Abstract
Obstructive sleep apnea (OSA) is a prevalent, underdiagnosed disease that imposes a significant impact on the health and wellbeing of patients and a financial burden on individuals, their families, and society. Development of new methods of testing other than an overnight sleep study, such as measurement of serum or plasma biomarkers, may provide an easier diagnostic process to identify patients with OSA and allow earlier initiation of treatment, which might prevent serious comorbidities. We conducted a systematic review and quality assessment of available meta-analyses regarding potential diagnostic and monitoring biomarkers of obstructive sleep apnea. A total of 14 sets of candidate biomarkers displayed differences in levels or concentrations in OSA patients compared to non-OSA controls, and decreased after OSA treatment: CRP, IL-6, TNF-α, Il-8, HCY, ICAM-1, VCAM-1, VEGF, TC, LDLc, HDLc, TG, leptin, MDA, ALT, AST, IGF-1, adiponectin, and cortisol. This review summarizes the evidence for OSA-associated potential biomarkers and demonstrates that the quality of available studies, as measured by AMSTAR2, is often low and associated with a high risk of bias.
1. Introduction
Obstructive sleep apnea (OSA) is a nocturnal disorder characterized by recurrent episodes of upper airway obstruction during sleep, associated with oxygen desaturation and sleep fragmentation [1,2,3,4,5]. It is an increasingly prevalent condition that greatly impacts public health. Epidemiologic data show it as a disorder with a high prevalence of 9% to 38% in the general adult population, from 13% to 33% in men, and from 6% to 19% in women [6].
Obstructive sleep apnea is responsible for the increased risks of cardiovascular and pulmonary illnesses, stroke, and operative and postoperative risks [7,8,9]. Patients with OSA often manifest daytime sleepiness and experience a high risk for workplace and traffic accidents [10]. There is a strong association between obstructive sleep apnea and traffic accidents. Apnea–hypopnea index (AHI) of greater than 15/hour is associated with multiple accidents in 5 years [11]. In patients with OSA, the risk of an accident is higher among those who had consumed alcohol on the day of the accident [12]. Patients with obstructive sleep apnea have significantly abnormal health-related quality of life (HRQoL) scores compared to healthy age- and gender-matched controls [13]. In summary, OSA has a significant impact on health and wellbeing and imposes a financial burden on individuals, their families, and society.
The pathogenesis of OSA is multifactorial and still not fully established. It involves a diverse range of mechanisms, including selective activation of inflammatory molecular pathways, endothelial dysfunction, metabolic dysregulation, and oxidative stress [14]. Endothelial dysfunction is often considered to be one of the earliest detectable and possibly reversible abnormalities during the development of atherosclerosis. Studies indicate an association between the presence of both coronary and systemic endothelial dysfunction and an increased risk for future cardiovascular morbidity and mortality in patients with OSA [15,16,17]. Consequently, the severity of endothelial dysfunction may depend on the severity of OSA. The development of cardiovascular morbidity and mortality may also occur secondary to other pathologies caused by OSA, such as hypertension and diabetes [18] (Figure 1).
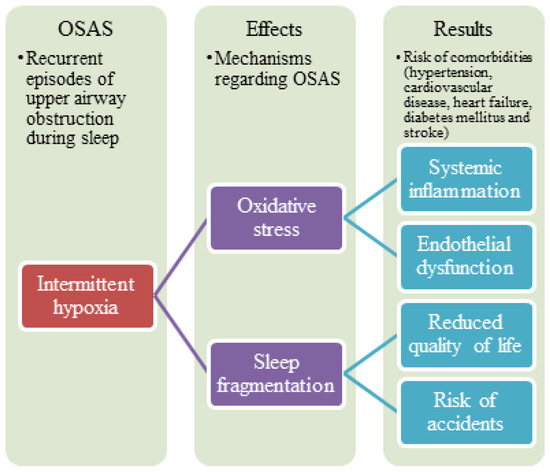
Figure 1.
Summary of mechanisms regarding obstructive sleep apnea syndrome.
Cardiovascular and pulmonary complications are thought to be caused by tissue injury associated with chronic intermittent hypoxia [19]. The mechanisms of these tissue injuries are not known. OSA stimulates, mainly through intermittent hypoxia, several mechanisms, including inflammation, endothelial dysfunction, and oxidative stress [20]. In intermittent hypoxia, the inflammatory signaling factors play essential roles in the transcriptional regulation of inflammatory cytokines. However, their role in OSA pathogenesis and the exact relationships between pathogenic mechanisms remain unclear.
Recurrent episodes of breathing cessation during sleep expose the cardiovascular system to cycles of significant hypoxia, exaggerated negative intrathoracic pressure, and arousals. In OSA cases, repetitive hypoxia and reoxygenation occur during sleep, inducing the oxidative stress response [20]. This oxidative stress not only damages endothelial cells in the peripheral vasculature but also contributes to the damage of alveolar epithelial cells [21]. Reduced alveolar trans-epithelial exchange rate for oxygen and carbon dioxide leads to worsened hypoxemia and hypercarbia. Increased permeability of the alveolar epithelium results in the development of intra-alveolar transudate and exudate. Moreover, the decreased level of pulmonary surfactants [22] leads to difficulty maintaining alveolar patency and preventing alveolar collapse and pulmonary atelectasis. Oxidative stress and chronic inflammatory state are the main characteristic pathophysiological changes in OSA contributing to neural, cardiovascular, and metabolic alterations. Thus, cardiovascular morbidity and mortality are increased in patients with OSA [23].
It is possible that OSA is associated with an increase or decrease of different compounds related to inflammation, oxidative stress, or changes in metabolism.
C-reactive protein (CRP), a key blood inflammatory marker, is generated in the liver and is predominantly regulated by the proinflammatory cytokine interleukin-6 (IL-6). Unlike other cytokines, CRP levels are rather steady in the same individual over 24 h and may indicate the extent of the inflammatory response. Epidemiological research has demonstrated that an elevated CRP level in the high–normal range (0.2 to 1.5 mg/dL) in seemingly healthy men and women is a reliable indicator of cardiovascular risk. A higher CRP level is related to future cardiovascular events in patients with stable angina pectoris, acute coronary artery disease, and a history of myocardial infarction. CRP may directly contribute to the onset and progression of atherosclerosis. Endothelial cells, vascular smooth muscle cells, and monocyte macrophages contain proinflammatory and proatherogenic features, and CRP levels are also connected with oxidative stress [24].
It is generally recognized that the acute phase response is mediated by IL-6, which is a cytokine that possesses multiple functions. In addition to its role in host defense, inflammation, and cancer, IL-6 is also thought to contribute to the proliferation and hypertrophy of individual cells [25]. Increases in vascular cell-derived IL-6 have been connected with multiple stimuli, such as inflammatory cytokines and growth factors. These variables have been shown to cause an increase in IL-6 production. The levels of IL-6 are often elevated in cardiovascular diseases such as atherosclerosis and hypertension. It is believed that IL-6 is responsible for the structural and functional changes that occur in arteries as a result of these diseases.
Tumor necrosis factor-alpha (TNF-α) is a proinflammatory cytokine that plays a crucial role in host defense and mediates the pathogenesis of several disease processes, including atherosclerosis, septic shock, and autoimmune disease [26]. It is implicated in multiple necroses- and apoptosis-related signaling pathways. It also regulates sleep and has been associated with excessive daytime drowsiness, disturbed nighttime sleep, and hypoxia [27].
The chemoattractant cytokine interleukin-8 (IL-8) is produced by some different types of tissue and blood cells. IL-8 is largely released by mononuclear macrophages. When stimulated appropriately, epithelial and endothelial cells can also produce IL-8 [28]. In inflammatory areas, IL-8 attracts neutrophils and activates them. To maintain inflammation, IL-8 can cause neutrophils to produce myeloperoxidase and attract other inflammatory cells. IL-8 binds to certain receptors on the surface of neutrophils, causing cell deformation, degranulation, and an increase in reactive oxygen species production. This procedure could cause lysosomes to release their contents and activate arachidonic acid, increasing vascular permeability and plasma protein exudation in the process, which can result in tissue damage, atherosclerosis, vascular inflammation, and other disorders [29]. OSA patients may experience a rapid mobilization of macrophage antigen 1 to the neutrophil surface by exposure to IL-8. As a result, upregulation of IL-8 in human bronchial epithelial cells has been observed in response to a vibration stimulation brought on by snoring [30]. IL-8 influences physiological sleep in healthy individuals and is connected to physiological secretory patterns [31]. Decreased IL-8 secretion is linked to good sleep at night and a healthy physical state the following day, whereas high IL-8 secretion may be linked to excessive daytime sleepiness and weariness [32].
Intercellular adhesion molecule-1 (ICAM1) is a ligand for lymphocyte-function-associated antigen-1 and an 80 to 110 kDa glycoprotein with five immunoglobulin-like domains. According to reports, ICAM-1 is crucial for leukocyte migration to inflamed tissue [33]. The injurious factors associated with OSA (repetitive hypoxia, sympathetic nervous system activation, hypertension, obesity, insulin resistance, and dyslipidemia) induce the release of primary proinflammatory cytokines (e.g., interleukin-1 and tumor necrosis factor-alpha), and this stimulates the production of adhesion molecules, procoagulants, and other mediators by endothelial and other cells. Leukocyte adherence to the endothelium is mediated by intercellular adhesion molecule-1 (ICAM1) and vascular cell adhesion molecule-1 (VCAM-1). VCAM-1 is a part of the immunoglobulin superfamily of adhesion molecules and binds for very late antigen-4, which is found on monocytes and lymphocytes but not on neutrophils [34]. The earliest indication of disease activity in both animal and human models of atherosclerosis is an elevation of adhesion molecules [35]. Adhesion molecules mediate the attachment of circulating leukocytes to the endothelium, in addition to their subsequent transmigration and accumulation in the arterial intima. ICAM-1 in particular has been reported to play an important role in the transmigration of leukocytes across the vascular endothelial wall [33,35,36]. The expression of adhesion molecules is an indicator of endothelial inflammation and is likely to be involved in the causal pathway leading to atherosclerosis [36]. According to several studies, OSA-induced repeated hypoxia may play a role in the pathophysiology of cardiovascular diseases by inducing inflammatory responses through increasing cytokine and adhesion molecule levels [37,38]. ICAM-1 levels have been strongly linked to cardiovascular-disease-related deaths [39]. Additionally, they have been associated with cardiovascular disease risk factors and subclinical cardiovascular disease findings [40,41]. In older men and women, circulating ICAM-1 has been associated with cardiovascular disease risk factors and fatal events [42].
Vascular endothelial growth factor (VEGF), a soluble angiogenic mitogen, can promote angiogenesis and increase tissue capillary density [43]. It may have an impact on the prognosis of cancer [44], the process of atherogenesis [45], the development of cardiovascular disorders [46], and other conditions. Hypoxia can stimulate the high expression of the VEGF gene through the hypoxia-inducible factor (HIF) [47]. Experimental studies have shown that intermittent hypoxia, a defining feature of OSA, is related to elevated VEGF [48,49]. However, there has not been a clear consensus about the VEGF levels in OSA patients in human research.
Homocysteine (HCY) is a sulfur amino acid that is metabolized through two different pathways: transsulfuration to cystathionine and remethylation to methionine [50]. Disrupted homocysteine metabolism leads to hyperhomocysteinemia, a condition that has been linked in recent epidemiological studies to a higher risk of many diseases—increased HCY is a specific risk factor for cardiovascular diseases, dementia, and Alzheimer’s disease [51,52]. Hyperhomocysteinemia maintains oxidative stress, weakens endothelial dysfunction, and increases the metabolism of harmful cysteine adducts [53]. A proportional risk of mortality is independently correlated with blood HCY levels [54]. Increased reactive oxygen species (ROS) could be linked to an inflammatory reaction occurring in OSA. Additionally, the local inflammatory reaction brought on by repeated upper airway collapse could potentially result in a systemic inflammatory reaction. Homocysteine has a highly reactive sulfhydryl group. The sulfhydryl group is readily oxidized by itself [55]. More than 98% of homocysteine is in an oxidized condition [56]. The vascular endothelium can sustain direct harm from activated ROS and white blood cells. The ability of oxidative stress in vivo diminishes as oxidative stress products increase in OSA patients [57]. Therefore, homocysteine is crucial in the development of oxidative stress in OSA patients.
Malondialdehyde (MDA) is an end-product of enzymatic or nonenzymatic decomposition of arachidonic acid and larger PUFAs [58]. During the formation of thromboxane A2, enzymatic pathways can produce MDA in vivo as a byproduct. Once generated, MDA can be processed by enzymes or react with DNA or proteins in cells and tissues to form adducts, which can cause biomolecular damage. The primary basis for MDA’s high reactivity is its electrophilicity, which renders it highly reactive toward nucleophiles such as basic amino acid residues (i.e., lysine, histidine, or arginine). Because MDA adducts can promote intramolecular or intermolecular protein/DNA crosslinking, which may cause significant alteration in the biochemical properties of biomolecules and accumulate during aging and in chronic diseases, they play an important role in secondary harmful reactions (such as cross-linking) [59,60,61]. Low-density lipoproteins, elastin, and collagen are examples of molecules that can be altered by MDA and may have effects on cardiovascular disease. The duration of nocturnal desaturation below 85% is linked with MDA concentrations. MDA plays a crucial role in the analysis of oxidative stress measurements in OSA because it is a significant component of thiobarbituric acid reactive chemicals [62,63].
The main source of circulating leptin is white adipose tissue. The main effects of leptin are to indicate satiety and to decrease the need to eat. Leptin and the leptin receptor are also implicated in the control of immunological function, inflammation, blood glucose metabolism, and energy expenditure [64]. When a person is obese, leptin levels in the blood are markedly elevated. The most important risk factor for the development of OSA is obesity.
IGF-1 is a representative of the peptide hormone family. Its structural makeup is remarkably similar to that of proinsulin. As a mitogenic and metabolic factor, it significantly affects cell growth and metabolism. IGF-1 is primarily produced in the liver [65]. Sex steroids are the primary regulators of local IGF-I synthesis in the reproductive system, while growth hormone, parathyroid hormone, and sex steroids control IGF-I production in bone. IGF-I has a major function in controlling growth after birth. At birth, the concentrations are modest; they rise significantly during childhood and puberty, then, starting in the third decade, they start to fall [66]. IGF-1 deficiency can cause significant problems in healthy growth. IGF-1 level is downregulated in T1DM, whereas in T2DM, it is upregulated. IGF-1 elevation in adulthood may be associated with a higher chance of developing cancer. Moreover, treating IGF-1 in sepsis can offer therapeutic protection [67].
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are liver transaminases aggregated in the cytosol of hepatocytes. In the serum, these enzymes are typically detectable at low concentrations of around 30 IU/L [68,69,70]. However, AST and ALT are released in greater quantities into the serum as a result of any procedure that results in the loss of hepatocyte membrane integrity or necrosis [71]. Most OSA patients are overweight, which places them at risk for fatty liver [5,72]. Numerous cases of ischemic hepatitis in severe OSA patients have been reported, and epidemiological studies have revealed that OSA is an independent risk factor for impairment of glucose homeostasis [73,74,75,76]. These data all point to the possibility that OSA, per se, could be a risk factor for liver injury independent of obesity. Therefore, direct liver hypoxia and OSA-induced insulin resistance may play a role in the development of liver disease linked to OSA.
Total cholesterol (TC), low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), and triglyceride (TG) are components of the lipid profile. The risk of coronary heart disease and LDL are strongly positively correlated. The risk of cardiac mortality, nonfatal myocardial infarction, ischemic stroke, and the requirement for revascularization treatments is decreased when LDL cholesterol is lowered with medication, according to randomized studies. Although a direct link between OSA and dyslipidemia has not yet been established, there is mounting evidence that chronic intermittent hypoxia, a major OSA component, is independently related to and may even be the underlying cause of dyslipidemia due to the production of stearoyl-coenzyme A desaturase-1 and reactive oxygen species, lipid peroxidation, and dysfunction of the sympathetic nervous system. Alterations in oxidative stress and immune–inflammatory response are promoted by intermittent hypoxia associated with sleep apnea. In comparison to controls, OSA patients exhibit greater levels of systemic inflammatory markers. Human vascular endothelial cells’ LDL metabolism may be altered by cytokines, particularly IL-1, which may also affect how cholesterol is metabolized by endothelial cells. These alterations in the metabolism of endothelial cells offer proof of the pivotal function of cytokines in atherogenesis and other comorbidities.
The biomarker is referred to as a specified property that is assessed as an indication of normal biological processes, pathogenic processes, or reactions to an exposure or intervention. This wide definition includes various measurements, which might be obtained from genetic, histologic, radiographic, or physiologic properties [77].
A diagnostic biomarker detects or verifies the existence of an illness or condition of interest, or identifies a person who has a certain disease subtype, such as sweat chloride, which is utilized as a diagnostic biomarker to confirm cystic fibrosis [78].
A biomarker is considered a monitoring biomarker when it can be evaluated repeatedly to determine the progression of a disease or medical condition, to look for signs of exposure to a medical product or environmental agent, or to identify a medical product’s or biological agent’s effects [77]. For example, the international normalized ratio (INR) or prothrombin time (PT) may be utilized in order to determine if the intended impact of anticoagulation has been achieved in warfarin-taking individuals [79].
The evaluation of a biomarker that can be tested with appropriate accuracy and reliability in a defined context of application remains difficult. The objective is to develop a validation procedure that ensures the biomarker can be tested reliably, accurately, and consistently at a low cost.
Disease specificity, obligatory presence in all affected patients (i.e., high sensitivity and specificity), reversibility upon correct therapy, and detectability before patients exhibit visible clinical signs are all important aspects of the perfect biomarker. In addition, ideal biomarkers should not only represent the severity of the disease but also provide insightful data over the disease’s cumulative history, as well as allow for a cut-off value with minimum overlap between normal and disease. Furthermore, an ideal diagnostic policy based on biomarkers would be expected to reduce the total cost and burden of diagnosing a patient, measurement costs, and misdiagnosis costs.
The standard diagnostic procedure for establishing the presence of obstructive sleep apnea (OSA) is overnight polysomnography (PSG). More than five scoreable respiratory events (e.g., apneas, hypopneas, RERAs) per hour of sleep need to be detected on PSG, as well as evidence of breathing effort for all or part of each respiratory event. Recent Clinical Practice Guidelines for OSA expressed the need for a new clinical screening or diagnostic tool for establishing the presence and severity of OSA [80]. More accurate and user-friendly screening and monitoring tools, such as serum and plasma biomarkers, may someday be the way to better diagnose OSA and assess its severity.
New methods of testing other than an overnight sleep study may provide an easier diagnostic process to identify patients with OSA and allow earlier dispense of treatment, e.g., positive airway pressure (PAP) therapy, thus preventing serious comorbidities. Additionally, a monitoring tool for treatment evaluation and possible disease complications would be an important asset for clinicians.
Aim of the Study
We conducted a systematic review of available meta-analyses (2a level of evidence according to Centre for Evidence-Based Medicine, Oxford) [81]. The aim is to clarify which potential biomarkers of OSA have diagnostic and monitoring potential and show different concentrations between OSA subjects and non-OSA controls in addition to a change after OSA treatment.
2. Methods
The criteria of the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) checklist were followed in conducting and reporting this systematic review of meta-analyses [82]. Our PICO (population, indicator, control, outcome) question is shown in Table 1.

Table 1.
PICO(s) question.
We searched PubMed and Scopus Library for meta-analysis articles concerning sleep apnea diagnostic and monitoring biomarkers. The search was performed using the words “sleep apnea”, “disordered breathing”, “biomarkers”, and “meta-analysis” in different combinations.
We searched the PubMed database using the following string: (sleep apnea) OR (OSAS) OR (disordered breathing) Filters: Meta-Analysis.
To obtain literature from the Scopus library, we used the following string:
TITLE-ABS-KEY (sleep AND apnea AND biomarker) OR (disordered AND breathing AND biomarker) AND (LIMIT-TO (SUBJAREA, “MEDI”)) AND (LIMIT-TO (EXACTKEYWORD, “Meta Analysis”) OR LIMIT-TO (EXACTKEYWORD, “Meta-Analysis”)).
Search results were exported to the Mendeley reference manager for the initial title and abstract screening of the records. Duplicate articles were removed by the “remove duplicates” function of Mendeley. The literature search was performed between 10 June 2022 and 21 June 2022 and again on 10 September 2022. To obtain articles not received from databases, bibliographies of published articles were manually reviewed to identify additional studies. Two authors independently performed the literature search and evaluated articles for inclusion. Discrepancies, if any, were resolved with discussion.
During the initial screening of titles and abstracts, the studies retrieved had to meet the following criteria for inclusion in full-text eligibility assessment: (1) meta-analysis papers; (2) papers concerning adult human subjects with OSA; (3) measurement of serum or plasma compound. Due to language limitations, only articles written in English were selected. Papers evaluating a solely pediatric population (i.e., age < 18 years) were excluded from the search. Before excluding a potential biomarker based on a lack of corresponding studies regarding its diagnostic or monitoring capabilities, a manual database search was conducted to ensure there were no unfounded exclusions. After the initial screening, full-text manuscripts were retrieved and independently assessed by two investigators. The process for selecting the studies is provided in the flow chart in Figure 2.
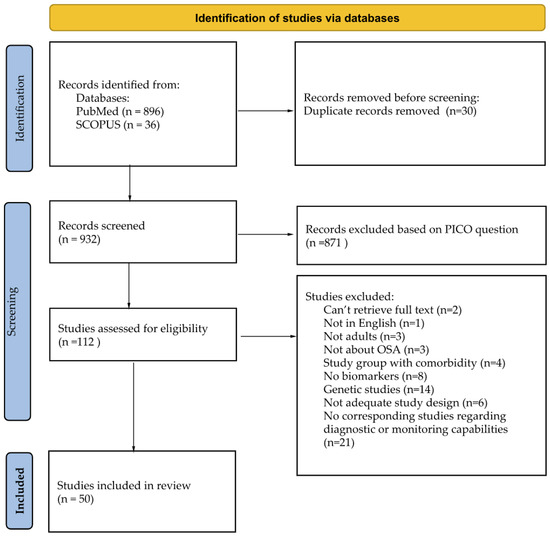
Figure 2.
Studies search and inclusion flow chart.
A quality score evaluation for studies was performed with AMSTAR2 (A MeaSurement Tool to Assess systematic Reviews) [83]. A total of 16 items were scored independently by 2 reviewers, and conflicting assessments were resolved by consensus. Items are listed in the Supplementary Material Table S1.
We selected a total of 50 meta-analyses of 14 potential sets of biomarkers (Table 2). ICAM-1, VCAM-1, ALT, and AST and lipid profile (TC, LDLc, HDLc, TG) were markers investigated in studies together, with similar results—in this review, they are described in conjunction.

Table 2.
Number of chosen meta-analyses regarding individual potential biomarkers.
2.1. Potential Biomarkers of OSA in Adults
2.1.1. CRP
CRP was recognized to be elevated in OSA patients compared to healthy controls as early as 2002 [84]. Shamsuzzaman et al. found that in 22 OSA patients, compared to 20 control subjects, CRP levels were independently associated with OSA severity. Since then, many studies investigated the relationship between CRP/high-sensitivity CRP (hs-CRP) in OSA patients compared to healthy control subjects, and significant decreases in CRP levels after CPAP treatment in OSA patients were reported. A summary of studies regarding CRP chosen in this review is shown in Table 3.

Table 3.
Meta-analyses of CRP studies in OSA adults.
Numerous studies have shown that CRP/hs-CRP levels are independently related to OSA. However, it is unknown whether there is a connection between the severity of OSA and elevated CRP or hs-CRP levels. Additionally, there was a strong correlation between OSA treatment, both CPAP therapy and sleep surgery, and CRP decrease.
According to a study by Yi et al., both CRP and TNF-α were shown to be elevated in OSA, and the severity of the condition affected these rising tendencies. Effective CPAP therapy can, albeit gradually, lower increased CRP and TNF-α levels. Mendelian randomization analysis also found a possible causal link between OSA and increased CRP. This recent discovery further cements CRP as a possible diagnostic marker for OSA.
2.1.2. Interleukin-6
In their 2015 scoping review, de Luca Canto et al. [97], after analyzing 117 biomarker studies in adults, concluded that IL-6 has a promising profile for screening and diagnosing patients with obstructive sleep apnea (OSA) syndrome. The summary of meta-analysis evidence of the IL-6 role in OSA is shown in Table 4.

Table 4.
Meta-analyses of IL-6 studies in OSA adults.
2.1.3. Tumor Necrosis Factor-Alpha
Blood monocytes from OSA patients have been shown to produce large quantities of TNF-α. In addition, various studies have demonstrated that these patients had greater levels of TNF-α in the morning and shortly after the onset of obstructive apnea. Most studies indicated that patients with OSA have higher concentrations of serum and plasma TNF-α. The concentrations of TNF-α are decreased after PAP therapy treatment and sleep surgery. The summary of meta-analysis evidence of the TNF-α role in OSA is shown in Table 5.

Table 5.
Meta-analyses of TNA-α studies.
2.1.4. Interleukin-8
The concentrations of IL-8 in the serum and plasma are higher in subjects with OSA, compared to the control group, and decrease after PAP therapy treatment. The summary of meta-analysis evidence of the IL-8 role in OSA is shown in Table 6.

Table 6.
Meta-analyses of IL-8 studies in OSA adults.
2.1.5. ICAM-1 and VCAM-1
ICAM-1 and VCAM-1 were two of the first potential biomarkers assessed by de Luca Canto et al. [97]. Subjects with OSA exhibit higher serum concentrations of ICAM-1 and VCAM-1, which are reduced after treatment. The summary of meta-analysis evidence of the role of ICAM-1 and VCAM-1 in OSA is shown in Table 7.

Table 7.
Meta-analyses of ICAM-1 and VCAM-1 studies in OSA adults.
2.1.6. VEGF
According to several studies, VEGF is higher in OSA patients than in healthy controls, and OSA is associated with higher VEGF regardless of confounding variables [102,103]; meanwhile, additional research did not demonstrate comparable results in the OSA population [104,105]. The concentrations of VEGF decrease after PAP therapy treatment. The summary of meta-analysis evidence of VEGF’s role in OSA is shown in Table 8.

Table 8.
Meta-analyses of VEGF studies in OSA adults.
2.1.7. Homocysteine
Studies have found higher concentrations of serum HCY in OSA subject in comparison to healthy controls. CPAP reduces HCY concentrations in OSA subjects. The summary of meta-analysis evidence of HCY’s role in OSA is shown in Table 9.

Table 9.
Meta-analyses of homocysteine studies in OSA adults.
2.1.8. Malondialdehyde
The concentrations of MDA in serum are higher in OSA patients compared to non-OSA controls. CPAP lowers serum and plasma concentrations of MDA in OSA patients. The summary of meta-analysis evidence of MDA’s role in OSA is shown in Table 10.

Table 10.
Meta-analyses of MDA studies in OSA adults.
2.1.9. Leptin
Subjects with OSA exhibit higher leptin concentrations in plasma. CPAP reduces leptin levels in OSA patients. The summary of meta-analysis evidence of leptin’s role in OSA is shown in Table 11.

Table 11.
Meta-analyses of leptin studies in OSA adults.
2.1.10. IGF-1
In comparison to healthy persons, OSA patients have considerably lower plasma/serum IGF-1 concentrations. Serum IGF-1 levels in patients with OSA may be influenced by the degree of the disease and ethnic variances. Additionally, the levels of plasma/serum IGF-1 correlated with minimal oxygen saturation and negatively correlated with the apnea–hypopnea index and oxygen desaturation index scores. This relation is independent of factors such as age, sample detection method, or study design [120].
Chen et al. suggested in their meta-analysis that in OSA patients, CPAP was linked to a statistically significant rise in IGF-1 [120]. Results of appropriate OSA studies regarding IGF-1 levels in comparison to healthy controls and change of IGF-1 levels after CPAP treatment are shown in Table 12.

Table 12.
Meta-analyses of IGF-1 studies.
2.1.11. ALT and AST
The concentrations of ALT and AST are higher in subjects with OSA compared to the control group and decrease after PAP therapy treatment. The summary of meta-analysis evidence of ALT’s and AST’s roles in OSA is shown in Table 13.

Table 13.
Meta-analyses of ALT and AST studies in OSA adults.
2.1.12. Lipid Profile
Patients with OSA have increased dyslipidemia compared to non-OSA controls. AHI had a substantial impact on LDL and TG, and BMI had a large impact on LDL and HDL concentration. CPAP and sleep surgery improved lipid profile. The summary of meta-analysis evidence of lipid profile’s role in OSA is shown in Table 14.

Table 14.
Meta-analyses of lipid profile studies in OSA adults.
2.1.13. Adiponectin
The concentrations of adiponectin in serum and plasma are lower in OSA patients compared to non-OSA controls. CPAP did not change the adiponectin levels in OSA patients after the therapy. The summary of meta-analysis evidence of adiponectin’s role in OSA is shown in Table 15.

Table 15.
Meta-analyses of adiponectin studies.
2.1.14. Cortisol
Although PAP treatment decreased plasma levels of cortisol in OSA subjects, available meta-analyses found that cortisol serum and plasma concentrations did not differ between OSA subjects and non-OSA controls. The summary of meta-analysis evidence of cortisol’s role in OSA is shown in Table 16.

Table 16.
Meta-analyses of cortisol studies.
2.2. Quality of Studies
We conducted a quality assessment of the studies using the AMSTAR2 tool. As written by the authors in the AMSTAR2 guidance document, AMSTAR2 is not intended to produce a final “score”. A high score could conceal significant flaws in a particular area, such as a poor literature search or a failure to consider the risk of bias (ROB) for each study that was included in a systematic review or meta-analysis.
No meta-analysis received the full score. The most common flaws were associated with the explanation and prior publication of the study protocol, insufficient study design, no justification of excluded studies, or incomplete individual studies ROB assessment. The final selection of studies with AMSTAR2 quality scores is shown in the Supplementary Material Table S2.
3. Discussion
Given its rising incidence and effects on the healthcare system, economy, and society, OSA has come to be recognized as a serious public health problem on a global scale. However, existing diagnostic techniques have shortcomings, which makes OSA clinical management difficult. Alternative approaches, such as biomarkers, are needed to direct medical decision-making. We aimed to gather possible OSA biomarkers for diagnostic and prognostic reasons in this systematic study.
Our review is an attempt to encapsulate the state of knowledge regarding probable new ways of OSA screening, diagnosing, monitoring, and prognosis. We summarized and evaluated the quality of available meta-analyses regarding potential diagnostic and monitoring biomarkers in OSA patients.
We included meta-analyses that inadvertently shared subject groups with other studies that were included, causing study overlap. There is a limited number of published studies, so performing a systematic review and meta-analysis of all the studies regarding a specific potential biomarker will result in investigating the patient group previously researched.
This is an umbrella review of the existing meta-analyses, and it does not combine data from the previously published meta-analyses or perform a new statistical analysis using the combined meta-analysis data. If possible, such work would require the identification of the individual studies that were included in each meta-analysis. To avoid over-representation of those studies, they should be removed after being included for the first time. Such work would require performing another meta-analysis, reaching, perhaps, a different conclusion, due to including a different set of original studies, but it would not be an umbrella review of the meta-analyses, as we conducted in this study.
In regards to the diagnostic capability of various biomarkers for OSA, most included studies reported differences between OSA subjects and healthy controls. We found only two meta-analyses comparing the sensitivity and specificity of using biomarkers to diagnose OSA. De Luca Canto et al. set out to compare the diagnostic significance of biological markers (exhaled breath condensate, blood, salivary, and urine) to the gold standard of nocturnal PSG in the diagnosis of OSA. They analyzed nine studies, four of which involved children and five of which involved adults, and concluded that only kallikrein-1, uromodulin, urocortin-3, and orosomucoid-1, when examined together, had sufficient accuracy to be an OSA diagnostic test in children. In adults, plasma levels of IL-6 and IL-10 may be useful indicators for determining whether OSA is present.
A follow-up systematic review by Gaspar et al. analyzed data from 16 studies with a total of 2156 individuals, of which 1369 had OSA diagnoses and 787 were healthy controls. Only two of the 38 biomarker candidates analyzed were examined in multiple studies. The most promising candidates for OSA diagnosis were identified by the mRNA levels of ADAM29, FLRT2, and SLC18A3 in PBMCs, Endocan, and YKL-40 in serum, and IL-6, and Vimentin in plasma.
Gaspar et al. found various limitations in current OSA diagnostic biomarker research. Many studies did not include clinical factors such as PSG results or clinical history and demographic data such as race/ethnicity. The majority of the included studies were single-center, had small sample sizes, and had a wide range of assessed/reported clinical and demographic characteristics. The majority of this research was either based solely or primarily on male cohorts or did not particularly address sex differences. They emphasized the urgent need for adopting better practice guidelines, for reporting to be improved, and for procedures to be standardized to increase the reproducibility and comparability of investigations.
The approach for OSA treatment is constantly developing. The golden standard is PAP therapy, the effect of which varies regarding patient compliance. There is also growing frequency and usage of soft-tissue surgical procedures for OSA, particularly in those with moderate to severe OSA who cannot use PAP therapy. Other forms of treatment, such as oral appliances or hypoglossal nerve stimulation therapy, are also increasingly common. For this review, we included meta-analyses that measured plasma and serum biomarkers after CPAP treatment and sleep surgery, due to the lack of research regarding other forms of treatment.
Although thorough, this review of meta-analyses has its limitations. Firstly, for the potential biomarker to be included, there must have been a meta-analysis study for comparison between OSA patients and the non-OSA control group, including regarding its change after treatment. This proves that many potential diagnostic and monitoring biomarkers are still in the early stage of research.
Moreover, meta-analyses included in this review are of varying quality, as scored in the AMSTAR2 assessment. More recent studies scored higher on average, possibly due to more frequent planning and preregistering protocols for systematic reviews and meta-analyses and tools for risk of bias assessment, such as the Newcastle–Ottawa scale or Cochrane ROBINS-I instrument.
Lastly, even though we tried to mitigate the risk of bias in our review regarding data search, selection and extraction, and quality assessment in our study by various means, there still may be a degree of bias. Studies might have been missed due to inadequate search strategy and not researching other databases during abstract screening or full-text reading.
4. Conclusions
There is a moderate level of evidence that OSA is associated with increased levels of serum and plasma inflammatory cytokines, oxidative stress indicators, adhesion molecules, adipose tissue hormones, abnormal lipid profile, and elevated liver enzymes, which can be decreased with CPAP treatment, namely, CRP, IL-6, TNF-α, Il-8, HCY, ICAM-1, VCAM-1, VEGF, TC, LDLc, HDLc, TG, leptin, adiponectin, cortisol, MDA, ALT, AST, and IGF-1. Individual potential biomarkers are reduced after sleep surgery treatment. Further, low-bias, high-quality studies and randomized control trials and meta-analyses of such are required to develop procedures that utilize serum or plasma biomarkers for diagnostic, prognostic, or monitoring purposes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12010060/s1, Table S1: Items included in AMSTAR2 instrument; Table S2: Summary of meta-analyses included with quality assessment.
Author Contributions
Conceptualization, P.F.; methodology, P.F.; software, P.F.; validation, P.F., E.O., A.P. and M.W.; formal analysis, P.F.; investigation, P.F.; resources, M.W.; data curation, M.W.; writing—original draft preparation, A.P., M.W. and P.F.; writing—review and editing, E.O.; visualization, P.F.; supervision, E.O.; project administration, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Olszewska, E.; Panek, J.; O’Day, J.; Rogowski, M. Usefulness of snoreplasty in the treatment of simple snoring and mild obstructive sleep apnea/hypopnea syndrome—Preliminary report. Otolaryngol. Pol. 2014, 68, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, E.; Sieskiewicz, A.; Rozycki, J.; Rogalewski, M.; Tarasow, E.; Rogowski, M.; Kulikowska, J. A comparison of cephalometric analysis using radiographs and craniofacial computed tomography in patients with obstructive sleep apnea syndrome: Preliminary report. Eur. Arch. Oto-Rhino-Laryngol. 2009, 266, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, E.; Rutkowska, J.; Czajkowska, A.; Rogowski, M. Selected surgical management in snoring and obstructive sleep apnea patients. Med. Sci. Monit. 2012, 18, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, E.; Woodson, B.T. Palatal anatomy for sleep apnea surgery. Laryngoscope Investig. Otolaryngol. 2019, 4, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.P.; Baptista, P.M.; Olszewska, E.; Braverman, I.; Carrasco-Llatas, M.; Kishore, S.; Chandra, S.; Yang, H.C.; Wang, C.M.Z.; Chan, Y.H.; et al. Does drug-induced sleep endoscopy affect surgical outcome? A multicenter study of 326 obstructive sleep apnea patients. Laryngoscope 2020, 130, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowea, A.J.; Campbella, B.E.; Mathesona, M.C.; Hamiltonde, G.S.; Dharmagea, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- McNicholas, W.T.; Bonsignore, M.R. Sleep apnoea as an independent risk for cardiovascular disease: Current evidence, basic mechanisms and research priorities. Eur. Respir. J. 2007, 29, 156–178. [Google Scholar] [CrossRef]
- Wang, J.; Yu, W.; Gao, M.; Zhang, F.; Gu, C.; Yu, Y.; Wei, Y. Impact of obstructive sleep apnea syndrome on endothelial function, arterial stiffening, and serum inflammatory markers: An updated meta-analysis and metaregression of 18 studies. J. Am. Heart Assoc. 2015, 4, e002454. [Google Scholar] [CrossRef]
- Chiang, C.L.; Chen, Y.T.; Wang, K.L.; Su, V.Y.-F.; Wu, L.-A.; Perng, D.-W.; Chang, S.-C.; Chen, Y.-M.; Chen, T.-J.; Chou, K.-T. Comorbidities and risk of mortality in patients with sleep apnea. Ann. Med. 2017, 49, 377–383. [Google Scholar] [CrossRef]
- Karimi, M.; Hedner, J.; Häbel, H.; Nerman, O.; Grote, L. Sleep apnea-related risk of motor vehicle accidents is reduced by continuous positive airway pressure: Swedish traffic accident registry data. Sleep 2015, 38, 341–349. [Google Scholar] [CrossRef]
- Teran-Santos, J.; Jimenez-Gomez, A.; Cordero-Guevara, J. The association between sleep apnea and the risk of traffic accidents. N. Engl. J. Med. 1999, 340, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Blustein, J.; Finn, L.; Palta, M. Sleepiness, Driving, and Accidents Sleep-Disordered Breathing and Motor Vehicle Accidents in a Population-Based Sample of Employed Adults. Sleep 1997, 20, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Lacasse, Y.; Godbout, C.; Sériès, F. Health-related quality of life in obstructive sleep apnoea. Eur. Respir. J. 2002, 19, 499–503. [Google Scholar] [CrossRef]
- Cofta, S.; Winiarska, H.M.; Płóciniczak, A.; Bielawska, L.; Brożek, A.; Piorunek, T.; Kostrzewska, T.M.; Wysocka, E. Oxidative Stress Markers and Severity of Obstructive Sleep Apnea. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2019; Volume 1222, pp. 27–35. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial dysfunction: A marker of atherosclerotic risk. Arter. Thromb. Vasc. Biol. 2003, 23, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J.; Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109 (Suppl. S23), III-27. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Gupta, V.; Modi, R.; Munnur, K.; Cameron, J.D.; Seneviratne, S.; Edwards, B.A.; Landry, S.A.; Joosten, S.A.; Hamilton, G.S.; et al. Severe obstructive sleep apnea is associated with significant coronary artery plaque burden independent of traditional cardiovascular risk factors. Int. J. Cardiovasc. Imaging 2020, 36, 347–355. [Google Scholar] [CrossRef]
- Zamarrón, C.; Valdés Cuadrado, L.; Álvarez-Sala, R. Pathophysiologic mechanisms of cardiovascular disease in obstructive sleep apnea syndrome. Pulm Med. 2013, 2013, 521087. [Google Scholar] [CrossRef]
- Morsy, N.E.; Farrag, N.S.; Zaki, N.F.W.; Badawy, A.Y.; Abdelhafez, S.A.; El-Gilany, A.-H.; Shafey, M.M.E.; Pandi-Perumal, S.R.; Spence, D.W.; BaHammam, A.S. Obstructive sleep apnea: Personal, societal, public health, and legal implications. Rev. Environ. Health 2019, 34, 153–169. [Google Scholar] [CrossRef]
- Fiedorczuk, P.; Stróżyński, A.; Olszewska, E. Is the oxidative stress in obstructive sleep apnea associated with cardiovascular complications? Systematic review. J. Clin. Med. 2020, 9, 3734. [Google Scholar] [CrossRef]
- Racanelli, A.C.; Kikkers, S.A.; Choi, A.M.K.; Cloonan, S.M. Autophagy and inflammation in chronic respiratory disease. Autophagy 2018, 14, 221–232. [Google Scholar] [CrossRef]
- Lu, D.; Abulimiti, A.; Wu, T.; Abudureyim, A.; Li, N. Pulmonary surfactant-associated proteins and inflammatory factors in obstructive sleep apnea. Sleep Breath. 2018, 22, 99–107. [Google Scholar] [CrossRef]
- Destors, M.; Tamisier, R.; Baguet, J.P.; Levy, P.; Pepin, J.L. Cardiovascular morbidity associated with obstructive sleep apnea syndrome. Rev. Mal. Respir. 2014, 31, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Mano, Y.; Anzai, T.; Kaneko, H.; Nagatomo, Y.; Nagai, T.; Anzai, A.; Maekawa, Y.; Takahashi, T.; Meguro, T.; Yoshikawa, T.; et al. Overexpression of human C-reactive protein exacerbatesleft ventricular remodeling in diabetic cardiomyopathy. Circ. J. 2011, 75, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Il-6 in inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Idriss, H.T.; Naismith, J.H. TNFα and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Rockstrom, M.D.; Chen, L.; Taishi, P.; Nguyen, J.T.; Gibbons, C.M.; Veasey, S.C.; Krueger, J.M. Tumor necrosis factor alpha in sleep regulation. Sleep Med. Rev. 2018, 40, 69–78. [Google Scholar] [CrossRef]
- Jiang, W.G.; Sanders, A.J.; Ruge, F.; Harding, K.G. Influence of interleukin-8 (IL-8) and IL-8 receptors on the migration of human keratinocytes, the role of plc-γ and potential clinical implications. Exp. Med. 2012, 3, 231–236. [Google Scholar] [CrossRef]
- Azagra-Calero, E.; Espinar-Escalona, E.; Barrera-Mora, J.M.; Llamas-Carreras, J.M.; Solano-Reina, E. Obstructive sleep apnea syndrome (OSAS). Review of the literature. Med. Oral. Patol. Oral. Cir. Bucal 2012, 17, e925. [Google Scholar] [CrossRef]
- Nadeem, R.; Molnar, J.; Madbouly, E.M.; Nida, M.; Aggarwal, S.; Sajid, H.; Naseem, J.; Loomba, R. Serum inflammatory markers in obstructive sleep apnea: A meta-analysis. J. Clin. Sleep Med. 2013, 9, 1003–1012. [Google Scholar] [CrossRef]
- Li, A.M.; Lam, H.S.; Chan, M.H.M.; So, H.K.; Ng, S.K.; Chan, I.H.S.; Lam, C.W.K.; Wing, Y.K. Inflammatory Cytokines and Childhood Obstructive Sleep Apnoea. Ann. Acad. Med. Singap. 2008, 37, 649–654. [Google Scholar]
- Yang, H.; Engeland, C.G.; King, T.S.; Sawyer, A.M. The relationship between diurnal variation of cytokines and symptom expression in mild obstructive sleep apnea. J. Clin. Sleep Med. 2020, 16, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Arnould, T.; Michiels, C.; Remacle, J. Increased PMN adherence on endothelial cells after hypoxia: Involvement of PAF, CDlt3/CDIlb, and ICAM. Am. J. Physiol. 1993, 264 Pt 1, C1102–C1110. [Google Scholar] [CrossRef] [PubMed]
- Springer, T.A. Adhesion receptors of the immune system. Nature 1990, 346, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cybulsky, M.I.; Gimbrone, M.A.; Libby, P. An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononuclear leukocyte adhesion molecule, in rabbit aortic endothelium. Arter. Thromb. 1993, 13, 197–204. [Google Scholar] [CrossRef]
- Amberger, A.; Maczek, C.; Jürgens, G.; Michaelis, D.; Schett, G.; Trieb, K.; Eberl, T.; Jindal, S.; Xu, Q.; Wick, G. Co-expression of ICAM-1, VCAM-1, ELAM-1 and Hsp60 in human arterial and venous endothelial cells in response to cytokines and oxidized low-density lipoproteins. Cell Stress Chaperones 1997, 2, 94–103. [Google Scholar] [CrossRef]
- Alberti, A.; Sarchielli, P.; Gallinella, E.; Floridi, A.; Floridi, A.; Mazzotta, G.; Gallai, V. Plasma cytokine levels in patients with obstructive sleep apnea syndrome: A preliminary study. J. Sleep Res. 2003, 12, 305–311. [Google Scholar] [CrossRef]
- Ciftci, T.U.; Kokturk, O.; Bukan, N.; Bilgihan, A. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine 2004, 28, 87–91. [Google Scholar] [CrossRef]
- Blankenberg, S.; Rupprecht, H.J.; Bickel, C.; Peetz, D.; Hafner, G.; Tiret, L.; Meyer, J.; the AtheroGene Investigators. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation 2001, 104, 1336–1342. [Google Scholar] [CrossRef]
- van der Meer, I.M.; de Maat, M.P.; Bots, M.L.; Breteler, M.M.; Meijer, J.; Kiliaan, A.J.; Hofman, A.; Witteman, J.C. Inflammatory mediators and cell adhesion molecules as indicators of severity of atherosclerosis: The Rotterdam Study. Arter. Thromb. Vasc. Biol. 2002, 22, 838–842. [Google Scholar] [CrossRef]
- Blann, A.D.; Lip, G.Y.; McCollum, C.N. Changes in von Willebrand factor and soluble ICAM, but not soluble VCAM, soluble E selectin or soluble thrombomodulin, reflect the natural history of the progression of atherosclerosis. Atherosclerosis 2002, 165, 389–391. [Google Scholar] [CrossRef]
- Jenny, N.S.; Arnold, A.M.; Kuller, L.H.; Sharrett, A.R.; Fried, L.P.; Psaty, B.M.; Tracy, R.P. Soluble intracellular adhesion molecule-1 is associated with cardiovascular disease risk and mortality in older adults. J. Thromb. Haemost. 2006, 4, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.W.; Cachianes, G.; Kuang, W.J.; Goeddel, D.V.; Ferrara, N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989, 246, 1306–1309. [Google Scholar] [CrossRef] [PubMed]
- Salven, P.; Mänpää, H.; Orpana, A.; Alitalo, K.; Joensuu, H. Serum vascular endothelial growth factor is often elevated in disseminated cancer. Clin. Cancer Res. 1997, 3, 647–651. [Google Scholar] [PubMed]
- Lin, T.-H.; Su, H.-M.; Wang, C.-L.; Voon, W.-C.; Shin, S.-J.; Lai, W.-T.; Sheu, S.-H. Vascular endothelial growth factor polymorphisms and extent of coronary atherosclerosis in Chinese population with advanced coronary artery disease. Am. J. Hypertens. 2010, 23, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Itoh, H.; Ueda, M.; Naruko, T.; Kojima, A.; Komatsu, R.; Doi, K.; Ogawa, Y.; Tamura, N.; Takaya, K.; et al. Vascular endothelial growth factor (VEGF) expression in human coronary atherosclerotic lesions: Possible pathophysiological significance of VEGF in progression of atherosclerosis. Circulation 1998, 98, 2108–2116. [Google Scholar] [CrossRef]
- Shweiki, D.; Itin, A.; Soffer, D.; Keshet, E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992, 359, 843–845. [Google Scholar] [CrossRef]
- Philippe, C.; Boussadia, Y.; Prulière-Escabasse, V.; Papon, J.F.; Clerici, C.; Isabey, D.; Coste, A.; Escudier, E.; D’Ortho, M.P. Airway cell involvement in intermittent hypoxia-induced airway inflammation. Sleep Breath. 2015, 19, 297–306. [Google Scholar] [CrossRef]
- Tekin, D.; Dursun, A.D.; Baştuǧ, M.; Karaorman, G.; Fiçicilar, H. The effects of acute and intermittent hypoxia on the expressions of HIF-1α and VEGF in the left and right ventricles of the rabbit heart. Anadolu Kardiyol. Derg. 2011, 11, 379–385. [Google Scholar] [CrossRef]
- Selhub, J. Homocysteine metabolism. Annu. Rev. Nutr. 1999, 19, 217–246. [Google Scholar] [CrossRef]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.; Wolf, P.A. Plasma Homocysteine as a Risk Factor for Dementia and Alzheimer’s Disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef]
- Danesh, J.; Lewington, S. Plasma homocysteine and coronary heart disease: Systematic review of published epidemiological studies. J. Cardiovasc. Risk 1998, 5, 229–232. [Google Scholar] [CrossRef]
- Chambers, J.C.; McGregor, A.; Jean-Marie, J.; Obeid, O.A.; Kooner, J.S. Demonstration of rapid onset vascular endothelial dysfunction after hyperhomocysteinemia: An effect reversible with vitamin C therapy. Circulation 1999, 99, 1156–1160. [Google Scholar] [CrossRef]
- Bostom, A.G.; Silbershatz, H.; Rosenberg, I.H.; Selhub, J.; D’Agostino, R.B.; Wolf, P.A.; Jacques, P.F.; Wilson, P.W.F. Nonfasting Plasma Total Homocysteine Levels and All-Cause and Cardiovascular Disease Mortality in Elderly Framingham Men and Women. Arch. Intern. Med. 1999, 159, 1077–1080. [Google Scholar] [CrossRef]
- Mcdowell, I.F.W.; Lang, D. Homocysteine and endothelial dysfunction: A link with cardiovascular disease. J. Nutr. 2000, 130, 369–372. [Google Scholar] [CrossRef]
- Jacobsen, D.W. Hyperhomocysteinemia and oxidative stress time for a reality check? Arter. Thromb. Vasc. Biol. 2000, 20, 1182–1184. [Google Scholar] [CrossRef]
- Katsoulis, K.; Kontakiotis, T.; Spanogiannis, D.; Vlachogiannis, E.; Kougioulis, M.; Gerou, S.; Daskalopoulou, E. Total antioxidant status in patients with obstructive sleep apnea without comorbidities: The role of the severity of the disease. Sleep Breath. 2011, 15, 861–866. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Negre-Salvayre, A.; Coatrieux, C.; Ingueneau, C.; Salvayre, R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br. J. Pharm. 2008, 153, 6–20. [Google Scholar] [CrossRef]
- Epizzimenti, S.; Ciamporcero, E.S.; Edaga, M.; Epettazzoni, P.; Earcaro, A.; Ecetrangolo, G.; Eminelli, R.; Edianzani, C.; Elepore, A.; Egentile, F.; et al. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front. Physiol. 2013, 4, 242. [Google Scholar] [CrossRef]
- Slatter, D.A.; Avery, N.C.; Bailey, A.J. Identification of a New Cross-link and Unique Histidine Adduct from Bovine Serum Albumin Incubated with Malondialdehyde. J. Biol. Chem. 2004, 279, 61–69. [Google Scholar] [CrossRef]
- Jordan, W.; Cohrs, S.; Degner, D.; Meier, A.; Rodenbeck, A.; Mayer, G.; Pilz, J.; Rüther, E.; Kornhuber, J.; Bleich, S. Evaluation of oxidative stress measurements in obstructive sleep apnea syndrome. J. Neural. Transm. 2006, 113, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Cofta, S.; Wysocka, E.; Piorunek, T.; Rzymkowska, M.; Batura-Gabryel, H.; Torlinski, L. Oxidative stress markers in the blood of persons with different stages of obstructive sleep apnea syndrome. J. Physiol. Pharm. 2008, 59, 183–190. [Google Scholar]
- Triantafyllou, G.A.; Paschou, S.A.; Mantzoros, C.S. Leptin and Hormones: Energy Homeostasis. Endocrinol. Metab. Clin. North Am. 2016, 45, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Ocrant, I.; Fielder, P.J.; Neely, E.; Gargosky, S.E.; Deal, C.I.; Ceda, G.; Youngman, O.; Pham, H.; Lamson, G.; et al. Insulin-like growth factors (IGFs): Implications for aging. Psychoneuroendocrinology 1992, 17, 335–342. [Google Scholar] [CrossRef]
- Le Roith, D. Seminars in medicine of the Beth Israel Deaconess Medical Center. Insulin-like growth factors. N. Engl. J. Med. 1997, 336, 633–640. [Google Scholar] [CrossRef]
- Bailes, J.; Soloviev, M. Insulin-like growth factor-1 (IGF-1) and its monitoring in medical diagnostic and in sports. Biomolecules 2021, 11, 217. [Google Scholar] [CrossRef]
- Kalra, A.; Yetiskul, E.; Wehrle, C.J.; Tuma, F. Physiology, Liver. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK535438 (accessed on 30 September 2022).
- Knell, A.J. Liver function and failure: The evolution of liver physiology. J. R. Coll. Physicians Lond. 1980, 14, 205–208. [Google Scholar]
- Lala, V.; Zubair, M.; Minter, D.A. Liver Function Tests; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482489 (accessed on 30 September 2022).
- Oh, R.C.; Hustead, T.R.; Ali, S.M.; Pantsari, M.W. Mildly Elevated Liver Transaminase Levels: Causes and Evaluation. Am. Fam. Physician 2017, 96, 709–715. [Google Scholar]
- Strollo, P.J.; Rogers, R. Obstructive sleep apnea. N. Engl. J. Med. 1996, 334, 99–104. [Google Scholar] [CrossRef]
- Henrion, J.; Colin, L.; Schapira, M.; Heller, F.R. Hypoxic hepatitis caused by severe hypoxemia from obstructive sleep apnea. J. Clin. Gastroenterol. 1997, 24, 245–249. [Google Scholar] [CrossRef]
- Stoohs, R.A.; Facchini, F.; Gullleminault, C. Insulin resistance and sleep-disordered breathing in healthy humans. Am. J. Respir. Crit. Care Med. 1996, 154, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, I.; Mcnamara, S.G.; Collins, F.L.; Grunstein, R.R.; Sullivan, C.E. “Syndrome Z”: The interaction of sleep apnoea, vascular risk factors and heart disease. Thorax 1998, 53 (Suppl. S3), S25–S28. [Google Scholar]
- Vgontzas, A.N.; Papanicolaou, D.A.; Bixler, E.O.; Hopper, K.; Lotsikas, A.; Lin, H.-M.; Kales, A.; Chrousos, G.P. Sleep Apnea and Daytime Sleepiness and Fatigue: Relation to Visceral Obesity, Insulin Resistance, and Hypercytokinemia. J. Clin. Endocrinol. Metab. 2000, 85, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.M.; Rosenstein, B.J.; White, T.B. Guidelines for Diagnosis of Cystic Fibrosis in Newborns through Older Adults: Cystic Fibrosis Foundation Consensus Report. J. Pediatr. 2008, 153, S4–S14. [Google Scholar] [CrossRef]
- Holbrook, A.; Schulman, S.; Witt, D.M.; Vandvik, P.O.; Fish, J.; Kovacs, M.J.; Svensson, P.J.; Veenstra, D.L.; Crowther, M.; Guyatt, G.H. Evidence-based management of anticoagulant therapy. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012, 141 (Suppl. S2), e152S–e184S. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American academy of sleep medicine clinical practice guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Howick, J.; Chalmers Iain (James Lind Library); Glasziou, P. OCEBM Table of Evidence Working Group “The Oxford 2011 Levels of Evidence”. Oxford Centre for Evidence-Based Medicine. Available online: http://www.cebm.net/index.aspx?o=5653 (accessed on 30 September 2022).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Shea, B.J.; Grimshaw, J.M.; A Wells, G.; Boers, M.; Andersson, N.; Hamel, C.; Porter, A.C.; Tugwell, P.; Moher, D.; Bouter, L.M. Development of AMSTAR: A measurement tool to assess the methodological quality of systematic reviews. BMC Med. Res. Methodol. 2007, 7, 10. [Google Scholar] [CrossRef]
- Shamsuzzaman, A.S.; Winnicki, M.; Lanfranchi, P.; Wolk, R.; Kara, T.; Accurso, V.; Somers, V.K. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation 2002, 105, 2462–2464. [Google Scholar] [CrossRef]
- Imani, M.; Sadeghi, M.; Farokhzadeh, F.; Khazaie, H.; Brand, S.; Dürsteler, K.; Brühl, A.; Sadeghi-Bahmani, D. Evaluation of blood levels of C-reactive protein marker in obstructive sleep apnea: A systematic review, meta-analysis and meta-regression. Life 2021, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Van der Touw, T.; Andronicos, N.M.; Smart, N. Is C-reactive protein elevated in obstructive sleep apnea? A systematic review and meta-analysis. Biomarkers 2019, 24, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wei, P.; Qin, Y.; Wei, Y. Is C-reactive protein a marker of obstructive sleep apnea? Medicine 2017, 96, e6850. [Google Scholar] [CrossRef] [PubMed]
- Yeo, B.S.Y.; Koh, J.H.; Tan, B.K.J.; Ding, Y.; Teo, Y.H.; Alkan, U.; See, A.; Loh, S.; Toh, S.T. Improved Inflammatory and Cardiometabolic Profile after Soft-Tissue Sleep Surgery for Obstructive Sleep Apnea: A Systematic Review and Meta-analysis. JAMA Otolaryngol. Head Neck Surg. 2022, 148, 862–869. [Google Scholar] [CrossRef]
- Yi, M.; Zhao, W.; Tan, Y.; Fei, Q.; Liu, K.; Chen, Z.; Zhang, Y. The causal relationships between obstructive sleep apnea and elevated CRP and TNF-α protein levels. Ann. Med. 2022, 54, 1578–1589. [Google Scholar] [CrossRef]
- Wang, Y.; Ni Lin, Y.; Zhang, L.Y.; Li, C.X.; Li, S.Q.; Li, H.P.; Zhang, L.; Li, N.; Yan, Y.R.; Li, Q.Y. Changes of circulating biomarkers of inflammation and glycolipid metabolism by CPAP in OSA patients: A meta-analysis of time-dependent profiles. Ther. Adv. Chronic Dis. 2022, 13, 20406223211070919. [Google Scholar] [CrossRef]
- Kang, K.; Yeh, T.; Hsu, Y.; Ko, J.; Lee, C.; Lin, M.; Hsu, W. Effect of Sleep Surgery on C-Reactive Protein Levels in Adults with Obstructive Sleep Apnea: A Meta-Analysis. Laryngoscope 2021, 131, 1180–1187. [Google Scholar] [CrossRef]
- Ning, Y.; Zhang, T.-S.; Wen, W.-W.; Li, K.; Yang, Y.-X.; Qin, Y.-W.; Zhang, H.-N.; Du, Y.-H.; Li, L.-Y.; Yang, S.; et al. Effects of continuous positive airway pressure on cardiovascular biomarkers in patients with obstructive sleep apnea: A meta-analysis of randomized controlled trials. Sleep Breath. 2019, 23, 77–86. [Google Scholar] [CrossRef]
- Guo, Y.; Pan, L.; Ren, D.; Xie, X. Impact of continuous positive airway pressure on C-reactive protein in patients with obstructive sleep apnea: A meta-analysis. Sleep Breath. 2013, 17, 495–503. [Google Scholar] [CrossRef]
- Baessler, A.; Nadeem, R.; Harvey, M.; Madbouly, E.; Younus, A.; Sajid, H.; Naseem, J.; Asif, A.; Bawaadam, H. Treatment for sleep apnea by continuous positive airway pressure improves levels of inflammatory markers-a meta-analysis. J. Inflamm. 2013, 10, 13. [Google Scholar] [CrossRef]
- Xie, X.M.; Pan, L.; Ren, D.Q.; Du, C.J.; Guo, Y.Z. Effects of continuous positive airway pressure therapy on systemic inflammation in obstructive sleep apnea: A meta-analysis. Sleep Med. 2013, 14, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Samuelson, C.G.; Hamilton, C.; Fisher, M.; Kelley, K.; Joseph, N.J.; Wang, P.-C.; Lin, H.-C. Effect of continuous positive airway pressure on c-reactive protein levels in sleep apnea: A meta-analysis. Otolaryngol. Head Neck Surg. 2012, 147, 423–433. [Google Scholar] [CrossRef] [PubMed]
- de Luca Canto, G.; Pachêco-Pereira, C.; Aydinoz, S.; Major, P.W.; Flores-Mir, C.; Gozal, D. Diagnostic capability of biological markers in assessment of obstructive sleep apnea: A systematic review and meta-analysis. J. Clin. Sleep Med. 2015, 11, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, L.S.; Santos-Carvalho, A.; Santos, B.; Carvalhas-Almeida, C.; Barros-Viegas, A.T.; Oliveiros, B.; Donato, H.; Santos, C.; Moita, J.; Cavadas, C.; et al. Peripheral biomarkers to diagnose obstructive sleep apnea in adults: A systematic review and meta-analysis. Sleep Med. Rev. 2022, 64, 101659. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Zhao, W.; Fei, Q.; Tan, Y.; Liu, K.; Chen, Z.; Zhang, Y. Causal analysis between altered levels of interleukins and obstructive sleep apnea. Front. Immunol. 2022, 13, 3937. [Google Scholar] [CrossRef]
- Imani, M.M.; Sadeghi, M.; Khazaie, H.; Emami, M.; Sadeghi Bahmani, D.; Brand, S. Evaluation of Serum and Plasma Interleukin-6 Levels in Obstructive Sleep Apnea Syndrome: A Meta-Analysis and Meta-Regression. Front. Immunol. 2020, 11, 1343. [Google Scholar] [CrossRef]
- Zhong, A.; Xiong, X.; Shi, M.; Xu, H. Roles of interleukin (IL)-6 gene polymorphisms, serum IL-6 levels, and treatment in obstructive sleep apnea: A meta-analysis. Sleep Breath. 2016, 20, 719–731. [Google Scholar] [CrossRef]
- Lee, C.H.; Hsu, W.C.; Yeh, T.H.; Ko, J.Y.; Lin, M.T.; Kang, K.T. Effect of Sleep Surgery on Inflammatory Cytokines in Adult Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Laryngoscope 2022, 132, 2275–2284. [Google Scholar] [CrossRef]
- Imani, M.M.; Sadeghi, M.; Khazaie, H.; Emami, M.; Bahmani, D.S.; Brand, S. Serum and plasma tumor necrosis factor alpha levels in individuals with obstructive sleep apnea syndrome: A meta-analysis and meta-regression. Life 2020, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Song, Y.; Ning, P.; Zhang, L.; Wu, S.; Quan, J.; Li, Q. Association between tumor necrosis factor alpha and obstructive sleep apnea in adults: A meta-analysis update. BMC Pulm. Med. 2020, 20, 215. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, X. Tumor necrosis factor alpha is a promising circulating biomarker for the development of obstructive sleep apnea syndrome: A meta-analysis. Oncotarget 2017, 8, 27616–27626. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, R.; Ren, X.; He, J. Interleukin-8 concentrations in obstructive sleep apnea syndrome: A systematic review and meta-analysis. Bioengineered 2021, 12, 10666–10681. [Google Scholar] [CrossRef]
- Tian, Z.; Xiao, J.; Kang, J.; Sun, H.; Mu, Z.; Tong, D.; Li, M. Effects of Continuous Positive Airway Pressure on Cell Adhesion Molecules in Patients with Obstructive Sleep Apnea: A Meta-Analysis. Lung 2021, 199, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Li, X.; Zhang, X.; Wang, W.; Chen, J.; Liu, Y.; Fang, X.; Ni, X.; Zhang, J.; Wang, S.; et al. Prothrombotic Factors in Obstructive Sleep Apnea: A Systematic Review with Meta-Analysis. Ear Nose Throat J. 2020, 101, NP412–NP421. [Google Scholar] [CrossRef] [PubMed]
- Zhang X bin Jiang, X.T.; Cai, F.R.; Zeng, H.Q.; Du, Y.P. Vascular endothelial growth factor levels in patients with obstructive sleep apnea: A meta-analysis. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.-C.; Zhang, L.; Li, H.; Zeng, H.; Ye, Y.; Wang, T.; Wu, Q.; Chen, L.; Xu, Q.; Zheng, Y.; et al. Impact of continuous positive airway pressure on vascular endothelial growth factor in patients with obstructive sleep apnea: A meta-analysis. Sleep Breath. 2019, 23, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, J.; Qin, Y.; Wei, Y.X. Association between Serum Homocysteine Level and Obstructive Sleep Apnea: A Meta-Analysis. Biomed Res Int 2017, 2017, 7234528. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Chen, X.; Xiao, Y.; Dong, J.; Zhang, R.; Lü, M.; Kong, W. The differences in homocysteine level between obstructive sleep apnea patients and controls: A meta-analysis. PLoS ONE 2014, 9, e95794. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Niu, X.; Xiao, Y.; Dong, J.; Zhang, R.; Lu, M.; Kong, W. Effect of continuous positive airway pressure on homocysteine levels in patients with obstructive sleep apnea: A meta-analysis. Sleep Breath. 2014, 18, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Fadaei, R.; Safari-Faramani, R.; Hosseini, H.; Koushki, M.; Ahmadi, R.; Rostampour, M.; Khazaie, H. Increased the circulating levels of malondialdehyde in patients with obstructive sleep apnea: A systematic review and meta-analysis. Sleep Breath. 2021, 25, 1753–1760. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, L.-D.; Chen, M.-X.; Wu, Y.-H.; Zeng, H.-X.; Hu, M.-F.; Zhang, W.-L.; Zheng, Y.-F.; Lin, Q.-C. The effect of continuous positive airway pressure on circulating malondialdehyde among obstructive sleep apnea patients: A meta-analysis. Sleep Breath. 2020, 24, 1407–1415. [Google Scholar] [CrossRef]
- Fadaei, R.; Koushki, M.; Sharafkhaneh, A.; Moradi, N.; Ahmadi, R.; Rostampour, M.; Khazaie, H. The impact of continuous positive airway pressure therapy on circulating levels of malondialdehyde: A systematic review and meta-analysis. Sleep Med. 2020, 75, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, J. The Association Between Serum/Plasma Leptin Levels and Obstructive Sleep Apnea Syndrome: A Meta-Analysis and Meta-Regression. Front. Endocrinol. 2021, 12, 696418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, J.; Long, S.; Xie, X.; Guo, Y. Association between continuous positive airway pressure and changes in serum leptin in patients with obstructive sleep apnoea: A meta-analysis. Sleep Breath. 2014, 18, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Niu, X.; Xiao, Y.; Dong, J.; Lu, M.; Kong, W. Effect of continuous positive airway pressure on leptin levels in patients with obstructive sleep apnea: A meta-analysis. Otolaryngol.-Head Neck Surg. 2015, 152, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Chen L da Lin, L.; Huang, J.F.; Chen, X.; Xu, Q.Z.; Liu, J.N. Effect of continuous positive airway pressure on insulin growth factor-1 in patients with obstructive sleep apnea: A meta-analysis. Growth Horm. IGF Res. 2015, 25, 75–79. [Google Scholar] [CrossRef]
- He, J.; Li, X.; Yu, M. The correlation of serum/plasma IGF-1 concentrations with obstructive sleep apnea hypopnea syndrome: A meta-analysis and meta-regression. Front. Endocrinol. 2022, 13, 922229. [Google Scholar] [CrossRef]
- Sookoian, S.; Pirola, C.J. Obstructive sleep apnea is associated with fatty liver and abnormal liver enzymes: A meta-analysis. Obes. Surg. 2013, 23, 1815–1825. [Google Scholar] [CrossRef]
- Chen, L.-D.; Lin, L.; Zhang, L.-J.; Zeng, H.-X.; Wu, Q.-Y.; Hu, M.-F.; Xie, J.-J.; Liu, J.-N. Effect of continuous positive airway pressure on liver enzymes in obstructive sleep apnea: A meta-analysis. Clin. Respir. J. 2018, 12, 373–381. [Google Scholar] [CrossRef]
- Nadeem, R.M.; Singh, M.M.; Nida, M.; Waheed, I.; Khan, A.; Ahmed, S.; Naseem, J.; Champeau, D. Effect of obstructive sleep apnea hypopnea syndrome on lipid profile: A meta-regression analysis. J. Clin. Sleep Med. 2014, 10, 475–489. [Google Scholar] [CrossRef]
- Lee, C.H.; Hsu, W.C.; Yeh, T.H.; Ko, J.Y.; Lin, M.T.; Kang, K.T. Effect of sleep surgery on lipid profiles in adults with obstructive sleep apnea: A meta-analysis. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 3811–3820. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, R.; Singh, M.; Nida, M.; Kwon, S.; Sajid, H.; Witkowski, J.; Pahomov, E.; Shah, K.; Park, W.; Champeau, D. Effect of CPAP treatment for obstructive sleep apnea hypopnea syndrome on lipid profile: A meta-regression analysis. J. Clin. Sleep Med. 2014, 10, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yi, H.; Guan, J.; Yin, S. Effect of continuous positive airway pressure on lipid profile in patients with obstructive sleep apnea syndrome: A meta-analysis of randomized controlled trials. Atherosclerosis 2014, 234, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Fang, F.; Wang, Z.; Wei, P.; Hu, C.; Wei, Y. Association between serum/plasma levels of adiponectin and obstructive sleep apnea hypopnea syndrome: A meta-analysis. Lipids Health Dis. 2019, 18, 30. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-D.; Liu, J.-N.; Lin, L.; Wu, Z.; Li, H.; Ye, Y.-M.; Xu, Q.-Z.; Lin, Q.-C. Effect of continuous positive airway pressure on adiponectin in patients with obstructive sleep apnea: A meta-analysis. PLoS ONE 2015, 10, e0136837. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, I.H.; Hoyos, C.M.; Phillips, C.L.; Magalang, U.J. Meta-analyses of the association of sleep apnea with insulin resistance, and the effects of CPAP on HOMA-IR, adiponectin, and visceral adipose fat. J. Clin. Sleep Med. 2015, 11, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Hecht, L.; Möhler, R.; Meyer, G. Effects of CPAP-respiration on markers of glucose metabolism in patients with obstructive sleep apnoea syndrome: A systematic review and meta-analysis. Ger. Med. Sci. 2011, 9, Doc20. [Google Scholar] [CrossRef] [PubMed]
- Imani, M.M.; Sadeghi, M.; Khazaie, H.; Sanjabi, A.; Brand, S.; Brühl, A.; Bahmani, D.S. Associations Between Morning Salivary and Blood Cortisol Concentrations in Individuals with Obstructive Sleep Apnea Syndrome: A Meta-Analysis. Front. Endocrinol. 2021, 11, 568823. [Google Scholar] [CrossRef]
- Ken-Dror, G.; Fry, C.H.; Murray, P.; Fluck, D.; Han, T.S. Changes in cortisol levels by continuous positive airway pressure in patients with obstructive sleep apnoea: Meta-analysis of 637 individuals. Clin. Endocrinol. 2021, 95, 909–917. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).