Abstract
Pulmonary tuberculosis (TB) is a known risk factor for lung cancer. However, a detailed analysis of lung cancer type, age, sex, smoking, and TB burden associated with geographic and socioeconomic status has not been performed previously. We systematically appraised relevant observational studies reporting an association between pulmonary TB and lung cancer. All studies were included in the primary analysis, and studies that used robust TB diagnostic methods, such as validated medical diagnostic codes, were included in the secondary analysis. Thirty-two articles were included. The association between the history of pulmonary TB and diagnosis of lung cancer was statistically significant (OR 2.09, 95% CI: 1.62–2.69, p < 0.001). There was a high heterogeneity (I2 = 95%), without any publication bias. The analysis indicated a high association in advanced articles describing stringent pulmonary TB diagnosis (OR 2.26, 95% CI: 1.29–3.94, p = 0.004). The subgroup analyses suggested a significant association in countries with medium or high TB burdens, from East Asia and the Pacific region, and upper-middle income countries. Heterogeneity within the subgroups remained high in a majority of the subgroup analyses. A meta-regression analysis revealed that younger patients showed a significantly higher association between TB and lung cancer (regression coefficient = 0.949, p < 0.001). The history of pulmonary TB is an independent risk factor for lung cancer, especially in younger patients diagnosed with pulmonary TB. Clinicians should be aware of this association while treating young patients with a history of pulmonary TB.
1. Introduction
Lung cancer is one of the most common malignancies, with approximately 2.09 million new diagnoses worldwide in 2018. It also accounts for approximately 18.4% of the total cancer-related deaths, the highest of all cancer types [1]. The prognosis of lung cancer is relatively unfavorable compared to that of other malignancies, and as a prognosis largely depends on the stage of onset, thus, the early diagnosis of lung cancer is very important.
Cigarette smoking has been a major causal factor for lung cancer since 1912 [2,3,4]. Environmental factors such as air pollution, nutrition, occupational exposure, and a family history of previous cancer are also related to lung cancer. With the recent development of molecular diagnosis, research on genetic or inflammatory factors that contribute to lung carcinogenesis is being actively conducted [5,6].
Chronic inflammation resulting in pathological changes is a major risk factor in carcinogenesis. Inflammation is known to play a key role in carcinogenesis, such as infection with hepatitis B and C viruses in hepatocellular carcinoma, Helicobacter pylori in gastric cancer, and human papilloma virus in gynecological cancers [7]. Several meta-analyses have shown that previous inflammatory diseases in the lungs, such as pneumonia, chronic bronchitis, and pulmonary tuberculosis (TB), may increase the risk of lung cancer (relative risk ratio 1.36–1.90), independent of cigarette smoking [8,9]. According to forty-nine studies, pulmonary and extra-pulmonary TB infections increase the risk of 10 cancer types, including head and neck cancer, leukemia, lymphoma, gastrointestinal cancer, kidney cancer, bladder cancer, and lung cancer [10]. Thus, TB infection may influence the pathogenesis of lung cancer with or without cigarette smoking. To prevent the emergence of airborne transmittable TB and its progression to cancer, the control and prevention of TB is very important.
A detailed analysis of lung cancer types, patient age, sex, smoking status, and TB burden associated with geographic and socioeconomic statuses has not been performed in previous studies. Therefore, this study aimed to clarify the association between previous pulmonary TB infection and lung cancer by performing a comprehensive review of selected high-quality studies. We systematically reviewed the relationship between TB and lung cancer and also assessed various subgroups of the study population to identify the factors that affect this causal relationship.
2. Materials and Methods
2.1. Literature Search Strategy and Eligibility Criteria
This study was prospectively registered at PROSPERO (registration number: CRD42020211014). Two researchers (J.Y.K. and S.Y.H.) independently searched the PubMed, EMBASE, and Cochrane databases on 30 August 2020, using “tuberculosis” and “lung cancer” (complete search strategy provided in Supplementary Table S1) as keywords. Observational studies reporting an association between a history of pulmonary TB and lung cancer were included; reviews, clinical trials, and case reports were excluded. Studies lacking an estimation of the relative risk or data necessary to calculate risk or that used a limited population with a history of environmental or occupational exposure such as asbestos and silica were omitted. Non-English articles and studies published before 1990 were also excluded. When there were two or more articles using overlapping data sources, the article with the largest number of participants was selected. Eligible studies were extracted by first screening the title and abstract, followed by the full text. References from the relevant articles were also reviewed for selecting eligible studies. Disagreements were resolved by discussions between S.Y.H., J.Y.K., and K.H.L.
2.2. Diagnosis of TB and Lung Cancer
Studies, regardless of TB diagnostic methods, such as historical interviews and imaging, such as chest radiography and computed tomography, were included in the primary analysis. We recognized the weakness of diagnosing TB by radiographic images, history, or questionnaire and tried an additional analysis with high-quality studies based on microbiological diagnosis. Thus, we defined studies as high-quality if they used robust diagnostic methods based on medical records, including International Classification of Diseases (ICD) codes: ICD-8 codes 011 (pulmonary TB) and 012 (other respiratory TB); ICD-9 codes 010 (primary tuberculous infection), 011 (pulmonary TB), 012 (other respiratory TB), and 018 (miliary TB); and ICD-10 codes A15 (respiratory TB, bacteriologically and histologically confirmed), A16 (respiratory TB, not confirmed bacteriologically or histologically), and A19 (miliary TB). Regardless of the lung cancer type, studies that diagnosed lung cancer based on validated records such as hospital medical records or national registries were included.
2.3. Data Extraction
From the eligible studies, we extracted the name of the first author; publication year; country; baseline population characteristics (mean age, sex, smoking history, comorbidities with diabetes, hypertension, and chronic obstructive pulmonary diseases); number of total participants; number of TB cases; number of lung cancer cases; diagnostic method of TB; lung cancer type (small cell carcinoma, adenocarcinoma, large cell carcinoma, and squamous cell carcinoma); study design; effect size metrics or data necessary for calculations; maximally adjusted effect estimate of the association; and covariates used for adjustment. The Newcastle–Ottawa Scale (NOS) was applied to assess the risk of bias in the observational studies [11]. Countries were classified by region and economic income as per the World Bank classifications [12]. The incidence, prevalence, and mortality of TB are published annually by the World Health Organization (WHO) using information gathered through surveillance systems. The TB burden was stratified by the WHO definition, and high-burden countries were defined based on the TB burden data during 2016–2020 as 20 countries with the highest estimated numbers of TB incidence plus the top 10 countries with the highest estimated TB incidence. Intermediate and low-burden countries were defined as countries with a TB incidence of >40 and <40 cases per 100,000 persons, respectively, according to the WHO TB burden estimates for 2019 [13].
2.4. Statistical Analysis
We pooled adjusted estimates from all eligible studies for the primary analysis and pooled adjusted estimates of high-quality studies for the secondary analysis. The random effects model was used to obtain the summary estimates [14]. Heterogeneity between the included studies was estimated by I2 statistics [15].
Subgroup analyses were performed by country group (geographic region, economic status, and TB burden); covariates used for estimate adjustment (age; sex; smoking status; hypertension; diabetes; and history of respiratory diseases, such as pneumonia, chronic obstructive pulmonary diseases (COPD), and emphysema); cohort type (population-based or hospital-based); study design (prospective cohort, retrospective cohort, or case–control study); and TB diagnostic method (medical record, imaging, and self-report or physical examination). Meta-regression analyses were performed using covariates of baseline population characteristics (mean age, sex, and history of COPD and smoking) and lung cancer type (adenocarcinoma, squamous cell carcinoma, small-cell lung cancer, and large-cell lung cancer).
Publication bias was examined through visual inspection of the funnel plot and Egger’s test [16]. In order to prevent overestimation due to data duplication when using overlapping data sources, articles with a high number of participants that meet the research purpose and have high statistical power were selected and then analyzed. Additionally, a sensitivity analysis was performed while changing the data source, but there was no significant difference in the results. All statistical tests were two-sided. Statistical analyses were performed using software R version 4.0.3 and its “metafor” package [17,18].
3. Results
3.1. Characteristics of Literature
In the initial search, 10,229 potentially eligible articles were identified, of which 138 studies were selected for text screening. Finally, 32 articles [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50] corresponding to 33 cohorts met the inclusion criteria (Table 1). The reasons for exclusion are shown in Figure 1. The included studies comprised a total of 982,797 participants, of whom 30,159 had pulmonary TB and 52,773 had lung cancer. The participating countries were from the East Asia and Pacific, Europe, and Central Asia and North America regions. All the countries had a high- or upper-middle income status, as low- and low-middle income countries were not included in these studies. Eight out of 32 articles were included in the high-quality TB diagnosis, followed by a secondary in-depth analysis.

Table 1.
Baseline characteristics of the studies included in the meta-analysis.
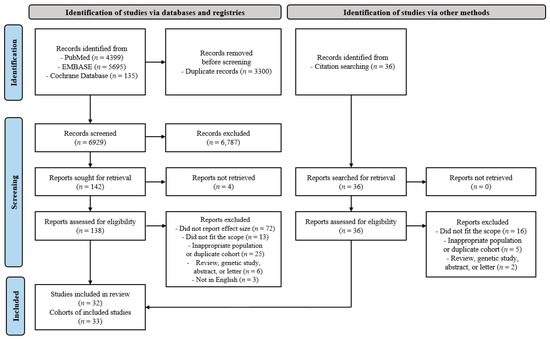
Figure 1.
Flow chart of the included studies.
The diagnosis of pulmonary TB is definitively established by the isolation of M. tuberculosis from a clinical specimen or tissue. An acid-fast bacilli smear or a polymerase chain reaction-based diagnosis are also used. However, radiographic image is just important supportive diagnostic tool. Based on the microbiological and pathological diagnoses, ICD code-related TB is then confirmed by the physician. Therefore, six out of the 32 enrolled articles presented an ICD code of tuberculosis for diagnosis, and two articles showed diagnosis by a medical expert based on the same criteria. These eight articles were included in the high-quality articles in this study. The remaining 24 articles were excluded from the final high-quality analysis, because they depended on interviews, memories of the participating patients, and medical images.
3.2. Pulmonary TB and Risk of Lung Cancer with All Eligible Studies
The overall association between a previous history of pulmonary TB and newly diagnosed lung cancer was statistically significant (odds ratio (OR): 2.09; 95% confidence interval (CI): 1.62–2.69, p < 0.001). There was high heterogeneity (I2 = 95%), no evidence of publication bias, the egger p-value was 0.447, and no visual asymmetry in the funnel plot (Figure 2A and Figure 3A). In the subgroup analysis by TB burden, the high-burden countries showed higher OR (2.57, 95% CI: 1.68–3.93, p < 0.001) than the medium-burden (OR: 2.48, 95% CI: 1.71–3.58, p < 0.001) and low-burden countries (OR: 1.77, 95% CI: 1.22–2.56, p = 0.003). Geographically, East Asia and the Pacific region showed a prominent risk (OR: 2.49, 95% CI: 1.83–3.39, p < 0.001) compared to the Europe and Central Asia (OR: 1.60, 95% CI: 0.80–3.22, p = 0.185) or North America (OR: 1.53, 95% CI: 1.11–2.12, p = 0.010) regions. The economic income statuses of the countries also reflected the characteristics of patients with TB, and the countries with upper-middle incomes (OR: 2.57, 95% CI: 1.68–3.93, p < 0.001) demonstrated a higher risk of lung cancer than high-income status countries (OR: 1.91, 95% CI: 1.41–2.59, p < 0.001). The association between pulmonary TB and newly developed lung cancer was statistically significant regardless of the adjustment for age, sex, smoking status, and cohort type or study design. The magnitude of association was similar regardless of whether pulmonary TB was diagnosed based on medical records (OR: 2.26, 95% CI: 1.29–3.94, p = 0.004), imaging (OR: 2.13, 95% CI: 1.16–3.92, p = 0.015), or self-report/physical examination (OR: 1.96, 95% CI: 1.56–2.47, p < 0.001). The heterogeneity within subgroups remained at a high level in a majority of the subgroup analyses (Table 2).
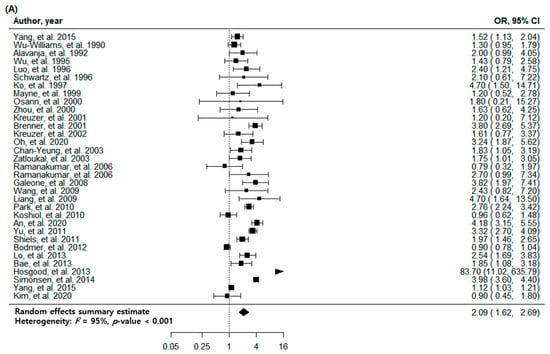
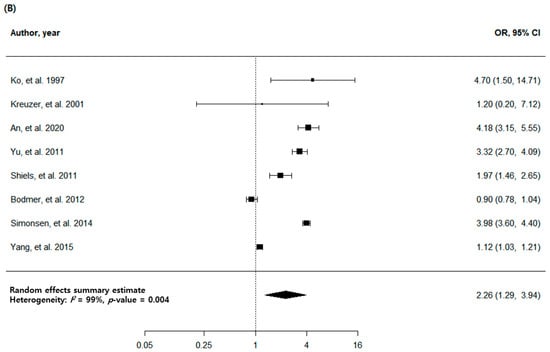
Figure 2.
Forest plots of risk estimates for the association between tuberculosis and lung cancer. (A) Meta-analysis of all eligible studies. (B) Meta-analysis of high-quality studies.
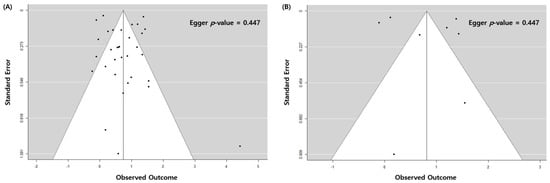
Figure 3.
Funnel plot of the study estimates. (A) All eligible studies. (B) High-quality studies.

Table 2.
Meta-analysis of 33 eligible cohorts to assess the association between pulmonary tuberculosis and lung cancer.
3.3. Pulmonary TB and Risk of Lung Cancer with High-Quality Studies
The analysis of eight high-quality studies showed a higher OR (2.26, 95% CI: 1.29–3.94, p = 0.004) than the analysis of all the studies. There was a high heterogeneity (I2 = 99%) with no publication bias, with Egger p = 0.621, and no visual asymmetry in the funnel plot (Figure 2B and Figure 3B). Of the eight articles, seven had cohorts from countries with a low TB burden, and only one had a cohort from a country with a medium TB burden. In the subgroup analysis with a TB burden, the medium-burden countries showed higher OR (4.18, 95% CI: 3.15–5.55, p < 0.001) than the low-burden countries (OR: 2.04, 95% CI: 1.12–3.73, p = 0.020). Geographically, the East Asia and the Pacific region showed a more prominent risk (OR: 2.79, 95% CI: 1.21–6.39, p = 0.016) compared to the Europe and Central Asia regions (OR: 1.79, 95% CI: 0.67–4.77, p = 0.244) (Table 3).

Table 3.
Meta-analysis of high-quality studies to assess the association between TB and lung cancer.
3.4. Stratified and Sensitivity Analysis
The quality of the 33 included articles was evaluated using the NOS. The quality assessment of 27 case–control studies is shown in Table 4 and that of six retrospective cohort studies is demonstrated in Table 5. We performed meta-regression analyses with continuous variables, such as the mean age at diagnosis of pulmonary TB, baseline characteristics including comorbidity, and pathological cell type of lung cancer. All the results are shown in Supplementary Table S2. Of these, patients with a low mean age at diagnosis of pulmonary TB showed a significant association between pulmonary TB and lung cancer. The primary analysis with all 32 articles estimated a regression coefficient of 0.949 (p < 0.001). The secondary analysis with eight high-quality studies with stringent TB diagnostic methods showed similar results (regression coefficient = 0.945, p < 0.001) (Figure 4).

Table 4.
Quality assessment of the included case–control studies using the Newcastle–Ottawa Scale.

Table 5.
Quality assessment of the included retrospective cohort studies using the Newcastle–Ottawa Scale.
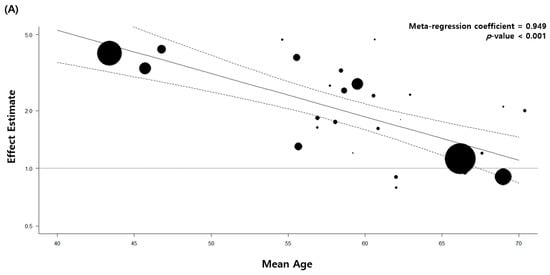
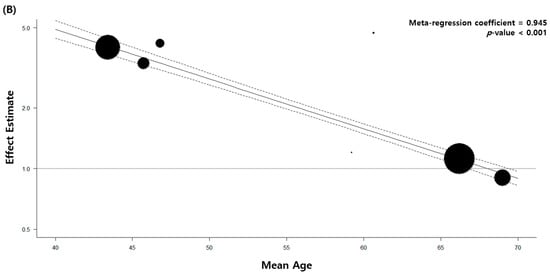
Figure 4.
Meta-regression analysis of the mean patient age and association between tuberculosis and lung cancer. (A) All eligible studies. (B) High-quality studies.
4. Discussion
This study assessed the association between previous pulmonary TB infection and the risk of lung cancer. The overall effect size of the eight high-quality studies was higher than that of the 32 total studies. The risk increased in patients diagnosed with pulmonary TB at a young age and in countries with a high TB burden, upper-middle income economic status, and the East Asian and Pacific regions.
Reverse causality between pulmonary TB and lung cancer is an unaddressed issue. To establish a temporal order from TB diagnosis to lung cancer diagnosis, we included case–control studies of cases with lung cancer and cohort studies following TB and non-TB populations. In addition, diagnostic misclassification between pulmonary TB and lung cancer occurs because of overlapping radiologic findings and the concomitant existence of the two entities [51]. Moreover, the similarity in clinical symptoms, such as cough, expectoration, fever, hemoptysis, and weight loss, as well as radiological similarities, contribute to misdiagnosis of the two diseases [52]. This has frequently led to the misdiagnosis of lung cancer as TB and prescribed medication for TB in patients with lung cancer in some countries [53]. Since extra-pulmonary TB is often misdiagnosed as cancer in its early stages, the difficulty of diagnosis is also applied in the opposite case [54]. To account for the potential bias due to the diagnostic misclassification of pulmonary TB with other respiratory diseases, we performed subgroup analyses of eight high-quality studies that diagnosed TB with validated medical codes; the analyses suggested that the observed association was similar to that in the main analysis.
The increased risk of lung cancer found in younger patients with TB was described by Wu et al. and An et al., both included in previous meta-analyses [20,55]. However, an overall unfavorable opinion on the association between age and increased risk warrants additional analysis.
The increased risk of pulmonary TB in East Asia and Pacific countries may be associated with socioeconomic determinants, such as hunger and limited public health infrastructure, that are related to poor TB outcomes [56,57]. The control of TB is poorer in these regions, with as a higher TB burden, or upper-middle income countries due to the difficulty in accessing healthcare. The burden of TB and the national income tend to be inversely proportional. Since these countries lack a necessary medical infrastructure, the early diagnosis of TB is difficult, the incidence of multidrug-resistant TB is high, and latent TB infection (LTBI) cannot be managed. A study in Indonesia suggested that the diagnosis of pulmonary TB was delayed in a majority of patients by >30 days after the onset of symptoms and that those patients were also unaware of the disease [58]. In 2019, the largest proportion of newly diagnosed TB cases (44%) was observed in the South-East Asia region, and the attributable risk factors were undernutrition, infection with human immunodeficiency virus, alcoholism, smoking, and diabetes [59].
Smoking is the most important environmental risk factor for lung cancer and is also known to increase the risk of TB [60]. However, in the present study, the current smoking status failed to correlate with an increase in the risk of lung cancer, confirming that smoking was not a confounding factor in this progression. The findings of the present study are consistent with those of a previous systematic review by Liang et al., which suggested that smoking is not an influential factor in the increased risk of lung cancer in patients with preexisting TB [61]. Moreover, there was no significant relationship with a history of common comorbidities, such as hypertension and diabetes.
There was no significant association between the incidence of TB and occurrence of a particular lung cancer type. This is in contrast to the results of a meta-analysis of 41 studies that showed a significant association between TB infection and adenocarcinoma [61]. Another study provided experimental evidence that chronic TB leads to the remodeling of lung tissue, evoking squamous cell metaplasia and a microenvironment accelerating squamous cell carcinogenesis [62]. However, the present study showed there was no meaningful association between TB and lung cancer type.
Several causal mechanisms have been suggested to explain this association. First, chronic inflammation induced by pulmonary TB may induce genetic mutations in lung parenchyma cells [53]; this hypothesis is supported by evidence that chronic TB induces cell dysplasia and the aggregation of squamous cells through Mycobacterium tuberculosis-infected macrophages [62]. This also applies to LTBI, which consists of chronic infection and inflammation, thus leading to an increased risk of lung cancer. The patients with LTBI showed a higher risk of future lung cancer (hazard ratio (HR) 2.69, 95% CI 0.66–11.07, p = 0.170). Additionally, among 136 TB contacts who received isoniazid prophylaxis, not one developed cancer. Therefore, the treatment of TB, including LTBI, is important in the further prevention of cancer by the suppression of chronic inflammation [63]. Second, the immunosuppressive role of TB by naturally occurring regulatory T cells may increase the risk of malignancy [64]. Finally, Mycobacterium tuberculosis can induce cellular DNA damage by increasing exposure to reactive oxygen and reactive nitrogen intermediates while entering macrophages [65]. Moreover, patients with TB showed frequent DNA damage, defects in cytokinesis, and a higher frequency of apoptotic and necrotic cells [66]. Additionally, repeated tissue damage cause fibrotic scar tissue formation; then, fibrosis could promote an enhanced tumorigenic potential [67].
The link between chronic inflammation in pulmonary TB and tumorigenesis of lung cancer possesses further findings applicable in clinical settings. Potential biomarkers such as nuclear protein MKI67, which regulates the genes related to tumorigenesis, can predict lung cancer, as it is shared by both TB and lung adenocarcinoma [68]. TB-induced EGFR mutations and epiregulin, a potent ligand for EGFR, may also contribute to carcinogenesis, and the treatment response of EGFR-TKIs differs in patients with pulmonary adenocarcinoma who previously had TB [62,69].
The present study had several limitations. First, this was a retrospective study that depended on ICD codes. Previous experimental studies have identified an association between pulmonary TB and progression to squamous cell lung cancer; however, the present retrospective study failed to provide any evidence on the correlation of a particular lung cancer type with TB. Thus, well-designed prospective studies are warranted to validate the association. Second, we could not identify individual studies from low-middle income or low-income countries, and in the analysis of the high-quality studies, the countries with a low TB burden accounted for seven out of eight studies. The absence of data from countries with low incomes and high TB burdens may have introduced bias in the summary estimate and lead to discrepancies in real-world estimates. Lastly, an analysis of treatment outcomes such as resistant TB, drug adherence, and treatment failure was not included. As this study reviewed articles analyzing the relationship between the risk factors of pulmonary tuberculosis and lung cancer, the focus was mainly on the “diagnosis”. In general, the incidence of resistant tuberculosis is high in countries with a low socioeconomic status, and in these areas, treatment failure and drug adherence are also poor. Therefore, this will also be a good additional analytic point in the future.
Nevertheless, the study had some strengths. Several studies have investigated the association between TB infection and lung cancer. However, the present study compared the results of all the studies and a few high-quality studies and obtained consistent results. Further, while screening, we excluded patient groups with specific diseases to maintain homogeneity in the complete population. We also merged the data of various subgroups to identify the characteristics that affected this association, and we obtained several significant results. Based on the socioeconomic factors and TB infection, which are inseparable, we elucidated that the patients in countries with high s TB burden, upper and middle incomes, and from the East Asia and Pacific regions had a higher risk of lung cancer than other patients. We believe that these findings will help design an approach for prioritizing and planning a strategy for worldwide TB control [70].
5. Conclusions
In conclusion, we found that the diagnosis of pulmonary TB at a young age is a risk factor for lung cancer, regardless of the underlying disease or smoking history. This trend is more pronounced in patients from countries with a high TB burden. This study is significant, as it summarized the results of several observational studies that described the association between pulmonary TB and lung cancer.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm11030765/s1. Table S1: Full search strategy. Table S2: Meta-regression analysis between pulmonary tuberculosis and lung cancer.
Author Contributions
K.H.L. designed the concept and supervised the study. S.Y.H. and J.Y.K. extracted the medical data. S.Y.H., J.Y.K., H.S.L. and S.L. performed the data analysis. D.K., S.K., J.H.H., J.I.S., S.H.H. and Y.G.S. interpreted the data with feedback. S.Y.H. and J.Y.K. drafted the main manuscript text. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no competing interest.
References
- World Health Organization. WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Doll, R.; Hill, A.B. Smoking and Carcinoma of the Lung. Br. Med. J. 1950, 2, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Wynder, E.L.; Graham, E.A. Etiologic Factors-in Bronchiogenic Carcinoma with Special Reference to Industrial Exposures. Report of Eight Hundred Fifty-Seven Proved Cases. AMA Arch. Ind. Hyg. Occup. Med. 1951, 4, 221–235. [Google Scholar] [PubMed]
- Adler, I. Primary Malignant Growths of the Lungs and Bronchi; Longmans, Green & Co.: London, UK, 1912. [Google Scholar]
- Gorlova, O.Y.; Zhang, Y.; Schabath, M.B.; Lei, L.; Zhang, Q.; Amos, C.I.; Spitz, M.R. Never Smokers and Lung Cancer Risk: A Case-Control Study of Epidemiological Factors. Int. J. Cancer 2006, 118, 1798–1804. [Google Scholar] [CrossRef] [PubMed]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and Cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Brenner, D.R.; Boffetta, P.; Duell, E.J.; Bickeböller, H.; Rosenberger, A.; McCormack, V.; Muscat, J.E.; Yang, P.; Wichmann, H.-E.; Brueske-Hohlfeld, I.; et al. Previous Lung Diseases and Lung Cancer Risk: A Pooled Analysis From the International Lung Cancer Consortium. Am. J. Epidemiol. 2012, 176, 573–585. [Google Scholar] [CrossRef]
- Brenner, D.R.; McLaughlin, J.R.; Hung, R.J. Previous Lung Diseases and Lung Cancer Risk: A Systematic Review and Meta-Analysis. PLoS ONE 2011, 6, e17479. [Google Scholar] [CrossRef]
- Leung, C.Y.; Huang, H.-L.; Rahman, M.; Nomura, S.; Abe, S.K.; Saito, E.; Shibuya, K. Cancer Incidence Attributable to Tuberculosis in 2015: Global, Regional, and National Estimates. BMC Cancer 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; Connell, D.O.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (Nos) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.Ohri.Ca/programs/clinical_epidemiology/oxford.Asp (accessed on 1 December 2020).
- The World Bank. World Bank Country and Lending Groups. Available online: https://datahelpdesk.Worldbank.Org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed on 1 December 2020).
- World Health Organization. Tuberculosis Data. Available online: https://www.Who.Int/teams/global-tuberculosis-programme/data (accessed on 1 December 2020).
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying Heterogeneity in a Meta-Analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. In R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Viechtbauer, W. Conducting Meta-Analyses in R with the Metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.Y.; Goo, J.M.; Kim, Y. Lung Cancer CT Screening and Lung-Rads in a Tuberculosis-Endemic Country: The Korean Lung Cancer Screening Project (K-LUCAS). Radiology 2020, 296, 181–188. [Google Scholar] [CrossRef] [PubMed]
- An, S.J.; Kim, Y.J.; Han, S.S.; Heo, J. Effects of Age on the Association between Pulmonary Tuberculosis and Lung Cancer in a South Korean Cohort. J. Thorac. Dis. 2020, 12, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.-M.; Roh, Y.-H.; Lim, D.; Kong, H.-J.; Cho, H.; Hwangbo, B.; Won, Y.-J.; Jung, K.-W.; Oh, K. Pulmonary Tuberculosis is Associated with Elevated Risk of Lung Cancer in Korea: The Nationwide Cohort Study. J. Cancer 2020, 11, 1899–1906. [Google Scholar] [CrossRef]
- Yang, L.; Lu, X.; Deng, J.; Zhou, Y.; Huang, N.; Qiu, F.; Yang, X.; Yang, R.; Fang, W.; Ran, P.; et al. Risk Factors Shared by COPD and Lung Cancer and Mediation Effect of COPD: Two Center Case–Control Studies. Cancer Causes Control 2014, 26, 11–24. [Google Scholar] [CrossRef]
- Yang, T.-Y.; Lin, W.-M.; Lin, C.-L.; Sung, F.-C.; Kao, C.-H. Correlation between Use of Simvastatin and Lovastatin and Female Lung Cancer Risk: A Nationwide Case-Control Study. Int. J. Clin. Pract. 2014, 69, 571–576. [Google Scholar] [CrossRef]
- Simonsen, D.F.; Farkas, D.K.; Søgaard, M.; Horsburgh, C.R.; Sørensen, H.T.; Thomsen, R.W. Tuberculosis and Risk of Cancer: A Danish Nationwide Cohort Study. Int. J. Tuberc. Lung Dis. 2014, 18, 1211–1219. [Google Scholar] [CrossRef]
- HosgoodIII, H.D.; Chapman, R.S.; He, X.; Hu, W.; Tian, L.; Liu, L.Z.; Lai, H.; Chen, W.; Rothman, N.; Lan, Q. History of Lung Disease and Risk of Lung Cancer in a Population with High Household Fuel Combustion Exposures in Rural China. Lung Cancer 2013, 81, 343–346. [Google Scholar] [CrossRef]
- Bae, J.-M.; Li, Z.-M.; Shin, M.-H.; Kim, D.-H.; Lee, M.-S.; Ahn, Y.-O. Pulmonary Tuberculosis and Lung Cancer Risk in Current Smokers: The Seoul Male Cancer Cohort Study. J. Korean Med. Sci. 2013, 28, 896–900. [Google Scholar] [CrossRef]
- Lo, Y.-L.; Hsiao, C.-F.; Chang, G.-C.; Tsai, Y.-H.; Huang, M.-S.; Su, W.-C.; Chen, Y.-M.; Hsin, C.-W.; Chang, C.-H.; Yang, P.-C.; et al. Risk Factors for Primary Lung Cancer among Never Smokers by Gender in a Matched Case–Control Study. Cancer Causes Control 2012, 24, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, M.; Becker, C.; Jick, S.S.; Meier, C.R. Metformin Does Not Alter the Risk of Lung Cancer: A Case–Control Analysis. Lung Cancer 2012, 78, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Shiels, M.S.; Albanes, D.; Virtamo, J.; Engels, E.A. Increased Risk of Lung Cancer in Men with Tuberculosis in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Liao, C.C.; Hsu, W.H.; Chen, H.J.; Liao, W.C.; Muo, C.H.; Sung, F.C.; Chen, C.Y. Increased Lung Cancer Risk among Patients with Pulmonary Tuberculosis: A Population Cohort study. J. Thorac. Oncol. 2011, 6, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Koshiol, J.; Rotunno, M.; Consonni, D.; Pesatori, A.C.; De Matteis, S.; Goldstein, A.M.; Chaturvedi, A.K.; Wacholder, S.; Landi, M.T.; Lubin, J.H.; et al. Lower Risk of Lung Cancer after Multiple Pneumonia Diagnoses. Cancer Epidemiol. Biomark. Prev. 2010, 19, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Cho, L.Y.; Yang, J.J.; Park, B.; Chang, S.H.; Lee, K.S.; Kim, H.; Yoo, K.Y.; Lee, C.T. Lung Cancer Risk and Cigarette Smoking, Lung Tuberculosis According to Histologic Type and Gender in a Population Based Case-Control Study. Lung Cancer 2010, 68, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Guan, P.; Yin, Z.; Li, X.; He, Q.; Zhou, B. Risk of Lung Cancer following Nonmalignant Respiratory Conditions among Nonsmoking Women Living in Shenyang, Northeast China. J. Womens Health 2009, 18, 1989–1995. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.R.; Yu, I.T.; Chiu, Y.L.; Qiu, H.; Fu, Z.; Goggins, W.; Au, J.S.; Tse, L.A.; Wong, T.W. Previous Pulmonary Disease and Family Cancer History Increase the Risk of Lung Cancer among Hong Kong Women. Cancer Causes Control 2009, 20, 757–763. [Google Scholar] [CrossRef]
- Galeone, C.; Pelucchi, C.; La Vecchia, C.; Negri, E.; Bosetti, C.; Hu, J. Indoor Air Pollution from Solid Fuel Use, Chronic Lung Diseases and Lung Cancer in Harbin, Northeast China. Eur. J. Cancer Prev. 2008, 17, 473–478. [Google Scholar] [CrossRef]
- Ramanakumar, A.V.; Parent, M.E.; Menzies, D.; Siemiatycki, J. Risk of Lung Cancer Following Nonmalignant Respiratory Conditions: Evidence from Two Case-Control Studies in Montreal, Canada. Lung Cancer 2006, 53, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Zatloukal, P.; Kubík, A.; Pauk, N.; Tomásek, L.; Petruzelka, L. Adenocarcinoma of the Lung among Women: Risk Associated with Smoking, Prior Lung Disease, Diet and Menstrual and Pregnancy History. Lung Cancer 2003, 41, 283–293. [Google Scholar] [CrossRef]
- Chan-Yeung, M.; Koo, L.C.; Ho, J.C.; Tsang, K.W.; Chau, W.S.; Chiu, S.W.; Ip, M.S.; Lam, W.K. Risk Factors Associated with Lung Cancer in Hong Kong. Lung Cancer 2003, 40, 131–140. [Google Scholar] [CrossRef]
- Kreuzer, M.; Heinrich, J.; Kreienbrock, L.; Rosario, A.S.; Gerken, M.; Wichmann, H.E. Risk Factors for Lung Cancer among Nonsmoking Women. Int. J. Cancer 2002, 100, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Brenner, A.V.; Wang, Z.; Kleinerman, R.A.; Wang, L.; Zhang, S.; Metayer, C.; Chen, K.; Lei, S.; Cui, H.; Lubin, J.H. Previous Pulmonary Diseases and Risk of Lung Cancer in Gansu Province, China. Int. J. Epidemiol. 2001, 30, 118–124. [Google Scholar] [CrossRef]
- Kreuzer, M.; Gerken, M.; Kreienbrock, L.; Wellmann, J.; Wichmann, H.E. Lung Cancer in Lifetime Nonsmoking Men-Results of a Case-Control Study in Germany. Br. J. Cancer 2001, 84, 134–140. [Google Scholar] [CrossRef]
- Zhou, B.S.; Wang, T.J.; Guan, P.; Wu, J.M. Indoor Air Pollution and Pulmonary Adenocarcinoma among Females: A Case-Control Study in Shenyang, China. Oncol. Rep. 2000, 7, 1253–1259. [Google Scholar] [CrossRef]
- Osann, K.E.; Lowery, J.T.; Schell, M.J. Small Cell Lung Cancer in Women: Risk Associated with Smoking, Prior Respiratory Disease, and Occupation. Lung Cancer 2000, 28, 1–10. [Google Scholar] [CrossRef]
- Mayne, S.T.; Buenconsejo, J.; Janerich, D.T. Previous Lung Disease and Risk of Lung Cancer among Men and Women Nonsmokers. Am. J. Epidemiol. 1999, 149, 13–20. [Google Scholar] [CrossRef]
- Ko, Y.C.; Lee, C.H.; Chen, M.J.; Huang, C.C.; Chang, W.Y.; Lin, H.J.; Wang, H.Z.; Chang, P.Y. Risk Factors for Primary Lung Cancer among Non-Smoking Women in Taiwan. Int. J. Epidemiol. 1997, 26, 24–31. [Google Scholar] [CrossRef]
- Schwartz, A.G.; Yang, P.; Swanson, G.M. Familial Risk of Lung Cancer among Nonsmokers and their Relatives. Am. J. Epidemiol. 1996, 144, 554–562. [Google Scholar] [CrossRef]
- Luo, R.X.; Wu, B.; Yi, Y.N.; Huang, Z.W.; Lin, R.T. Indoor Burning Coal Air Pollution and Lung Cancer—A Case-Control Study in Fuzhou, China. Lung Cancer 1996, 14 (Suppl. 1), S113–S119. [Google Scholar] [CrossRef]
- Wu, A.H.; Fontham, E.T.; Reynolds, P.; Greenberg, R.S.; Buffler, P.; Liff, J.; Boyd, P.; Henderson, B.E.; Correa, P. Previous Lung Disease and Risk of Lung Cancer among Lifetime Nonsmoking Women in the United States. Am. J. Epidemiol. 1995, 141, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Alavanja, M.C.; Brownson, R.C.; Boice, J.D., Jr.; Hock, E. Preexisting Lung Disease and Lung Cancer among Nonsmoking Women. Am. J. Epidemiol. 1992, 136, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Wu-Williams, A.H.; Dai, X.D.; Blot, W.; Xu, Z.Y.; Sun, X.W.; Xiao, H.P.; Stone, B.J.; Yu, S.F.; Feng, Y.P.; Ershow, A.G.; et al. Lung Cancer among Women in North-East China. Br. J. Cancer 1990, 62, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Kouranos, V.D.; Athanassa, Z.; Kopterides, P. Tuberculosis and Malignancy. QJM Int. J. Med. 2010, 103, 461–487. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, M.; Kant, S.; Bhaskar, R. Pulmonary Tuberculosis as Differential Diagnosis of Lung Cancer. South Asian J. Cancer 2012, 1, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Keikha, M.; Esfahani, B.N. The Relationship between Tuberculosis and Lung Cancer. Adv. Biomed. Res. 2018, 7, 58. [Google Scholar] [PubMed]
- Aisenberg, G.M.; Jacobson, K.; Chemaly, R.F.; Rolston, K.V.; Raad, I.I.; Safdar, A. Extrapulmonary Tuberculosis aActive Infection Misdiagnosed as Cancer: Mycobacterium Tuberculosis Disease in Patients at a Comprehensive Cancer Center (2001–2005). Cancer 2005, 104, 2882–2887. [Google Scholar] [CrossRef]
- Wu, C.Y.; Hu, H.Y.; Pu, C.Y.; Huang, N.; Shen, H.C.; Li, C.P.; Chou, Y.J. Pulmonary Tuberculosis Increases the Risk of Lung Cancer: A Population-Based Cohort Study. Cancer 2011, 117, 618–624. [Google Scholar] [CrossRef]
- Wu, J.; Dalal, K. Tuberculosis in Asia and the Pacific: The Role of Socioeconomic Status and Health System Development. Int. J. Prev. Med. 2012, 3, 8–16. [Google Scholar]
- Vermund, S.H.; Yamamoto, N. Co-Infection with Human Immunodeficiency Virus and Tuberculosis in Asia. Tuberculosis 2007, 87, S18–S25. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rintiswati, N.; Mahendradhata, Y.; Subronto, Y.; Varkevisser, C.M.; Van Der Werf, M.J. Journeys to Tuberculosis Treatment: A Qualitative Study of Patients, Families and Communities in Jogjakarta, Indonesia. BMC Public Health 2009, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2020: Executive Summary; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Alavi-Naini, R.; Sharifi-Mood, B.; Metanat, M. Association between Tuberculosis and Smoking. Int. J. High Risk Behav. Addict. 2012, 1, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.Y.; Li, X.L.; Yu, X.S.; Guan, P.; Yin, Z.H.; He, Q.C.; Zhou, B.S. Facts and Fiction of the Relationship between Preexisting Tuberculosis and Lung Cancer Risk: A Systematic Review. Int. J. Cancer 2009, 125, 2936–2944. [Google Scholar] [CrossRef]
- Nalbandian, A.; Yan, B.S.; Pichugin, A.; Bronson, R.T.; Kramnik, I. Lung Carcinogenesis Induced by Chronic Tuberculosis Infection: The Experimental Model and Genetic Control. Oncogene 2009, 28, 1928–1938. [Google Scholar] [CrossRef] [PubMed]
- Su, V.Y.; Yen, Y.F.; Pan, S.W.; Chuang, P.H.; Feng, J.Y.; Chou, K.T.; Chen, Y.M.; Chen, T.J.; Su, W.J. Latent Tuberculosis Infection and the Risk of Subsequent Cancer. Medicine 2016, 95, e2352. [Google Scholar] [CrossRef]
- Roberts, T.; Beyers, N.; Aguirre, A.; Walzl, G. Immunosuppression during Active Tuberculosis is Characterized by Decreased Interferon-γ Production and CD25 Expression with Elevated Forkhead Box P3, Transforming Growth Factor-β, and Interleukin-4 mRNA Levels. J. Infect. Dis. 2007, 195, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.B.; Dinauer, M.C.; Morgenstern, D.E.; Krahenbuhl, J.L. Comparison of the Roles of Reactive Oxygen and Nitrogen Intermediates in the Host Response to Mycobacterium Tuberculosis Using Transgenic Mice. Tuber. Lung Dis. 1997, 78, 237–246. [Google Scholar] [CrossRef]
- da Silva, A.L.G.; Bresciani, M.J.; Karnopp, T.E.; Weber, A.F.; Ellwanger, J.H.; Henriques, J.A.P.; Valim, A.R.d.M.; Possuelo, L.G. DNA Damage and Cellular Abnormalities in Tuberculosis, Lung Cancer and Chronic Obstructive Pulmonary Disease. Multidiscip. Respir. Med. 2015, 10, 38. [Google Scholar] [CrossRef]
- Woo, S.J.; Kim, Y.; Jung, H.; Lee, J.J.; Hong, J.Y. Tuberculous Fibrosis Enhances Tumorigenic Potential via the NOX4-Autophagy Axis. Cancers 2021, 13, 687. [Google Scholar] [CrossRef]
- Chai, Q.; Lu, Z.; Liu, Z.; Zhong, Y.; Zhang, F.; Qiu, C.; Li, B.; Wang, J.; Zhang, L.; Pang, Y.; et al. Lung Gene Expression Signatures Suggest Pathogenic Links and Molecular Markers for Pulmonary Tuberculosis, Adenocarcinoma and Sarcoidosis. Commun. Biol. 2020, 3, 604. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.K.; Paik, S.S.; Lee, S.H. Impact of Pulmonary Tuberculosis on the EGFR Mutational Status and Clinical Outcome in Patients with Lung Adenocarcinoma. Cancer Res. Treat. 2019, 51, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.J.; Arinaminpathy, N.; Bloom, A.; Bloom, B.R.; Boehme, C.; Chaisson, R.; Chin, D.P.; Churchyard, G.; Cox, H.; Ditiu, L. Building a Tuberculosis-Free World: The Lancet Commission on Tuberculosis. Lancet 2019, 393, 1331–1384. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).