Cancer 2020, 135, 31–38. [Google Scholar] [CrossRef]
- Häfliger, E.M.; Ramelyte, E.; Mangana, J.; Kunz, M.; Kazakov, D.; Dummer, R.; Cheng, P. Metastatic acral lentiginous melanoma in a tertiary referral center in Switzerland: A systematic analysis. Melanoma Res. 2018, 28, 442–450. [Google Scholar] [CrossRef]
- Fujimura, T.; Yoshino, K.; Kato, H.; Fujisawa, Y.; Nakamura, Y.; Yamamoto, Y.; Kunimoto, K.; Ito, T.; Matsushita, S.; Maekawa, T.; et al. Case series of BRAF-mutated advanced melanoma treated with encorafenib plus binimetinib combination therapy. J. Dermatol. 2020, 48, 397–400. [Google Scholar] [CrossRef]
- Bai, X.; Mao, L.L.; Chi, Z.H.; Sheng, X.N.; Cui, C.L.; Kong, Y.; Dai, J.; Wang, X.; Li, S.M.; Tang, B.X.; et al. BRAF inhibitors: Efficacious and tolerable in BRAF-mutant acral and mucosal melanoma. Neoplasma 2017, 64, 626–632. [Google Scholar] [CrossRef]
- Nathan, P.; Hassel, J.C.; Rutkowski, P.; Baurain, J.-F.; Butler, M.O.; Schlaak, M.; Sullivan, R.J.; Ochsenreither, S.; Dummer, R.; Kirkwood, J.M.; et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N. Engl. J. Med. 2021, 385, 1196–1206. [Google Scholar] [CrossRef]
- Barros, L.R.C.; Couto, S.C.F.; Santurio, D.D.S.; Paixão, E.A.; Cardoso, F.; da Silva, V.J.; Klinger, P.; Ribeiro, P.D.A.C.; Rós, F.A.; Oliveira, T.G.M.; et al. Systematic review of available CAR-T cell trials around the world. Cancers 2022, 14, 2667. [Google Scholar] [CrossRef] [PubMed]
- Soltantoyeh, T.; Akbari, B.; Karimi, A.; Chalbatani, G.M.; Ghahri-Saremi, N.; Hadjati, J.; Hamblin, M.; Mirzaei, H. Chimeric antigen receptor (CAR) T cell therapy for metastatic melanoma: Challenges and road ahead. Cells 2021, 10, 1450. [Google Scholar] [CrossRef] [PubMed]
- Esnault, C.; Schrama, D.; Houben, R.; Guyétant, S.; Desgranges, A.; Martin, C.; Berthon, P.; Viaud-Massuard, M.-C.; Touzé, A.; Kervarrec, T.; et al. Antibody–drug conjugates as an emerging therapy in oncodermatology. Cancers 2022, 14, 778. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.; Johnson, D.B. Antibody-drug conjugates for melanoma and other skin malignancies. Curr. Treat. Options Oncol. 2022, 23, 1428–1442. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Ito, T.; Tanaka, Y.; Kaku-Ito, Y.; Furue, M. NECTIN4 expression in extramammary paget’s disease: Implication of a new therapeutic target. Int. J. Mol. Sci. 2020, 21, 5891. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Murata, M.; Oda, Y.; Furue, M.; Ito, T. Nectin cell adhesion molecule 4 (NECTIN4) expression in cutaneous squamous cell carcinoma: A new therapeutic target? Biomedicines 2021, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Hashimoto, H.; Tanaka, Y.; Tanegashima, K.; Murata, M.; Oda, Y.; Kaku-Ito, Y. NECTIN4 expression in sebaceous and sweat gland carcinoma. Eur. J. Dermatol. 2022, 32, 181–186. [Google Scholar] [CrossRef]
- Tanaka, Y.; Murata, M.; Shen, C.-H.; Furue, M.; Ito, T. NECTIN4: A novel therapeutic target for melanoma. Int. J. Mol. Sci. 2021, 22, 976. [Google Scholar] [CrossRef]
- Tanaka, Y.; Murata, M.; Tanegashima, K.; Oda, Y.; Ito, T. Nectin cell adhesion molecule 4 regulates angiogenesis through Src signaling and serves as a novel therapeutic target in angiosarcoma. Sci. Rep. 2022, 12, 1–16. [Google Scholar] [CrossRef]
- Hashimoto, H.; Tanaka, Y.; Murata, M.; Ito, T. Nectin-4: A novel therapeutic target for skin cancers. Curr. Treat. Options Oncol. 2022, 23, 578–593. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Tanegashima, K.; Tanaka, Y.; Hashimoto, H.; Murata, M.; Oda, Y.; Kaku-Ito, Y. Trop2 expression in extramammary paget’s disease and normal skin. Int. J. Mol. Sci. 2021, 22, 7706. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Hashimoto, H.; Tanaka, Y.; Tanegashima, K.; Murata, M.; Ichiki, T.; Iwasaki, T.; Oda, Y.; Kaku-Ito, Y. TROP2 expression in sebaceous and sweat gland carcinoma. J. Clin. Med. 2022, 11, 607. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Ito, T.; Kaku-Ito, Y.; Tanegashima, K.; Tsuji, G.; Kido-Nakahara, M.; Oda, Y.; Nakahara, T. Human epidermal growth factor receptor 3 serves as a novel therapeutic target for acral melanoma. Cell Death Discov. 2023, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).