Age-Related Effects of COVID-19 Pandemic on Mechanical Reperfusion and 30-Day Mortality for STEMI: Results of the ISACS-STEMI COVID-19 Registry
Abstract
1. Background
2. Study Design and Population
3. Results
4. Baseline Demographic and Clinical Characteristics
5. Procedural Characteristics
6. In-Hospital and 30-Day Mortality
7. Discussion
8. Limitations
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCI | Percutaneous coronary intervention |
| STEMI | ST-segment elevation myocardial infarction |
| DTB | Door-to-balloon time |
| IRR | Incidence rate ratio |
| ACS | Acute coronary syndrome |
| DES | Drug-eluting stent |
| RASI | Renin-angiotensin system inhibitors |
References
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020, 26, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Wood, S. The Mystery of the Missing STEMIs during the COVID-19 Pandemic. tctMD: 2020. Available online: https://www.tctmd.com/news/mystery-missing-stemis-during-covid-19-pandemic (accessed on 24 June 2021).
- Garcia, S.; Albaghdadi, M.S.; Meraj, P.M.; Schmidt, C.; Garberich, R.; Jaffer, F.A.; Dixon, S.; Rade, J.J.; Tannenbaum, M.; Chambers, J.; et al. Reduction in ST-Segment Elevation Cardiac Catheterization Laboratory Activations in the United States during COVID-19 Pandemic. J. Am. Coll. Cardiol. 2020, 75, 34913–34915. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.F.; Cheung, K.S.; Lam, S.; Wong, A.; Yung, A.; Sze, M.; Lam, Y.M.; Chan, C.; Tsang, T.C.; Tsui, M.; et al. Impact of Coronavirus Disease 2019 (COVID-19) Outbreak on ST-Segment-Elevation Myocardial Infarction Care in Hong Kong, China. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e006631. [Google Scholar] [CrossRef]
- Piccolo, R.; Bruzzese, D.; Mauro, C.; Aloia, A.; Baldi, C.; Boccalatte, M.; Bottiglieri, G.; Briguori, C.; Caiazzo, G.; Calabrò, P.; et al. Population Trends in Rates of Percutaneous Coronary Revascularization for Acute Coronary Syndromes Associated with the COVID-19 Outbreak. Circulation 2020, 141, 2035–2037. [Google Scholar] [CrossRef] [PubMed]
- Roffi, M.; Guagliumi, G.; Ibanez, B. The Obstacle Course of Reperfusion for STEMI in the COVID-19 Pandemics. Circulation 2020, 141, 1951–1953. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Spaccarotella, C.; Basso, C.; Calabrò, M.P.; Curcio, A.; Filardi, P.P.; Mancone, M.; Mercuro, G.; Muscoli, S.; Nodari, S.; et al. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Società Italiana di Cardiologia and the CCU Academy investigators group. Eur. Heart J. 2020, 41, 2083–2088. [Google Scholar] [CrossRef]
- Xiang, D.; Xiang, X.; Zhang, W.; Yi, S.; Zhang, J.; Gu, X.; Xu, Y.; Huang, K.; Su, X.; Yu, B.; et al. Management and Outcomes of Patients with STEMI during the COVID-19 Pandemic in China. J. Am. Coll. Cardiol. 2020, 76, 1318–1324. [Google Scholar] [CrossRef]
- De Luca, G.; Verdoia, M.; Cercek, M.; Jensen, L.O.; Vavlukis, M.; Calmac, L.; Johnson, T.; Ferrer, G.R.; Ganyukov, V.; Wojakowski, W.; et al. Impact of COVID-19 Pandemic on Mechanical Reperfusion for Patients With STEMI. J. Am. Coll. Cardiol. 2020, 76, 2321–2330. [Google Scholar] [CrossRef]
- De Luca, G.; Cercek, M.; Jensen, L.O.; Vavlukis, M.; Calmac, L.; Johnson, T.; Roura I Ferrer, G.; Ganyukov, V.; Wojakowski, W.; von Birgelen, C.; et al. Impact of COVID-19 pandemic and diabetes on mechanical reperfusion in patients with STEMI: Insights from the ISACS STEMI COVID 19 Registry. Cardiovasc. Diabetol. 2020, 19, 215. [Google Scholar] [CrossRef]
- De Luca, G.; Suryapranata, H.; Ottervanger, J.P.; Antman, E.M. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: Every minute of delay counts. Circulation 2004, 109, 1223–1225. [Google Scholar] [CrossRef]
- De Luca, G.; van’t Hof, A.W.; de Boer, M.J.; Ottervanger, J.P.; Hoorntje, J.C.; Gosselink, A.T.; Dambrink, J.H.; Zijlstra, F. Suryapranata HTime-to-treatment significantly affects the extent of ST-segment resolution and myocardial blush in patients with acute myocardial infarction treated by primary angioplasty. Eur. Heart J. 2004, 25, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. ESC Scientific Document Group.2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar]
- Gelman, A.; Hill, J. Data Analysis Using Regression and Multilevel/Hierarchical Models; Cambridge University Press: New York, NY, USA, 2007. [Google Scholar]
- Madjid, M.; Safavi-Naeini, P.; Solomon, S.D.; Vardeny, O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020, 5, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Madjid, M.; Vela, D.; Khalili-Tabrizi, H.; Casscells, S.W.; Litovsky, S. Systemic infections cause exaggerated local inflammation in atherosclerotic coronary arteries: Clues to the triggering effect of acute infections on acute coronary syndromes. Tex. Heart Inst. J. 2007, 34, 11–18. [Google Scholar] [PubMed]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Baldi, E.; Sechi, G.M.; Mare, C.; Canevari, F.; Brancaglione, A.; Primi, R.; Klersy, C.; Palo, A.; Contri, E.; Ronchi, V.; et al. Lombardia CARe Researchers. Out-of-Hospital Cardiac Arrest during the COVID-19 Outbreak in Italy. N. Engl. J. Med. 2020, 383, 496–498. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; van’t Hof, A.W.; Ottervanger, J.P.; Hoorntje, J.C.; Gosselink, A.T.; Dambrink, J.H.; de Boer, M.J.; Suryapranata, H. Ageing, impaired myocardial perfusion, and mortality in patients with ST-segment elevation myocardial infarction treated by primary angioplasty. Eur. Heart J. 2005, 26, 662–666. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; van’t Hof, A.W.; Huber, K.; Gibson, C.M.; Bellandi, F.; Arntz, H.R.; Maioli, M.; Noc, M.; Zorman, S.; Secco, G.G.; et al. Impact of advanced age on myocardial perfusion, distal embolization, and mortality patients with ST-segment elevation myocardial infarction treated by primary angioplasty and glycoprotein IIb-IIIa inhibitors. Heart Vessel. 2014, 29, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Verdoia, M.; Pergolini, P.; Nardin, M.; Rolla, R.; Tonon, F.; Kedhi, E.; Suryapranata, H.; Carriero, A.; De Luca, G. Novara Atherosclerosis Study Group (NAS). Impact of aging on platelet reactivity in diabetic patients receiving dual antiplatelet therapy. J. Thromb. Thrombolysis 2019, 48, 413–421. [Google Scholar] [CrossRef]
- Verdoia, M.; Pergolini, P.; Rolla, R.; Nardin, M.; Schaffer, A.; Barbieri, L.; Marino, P.; Bellomo, G.; Suryapranata, H.; De Luca, G. Advanced age and high-residual platelet reactivity in patients receiving dual antiplatelet therapy with clopidogrel or ticagrelor. J. Thromb. Haemost. 2016, 14, 57–64. [Google Scholar] [CrossRef]
- Silverio, A.; Di Maio, M.; Citro, R.; Esposito, L.; Iuliano, G.; Bellino, M.; Baldi, C.; De Luca, G.; Ciccarelli, M.; Vecchione, C.; et al. Cardiovascular risk factors and mortality in hospitalized patients with COVID-19: Systematic review and meta-analysis of 45 studies and 18,300 patients. BMC Cardiovasc. Disord. 2021, 21, 23. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, G.G.; Montorfano, M.; Trabattoni, D.; Andreini, D.; Ferrante, G.; Ancona, M.; Metra, M.; Curello, S.; Maffeo, D.; Pero, G.; et al. ST-Elevation Myocardial Infarctionin Patients with COVID-19: Clinical and Angiographic Outcomes. Circulation 2020, 141, 2113–2116. [Google Scholar] [CrossRef] [PubMed]
- Marijon, E.; Karam, N.; Jost, D.; Perrot, D.; Frattini, B.; Derkenne, C.; Sharifzadehgan, A.; Waldmann, V.; Beganton, F.; Narayanan, K.; et al. Out-of-hospital cardiac arrest during the COVID-19 pandemic in Paris, France: A population-based, observational study. Lancet Public Health 2020, 5, e437–e443. [Google Scholar] [CrossRef] [PubMed]
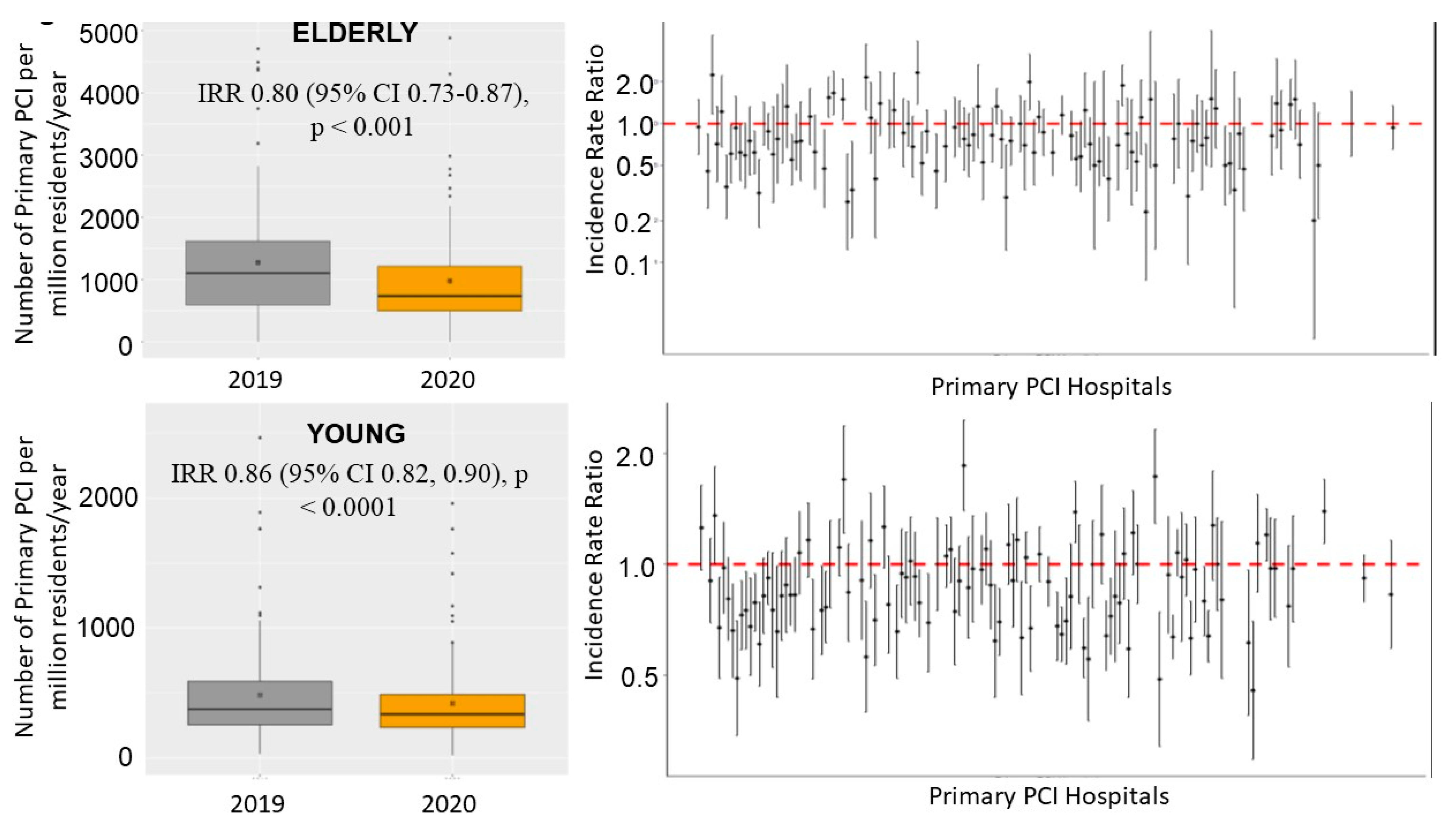

| Elderly 2019 (n = 1682) | Elderly 2020 (n = 1365) | p Value | Young 2019 (n = 7016) | Young 2020 (n = 6020) | p Value | |
|---|---|---|---|---|---|---|
| Age (median, IQR) | 81 (77–85) | 81 (77–85) | 0.97 | 60 (52–66) | 59 (52–66) | 0.33 * |
| Male gender—n (%) | 967 (57.5) | 805 (59.0) | 0.409 | 5604 (79.9) | 4788 (79.5) | 0.631 |
| Medical History | ||||||
| Hypertension—n (%) | 1212 (72.1) | 987 (72.3) | 0.878 | 3533 (50.4) | 3081 (51.2) | 0.349 |
| Diabetes mellitus—n (%) | 490 (29.1) | 390 (28.6) | 0.734 | 1548 (22.1) | 1384 (23.0) | 0.207 |
| Hypercholesterolemia—n (%) | 721 (42.9) | 611 (44.8) | 0.294 | 2724 (38.8) | 2297 (38.2) | 0.434 |
| Active smoker—n (%) | 515 (30.6) | 408 (29.9) | 0.664 | 4314 (61.5) | 3549 (59.0) | 0.003 |
| Family history of CAD—n (%) | 200 (11.9) | 128 (9.4) | 0.026 | 1635 (23.3) | 1335 (22.2) | 0.126 |
| Previous STEMI—n (%) | 195 (11.6) | 153 (11.2) | 0.740 | 637 (9.1) | 558 (9.3) | 0.708 |
| Previous PCI—n (%) | 245 (14.6) | 211 (15.5) | 0.493 | 793 (11.3) | 744 (12.4) | 0.062 |
| Previous CABG—n (%) | 63 (3.7) | 50 (3.7) | 0.905 | 81 (1.2) | 78 (1.3) | 0.464 |
| Geographic area | 0.038 | <0.001 | ||||
| Europe—n (%) | 1476 (87.8) | 1176 (86.2) | 5507 (78.5) | 4655 (77.3) | ||
| Latin America—n (%) | 89 (5.3) | 106 (7.8) | 541 (7.7) | 614 (10.2) | ||
| Southeast Asia—n (%) | 92 (5.5) | 67 (4.9) | 614 (8.8) | 520 (8.6) | ||
| North Africa—n (%) | 25 (1.5) | 16 (1.2) | 354 (5.0) | 231 (3.8) | ||
| Referral to Primary PCI Hospital | ||||||
| Type | 0.212 | 0.755 | ||||
| Ambulance (from community)—n (%) | 848 (50.4) | 720 (52.7) | 3314 (47.2) | 2856 (47.4) | ||
| Direct access to hub—n (%) | 439 (26.1) | 319 (23.4) | 2010 (28.6) | 1745 (29.0) | ||
| Transfer from spoke—n (%) | 395 (23.5) | 326 (23.9) | 1692 (24.1) | 1419 (23.6) | ||
| Time delays | ||||||
| Ischemia time, median (25–75th) | 225 (140–375) | 244 (150–430) | <0.0001 | 190 (120–345) | 220 (130–402) | <0.0001 * |
| Total ischemia time | 0.009 | <0.001 | ||||
| <6 h—n (%) | 1257 (74.7) | 945 (69.2) | 5365 (76.5) | 4355 (72.3) | ||
| 6–12 h—n (%) | 249 (14.8) | 243 (17.8) | 1035 (14.8) | 972 (16.1) | ||
| 12–24 h—n (%) | 113 (6.7) | 110 (8.1) | 424 (6.0) | 441 (7.3) | ||
| >24 h—n (%) | 63 (3.7) | 67 (4.9) | 192 (2.7) | 252 (4.2) | ||
| Total ischemia time > 12 h—n (%) | 176 (10.5) | 177 (13.0) | 0.032 | 616 (8.8) | 693 (11.5) | <0.001 * |
| Door-to-balloon time, median (25–75th) | 40 (25–70) | 40 (26–74) | 0.071 | 40 (25–62) | 40 (25–70) | 0.001 * |
| Door-to-balloon time | 0.428 | 0.001 | ||||
| <30 min—n (%) | 675 (40.1) | 517 (37.9) | 2904 (41.4) | 2337 (38.8) | ||
| 30–60 min—n (%) | 527 (31.3) | 438 (32.1) | 2318 (33.0) | 1976 (32.8) | ||
| >60 min—n (%) | 480 (28.5) | 410 (30.0) | 1794 (25.6) | 1707 (28.4) | ||
| Door-to-balloon time > 30 min—n (%) | 1007 (59.9) | 848 (62.1) | 0.205 | 4112 (58.6) | 3683 (61.2) | 0.003 |
| Clinical Presentation | ||||||
| Anterior STEMI—n (%) | 803 (47.7) | 654 (47.9) | 0.925 | 3183 (45.4) | 2806 (46.6) | 0.155 |
| Out-of-hospital cardiac arrest—n (%) | 87 (5.2) | 63 (4.6) | 0.480 | 428 (6.1) | 378 (6.3) | 0.510 |
| Cardiogenic shock—n (%) | 170 (10.1) | 148 (10.8) | 0.509 | 455 (6.5) | 395 (6.6) | 0.550 |
| Rescue PCI for failed thrombolysis—n (%) | 82 (4.9) | 57 (4.2) | 0.358 | 523 (7.5) | 437 (7.3) | 0.670 |
| Elderly 2019 (n = 1682) | Elderly 2020 (n = 1365) | p Value | Young 2019 (n = 7016) | Young 2020 (n = 6020) | p Value | |
|---|---|---|---|---|---|---|
| Radial Access (%) | 1221 (72.6) | 1031 (75.5) | 0.066 | 5302 (75.6) | 4714 (78.3) | <0.001 |
| Culprit vessel | 0.707 | 0.521 | ||||
| Left main—n (%) | 38 (2.3) | 24 (1.8) | 103 (1.5) | 87 (1.4) | ||
| Left anterior descending artery—n (%) | 805 (47.9) | 629 (46.1) | 3182 (45.4) | 2742 (45.5) | ||
| Circumflex—n (%) | 206 (12.2) | 183 (13.4) | 1040 (14.8) | 921 (15.3) | ||
| Right coronary artery—n (%) | 612 (36.4) | 511 (37.4) | 2648 (37.7) | 2230 (37.0) | ||
| Anterolateral branch—n (%) | 4 (0.2) | 2 (0.1) | 21 (0.3) | 14 (0.2) | ||
| SVG—n (%) | 17 (1.0) | 16 (1.2) | 20 (0.3) | 26 (0.4) | ||
| Proximal lesion location—n (%) | ||||||
| In-stent thrombosis—n (%) | 67 (4.0) | 69 (5.1) | 0.154 | 272 (3.9) | 224 (3.7) | 0.643 |
| Multivesseldisease—n (%) | 928 (55.2) | 775 (56.8) | 0.463 | 3308 (47.1) | 2875 (47.8) | 0.224 |
| Preprocedural TIMI 0 flow—n (%) | 1028 (61.1) | 869 (63.7) | 0.149 | 4738 (67.5) | 4096 (68.0) | 0.536 |
| Thrombectomy—n (%) | 242 (14.4) | 180 (13.2) | 0.34 | 1160 (16.5) | 981 (16.3) | 0.715 |
| Stenting—n (%) | 1491 (88.6) | 1203 (88.1) | 0.66 | 6507 (92.7) | 5565 (92.4) | 0.459 |
| Drug-elutingstent—n (%) | 1448 (86.1) | 1176 (86.2) | 0.958 | 6208 (88.5) | 5422 (90.1) | 0.004 |
| Postprocedural TIMI 3 flow—n (%) | 1500 (89.2) | 1204 (88.2) | 0.397 | 6530 (93.1) | 5587 (92.8) | 0.555 |
| Gp IIb-IIIa inhibitors/cangrelor—n (%) | 248 (14.7) | 229 (16.8) | 0.125 | 1505 (21.5) | 1285 (21.3) | 0.884 |
| Bivalirudin—n (%) | 4 (0.2) | 3 (0.2) | 0.918 | 30 (0.4) | 15 (0.2) | 0.083 |
| Mechanical support—n (%) | 57 (3.4) | 54 (4.0) | 0.406 | 189 (2.7) | 197 (3.3) | 0.052 |
| Additional PCI | 0.444 | 0.001 | ||||
| During the index procedure—n (%) | 167 (9.9) | 152 (11.1) | 620 (8.8) | 637 (10.6) | ||
| Staged—n (%) | 171 (10.2) | 147 (10.8) | 715 (10.2) | 653 (10.8) | ||
| DAPT therapy—n (%) | 1647 (97.9) | 1347 (98.7) | 0.11 | 6946 (99.0) | 5965 (99.1) | 0.623 |
| In-hospital RASI—n (%) | 839 (49.9) | 752 (55.1) | 0.004 | 3787 (54.0) | 3519 (58.5) | <0.001 |
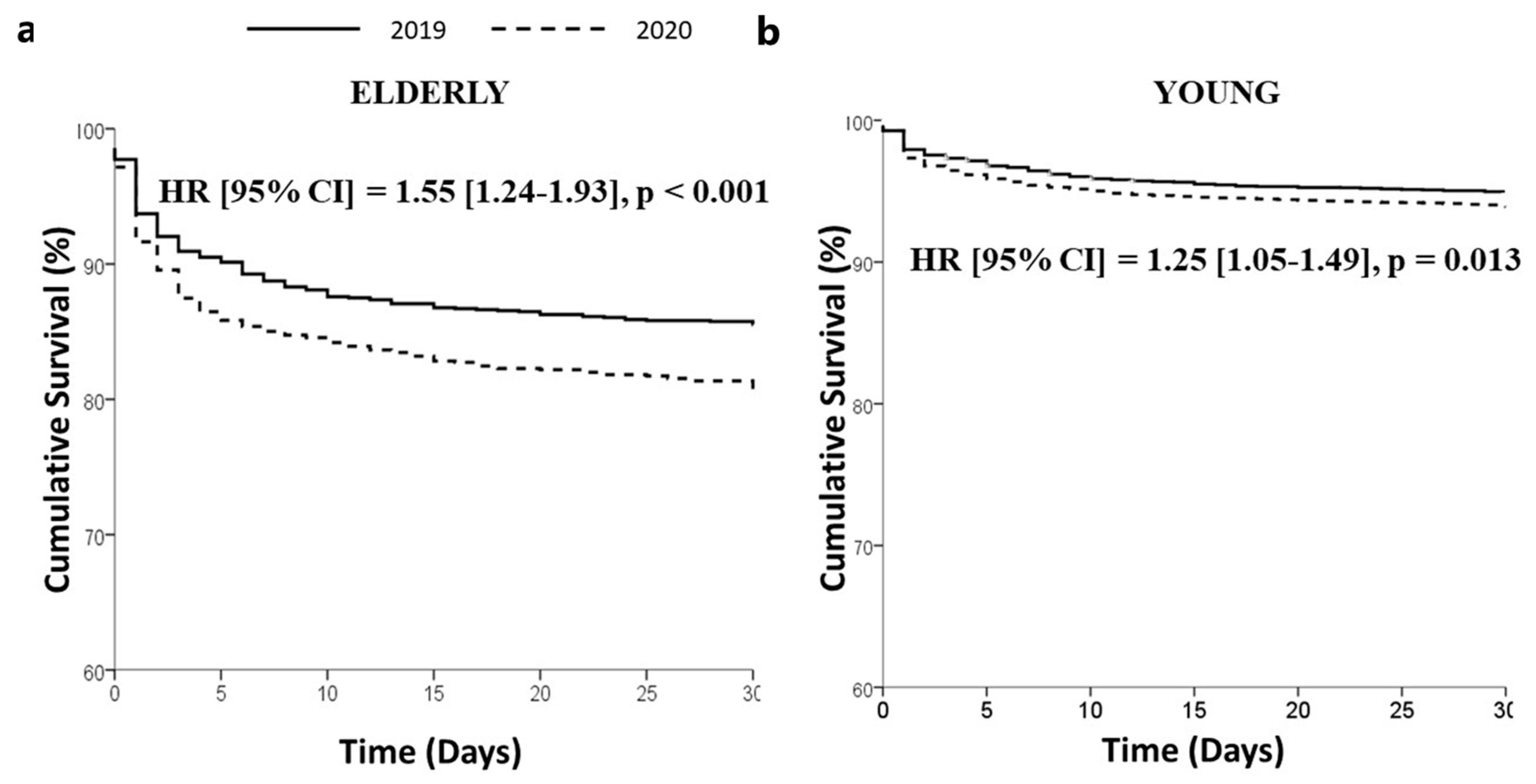
| In-hospital death—n (%) | 180 (10.7) | 200 (14.7) | 0.001 | 277 (3.9) | 281 (4.7) | 0.043 |
| Death—n (%) | 201 (13.6) | 215 (17.9) | 0.002 | 303 (4.8) | 308 (5.7) | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, G.; Algowhary, M.; Uguz, B.; Oliveira, D.C.; Ganyukov, V.; Busljetik, O.; Cercek, M.; Jensen, L.O.; Loh, P.H.; Calmac, L.; et al. Age-Related Effects of COVID-19 Pandemic on Mechanical Reperfusion and 30-Day Mortality for STEMI: Results of the ISACS-STEMI COVID-19 Registry. J. Clin. Med. 2023, 12, 2116. https://doi.org/10.3390/jcm12062116
De Luca G, Algowhary M, Uguz B, Oliveira DC, Ganyukov V, Busljetik O, Cercek M, Jensen LO, Loh PH, Calmac L, et al. Age-Related Effects of COVID-19 Pandemic on Mechanical Reperfusion and 30-Day Mortality for STEMI: Results of the ISACS-STEMI COVID-19 Registry. Journal of Clinical Medicine. 2023; 12(6):2116. https://doi.org/10.3390/jcm12062116
Chicago/Turabian StyleDe Luca, Giuseppe, Magdy Algowhary, Berat Uguz, Dinaldo C. Oliveira, Vladimir Ganyukov, Oliver Busljetik, Miha Cercek, Lisette Okkels Jensen, Poay Huan Loh, Lucian Calmac, and et al. 2023. "Age-Related Effects of COVID-19 Pandemic on Mechanical Reperfusion and 30-Day Mortality for STEMI: Results of the ISACS-STEMI COVID-19 Registry" Journal of Clinical Medicine 12, no. 6: 2116. https://doi.org/10.3390/jcm12062116
APA StyleDe Luca, G., Algowhary, M., Uguz, B., Oliveira, D. C., Ganyukov, V., Busljetik, O., Cercek, M., Jensen, L. O., Loh, P. H., Calmac, L., Ferrer, G. R. i., Quadros, A., Milewski, M., Scotto D’Uccio, F., von Birgelen, C., Versaci, F., Ten Berg, J., Casella, G., Wong Sung Lung, A., ... Verdoia, M. (2023). Age-Related Effects of COVID-19 Pandemic on Mechanical Reperfusion and 30-Day Mortality for STEMI: Results of the ISACS-STEMI COVID-19 Registry. Journal of Clinical Medicine, 12(6), 2116. https://doi.org/10.3390/jcm12062116












