Abstract
Tranexamic acid (TXA) is an antifibrinolytic pharmacological agent, but its use in gastrointestinal bleeding remains contentious. Moreover, studies on the timing of TXA administration are limited. We examined whether early TXA administration reduced the risk of mortality in patients with gastrointestinal bleeding in a Taiwanese population. We used the National Health Insurance Research Database to identify patients diagnosed with gastrointestinal bleeding with early and late TXA treatment. We defined early treatment as initial TXA treatment in an emergency department and late treatment as initial TXA treatment after hospitalization. Mortality within 52 weeks was the primary outcome. A multivariable analysis using a multiple Cox regression model was applied for data analysis. Propensity score matching (PSM) was performed to reduce the potential for bias caused by measured confounding variables. Of the 52,949 selected patients with gastrointestinal bleeding, 5127 were assigned to either an early or late TXA treatment group after PSM. The incidence of mortality was significantly decreased during the first and fourth weeks (adjusted HR (aHR): 0.65, 95% CI: 0.56–0.75). A Kaplan–Meier curve revealed a significant decrease in cumulative incidence of mortality in the early TXA treatment group (log-rank test: p < 0.0001). Multiple Cox regression analysis revealed significantly lower mortality in the early TXA treatment group compared with the late treatment group (aHR: 0.64, 95% CI: 0.57–0.73). Thromboembolic events were not significantly associated with early or late TXA treatment (aHR: 1.03, 95% CI: 0.94–1.12). A Kaplan–Meier curve also revealed no significant difference in either venous or arterial events (log-rank test: p = 0.3654 and 0.0975, respectively). In conclusion, early TXA treatment was associated with a reduced risk of mortality in patients with gastrointestinal bleeding compared with late treatment, without an increase in thromboembolic events. The risk of rebleeding and need for urgent endoscopic intervention require further randomized clinical trials.
1. Introduction
Acute gastrointestinal bleeding is a common cause of morbidity and mortality worldwide [1] with a reported mortality rate of 2–10% [2,3]. The bleeding can arise from both the upper and lower gastrointestinal tracts, including from peptic ulcers, esophageal or gastric varices, diverticulitis, colitis, or malignancy in the gastrointestinal tract. Clinical symptoms of acute gastrointestinal bleeding typically include hematemesis, melaena, or hematochezia. The initial management of gastrointestinal bleeding in emergency departments includes triage, supportive management, blood transfusion, fluid resuscitation, and endoscopic therapy, depending on the severity and hemodynamic status of patients.
Tranexamic acid (TXA) is an antifibrinolytic agent that reversibly inhibits the conversion of plasminogen to plasmin, resulting in a reduction in fibrinolysis. First introduced for menorrhagia in 1968 [4], TXA has the ability to reduce postoperative hemorrhage [5,6], postpartum hemorrhage [7], and mortality in patients with traumatic hemorrhage [8]. The role of TXA in acute gastrointestinal bleeding remains under debate, without clear recommendations for its clinical use.
However, studies have demonstrated its efficacy in reducing the mortality rate of patients with acute gastrointestinal bleeding [9,10]. A recent systemic review and meta-analysis including 13 randomized trials with a total of 2271 patients with acute gastrointestinal bleeding revealed that TXA significantly reduced the mortality rate (relative risk (RR) = 0.60; 95% CI, 0.45–0.80) and rates of continued bleeding (RR = 0.60; 95% CI, 0.43–0.84) [11]. In contrast, another randomized controlled trial (HALT-IT trial) revealed that TXA did not significantly reduce mortality in patients with gastrointestinal bleeding (RR = 0.99, 95% CI, 0.82–1.18) [12]. However, adverse venous thromboembolic events were higher in patients using TXA than in those not using it.
A possible confounding factor is the timing of TXA administration, which has rarely been considered in studies and could affect study outcomes. Hence, this nationwide cohort study aimed to identify whether early or late use of TXA reduced the mortality of patients with gastrointestinal bleeding in Taiwan. We hypothesized that patients with gastrointestinal bleeding receiving TXA early would have lower risk of mortality.
2. Materials and Methods
2.1. Study Design and Population
In this retrospective cohort study, we analyzed the administration of TXA for gastrointestinal bleeding and the risk of all-cause mortality. Taiwan adopted a National Health Insurance system in 1995, this system claims data as the National Health Insurance Research Database (NHIRD). The NHIRD provides real-world evidence for exploring the risk factors or effects of an intervention for specific diseases, and contains the insurance claims data of more than 99% of Taiwan’s population. We used NHIRD data from between January 2000 and December 2017 to evaluate the risk of all-cause mortality among patients with gastrointestinal bleeding who received early or late TXA treatment. This study was approved by the Institutional Review Board of the Chung Shan Medical University Hospital (approval number CS2-20036).
2.2. Study Population
We initially included 52,949 hospitalized patients who went to an emergency department for gastrointestinal bleeding, as defined by the following International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes: 530.1, 530.2, 530.7, 531.0, 531.4, 531.9, 532.0, 532.4, 532.9, and 578. In addition, the following ICD-10-CM codes were applied: K20.0, K20.8, K20.9, K21.0, K22.10, K22.11, K22.6, K25.0, K25.4, K25.9, K26.0, K26.4, K26.9, K92.0, K92.1, and K92.2 (Supplementary Table S1). All the selected patients had a subsequent hospitalization record within 1 day of emergency care. The exclusion criteria were as follows: (1) an index date before 2000 or after 2016 (n = 6670); (2) lack of TXA treatment (ATC code: B02AA02) during emergency treatment or admission (n = 27,436); (3) cancer diagnosis before the index date (n = 4151); and (4) death before the index date (n = 6). This study included 14,686 patients with gastrointestinal bleeding who had received TXA treatment; among these patients, 9513 received early treatment, defined as initial TXA treatment in an emergency department, and 5173 received late treatment, defined as initial TXA treatment after hospitalization.
2.3. Characteristics, Comorbidities, and Study Outcomes
We identified the baseline (within 180 days of the index date) demographic characteristics, such as age and sex, and the comorbidities and medication of each participant to evaluate their health status. Comorbidities included hypertension, diabetes mellitus, hyperlipidemia, kidney disease, chronic pulmonary diseases, liver disease, ischemic heart diseases, ischemic stroke, hemorrhagic stroke, atrial fibrillation, congestive heart failure, dementia, and peripheral vascular disease. Medications included proton-pump inhibitors, hemostatic agents, drugs for constipation, furosemide, metoclopramide, silicon, magnesium oxide, aspirin, clopidogrel or ticagrelor, and nonsteroidal anti-inflammatory drugs.
The study’s primary outcome was all-cause mortality within 52 weeks of the index date. The secondary outcomes were thromboembolic events (deep-vein thrombosis, pulmonary embolism, acute myocardial infarction, hemorrhagic stroke, and ischemic stroke). All the patients in the study were followed up from the index date until their withdrawal from the National Health Insurance program, the occurrence of a study event, or 52 weeks after the index date.
2.4. Statistical Analysis
Statistical analysis was performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA), and a p value of < 0.05 was considered significant. The propensity score was the odds of gastrointestinal bleeding according to demographic data, including birth year, sex, age (±1 year) on the index date, index year, comorbidities, and medication. To reduce potential confounding bias caused by measured factors, 1:1 propensity score matching (PSM) was performed using greedy nearest neighbor non-replacement matching with a caliper width of 0.1. The difference in covariates between the 2 study groups was evaluated using the absolute standardized difference (ASD), as an absolute ASD value of ASD < 0.1 indicated that the groups were balanced with their matched control.
Categorical data are presented as numbers and percentages, and the differences in categorical variables were compared using a chi-square test. The incidence rate with the corresponding CIs and crude hazard ratios (HRs) were calculated using Poisson regression. After the proportional hazards assumption was tested, a Cox proportional hazards model analysis was performed to estimate the HRs for mortality and 95% CIs. The cumulative probabilities of mortality were assessed using a Kaplan–Meier analysis, with statistical significance being determined using the results of a log-rank test.
3. Results
3.1. Characteristics of the Participants
The study flowchart is presented in Figure 1. Of the patients with gastrointestinal bleeding administered TXA, 9513 received early treatment and 5173 received late treatment. In total, 67% of the patients were male and 32% were female, and more than 40% were aged ≥ 71 years. Before PSM, the statistically significant differences between the two groups were index year and medication (including hemostatic agents, drugs for constipation, furosemide, metoclopramide, and silicon). After PSM, the two groups were balanced, as indicated by the ASD of the covariates (Table 1).
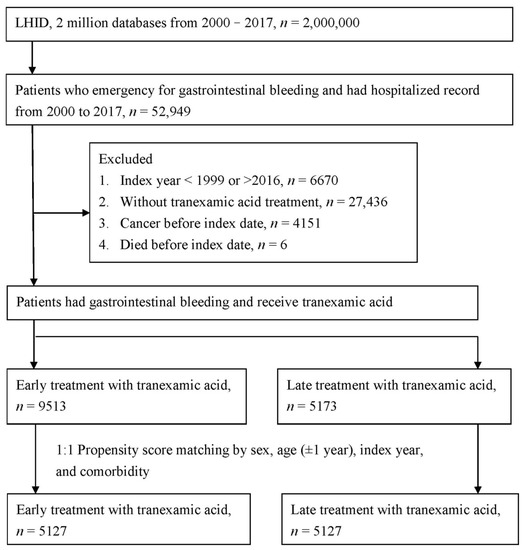
Figure 1.
Study flowchart.

Table 1.
Baseline characteristics among study groups.
3.2. The Risk of Mortality in the TXA Treatment Group
Figure 2 presents the mortality incidence density (per 100 person month), which were 1.22 (95% CI: 1.09–1.37) and 1.86 (95% CI: 1.70–2.04) in the PSM early and late treatment groups, respectively; the adjusted HR for early treatment was 0.65 (95% CI: 0.56–0.75) during the first and fourth weeks. During the 13th and 52nd weeks, the mortality incidence density (per 100 person month) was 0.23 (95% CI: 0.21–0.25) and 0.25 (95% CI: 0.23–0.27) in the early and late treatment groups, respectively; the adjusted HR (aHR) for early treatment was 0.90 (95% CI: 0.80–1.00). A Kaplan–Meier survival analysis revealed significantly lower cumulative incidence of mortality in the early treatment group (log-rank test: p < 0.0001; Figure 3).
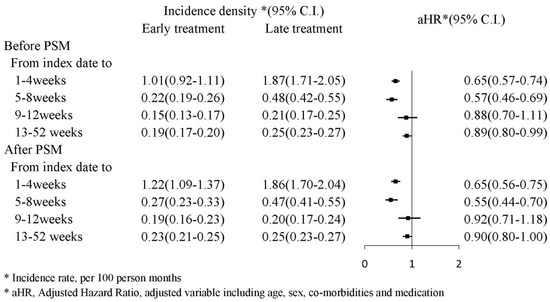
Figure 2.
Incidence density of mortality.
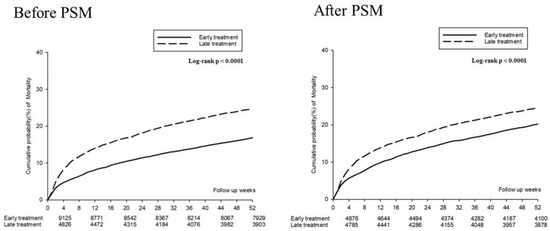
Figure 3.
Kaplan–Meier curves for the 52 week mortality risk.
The patients who received early TXA treatment had a significantly lower risk of mortality during the first and eighth weeks compared with those who received late treatment (aHR: 0.64, 95% CI: 0.57–0.73). Other significant risk factors of mortality were age; comorbid kidney disease, liver disease, and hemorrhagic stroke; and prescription for hemostatic agents, drugs for constipation, furosemide, and metoclopramide (Table 2).

Table 2.
Multiple Cox regression to estimate the hazard ratio for the 52 week mortality risk.
We also classified the TXA treatment into three groups: (1) early TXA treatment in an emergency department; (2) late TXA treatment after hospitalization; and (3) both early and late TXA treatment. Figure 4 presents the risk of mortality in those who received early TXA treatment compared with those who only received late TXA treatment. Regarding risk during the first and fourth weeks, the aHR for early treatment was 0.55 (95% CI: 0.46–0.65), and the aHR for both early and late treatment was 0.74 (95% CI: 0.63–0.86). Regarding risk during the 13th and 52nd weeks, the aHR for early treatment was 0.89 (95% CI: 0.78–1.02), and the aHR for both early and late treatment was 0.89 (95% CI: 0.78–1.01).
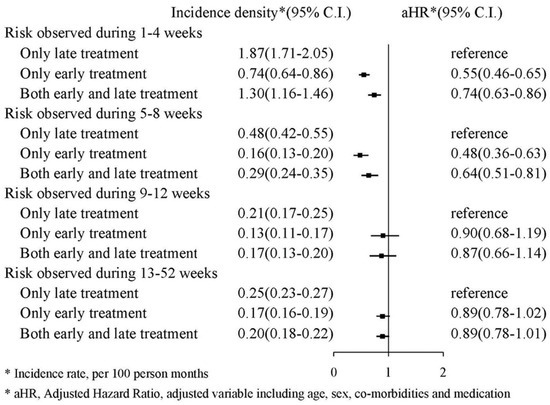
Figure 4.
Incidence density of mortality.
3.3. Thromboembolic Events in the TXA Treatment
The thromboembolic events (including deep-vein thrombosis, pulmonary embolism, acute myocardial infarction, hemorrhagic stroke, and ischemic stroke) are presented in Figure 5. The forest plot analysis indicated no significant association between thromboembolic events and early or late TXA treatment (aHR: 1.03, 95% CI: 0.94–1.12). The Kaplan–Meier survival analysis also identified no significant increased cumulative probability of either venous or arterial events (log-rank: p = 0.3654 and 0.0975, respectively, Figure 6).
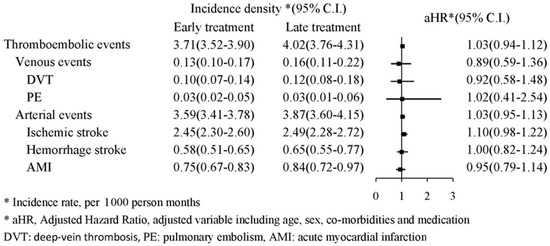
Figure 5.
Flowchart of patient selection.
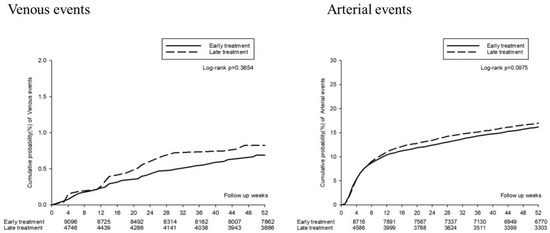
Figure 6.
Kaplan–Meier curves for the 52 week thromboembolic events.
4. Discussion
In this population-based trial, which included 10,254 patients with gastrointestinal bleeding, early TXA treatment was associated with 36% and 12% lower mortality for a follow-up period of 1 to 8 and 9 to 52 weeks, respectively. Moreover, early TXA treatment did not increase the risk of either venous or arterial thromboembolic events compared with late treatment.
TXA use for gastrointestinal bleeding has been evaluated in randomized clinical trials. In the HALT-IT trial, compared with the equivalent infusion of saline, TXA administration resulted in a lower risk of death caused by bleeding and rebleeding within 24 h, 5 days, and 28 days. However, an increased risk of venous thromboembolic events was observed [12]. Another randomized clinical trial, focusing on lower gastrointestinal bleeding, revealed that TXA had no benefits in relation to blood loss and clinical outcomes [13]. However, the results for TXA and outcomes for gastrointestinal bleeding have been inconsistent in recent systematic reviews and meta-analyses. Burke et al. performed a systemic review of 8 studies that included 12,994 patients with upper gastrointestinal bleeding. Although no effect on mortality was noted, the beneficial effect of TXA on lower rebleeding risk and a decreased need for surgery was observed [14]. Another systematic review and meta-analysis, including 13 relevant randomized clinical trials and a total of 2271 patients, demonstrated lower mortality and continued bleeding and less need for urgent endoscopic intervention [11].
Most of the aforementioned studies failed to consider a potential confounding factor: the timing of TXA administration. TXA can be prescribed for patients with gastrointestinal bleeding in an emergency department along with other initial treatments, or during hospitalization. For traumatic or postpartum hemorrhage, immediate TXA administration is recommended for improved survival [15]. Similarly, the timing of TXA treatment might affect its benefits for gastrointestinal bleeding, and trial results may be influenced by this confounding factor. Furthermore, current TXA treatment guidelines for gastrointestinal bleeding include no clear timings [16,17].
Hence, in our study design, we defined early treatment as TXA administered in an emergency department and late treatment as TXA administered after hospitalization. The results demonstrated a significant decrease in mortality rates for early TXA treatment in patients with gastrointestinal bleeding compared with late treatment for both short-term and long-term follow-up, which is compatible with the results of a systematic review and meta-analysis [11]. In addition to reducing mortality, early TXA administration in an emergency department was associated with a significant decrease in the need for urgent endoscopy in a randomized clinical trial exploring the effect of TXA on the urgent endoscopy rate for gastrointestinal bleeding [18].
The length of hospital stay is also a key clinical outcome. Our study demonstrated that early TXA treatment and both early and late treatment resulted in a shorter length of hospital stay compared with late treatment alone. Similar to our results, Miyamoto et al., who conducted a nationwide observational study in Japan, reported that TXA reduced the length of stay for patients with colonic diverticular bleeding [19]. Other retrospective studies have also revealed a decreased hospital stay following TXA administration for patients with vascular trauma [20] or intraoperative administration [21].
In addition to the common adverse effects of nausea, diarrhea, and stomach pain, the risk of thromboembolic events is a major adverse effect of TXA use in clinical practice [22,23]. The HALT-IT trial revealed an increase in venous thromboembolic events with TXA use, but primarily in patients with underlying liver diseases [12]. In contrast, the CRASH-3 trial demonstrated that TXA reduced the risk of death, with a similar risk of thromboembolic events compared with placebo groups [8]. Regarding the timing of TXA treatment, our study revealed a similar risk of thromboembolic events in early and late TXA treatment.
Our study also demonstrated that patients older than 71 years and those with liver and kidney disease all had a significantly increased risk of mortality from gastrointestinal bleeding. These results are consistent with those of a UK population-based study, which reported that older age was the most crucial prognostic factor for gastrointestinal bleeding, with a mortality rate 53 times higher for patients aged over 85 years. Liver and renal comorbidities were also associated with a 7.9 and 3.9 times higher mortality rate [24]. Another meta-analysis evaluated the relationship between kidney disease and outcomes of gastrointestinal bleeding, revealing a higher mortality in the chronic kidney disease group (odds ratio (OR): 1.786, 95% CI: 1.689–1.888, p < 0.001) and the end-stage renal disease group (OR: 2.530, 95% CI: 1.386–4.616, p = 0.002) [25].
Studies have demonstrated that TXA use leads to improved clinical outcomes for other hemorrhagic conditions, such as traumatic [8,26], major obstetric [27], postpartum [7], and surgical hemorrhage [5,28,29,30]. Studies have also considered the effect of TXA use on cerebral hemorrhage. A systematic review and meta-analysis including 14 randomized controlled trials with 4703 patients with cerebral hemorrhage demonstrated no improvement in mortality by day 90 in patients receiving TXA (OR: 0.99, 95% CI: 0.84–1.18, p = 0.95) [31]. However, the risk of rebleeding and hematoma expansion was reduced and thromboembolic events were not increased. Furthermore, Rowell et al. performed a double-blinded, randomized clinical trial of out-of-hospital TXA use within 2 h for neurological outcomes in patients with severe traumatic brain injury [32]. No significant difference in neurologic function at 6 months or mortality and progression of intracranial hemorrhage was observed.
This study has some limitations. First, data on personal behaviors, such as smoking and alcohol consumption, are not available in the NHIRD; such personal behaviors are potential confounders. However, to address these factors, we included related comorbidities and performed PSM. Second, the cause and location of the gastrointestinal bleeding, disease severity, endoscopic intervention and TXA dosage are not included in the NHIRD. Different hemorrhage severity, endoscopic intervention and TXA dosages are potential confounders of our results. Third, no control group of patients with gastrointestinal bleeding but without TXA treatment was included because our study evaluated the timing of TXA treatment. Finally, further randomized clinical trials with a sufficient sample size, rigorous patient selection, and controlled intervention are required.
5. Conclusions
In this Taiwan population-based study, early TXA treatment was associated with lower mortality without increased thromboembolic events compared with late treatment in patients with gastrointestinal bleeding. Future research is required to clarify the outcomes in terms of continued bleeding, rebleeding, blood transfusion, and the need for urgent endoscopic intervention.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11061741/s1, Table S1: Definition of study diseases related to ICD-9-CM and ICD-10-CM.
Author Contributions
Conceptualization, K.-H.T., Y.-Y.C. and C.-B.Y.; formal analysis, B.-H.S., S.-F.Y., P.-L.L. and J.-Y.H.; writing—original draft preparation, K.-H.T., Y.-Y.C. and C.-B.Y.; writing—review and editing, K.-H.T., Y.-Y.C. and C.-B.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethical Review Board of the Chung Shan Medical University Hospital (CS2-20036) approved our study.
Informed Consent Statement
Patient consent was waived by both the National Health Insurance Administration and the Institutional Review Board of Chung Shan Medical University Hospital due to the database-processing nature of the current study.
Data Availability Statement
Restrictions apply to the availability of these data. Data was obtained from National Health Insurance database and are available from the authors with the permission of National Health Insurance Administration of Taiwan.
Acknowledgments
This study was supported by research grants from the Chung Shan Medical University Hospital, Taiwan (CSH-2022-C-010). This study was partly based on data from the NHIRD provided by the NHI Administration, Ministry of Health and Welfare, and managed by the Health and Welfare Data Science Center (HWDC) in Taiwan. The interpretation and conclusions contained herein do not represent those of the NHI Administration, Ministry of Health and Welfare, or National Health Research Institutes.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Van Leerdam, M.E. Epidemiology of acute upper gastrointestinal bleeding. Best Pract. Res. Clin. Gastroenterol. 2008, 22, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Rockall, T.A.; Logan, R.F.A.; Devlin, H.B.; Northfield, T.C. Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom. BMJ 1995, 311, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Sostres, C.; Lanas, A. Epidemiology and Demographics of Upper Gastrointestinal Bleeding: Prevalence, Incidence, and Mortality. Gastrointest. Endosc. Clin. N. Am. 2011, 21, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Vermylen, J.; Verhaegen-Declercq, M.L.; Fierens, F.; Verstraete, M. A double blind study of the effect of tranexamic acid in essential menorrhagia. Bull. Soc. R. Belg. Gynecol. Obs. 1968, 38, 385–390. [Google Scholar] [CrossRef]
- Brown, J.R.; Birkmeyer, N.J.; O’Connor, G.T. Meta-analysis comparing the effectiveness and adverse outcomes of antifibrinolytic agents in cardiac surgery. Circulation 2007, 115, 2801–2813. [Google Scholar] [CrossRef] [PubMed]
- Myles, P.S.; Smith, J.A.; Forbes, A.; Silbert, B.; Jayarajah, M.; Painter, T.; Cooper, D.J.; Marasco, S.; McNeil, J.; Bussières, J.S.; et al. Tranexamic Acid in Patients Undergoing Coronary-Artery Surgery. N. Engl. J. Med. 2016, 376, 136–148. [Google Scholar] [CrossRef]
- Shakur, H.; Roberts, I.; Fawole, B.; Chaudhri, R.; El-Sheikh, M.; Akintan, A.; Qureshi, Z.; Kidanto, H.; Vwalika, B.; Abdulkadir, A.; et al. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): An international, randomised, double-blind, placebo-controlled trial. Lancet 2017, 389, 2105–2116. [Google Scholar] [CrossRef] [Green Version]
- The CRASH-3 Trial Collaborators. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): A randomised, placebo-controlled trial. Lancet 2019, 394, 1713–1723. [Google Scholar] [CrossRef] [Green Version]
- Barer, D.; Ogilvie, A.; Henry, D.; Dronfield, M.; Coggon, D.; French, S.; Ellis, S.; Atkinson, M.; Langman, M. Cimetidine and tranexamic acid in the treatment of acute upper-gastrointestinal-tract bleeding. N. Engl. J. Med. 1983, 308, 1571–1575. [Google Scholar] [CrossRef]
- Bennett, C.; Klingenberg, S.L.; Langholz, E.; Gluud, L.L. Tranexamic acid for upper gastrointestinal bleeding. Cochrane Database Syst. Rev. 2014, 2014, Cd006640. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.-L.; Yang, K.-S.; Tsai, H.-W.; Hou, S.-K.; Kang, Y.-N.; Chang, C.-C. Tranexamic acid for gastrointestinal bleeding: A systematic review with meta-analysis of randomized clinical trials. Am. J. Emerg. Med. 2021, 45, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, H.-I.T. Effects of a high-dose 24-h infusion of tranexamic acid on death and thromboembolic events in patients with acute gastrointestinal bleeding (HALT-IT): An international randomised, double-blind, placebo-controlled trial. Lancet 2020, 395, 1927–1936. [Google Scholar] [CrossRef]
- Smith, S.R.; Murray, D.; Pockney, P.G.; Bendinelli, C.; Draganic, B.D.; Carroll, R. Tranexamic Acid for Lower GI Hemorrhage: A Randomized Placebo-Controlled Clinical Trial. Dis. Colon Rectum 2018, 61, 99–106. [Google Scholar] [CrossRef]
- Burke, E.; Harkins, P.; Ahmed, I. Is There a Role for Tranexamic Acid in Upper GI Bleeding? A Systematic Review and Meta-Analysis. Surg. Res. Pract. 2021, 2021, 8876991. [Google Scholar] [CrossRef]
- Gayet-Ageron, A.; Prieto-Merino, D.; Ker, K.; Shakur, H.; Ageron, F.-X.; Roberts, I.; Kayani, A.; Geer, A.; Ndungu, B.; Fawole, B.; et al. Effect of treatment delay on the effectiveness and safety of antifibrinolytics in acute severe haemorrhage: A meta-analysis of individual patient-level data from 40 138 bleeding patients. Lancet 2018, 391, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Gralnek, I.M.; Dumonceau, J.-M.; Kuipers, E.J.; Lanas, A.; Sanders, D.S.; Kurien, M.; Rotondano, G.; Hucl, T.; Dinis-Ribeiro, M.; Marmo, R.; et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015, 47, a1–a46. [Google Scholar] [CrossRef] [Green Version]
- Stanley, A.J.; Laine, L. Management of acute upper gastrointestinal bleeding. BMJ 2019, 364, l536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavakoli, N.; Mokhtare, M.; Agah, S.; Azizi, A.; Masoodi, M.; Amiri, H.; Sheikhvatan, M.; Syedsalehi, B.; Behnam, B.; Arabahmadi, M.; et al. Comparison of the efficacy of intravenous tranexamic acid with and without topical administration versus placebo in urgent endoscopy rate for acute gastrointestinal bleeding: A double-blind randomized controlled trial. United Eur. Gastroenterol. J. 2017, 6, 46–54. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Ohbe, H.; Ishimaru, M.; Matsui, H.; Fushimi, K.; Yasunaga, H. Effect of tranexamic acid in patients with colonic diverticular bleeding: A nationwide inpatient database study. J. Gastroenterol. Hepatol. 2021, 36, 999–1005. [Google Scholar] [CrossRef]
- GnanaDev, R.; Dong, F.; Ali, A.; Makkar, G.; Esiobu, P.; Vara, R.; Wong, D.; Neeki, M. RS12. Comparing Mortality and Hospital Length of Stay in the Setting of Truncal and Peripheral Vascular Trauma in Patients Treated with Tranexamic Acid on Initial Presentation. J. Vasc. Surg. 2019, 69, e188–e189. [Google Scholar] [CrossRef] [Green Version]
- Saad, B.N.; Menken, L.G.; Elkattaway, S.; Liporace, F.A.; Yoon, R.S. Tranexamic acid lowers transfusion requirements and hospital length of stay following revision total hip or knee arthroplasty. Patient Saf. Surg. 2021, 15, 21. [Google Scholar] [CrossRef]
- Calapai, G. Systematic Review of Tranexamic Acid Adverse Reactions. J. Pharmacovigil. 2015, 3, 171. [Google Scholar] [CrossRef]
- Murdaca, G.; Greco, M.; Vassallo, C.; Gangemi, S. Tranexamic acid adverse reactions: A brief summary for internists and emergency doctors. Clin. Mol. Allergy 2020, 18, 16. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.E.; Button, L.A.; Williams, J.G. Prognosis following Upper Gastrointestinal Bleeding. PLoS ONE 2012, 7, e49507. [Google Scholar] [CrossRef] [PubMed]
- Hágendorn, R.; Farkas, N.; Vincze, Á.; Gyöngyi, Z.; Csupor, D.; Bajor, J.; Erőss, B.; Csécsei, P.; Vasas, A.; Szakács, Z.; et al. Chronic kidney disease severely deteriorates the outcome of gastrointestinal bleeding: A meta-analysis. World J. Gastroenterol. 2017, 23, 8415–8425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, I. Tranexamic acid in trauma: How should we use it? J. Thromb. Haemost. 2015, 13, S195–S199. [Google Scholar] [CrossRef] [PubMed]
- Wellington, K.; Wagstaff, A.J. Tranexamic Acid. Drugs 2003, 63, 1417–1433. [Google Scholar] [CrossRef]
- Cheriyan, T.; Maier, S.P., II; Bianco, K.; Slobodyanyuk, K.; Rattenni, R.N.; Lafage, V.; Schwab, F.J.; Lonner, B.S.; Errico, T.J. Efficacy of tranexamic acid on surgical bleeding in spine surgery: A meta-analysis. Spine J. 2015, 15, 752–761. [Google Scholar] [CrossRef]
- Ker, K.; Edwards, P.; Perel, P.; Shakur, H.; Roberts, I. Effect of tranexamic acid on surgical bleeding: Systematic review and cumulative meta-analysis. BMJ Br. Med. J. 2012, 344, e3054. [Google Scholar] [CrossRef] [Green Version]
- Sprigg, N.; Flaherty, K.; Appleton, J.P.; Salman, R.A.-S.; Bereczki, D.; Beridze, M.; Christensen, H.; Ciccone, A.; Collins, R.; Czlonkowska, A.; et al. Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): An international randomised, placebo-controlled, phase 3 superiority trial. Lancet 2018, 391, 2107–2115. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Xin, Y.; Chen, X.; Song, Z.; He, Z.; Zhao, Y. Tranexamic Acid in Cerebral Hemorrhage: A Meta-Analysis and Systematic Review. CNS Drugs 2019, 33, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Rowell, S.E.; Meier, E.N.; McKnight, B.; Kannas, D.; May, S.; Sheehan, K.; Bulger, E.M.; Idris, A.H.; Christenson, J.; Morrison, L.J.; et al. Effect of Out-of-Hospital Tranexamic Acid vs Placebo on 6-Month Functional Neurologic Outcomes in Patients with Moderate or Severe Traumatic Brain Injury. JAMA 2020, 324, 961–974. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).