Abstract
Background: Neurocognitive alterations in the perioperative period might be caused by a wide variety of factors including pain, blood loss, hypotension, hypoxia, micro- and macroemboli, cardiopulmonary bypass (CPB), reperfusion damage, and surgery itself, and all are risk factors for developing postoperative delirium (POD) and postoperative cognitive dysfunction (POCD). The objective of this study was to evaluate the effect of ketamine on neurocognitive dysfunction after anesthesia. Methods: We conducted a meta-analysis of randomized controlled trials (RCTs) comparing ketamine use (experimental group) with placebo (controls). Results: The model favors the control group over the experimental group in terms of frequency of hallucinations (the risk ratio with 95% CI is 1.54 [1.09, 2.19], p-value = 0.02), the number of patients readmitted within 30 days (RR with 95% CI is 0.25 [0.09, 0.70]), and the number of adverse events (overall RR with 95% CI is 1.31 [1.06, 1.62]). In terms of morphine consumption, the model favors the experimental group. Conclusion: There was no statistically significant difference in incidences of postoperative delirium, vasopressor requirement, and fentanyl consumption between the ketamine and control groups. However, hallucinations were more frequently reported in the ketamine group.
1. Introduction
Neurocognitive alterations in the perioperative period might be caused by a wide variety of factors including anesthesia and surgery itself [1]. Many perioperative factors, including pain, blood loss, hypotension, hypoxia, micro- and macroemboli, cardiopulmonary bypass (CPB), reperfusion damage, and surgery itself are risk factors for developing postoperative delirium (POD) and postoperative cognitive dysfunction (POCD) [2]. The incidence of POD varies from 10% to 80% and usually occurs during the first 72 h following surgery. Only 4% of ageing patients who developed POD fully recover at discharge, and up to 80% still have residual impairment at 6 months or later [2]. The incidence of POCD in older patients undergoing cardiac surgeries has been reported to range from 20% to 50% three months after surgery and may reach 55% in those undergoing some other major surgeries [2,3,4].
Management of POD is still empirical. There is some evidence that ketamine might be an option to reduce the risk of POD and POCD in surgical patients. Ketamine is a N-methyl-D-aspartic acid (NMDA) antagonist and has strong anti-nociceptive properties by impacting central sensitization and pain modulation [3,4]. Previous studies have found that ketamine given during subanesthetic intraoperative anesthesia lowers the levels of postoperative inflammation markers, opioid use, and pain. Despite reported hallucinogenic effects that might induce or exacerbate postoperative delirium, ketamine has been recently reported to have protective effects against postoperative neurocognitive dysfunction [3,4,5]. In both human and animal trials, ketamine has demonstrated substantial anti-inflammatory effects and the potential to lessen postoperative delirium [3,4,5]. A recent study showed that ketamine added to common anesthetics might lower POD in cardiac surgery to 3%, as opposed to 31% in the placebo group [6]. Additionally, by reducing the inflammation after surgery simultaneously with the central nervous system, ketamine may provide neuroprotection [4,7]. Contrarily, a large-scale trial in which a single dosage of ketamine was administered during induction during both non-cardiac and cardiac surgery found no difference in the results [6]. Especially when administered alone, the usage of ketamine might be exacerbated by side effects such as disorientation, euphoria, cognitive impairment, and perceptual problems [8].
This systematic review and meta-analysis aimed to compare the effect of ketamine and other anesthesia methods on neurocognitive dysfunction after anesthesia.
2. Materials and Methods
2.1. Protocol
This systematic review and meta-analysis was planned, performed, and reported according to the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)” guidelines [9]. The protocol was registered in Open Science Framework. We searched for randomized controlled trials (RCTs) published in English, which studied the effect of ketamine on neurocognitive dysfunction after surgery.
We searched for relevant articles in PubMed, Scopus, and the Cochrane Library published before March 2023 (Figure 1). The following search terms or their combinations were used during the search: ((((ketamine) AND (anesthesia)) AND (surgery)) AND (postoperative delirium)) AND (postoperative cognitive dysfunction); (“esketamine” OR “esketamine” OR “ketamine” OR “ketamine” OR “ketamin” OR “ketamine s” OR “ketamines”) AND (“anaesthesia” OR “anesthesia” OR “anesthesia” OR “anaesthesias” OR “anesthesias”) AND (“surgery” OR “surgery” OR “surgical procedures, operative” OR (“surgical” AND “procedures” AND “operative”) OR “operative surgical procedures” OR “general surgery” OR (“general” AND “surgery”) OR “general surgery” OR “surgery” OR “surgerys” OR “surgeries”) AND ((“postoperative period” OR (“postoperative” AND “period”) OR “postoperative period” OR “postop” OR “postoperative” OR “postoperatively” OR “postoperatives”) AND (“delirium” OR “delirium” OR “deliriums”)) AND (“postoperative cognitive complications” OR (“postoperative” AND “cognitive” AND “complications”) OR “postoperative cognitive complications” OR (“postoperative” AND “cognitive” AND “dysfunction”) OR “postoperative cognitive dysfunction”).
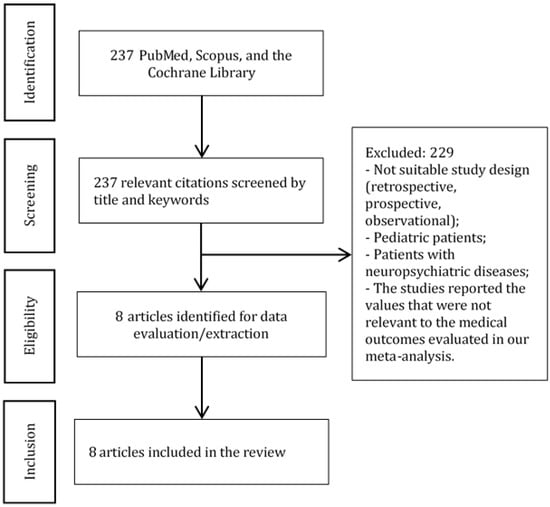
Figure 1.
PRISMA diagram. The diagram shows the study selection process.
2.2. Participants and Population
Inclusion criteria:
- Study types: RCTs;
- Study arms: comparison of ketamine and placebo;
- Age of patients: 18 years and older;
- Surgery: any type of surgery;
- Articles published in English.
2.3. Outcomes
The primary outcomes of our meta-analysis were the effect of ketamine on postoperative delirium and hallucinations. The secondary outcomes included hemodynamic stability, pain scores, total dose of opioids, and side effects.
2.4. Data Extraction and Statistical Methods
Data were extracted using a standardized Excel form. Study characteristics, such as first author, country, year of publication, patient population, intervention, study design, sample size, and outcomes, were extracted into Table 1.

Table 1.
Characteristics of the included studies. Abbreviations: CABG, coronary artery bypass graft; IV, intravenous(ly).
2.5. Assessment of Methodological Quality
First, the methodological quality of the included studies was assessed using the Oxford quality scoring system (Jadad Scale). The quality of the studies was graded within “the range from 1 (min) to 5 (max) as low (<3), acceptable (3), good (4), and excellent (5)” [12]. Then, each study was evaluated using the Cochrane risk of bias tool as “high risk”, “some concerns”, or “low risk” [13]. Finally, each outcome was examined for the certainty of evidence using GRADE as “high”, “moderate”, “low”, or “very low” [14].
3. Results
We have found 237 citations that matched our search criteria (Figure 1). Eight articles [1,2,3,4,5,7,10,11] with 896 patients were selected for the meta-analysis (Table 1 and Table 2).

Table 2.
Abbreviations: RCT, randomized controlled trial.
Data analysis was conducted using the “Review Manager (RevMan) [Computer program]. Version 5.4. The Cochrane Collaboration, 2020”. Heterogeneity was estimated by the I2 statistic. Whenever needed, we used mathematical methods for estimating the sample mean and standard deviation [15,16].
3.1. Outcomes
3.1.1. Incidence of Delirium
There is no statistically significant difference in the frequency of delirium between the experimental (ketamine) and control (placebo) groups (Figure 2).

Figure 2.
Incidence of delirium. The forest plot shows the pooled risk ratio of the incidence of delirium after ketamine versus placebo use [2,5,10].
3.1.2. Incidence of Hallucinations
The model favors the control group over the experimental group in terms of the frequency of hallucinations (Figure 3). However, the result is sensitive to the exclusion of a study by Avidan et al., 2017 [5] in which case there will be no difference between the groups.

Figure 3.
Incidence of hallucinations. The forest plot shows the pooled risk ratio of the incidence of hallucinations after ketamine versus placebo use [5,11].
3.1.3. Vasopressor Use
The model does not favor either group in terms of the number of patients requiring vasopressors (Figure 4).

Figure 4.
Vasopressor use. The forest plot shows the pooled risk ratio of the incidence of vasopressor use after ketamine versus placebo use [2,4].
3.1.4. Fentanyl Consumption (μg)
The model does not favor either group in terms of fentanyl consumption (μg) at p = 0.13 (Figure 5). We should note that Urban et al., 2008 [11] reported data values in mcg/kg, intraoperatively, so we have not included this study here.
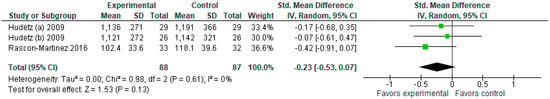
Figure 5.
Fentanyl consumption (μg). The forest plot shows the standardized mean difference in fentanyl consumption between the ketamine and the control groups [1,2,4].
3.1.5. Morphine Consumption (mg)
In terms of morphine consumption (Figure 6), the model favors the experimental group (SMD with 95% CI is −0.19 [−0.35, −0.02]).
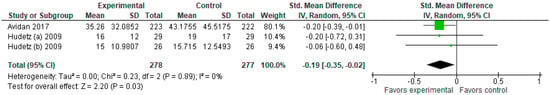
Figure 6.
Morphine consumption (mg). The forest plot shows the standardized mean difference in morphine consumption between the ketamine and the control groups [2,4,5].
3.1.6. Adverse Events
The model tends to favor the control group over the ketamine group. As shown in the forest plot (Figure 7), the overall risk ratio with a 95% CI is 1.31 [1.06, 1.62]. However, the model does not strongly favor the control group over the experimental group in any of the subgroups. The sensitivity analysis shows that the result in incidences of nausea is sensitive to the exclusion of a study by Avidan et al. [5], while the result in pain outcomes is sensitive to the exclusion of Rascon-Martinez et al. [1] and Salehi et al. [10].

Figure 7.
Adverse events. The forest plot shows the pooled risk ratio of the incidence of adverse events (blood transfusion, nausea, mild/moderate pain) after ketamine versus placebo use [1,2,4,5,7,10].
3.1.7. Surgery Duration (min)
The model does not favor the ketamine group over the control group in terms of surgery time (Figure 8).

Figure 8.
Surgery duration (min). The forest plot shows the standardized mean difference in surgery duration between the ketamine and the control groups [1,2,3,4,7,11].
3.1.8. Readmission within 30 Days
The model (Figure 9) favors the ketamine group over the control group in terms of the number of patients readmitted within 30 days, RR with a 95% CI is 0.25 [0.09, 0.70].
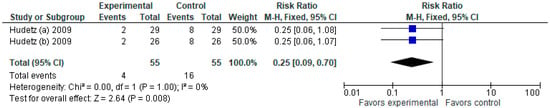
Figure 9.
Readmission within 30 days. The forest plot shows the pooled risk ratio of readmission within 30 days after ketamine versus placebo use [2,4].
3.2. Assessment of Methodological Quality
As evident from Table 3, of the eight studies, five were rated as “excellent”, two as “good”, and one as “acceptable” quality based on the Oxford quality scoring system (Jadad Scale). Based on the Cochrane Risk of Bias (Table 4), all the included studies had a “low risk” of bias. Based on GRADE (Table 5), the certainty of evidence ranged from “low” to “high”. Appendix A provides the Evidence profile of the studied outcomes.

Table 3.
Methodological quality of the studies (Jadad scale).

Table 4.
Cochrane risk of bias.

Table 5.
Summary of findings.
4. Discussion
Although we found no difference between ketamine and control in the incidence of postoperative delirium, the incidence of hallucinations was higher in the ketamine group. We must note that the result is sensitive to the exclusion of the study by Avidan et al. [5], in which case there will be no difference between the groups.
Since postoperative neurocognitive disorders can be induced by numerous factors, many secondary outcomes of this meta-analysis might be important. Despite ketamine is known to provide more stable hemodynamics compared to other intravenous anesthetics, there were no differences in terms of the number of patients requiring vasopressors. Interestingly, there is also a trend to favor control (not statistically significant) in terms of requirement in blood transfusion. We suppose that patients with blood loss were administered ketamine to maintain more stable hemodynamics. There was no difference between the ketamine and control groups in postoperative pain intensity and nausea. Another important finding was that there was a lower readmission rate in the ketamine group.
The conflicting results might be due to the failure of large studies to replicate the results of small studies [5]. Although meta-analyses of small studies might also contradict the findings of large trials, the results of the current meta-analysis support the findings of the large trials. More importantly, POD and POCD are multifactorial conditions, which might be caused by the following factors [17,18]:
- (1)
- Preoperative (advanced age, neurocognitive deterioration, depression, use of anti-depressants);
- (2)
- Intraoperative (blood loss, hypotension, pain, hypernatremia, hyponatremia, deep anesthesia (if the doses of anesthesia are higher than required, prolonged duration of anesthesia and surgery);
- (3)
- Postoperative factors (sleep deprivation, severe pain, electrolyte dysbalance, cerebrovascular events).
Therefore, it is challenging to consider all these factors during the study and patient enrollment.
It is also important to mention that ketamine has several effects, such as anesthetic, analgesic, antidepressant, anti-inflammatory, and neuroprotective [5,19,20,21]. Ketamine is known for inducing general (dissociative) anesthesia. Ketamine can be also used for local anesthesia as an adjunct to local anesthetics. Dissociative anesthesia is a type of anesthesia lacking complete unconsciousness and characterized by catalepsy, catatonia, and amnesia. Adequate analgesia can be achieved by using low subanesthetic doses (0.15–0.25 mg/kg) for the reduction of acute and chronic pain. Ketamine administration in pre- or intraoperative periods has been used to improve postoperative outcomes due to its ability to reduce the excessive production of proinflammatory cytokines, such as tumor necrosis factor-α, nuclear factor-kB, C-reactive protein, interleukin 6 (IL-6), and inducible nitric oxide synthase. Moreover, the anti-inflammatory effects of ketamine were attributed not only to local but also to systemic anti-inflammatory action [21]. Ketamine also has a preconditioning effect via the inhibition of NMDA receptors, and it was also shown to reduce post-ischemic cortical neuronal loss associated with glutamate-induced calcium overload [7]. Some previous studies also showed that the NMDA receptor through anti-inflammatory mechanisms plays a direct role in the recognition and short-term memory; however, other reports assign this effect to improved cerebral blood flow [22,23,24]. This binding of ketamine to the NMDA receptor suppresses the expression of the factor kβ2 that is involved in the transcription of genes that affects proinflammatory cytokines, including interleukins 6 and 8, and tumor necrosis factor α leading to reduced neuronal apoptosis. Subsequently, ketamine also activates the sympathetic nervous system leading to increased cerebral perfusion pressure [25,26]. Since elevated levels of some cytokines, such as IL-6, have been associated with poor postoperative outcomes, this effect of ketamine seems promising, but more RCTs are required. Ketamine has been reported to modulate abnormal inflammatory substances in major depressive disorder. Ketamine acts as an immunomodulatory but not as an immunosuppressive agent, which might be of particular importance since ketamine is usually used during the induction of anesthesia.
Although ketamine might have promising positive effects, it also has several psychotomimetic and dissociative adverse effects. Ketamine broadly influences consciousness and perception. Some patients report dissociative and extracorporeal experiences and illusions (being out-of-body) [21]. The most common psychoactive effects are dissociative effects (visual, auditory, and somatosensory stimuli), positive psychotomimetic (hallucinations, conceptual disorganization, unusual thought content, suspiciousness), as well as negative psychotomimetic (emotional withdrawal, motor retardation, and blunted affect). Memory and cognitive impairment effects of ketamine were also reported [5,19,20,21].
This meta-analysis has several important limitations. The first limitation of this meta-analysis was the inclusion of the original studies with small sample sizes. The second limitation was heterogeneity in reporting rubrics; therefore, we could not incorporate the results of these studies in the forest plots. The next limitation was no proper assessment or reporting of the side effects. For example, the side effects were observed within 24 h after surgery. However, some side effects might occur later, but they were not assessed or reported.
5. Conclusions
The incidence of postoperative delirium did not differ significantly between the ketamine and placebo groups. However, hallucinations were more frequently reported in the ketamine group. There was no statistically significant difference in fentanyl consumption and vasopressor requirement between the ketamine and control groups. Due to the limitations of the existing trials, future large RCTs are warranted to establish more convincing evidence regarding the protective effects of ketamine against neurocognitive dysfunction.
Author Contributions
Conceptualization, D.V.; methodology, D.V., F.B. and R.B.; data extraction, M.A.; formal analysis, F.N. and Y.A.; quality assessment, M.A. and N.Y.; writing—original draft preparation, D.V. and Y.A.; writing—review and editing, D.V., Y.A., M.A., F.N., N.Y., F.B. and R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by Nazarbayev University Faculty Development Competitive Research Grants No. 021220FD2851 and 11022021FD2906.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Evidence profile.
Table A1.
Evidence profile.
| Incidences of Delirium | Incidences of Hallucinations | Vasopressor Use | Fentanyl Consumption (μg) | Morphine Consumption (mg) | Overall Adverse Events | Readmissions within 30 Days | |
|---|---|---|---|---|---|---|---|
| Risk of bias | Not serious | Not serious | Not serious | Serious | Not serious | Not serious | Not serious |
| Lack of allocation concealment | No | No | No | No | No | No | No |
| Lack of blinding | No | No | No | No | No | No | No |
| Incomplete accounting of patients and outcome events | No | No | No | Yes | No | No | No |
| Selective outcome reporting | No | No | No | No | No | No | No |
| Other limitations | No | No | No | No | No | No | No |
| Inconsistency | Very serious | Serious | Not serious | Not serious | Not serious | Not serious | Not serious |
| I2 (unexplained heterogeneity of results) | Yes | No | No | No | No | No | No |
| Wide variance of point estimates | Yes | No | No | No | No | Yes | No |
| Confidence intervals (CIs) do not overlap | Yes | No | No | No | No | No | No |
| Indirectness | Not serious | Not serious | Not serious | Not serious | Not serious | Not serious | Not serious |
| Differences in population | No | No | No | No | No | No | No |
| Differences in interventions | No | No | No | No | No | No | No |
| Differences in outcome measures | No | No | No | No | No | No | No |
| Indirect comparisons | No | No | No | No | No | No | No |
| Imprecision | Serious | Serious | Very serious | Serious | Not serious | Not serious | Serious |
| Few patients | No | No | Yes | Yes | No | No | Yes |
| Wide confidence interval (CI) | Yes | No | Yes | No | No | No | No |
| Other considerations | None | None | None | None | None | None | Not serious |
| RR > 2 or RR < 0.5 RR > 5 or RR < 0.2 | No | No | No | No | No | No | No |
| Dose-response gradient | No | No | No | No | No | No | No |
| Effect of plausible residual confounding | No | No | No | No | No | No | No |
References
- Rascón-Martínez, D.M.; Fresán-Orellana, A.; Ocharán-Hernández, M.E.; Genis-Zarate, J.H.; Castellanos-Olivares, A. The Effects of Ketamine on Cognitive Function in Elderly Patients Undergoing Ophthalmic Surgery: A Pilot Study. Anesth. Analg. 2016, 122, 969–975. [Google Scholar] [CrossRef]
- Hudetz, J.A.; Patterson, K.M.; Iqbal, Z.; Gandhi, S.D.; Byrne, A.J.; Hudetz, A.G.; Warltier, D.C.; Pagel, P.S. Ketamine Attenuates Delirium after Cardiac Surgery with Cardiopulmonary Bypass. J. Cardiothorac. Vasc. Anesth. 2009, 23, 651–657. [Google Scholar] [CrossRef]
- Bornemann-Cimenti, H.; Wejbora, M.; Michaeli, K.; Edler, A.; Sandner-Kiesling, A. The Effects of Minimal-Dose versus Low-Dose S-Ketamine on Opioid Consumption, Hyperalgesia, and Postoperative Delirium: A Triple-Blinded, Randomized, Active- and Placebo-Controlled Clinical Trial. Minerva Anestesiol. 2016, 82, 1069–1076. [Google Scholar]
- Hudetz, J.A.; Iqbal, Z.; Gandhi, S.D.; Patterson, K.M.; Byrne, A.J.; Hudetz, A.G.; Pagel, P.S.; Warltier, D.C. Ketamine Attenuates Post-Operative Cognitive Dysfunction after Cardiac Surgery. Acta Anaesthesiol. Scand. 2009, 53, 864–872. [Google Scholar] [CrossRef]
- Avidan, M.S.; Maybrier, H.R.; Abdallah, A.B.; Jacobsohn, E.; Vlisides, P.E.; Pryor, K.O.; Veselis, R.A.; Grocott, H.P.; Emmert, D.A.; Rogers, E.M.; et al. Intraoperative Ketamine for Prevention of Postoperative Delirium or Pain after Major Surgery in Older Adults: An International, Multicentre, Double-Blind, Randomised Clinical Trial. Lancet 2017, 390, 267–275. [Google Scholar] [CrossRef]
- Siripoonyothai, S.; Sindhvananda, W. Comparison of Postoperative Delirium within 24 Hours between Ketamine and Propofol Infusion during Cardiopulmonary Bypass Machine: A Randomized Controlled Trial. Ann. Card. Anaesth. 2021, 24, 294. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, J.Y.; Kim, J.W.; Park, J.S.; Lee, K.W.; Jeon, S.Y. Influence of Ketamine on Early Postoperative Cognitive Function After Orthopedic Surgery in Elderly Patients. Anesthesiol. Pain Med. 2015, 5, e28844. [Google Scholar] [CrossRef]
- Loo, C.K.; Katalinic, N.; Garfield, J.B.B.; Sainsbury, K.; Hadzi-Pavlovic, D.; Mac-Pherson, R. Neuropsychological and Mood Effects of Ketamine in Electroconvulsive Therapy: A Randomised Controlled Trial. J. Affect. Disord. 2012, 142, 233–240. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Salehi, B.; Mohammadbeigi, A.; Kamali, A.; Taheri-Nejad, M.; Moshiri, I. Impact Comparison of Ketamine and Sodium Thiopental on Anesthesia during Electroconvulsive Therapy in Major Depression Patients with Drug-Resistant; a Double-Blind Randomized Clinical Trial. Ann. Card. Anaesth. 2015, 18, 486. [Google Scholar] [CrossRef]
- Urban, M.K.; Ya Deau, J.T.; Wukovits, B.; Lipnitsky, J.Y. Ketamine as an Adjunct to Postoperative Pain Management in Opioid Tolerant Patients after Spinal Fusions: A Prospective Randomized Trial. HSS J. ® Musculoskelet. J. Hosp. Spec. Surg. 2008, 4, 62–65. [Google Scholar] [CrossRef]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Schünemann, H.J.; Tugwell, P.; Knottnerus, A. GRADE Guidelines: A New Series of Articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. 2011, 64, 380–382. [Google Scholar] [CrossRef]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally Estimating the Sample Mean from the Sample Size, Median, Mid-Range, and/or Mid-Quartile Range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Viderman, D.; Brotfain, E.; Bilotta, F.; Zhumadilov, A. Risk factors and mechanisms of postoperative delirium after intracranial neurosurgical procedures. Asian J. Anesthesiol. 2020, 58, 5–13. [Google Scholar]
- Viderman, D.; Nabidollayeva, F.; Aubakirova, M.; Yessimova, D.; Badenes, R.; Abdildin, Y. Postoperative Delirium and Cognitive Dysfunction after General and Regional Anesthesia: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 3549. [Google Scholar] [CrossRef]
- Glumac, S.; Kardum, G.; Karanovic, N. Postoperative Cognitive Decline After Cardiac Surgery: A Narrative Review of Current Knowledge in 2019. Med. Sci. Monit. 2019, 25, 3262–3270. [Google Scholar] [CrossRef]
- Dale, O.; Somogyi, A.A.; Li, Y.; Sullivan, T.; Shavit, Y. Does Intraoperative Ketamine Attenuate Inflammatory Reactivity Following Surgery? A Systematic Review and Meta-Analysis. Anesth. Analg. 2012, 115, 934–943. [Google Scholar] [CrossRef]
- Zanos, P.; Moaddel, R.; Morris, P.J.; Riggs, L.M.; Highland, J.N.; Georgiou, P.; Pereira, E.F.R.; Albuquerque, E.X.; Thomas, C.J.; Zarate, C.A.; et al. Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol. Rev. 2018, 70, 621–660. [Google Scholar] [CrossRef]
- Malhotra, A.K.; Pinals, D.A.; Weingartner, H.; Sirocco, K.; Missar, C.D.; Pickar, D.; Breier, A. NMDA Receptor Function and Human Cognition: The Effects of Ketamine in Healthy Volunteers. Neuropsychopharmacology 1996, 14, 301–307. [Google Scholar] [CrossRef]
- Holcomb, H.H.; Lahti, A.C.; Medoff, D.R.; Weiler, M.; Tamminga, C.A. Sequential Regional Cerebral Blood Flow Brain Scans Using PET with H215O Demonstrate Ketamine Actions in CNS Dynamically. Neuropsychopharmacology 2001, 25, 165–172. [Google Scholar] [CrossRef]
- Sakai, T.; Ichiyama, T.; Whitten, C.W.; Giesecke, A.H.; Lipton, J.M. Ketamine Suppresses Endotoxin-Induced NF-KappaB Expression. Can. J. Anaesth. J. Can. Anesth. 2000, 47, 1019–1024. [Google Scholar] [CrossRef]
- Baldwin, A.S. The NF-Kappa B and I Kappa B Proteins: New Discoveries and Insights. Annu. Rev. Immunol. 1996, 14, 649–683. [Google Scholar] [CrossRef]
- Långsjö, J.W.; Kaisti, K.K.; Aalto, S.; Hinkka, S.; Aantaa, R.; Oikonen, V.; Sipilä, H.; Kurki, T.; Silvanto, M.; Scheinin, H. Effects of Subanesthetic Doses of Ketamine on Regional Cerebral Blood Flow, Oxygen Consumption, and Blood Volume in Humans. Anesthesiology 2003, 99, 614–623. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).