Abstract
Consideration for oversizing the proximal stent graft is suggested in endovascular aortic repair. However, a special recommendation for the proximal oversizing ratio (OSR) in patients with ruptured abdominal aortic aneurysm (rAAA) is ambiguous. This study aims to evaluate the effect of different degrees of the proximal oversizing ratio (OSR) on risk of type IA endoleak (TIAEL) in hemodynamically stable and unstable patients with rAAA undergoing emergency endovascular aortic repair (EVAR). Our study included 134 rAAA patients undergoing emergent EVAR, and we did not observe a significant association between hemodynamic instability and risk of T1AEL (HR 3.89, 95%CI 0.40–37.75, p = 0.24). All three T1AELs in the hemodynamically unstable subgroup were observed in patients with OSR ≤ 30%, but no significant difference was found regarding T1AEL between patients with OSR > 30% and OSR ≤ 30% (0.00% vs. 11.11%, p = 0.19). As for hemodynamically stable patients, OSR > 20% was associated with a significantly decreased risk of T1AEL (HR 0.03, 95%CI 0.01–0.53, p = 0.016). In conclusion, a proximal OSR > 20% is associated with a decreased risk of T1AEL in hemodynamically stable patients, while an OSR > 30% did not add an additional advantage of lowering the risk of T1AEL in hemodynamically unstable patients.
1. Introduction
Since the introduction of endovascular aneurysm repair (EVAR) in 1991 [1], ruptured abdominal aortic aneurysm (rAAA) has increasingly been treated with EVAR owing to its potential short-term benefits on survival and morbidity [2]. Data from individual-patient meta-analysis of three randomized controlled trials showed comparable survival but earlier discharge after EVAR in rAAA patients. Thus EVAR is now widely accepted in appropriately selected rAAA patients when adequate personnel and resources are present [3,4]. However, a relatively higher incidence of aneurysm-related complications and reinterventions remains the main drawback of endovascular repair [5]. Notably, the most disturbing complication is considered to be type IA endoleak (T1AEL), which can cause considerable blood flow leakage into the aneurysmal sac and remains the most important reason for late aneurysm rupture after EVAR [6,7].
To ensure the long-term stability of the stent graft, sufficient fixation and sealing of the proximal anchoring segments are essential. According to current guidelines and most instructions for the use of elective EVAR, stent grafts are often oversized by 10% to 25% to provide adequate radial support force for proximal neck anchoring [8,9]. However, there is ambiguity regarding the specific recommendation for the proximal oversizing ratio (OSR) in rAAA patients undergoing EVAR.
To avoid intraoperative or late type IA endoleak (T1AEL), the recent ESVS guideline suggests that a 30% oversizing of the stent graft in the proximal landing zone is preferable for patients undergoing emergency EVAR [8]. However, this recommendation was only inferred from a case report and a clinical observation of aortic diameter changes in trauma patients [10,11]. On the other hand, in vitro research indicated that excessive oversizing should also be avoided in the proximal anchoring, because oversizing >30% can lead to graft folding and poor barb penetration [12]. Clinical evidence also suggests endograft oversizing of >30% can significantly raise the risk of device migration in the midterm after EVAR [13,14]. Given the current rare and conflicting data, the investigation of the appropriate degree of proximal stent graft oversizing is necessary in emergency EVAR.
Thus, the purpose of our study was to investigate the influence of different degrees of proximal OSR on the risk of T1AEL in hemodynamically stable and unstable rAAA patients undergoing emergency EVAR.
2. Materials and Methods
2.1. Study Design and Approvals
A retrospective cohort study was conducted based on a database of patients who underwent EVAR at a tertiary academic hospital with a high volume of aortic surgeries. This study was reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement of cohort studies [15]. The STROBE checklist is shown in Table S1. Ethical approval for this study was obtained from the Ethics Committee of West China Hospital of Sichuan University (approval number: 2023-1705).
2.2. Participants
All consecutive patients undergoing emergency EVAR for impending rupture or ruptured AAA between July 2010 and December 2020 were included. Ruptured AAA was defined as the presence of retroperitoneal and/or intraperitoneal hematoma adjacent to the aneurysm sac in a preoperative computed tomography angiogram (CTA). Impending rupture was defined as those who had an acute onset or exacerbation of abdominal pain, with at least one of the following CTA findings: draped aorta sign, well-defined peripheral crescent of increased attenuation within the thrombus of a large AAA, focal discontinuity in circumferential wall calcifications, or a collection of fluid around the aneurysm sac [16]. Ruptured abdominal aortic aneurysm mostly presents as retroperitoneal hematoma and abdominal aortic aneurysm. Blood around the abdominal aorta may extend into the perirenal space and pararenal space. A localized gap of discontinuity of the aortic wall or continuous calcification of the surrounding wall may point to the site of rupture [16]. All the diagnoses of rAAA were confirmed by agreement between two vascular surgeons based on CTA findings. Patients were excluded if they met the following criteria: (1) juxtarenal aortic aneurysm repaired by hybrid or chimney techniques; (2) pseudoaneurysms from trauma or infection; (3) reintervention for endoleaks of prior endovascular procedures; (4) proximal oversizing was not available due to the absence of preoperative CTA.
2.3. Exposures
Proximal oversizing ratio (OSR) of the stent graft was defined as the ratio of the diameter difference between the aneurysm neck and proximal stent graft to the diameter of the aneurysm neck. The stent graft adopted in our study was from one manufacturer, Endurant II or IIs Stent Graft (Medtronic, Minneapolis, MN, USA). All measurements of preoperative proximal sizing were performed by two experienced vascular surgeons on preoperative CTA.
All included patients were divided into two subgroups based on the hemodynamic stability assessed by mean arterial pressure (MAP) and heart rate. Hemodynamic instability was defined as MAP ≤ 90 mmHg and heart rate ≥ 100 beats/min. The cutting points of proximal OSR of stent grafts were set as 20% and 30% in hemodynamically stable and unstable patients, respectively.
During the treatment, patients received either local or general anesthesia and systemic heparinization (intravenous injection of unfractionated heparin at a dose of 0.5 mg/kg). A vascular access sheath was inserted through percutaneous puncture or a femoral artery incision. Abdominal aortography was performed to evaluate the neck and morphology of the aneurysm. A 0.035 ‘Lunderquist® (COOK®, Inc., Bloomington, IN, USA) superhard guidewire was inserted into the abdominal aortic aneurysm main stent delivery device, and the proximal end of the stent was placed horizontally on the lower edge of the lower renal artery for release. Angiography was repeated above the level of the renal artery to confirm the distance between the main bifurcation and the common iliac bifurcation. The iliac stent transporter was placed along a 0.035 ‘Lunderquist® (COOK®, Inc., Bloomington, IN, USA) superhard guidewire. The proximal flat stent body bifurcation was marked, and the distal position was confirmed to be appropriate before releasing the iliac stent. Angiography was reviewed to confirm the presence of endoleak, and if necessary, a Reliant AB46 (Medtronic®, Inc., Minneapolis, MN, USA) balloon was applied to the neck, iliac branch, or stent junction, and cuff stents were added at the discretion of the surgeon. After confirming angiography, the guidewire catheter was withdrawn, and the blood flow of both lower limbs was opened to check the distal arterial pulse, comparing with that before the operation. All patients received fluid infusion and antiplatelet therapy with aspirin 100 mg/d after the operation.
2.4. Outcomes of Interest and Follow-Up Protocols
Patients undergoing EVAR were regularly followed up in outpatient clinics with vascular contrast-enhanced ultrasonography at 1, 6, and 12 months, and annually thereafter. CTA was performed if ultrasound observed T1AEL or other adverse aneurysm-related complications. Telephone calls were used to check the status and follow-up details of patients if they failed to come.
The primary outcome of interest was the rate of T1AEL after EVAR, identified by either duplex ultrasound or CTA during follow-up. Secondary outcomes included 30-day mortality, aneurysm-related reintervention, other types of endoleaks, and overall survival.
2.5. Variables
Demographics, comorbidities, vital signs on admission, aortic neck characteristics, and aneurysm-related parameters were recorded to address the potential confounding effect of covariates. The Charlson comorbidity index (CCI) was used to evaluate the severity of comorbidities in included patients [17]. Aortic neck parameters involved the aneurysmal neck diameter, neck length of the aneurysm, neck angulation (α and β angle according to previous definitions, [18]), and neck calcification of the aneurysm. CTA examinations in this study were acquired using a Philips Brilliance 64-slice spiral CT scanner. Data were post-processed on a Siemens workstation (Leonardo, Siemens Medical Solutions, Erlangen, Germany), including CT vascular three-dimensional reconstruction. All diameters were measured in the minor axis of axial cuts or planes perpendicular to the centerline of reformatted slices from adventitia to adventitia. Aneurysmal neck diameter was measured at the level immediately below the lowest renal artery. Neck calcification was defined as the circumferential proportion of calcified vessel wall at the aneurysm neck, and was estimated from the cross-sectional images. The maximum diameter of the aneurysm and concomitant common iliac artery aneurysm (CIAA) were also recorded. CIAA was defined as any aneurysmal dilation of the common iliac arteries with a diameter larger than 24 mm.
2.6. Statistical Analyses
All statistical analyses were performed using R Studio Version 1.2.1335 (http://www.R-project.org, accessed on 11 October 2023) and Empower(R) (www.empowerstats.com, accessed on 11 October 2023, X&Y solutions, Inc., Boston, MA, USA). Continuous data were compared with Student t test or Mann–Whitney U test and categorical data were compared using Fisher’s exact test or χ2 test. The Kaplan–Meier method was used to estimate the cumulative rate of time-to-event outcomes after EVAR, and log-rank tests were used to compare the differences in the estimated incidence of outcomes between groups.
To address the confounding effect of covariates, multivariate logistic regression analysis was performed to evaluate the association between OSR and 30-day mortality, and Cox proportional hazard regression analysis was performed to evaluate the effect of OSR on time-to-event outcomes. Covariate selection in the multivariate models was based on the change-in-estimate criteria and clinical relevance [19]. Missing data were handled with multiple imputation. Two subgroup analyses were performed in hemodynamically stable and unstable patients. A p value < 0.05 was considered statistically significant.
3. Results
3.1. Baseline Characteristics
There were 134 patients who underwent emergent EVAR for ruptured or impending rupture AAA. The detailed process of patient selection is displayed in Figure 1. Of these, 44 patients were considered hemodynamically unstable, with a mean MAP of 80.61 ± 9.30 mmHg and a mean heart rate of 103.82 ± 5.96 beats/min. The placement of the stent within indications for use was similar between hemodynamically stable and unstable patients (41.11% vs. 47.73%, p = 0.46). The mean age was comparable between hemodynamically stable and unstable patients (70.41 ± 9.89 vs. 72.55 ± 8.24, p = 0.22), while females were more common in hemodynamically unstable patients (36.36% vs. 18.89%, p = 0.027). The neck morphology was comparable between groups, except for hemodynamically unstable patients had larger α angles [27.66 (12.93–45.42) vs. 32.50 (10.77–66.73), p = 0.025]. The proximal OSR was significantly larger in hemodynamically unstable patients (25% (22–32%) vs. 22% (20–27%), p = 0.011). The baseline characteristics of included patients are summarized in Table 1.
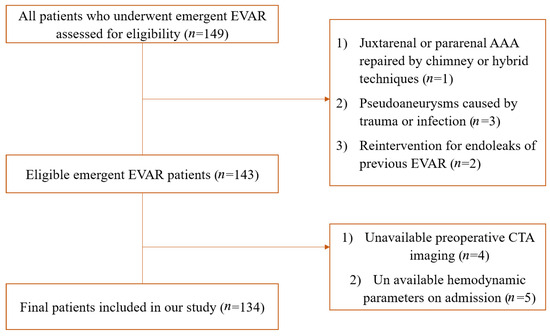
Figure 1.
Flow diagram of selection of eligible patients.

Table 1.
Baseline characteristics of included patients.
3.2. Risk Factors of T1AEL in Emergent EVAR
During a median imaging follow-up of 13 (3.50–34.00) months, eight patients were found to have T1AEL. The results of univariate regression analysis suggested that larger neck diameter was associated with a significantly increased risk of T1AEL (HR 1.27, 95%CI 1.03–1.56, p = 0.028), while OSR > 20% (HR 0.06, 95%CI 0.01–0.31, p = 0.001) and longer neck length (HR 0.83, 95%CI 0.72–0.95, p = 0.009) was associated with a significantly decreased risk of T1AEL. After adjusted for gender, age, and neck angulation, only OSR > 20% (HR 0.06, 95%CI 0.01–0.72, p = 0.026) and longer neck length (HR 0.74, 95%CI 0.56–0.98, p = 0.033) remained significantly associated with the risk of T1AEL (Table 2).

Table 2.
Univariate and multivariate regression analyses of risk factors of type IA endoleak.
3.3. Comparison of Outcomes in Hemodynamically Unstable and Stable Patients
The median survival (24.5 months vs. 33.0 months, p = 0.22) and imaging (10.0 months vs. 15.0 months, p = 0.24) follow-up time were similar between hemodynamically unstable and stable patients. We observed T1AEL in 3 (7.32%) hemodynamically unstable and 5 (5.56%) hemodynamically stable patients. As is shown in Table S2, no significant association was found between hemodynamic instability and the risk of T1AEL (HR 3.89, 95%CI 0.40–37.75, p = 0.24), as well as the risk of reintervention (HR 0.92, 95%CI 0.22–3.76, p = 0.90). However, hemodynamic instability was associated with a significantly increased risk of all-cause mortality (HR 2.12, 95%CI 1.06–4.27, p = 0.034). Higher MAP was associated with a decreased risk of 30-day mortality (β = 0.92, 95%CI 0.85–1.00, p = 0.038).
3.4. OSR > 30% versus OSR ≤ 30% in Hemodynamically Unstable Patients
Among the 44 hemodynamically unstable patients, 14 (31.82%) patients had a proximal stent graft OSR over 30%. The mean proximal OSR was 26% ± 5%, range from 17% to 39%. The median imaging follow-up time was similar between patients with OSR ≤ 30% and OSR > 30% (10.50 (1.25–32.50) months vs. 10.00 (1.00–39.50), p = 0.87). All three T1AELs occurred in patients with OSR ≤ 30%, but we did not observe a significant difference in T1AEL between patients with OSR > 30% and OSR ≤ 30% (0.00% vs. 11.11%, p = 0.19). There was no early T1AEL; all the three T1AELs in hemodynamically unstable patients were observed after 36 months postintervention (Figure 2). Additionally, no significant difference was found regarding the rates of other types of endoleaks, reintervention, and survival outcomes (Table 3).
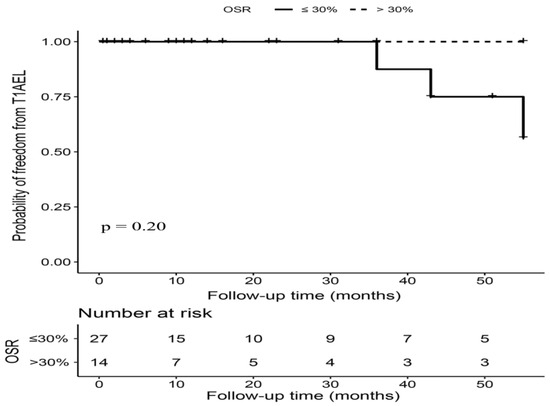
Figure 2.
Kaplan–Meier curves of freedom from T1AEL in hemodynamically unstable patients with OSR > 30% versus OSR ≤ 30%. Footnote: T1AEL = type IA endoleak, OSR = oversizing ratio.

Table 3.
Rates of adverse outcomes after emergent endovascular repair of patients with ruptured or impending rupture abdominal aortic aneurysm.
3.5. OSR > 20% versus OSR ≤ 20% in Hemodynamically Stable Patients
The proximal OSR ranged from 16% to 34%, with 66 (73.33%) patients having an OSR over 20%. Patients with OSR > 20% and OSR ≤ 20% had a similar length of imaging follow-up time (15.00 (5.25–34.50) months vs. 16.50 (5.00–29.00) months, p = 0.71). As is displayed in Figure 3, five T1AELs were observed after 24 months postintervention during the follow-up, and the incidence of T1AEL was significantly higher in patients with OSR ≤ 20% (16.67% vs. 1.52%, p = 0.006). After adjusted for age, gender, and neck angulation, OSR > 20% was associated with a significantly decreased risk of T1AEL in hemodynamically stable patients (HR 0.03, 95%CI 0.01–0.53, p = 0.016). The rates of other types of endoleaks, reintervention, and survival outcomes were comparable between two OSR groups (Table 3).
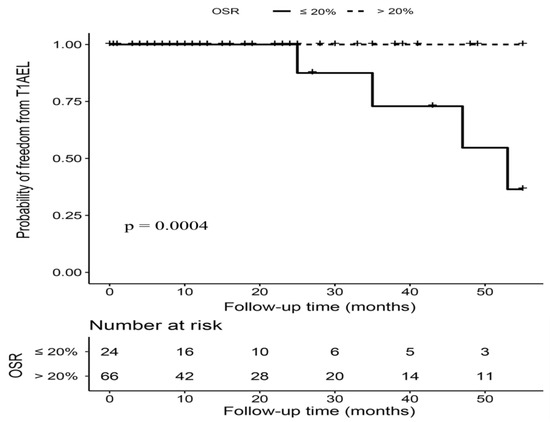
Figure 3.
Kaplan–Meier curves of freedom from type IA endoleak (T1AEL) in hemodynamically stable patients with OSR > 20% versus OSR ≤ 20%. Footnote: T1AEL = type IA endoleak, OSR = oversizing ratio.
4. Discussion
This cohort study reviewed all patients who underwent emergent EVAR in a high-volume center in the past decade. The results suggested that a proximal OSR over 20% was associated with a significantly decreased risk of T1AEL after emergent EVAR. With a mean OSR of 26%, hemodynamic instability was not associated with a significantly increased risk of T1AEL. As for hemodynamically unstable patients, a proximal OSR over 30% did not seem to exert additional benefit in lowering the risk of T1AEL. Most T1AEL cases were observed after 36 months postintervention, which indicated the reason T1AEL may not have inadequate sealing due to hypovolemia.
The concern about increasing the proximal OSR of aortic endografts originated from the effect of hemodynamic instability on aortic size. A retrospective study analyzed the difference in aortic diameters in trauma patients between admission and another stable moment, and the results suggested that the aortic diameter can decrease up to 12.6% in patients with a pulse over 130 beats/min [11]. Based on this data, current guidelines speculate that the proximal OSR may be raised to over 30% during emergent EVAR to avoid future type IA endoleak after the circulating blood volume is back to normal. Studies have suggested that CTA might underestimate aortic diameter when assessing type B aortic dissection or thoracic aorta injuries [20,21], which might contribute to late endoleak. However, recent research has indicated no significant differences in aortic measurements between intravascular ultrasound (IVUS) and CTA among non-ruptured AAA patients, particularly in proximal diameter measurements [22]. Moreover, there were no apparent differences observed in reinterventions [23]. Nevertheless, it remains unexplored whether differences exist in cases of rAAA or impending rupture AAA, and whether they have an impact on late endoleak and reinterventions. The mean MAP and heart rate of hemodynamically unstable patients in our study were 80.61 mmHg and 103.82 beats/min, which may correspond to nearly a 10% decrease in aortic diameter, according to the findings from Jonker FH and colleagues [11]. However, the change in aortic diameters may be less evident in hemodynamically unstable rAAA patients; AAA patients were much older, and the aortas were generally more calcified and stiffer than trauma patients.
We observed three T1AELs in hemodynamically unstable patients, and all of them were found after 24 months after the initial intervention. Although all three T1AELs were found in patients with a proximal OSR smaller than 30%, the late occurrence of T1AELs dispensed with the probability of inadequate sealing due to fluctuation of aortic hemodynamics, indicating that the average proximal OSR of 26% in hemodynamically unstable patients of our study was optimal. For example, for patients with an aortic neck diameter of 22 mm, choosing a 25 mm stent graft provides a standard OSR of 13.6%, which may be acceptable in elective AAA patients, but insufficient sealing of OSR smaller than 10% can occur if the collapsed aorta expands to normal size. In this circumstance, a 28 mm stent graft would be more appropriate and can provide a sufficient OSR between 15% and 27% when blood pressure fluctuates. Given the concern about the potential risk of stent migration, a proximal OSR greater than 35% may not be considered, since the diameters of some heavily atherosclerotic or calcified aortas may not be spectacular under the range of blood volume. In addition, previous experiments revealed that the dislodgement forces of thoracic aortic stents declined from 22.7 n to 9.0 n as the OSR increased from 10% to 20% [24], which suggested excessive OSR should also be avoided. Combined with the results of our study, an oversizing between 25% and 35% may be appropriate for hemodynamically unstable rAAA patients undergoing emergent EVAR. In addition, our results showed that both OSR > 20% vs. OSR ≤ 20% and OSR >30% vs. OSR ≤ 30% were not associated with the risk of reintervention in patients undergoing emergency EVAR. However, it is worth noting that the result was based on ratios rather than continuous variables. Further research is needed to determine whether a specific OSR value is associated with reintervention.
Sizing considerations should also be judged individually based on the aortic anatomy and preoperative aortic hemodynamics of the patients. A single center experience of C3 Gore Excluder stent graft suggested that a mean OSR of 23.5% was appropriate in patients with proximal aortic angulation over 60 degrees, and was not associated with an increased risk of T1AEL [25]. The latest study on EVAR for AAA using the Endurant stent graft indicates that the occurrence rate of type IA endoleaks is higher in the outside indications for use group than in those patients within the indications for use. The most common cause of endoleak in patients classified as outside indications for use is identified as having an angle or diameter issue in the proximal neck [26]. Based on their results, proper elevation of proximal OSR may be considered in patients with severe neck angulation. In addition to neck anatomy, the effect of preoperative circulating blood hemodynamics on oversizing consideration is also fundamental. The results of the IMPROVE trial suggested that the minimum threshold of 70 mmHg was too low for permissive hypotension for ruptured aneurysm, for it was associated with increased risk of 30-day mortality [27]. However, the authors did not record any increased risk of T1AEL in hemodynamically unstable patients due to inadequate sealing. Nevertheless, the results of IMPROVE trial recommended sufficient fluid resuscitation to raise mean arterial pressure before surgery, which can not only improve the survival of rAAA patients but also avoid unpredictable changes of aortic diameters due to dramatic change of blood volumes.
Limitations
The findings of our study should be interpreted with several limitations. First of all, the vital signs of included patients were recorded on admission, and the exact value of blood pressure before stent graft implantation was not available in a retrospective setting. The potential difference of aortic hemodynamics influenced by fluid resuscitation may affect aortic diameters. Thus, future prospective studies involving the exact vital signs before stent graft implantation are needed to provide more precise insight into the effect of hemodynamic instability on OSR choice in rAAA patients. Second, as most included patients in our study were followed up by duplex ultrasound, hence it was challenging to obtain changes of aortic size before and after stent graft implantation. In spite of this, the result of T1AEL is the final observation target, which also shed light on the alterations of aortic diameters. Third, the sample size of our study is relatively small, but it is still one of the largest single-center studies regarding OSR consideration in rAAA patients. Multicenter prospective cohort studies are warranted in the future. Fourth, our center only involved the Endurant Medtronic stent graft, so the OSR recommendation from our study may not be suitable to other devices.
5. Conclusions
After review of the rAAA patients undergoing emergent EVAR in a single center during the past decade, this cohort study suggested a proximal OSR over 20% was associated with a decreased risk of T1AEL in hemodynamically stable patients, while there is no difference between OSR > 30% and OSR ≤ 30%. However, we cannot adequately assess the specific impact of exact OSR values on the clinical outcomes after emergency EVAR, such as whether a specific OSR value significantly affects the occurrence of type IA endoleak or the need for reintervention. Prospective studies need to further evaluate the relationship between oversizing consideration of the proximal stent and the risk of endoleak after EVAR.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12237500/s1, Table S1: Strengthening the reporting of observational studies in epidemiology (STROBE) checklist of cohort studies; Table S2: Univariate and multivariate analyses of outcomes of interest in patients undergoing emergent endovascular aortic repair.
Author Contributions
Conceptualization, J.Z. and B.H.; methodology, Y.Q., C.W. and D.Y.; software, Y.Q. and C.W.; validation, Y.Q., C.W., T.W., D.Y., Y.M., Y.Y., J.Z. and B.H.; formal analysis, Y.Q. and C.W.; investigation, Y.M. and Y.Y.; resources, T.W. and D.Y.; data curation, Y.M. and Y.Y.; writing—original draft preparation, Y.Q. and C.W.; writing—review and editing, J.Z. and B.H.; visualization, Y.M. and Y.Y.; supervision, T.W. and D.Y.; project administration, J.Z. and B.H.; funding acquisition, T.W. and D.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was granted by the National Natural Science Foundation of China (82302152, 82300542), Sichuan Province Science and Technology Support Program (grant number: 2022YFS0366, 2022YFS0187, 2022YFS0359).
Institutional Review Board Statement
Approval from the institutional review board committee was obtained for this project.
Informed Consent Statement
Because of preexisting and de-identified data in our retrospective study, patient informed consent was waived.
Data Availability Statement
The dataset generated and analyzed is available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parodi, J.C.; Palmaz, J.C.; Barone, H.D. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann. Vasc. Surg. 1991, 5, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Investigators, I.T. Comparative clinical effectiveness and cost effectiveness of endovascular strategy v open repair for ruptured abdominal aortic aneurysm: Three year results of the IMPROVE randomised trial. BMJ 2017, 359, j4859. [Google Scholar] [CrossRef] [PubMed]
- Sweeting, M.J.; Balm, R.; Desgranges, P.; Ulug, P.; Powell, J.T.; Koelemay, M.J.W.; Idu, M.M.; Kox, C.; Legemate, D.A.; Huisman, L.C.; et al. Individual-patient meta-analysis of three randomized trials comparing endovascular versus open repair for ruptured abdominal aortic aneurysm. Br. J. Surg. 2015, 102, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Mastracci, T.M.; Garrido-Olivares, L.; Cinà, C.S.; Clase, C.M. Endovascular repair of ruptured abdominal aortic aneurysms: A systematic review and meta-analysis. J. Vasc. Surg. 2008, 47, 214–221. [Google Scholar] [CrossRef]
- Patel, R.; Sweeting, M.J.; Powell, J.T.; Greenhalgh, R.M. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): A randomised controlled trial. Lancet 2016, 388, 2366–2374. [Google Scholar] [CrossRef]
- Major, M.; Long, G.W.; Eden, C.L.; Studzinski, D.M.; Callahan, R.E.; Brown, O.W. Long-term outcomes and interventions of postoperative type 1a endoleak following elective endovascular aortic aneurysm repair. J. Vasc. Surg. 2021, 75, 136–143.e1. [Google Scholar] [CrossRef]
- Oliveira, N.F.; Oliveira-Pinto, J.; van Rijn, M.J.; Baart, S.; Raa, S.T.; Hoeks, S.E.; Gonçalves, F.B.; Verhagen, H.J. Risk Factors, Dynamics, and Clinical Consequences of Aortic Neck Dilatation after Standard Endovascular Aneurysm Repair. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 26–35. [Google Scholar] [CrossRef]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef]
- Chaikof, E.L.; Dalman, R.L.; Eskandari, M.K.; Jackson, B.M.; Lee, W.A.; Mansour, M.A.; Mastracci, T.M.; Mell, M.; Murad, M.H.; Nguyen, L.L.; et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 2018, 67, 2–77.e2. [Google Scholar] [CrossRef]
- Gonthier, C.; Deglise, S.; Brizzi, V.; Ducasse, E.; Midy, D.; Lachat, M.; Berard, X. Hemodynamic Conditions may Influence the Oversizing of Stent Grafts and the Postoperative Surveillance of Patients with Ruptured Abdominal Aortic Aneurysm Treated by EVAR. Ann. Vasc. Surg. 2016, 30, 308.e5–308.e10. [Google Scholar] [CrossRef]
- Jonker, F.H.; Verhagen, H.J.; Mojibian, H.; Davis, K.A.; Moll, F.L.; Muhs, B.E. Aortic endograft sizing in trauma patients with hemodynamic instability. J. Vasc. Surg. 2010, 52, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Kratzberg, J.A.; Golzarian, J.; Raghavan, M.L. Role of graft oversizing in the fixation strength of barbed endovascular grafts. J. Vasc. Surg. 2009, 49, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Sternbergh, W.C., 3rd; Money, S.R.; Greenberg, R.K.; Chuter, T.A.; Zenith Investigators. Influence of endograft oversizing on device migration, endoleak, aneurysm shrinkage, and aortic neck dilation: Results from the Zenith Multicenter Trial. J. Vasc. Surg. 2004, 39, 20–26. [Google Scholar] [CrossRef] [PubMed]
- van Prehn, J.; Schlösser, F.J.; Muhs, B.E.; Verhagen, H.J.; Moll, F.L.; van Herwaarden, J.A. Oversizing of aortic stent grafts for abdominal aneurysm repair: A systematic review of the benefits and risks. Eur. J. Vasc. Endovasc. Surg. 2009, 38, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Rakita, D.; Newatia, A.; Hines, J.J.; Siegel, D.N.; Friedman, B. Spectrum of CT findings in rupture and impending rupture of abdominal aortic aneurysms. Radiographics 2007, 27, 497–507. [Google Scholar] [CrossRef]
- Sundararajan, V.; Henderson, T.; Perry, C.; Muggivan, A.; Quan, H.; Ghali, W.A. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J. Clin. Epidemiol. 2004, 57, 1288–1294. [Google Scholar] [CrossRef]
- Oliveira, N.F.; Bastos Gonçalves, F.M.; de Vries, J.P.; Ultee, K.; Werson, D.; Hoeks, S.; Moll, F.; van Herwaarden, J.; Verhagen, H. Mid-Term Results of EVAR in Severe Proximal Aneurysm Neck Angulation. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 19–27. [Google Scholar] [CrossRef]
- Lee, P.H.; Burstyn, I. Identification of confounder in epidemiologic data contaminated by measurement error in covariates. BMC Med. Res. Methodol. 2016, 16, 54. [Google Scholar] [CrossRef]
- Belkin, N.; Jackson, B.M.; Foley, P.J.; Damrauer, S.M.; Kalapatapu, V.; Golden, M.A.; Fairman, R.M.; Wang, G.J. The use of intravascular ultrasound in the treatment of type B aortic dissection with thoracic endovascular aneurysm repair is associated with improved long-term survival. J. Vasc. Surg. 2020, 72, 490–497. [Google Scholar] [CrossRef]
- Rodriguez, M.C.; Realyvasquez, A.; Galante, J.; Pevec, W.; Humphries, M. Differences in Aortic Diameter Measurements with Intravascular Ultrasound and Computed Tomography After Blunt Traumatic Aortic Injury. J. Vasc. Surg. 2016, 64, 545. [Google Scholar] [CrossRef]
- Taalab, M.A.; Kamal, A.M.; Mohammad, A.F.; Zaki, M.M. Intravascular Ultrasound Versus Computed Tomography Angiography in Sizing and Operative Management of Endovascular Aortic Aneurysm Repair. J. Endovasc. Ther. 2023. [Google Scholar] [CrossRef] [PubMed]
- Illuminati, G.; Pacilè, M.A.; Ceccanei, G.; Ruggeri, M.; La Torre, G.; Ricco, J.-B. Peroperative intravascular ultrasound for endovascular aneurysm repair versus peroperative angiography: A pilot study in fit patients with favorable anatomy. Ann. Vasc. Surg. 2020, 64, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-H.; Yeh, M.-L.; Chen, W.-L.; Kan, C.-D. Apparatus for Comparison of Pullout Forces for Various Thoracic Stent Grafts at Varying Neck Angulations and Oversizes. Ann. Vasc. Surg. 2016, 31, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Shiraev, T.; Agostinho, N.; Dubenec, S. Sizing Considerations for GORE Excluder in Angulated Aortic Aneurysm Necks. Ann. Vasc. Surg. 2018, 49, 152–157. [Google Scholar] [CrossRef]
- Mwipatayi, B.P.; Faraj, J.; Oshin, O.; on behalf of the ENGAGE REGISTRY co-investigators. Endurant Stent Graft Demonstrates Promising Outcomes in Challenging Abdominal Aortic Aneurysm Anatomy. J. Vasc. Surg. 2021, 73, 69–80. [Google Scholar] [CrossRef]
- IMPROVE Trial Investigators; Powell, J.T.; Hinchliffe, R.J.; Thompson, M.M.; Sweeting, M.J.; Ashleigh, R.; Bell, R.; Gomes, M.; Greenhalgh, R.M.; Grieve, R.J.; et al. Observations from the IMPROVE trial concerning the clinical care of patients with ruptured abdominal aortic aneurysm. Br. J. Surg. 2014, 101, 216–224. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).