Associations of IL-18 with Altered Cardiovascular Risk Profile in Psoriatic Arthritis and Ankylosing Spondylitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Methods
2.3. ELISAs
2.4. Statistical Analysis
3. Results
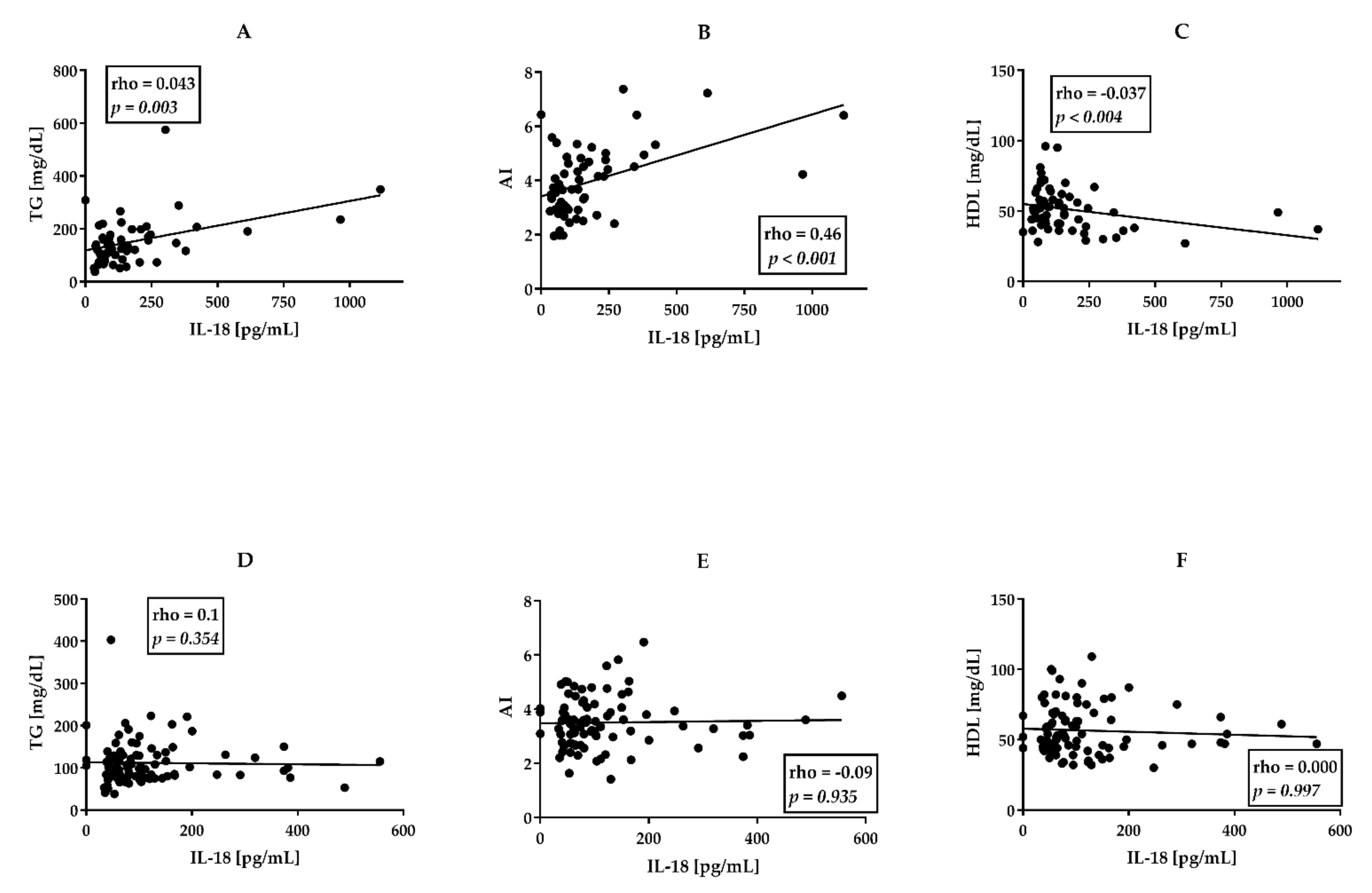
3.1. Higher Proatherogenic Profile and Serum IL-18 Levels in PsA Than AS Patients
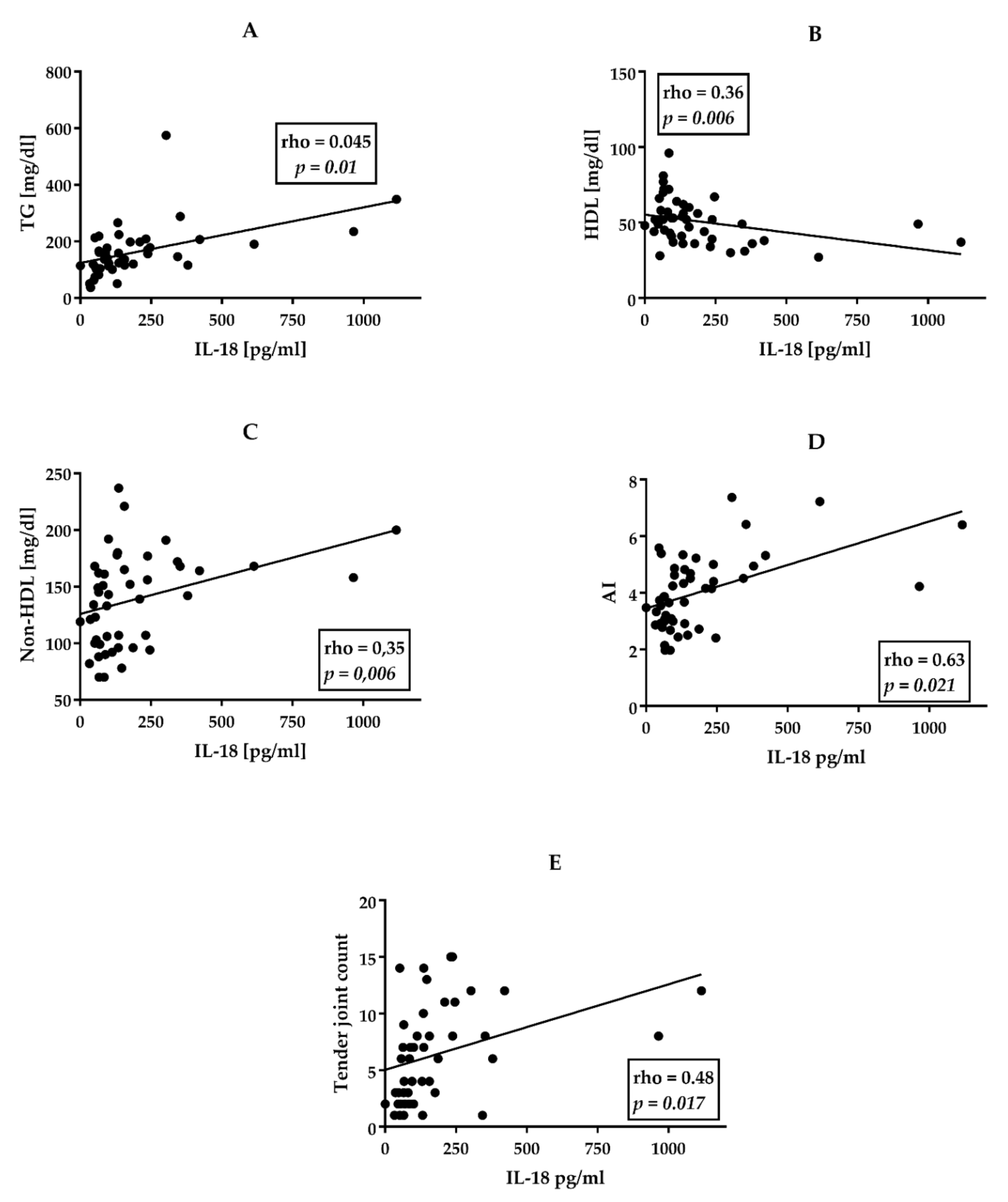
3.2. perPsA Patients Are Characterized by the Highest Cardiovascular Risk, Frequency of Hypertriglyceridemia and IHD, and IL-18 Serum Levels
3.3. Associations between Clinical Data, CVD Risk Factors, and Serum Cytokine Levels in perPsA Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kristensen, S.L.; McInnes, I.B.; Sattar, N. Psoriasis, psoriatic arthritis and cardiovascular risk: Are we closer to a clinical recommendation? Ann. Rheum. Diseases. 2015, 74, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Bahce-Altuntas, A.; Mowrey, W.; Broder, A. Active peripheral inflammation is associated with pro-atherogenic lipid profile in psoriatic arthritis. Semin. Arthritis Rheum. 2016, 46, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.H.M.; Cheng, I.T.; Li, E.K.; Wong, P.; Lee, J.; Yip, R.M.L.; Yim, C.W.; Ying, S.K.; Li, M.; Li, T.K.; et al. DAPSA, carotid plaque and cardiovascular events in psoriatic arthritis: A longitudinal study. Ann. Rheum. Dis. 2020, 79, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.H. Systemic inflammation and cardiovascular comorbidity in psoriasis patients: Causes and consequences. Front. Immunol. 2018, 9, 579. [Google Scholar] [CrossRef]
- Shimoura, N.; Nagai, H.; Fujiwara, S.; Jimbo, H.; Yoshimoto, T.; Nishigori, C. Interleukin (IL)-18, cooperatively with IL-23, induces prominent inflammation and enhances psoriasis-like epidermal hyperplasia. Arch. Dermatol. Res. 2017, 309, 315–321. [Google Scholar] [CrossRef]
- Blauvelt, A.; Chiricozzi, A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin. Rev. Allergy Immunol. 2018, 55, 379–390. [Google Scholar] [CrossRef] [Green Version]
- Kumthekar, A.; Ogdie, A. Obesity and psoriatic arthritis: A narrative review. Rheumatol. Ther. 2020, 7, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Cerdeira, C.; Cordeiro-Rodriguez, M.; Carnero-Gregorio, M.; Lopez-Barcenas, A.; Martinez-Herrera, E.; Fabbrocini, G.; Sinani, A.; Arenas-Guzmán, R.; González-Cespón, J.L. Biomarkers of inflammation in obesity-psoriatic patients. Mediat. Inflamm. 2019, 2019, 7353420. [Google Scholar] [CrossRef]
- Hu, S.C.; Lan, C.E. Psoriasis and cardiovascular comorbidities: Focusing on severe vascular events, cardiovascular risk factors and implications for treatment. Int. J. Mol. Sci. 2017, 18, 2211. [Google Scholar] [CrossRef] [Green Version]
- Horwood, N.J.; Udagawa, N.; Elliott, J.; Grail, D.; Okamura, H.; Kurimoto, M.; Dunn, A.R.; Martin, T.; Gillespie, M.T. Interleukin 18 inhibits osteoclast formation via T cell production of granulocyte macrophage colony-stimulating factor. J. Clin. Investig. 1998, 101, 595–603. [Google Scholar] [CrossRef] [Green Version]
- Olee, T.; Hashimoto, S.; Quach, J.; Lotz, M. IL-18 is produced by articular chondrocytes and induces proinflammatory and catabolic responses. J. Immunol. 1999, 162, 1096–1100. [Google Scholar] [PubMed]
- Rasmy, H.; Mikhael, N.; Ismail, S. Interleukin-18 expression and the response to treatment in patients with psoriasis. Arch. Med Sci. 2011, 7, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Klop, B.; Elte, J.W.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [Green Version]
- Tang, X. Analysis of interleukin-17 and interleukin-18 levels in animal models of atherosclerosis. Exp. Ther. Med. 2019, 18, 517–522. [Google Scholar] [CrossRef] [Green Version]
- McGonagle, D.G.; McInnes, I.B.; Kirkham, B.W.; Sherlock, J.; Moots, R. The role of IL-17A in axial spondyloarthritis and psoriatic arthritis: Recent advances and controversies. Ann. Rheum. Dis. 2019, 78, 1167–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinarello, C.A.; Novick, D.; Kim, S.; Kaplanski, G. Interleukin-18 and IL-18 binding protein. Front. Immunol. 2013, 4, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, S.M.; Nishioka, K.; Yudoh, K. Interleukin (IL) 18 stimulates osteoclast formation through synovial T cells in rheumatoid arthritis: Comparison with IL1 beta and tumour necrosis factor alpha. Ann. Rheum. Dis. 2004, 63, 1379–1386. [Google Scholar] [CrossRef]
- Makiishi-Shimobayashi, C.; Tsujimura, T.; Iwasaki, T.; Yamada, N.; Sugihara, A.; Okamura, H.; Hayashi, S.I.; Terada, N. Interleukin-18 up-regulates osteoprotegerin expression in stromal/osteoblastic cells. Biochem. Biophys. Res. Commun. 2001, 281, 361–366. [Google Scholar] [CrossRef]
- Weitzmann, M.N. The role of inflammatory cytokines, the RANKL/OPG axis, and the immunoskeletal interface in physiological bone turnover and osteoporosis. Scientifica 2013, 2013, 125705. [Google Scholar] [CrossRef]
- Nozaki, Y.; Ri, J.; Sakai, K.; Niki, K.; Kinoshita, K.; Funauchi, M.; Matsumura, I. Inhibition of the IL-18 receptor signaling pathway ameliorates disease in a murine model of rheumatoid arthritis. Cells 2019, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, Q.; Su, H.; Cheng, J. Exosomes from adipose derived mesenchymal stem cells alleviate diabetic osteoporosis in rats through suppressing NLRP3 inflammasome activation in osteoclasts. J. Biosci. Bioeng. 2021, 131, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Min, H.K.; Kim, S.; Lee, J.Y.; Kim, K.W.; Lee, S.H.; Kim, H.R. IL-18 binding protein suppresses IL-17-induced osteoclastogenesis and rectifies type 17 helper T cell/regulatory T cell imbalance in rheumatoid arthritis. J. Transl. Med. 2021, 19, 392. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.; Gladman, D.; Helliwell, P.; Marchesoni, A.; Mease, P.; Mielants, H. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2006, 54, 2665–2673. [Google Scholar] [CrossRef] [PubMed]
- Rudwaleit, M.; Van Der Heijde, D.; Landewé, R.; Listing, J.; Akkoc, N.; Brandt, J.; Braun, J.; Chou, C.T.; Collantes-Estevez, E.; Dougados, M.; et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann. Rheum. Dis. 2009, 68, 777–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagenmakers, E.J.; Farrell, S. AIC model selection using Akaike weights. Psychon. Bull. Rev. 2004, 11, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, M.V.; Simon, D.; Nas, K.; Zaiss, M.M.; Luo, Y.; Zhao, Y.; Rech, J.; Schett, G. A set of serum markers detecting systemic inflammation in psoriatic skin, entheseal, and joint disease in the absence of C-reactive protein and its link to clinical disease manifestations. Arthritis Res. Ther. 2020, 22, 26. [Google Scholar] [CrossRef] [Green Version]
- Braun, J.; Deodhar, A.; Landewé, R.; Baraliakos, X.; Miceli-Richard, C.; Sieper, J.; Quebe-Fehling, E.; Martin, R.; Porter, B.; Gandhi, K.K.; et al. Impact of baseline C-reactive protein levels on the response to secukinumab in ankylosing spondylitis: 3-year pooled data from two phase III studies. RMD Open 2018, 4, e000749. [Google Scholar] [CrossRef] [Green Version]
- Landgren, A.J.; Dehlin, M.; Jacobsson, L.; Bergsten, U.; Klingberg, E. Cardiovascular risk factors in gout, psoriatic arthritis, rheumatoid arthritis and ankylosing spondylitis: A cross-sectional survey of patients in Western Sweden. RMD Open 2021, 7, e001568. [Google Scholar] [CrossRef]
- Bengtsson, K.; Forsblad-d’Elia, H.; Lie, E.; Klingberg, E.; Dehlin, M.; Exarchou, S.; Lindström, U.; Askling, J.; Jacobsson, L.T. Are ankylosing spondylitis, psoriatic arthritis and undifferentiated spondyloarthritis associated with an increased risk of cardiovascular events? A prospective nationwide population-based cohort study. Arthritis Res. Ther. 2017, 19, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puche-Larrubia, M.Á.; Ladehesa-Pineda, L.; Font-Ugalde, P.; Escudero-Contreras, A.; Moltó, A.; López-Medina, C.; Collantes-Estévez, E. Distribution of comorbidities in spondyloarthritis with regard to the phenotype and psoriasis: Data from the ASAS-COMOSPA study. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X211045263. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Krishan, P.; Syngle, A. Atherosclerosis in psoriatic arthritis: A multiparametric analysis using imaging technique and laboratory markers of inflammation and vascular function. Int. J. Angiol. 2016, 25, 222–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brembilla, N.C.; Senra, L.; Boehncke, W.H. The IL-17 family of cytokines in psoriasis: IL-17A and beyond. Front. Immunol. 2018, 9, 1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chehimi, M.; Vidal, H.; Eljaafari, A. Pathogenic role of IL-17-producing immune cells in obesity, and related inflammatory diseases. J. Clin. Med. 2017, 6, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veldhuijzen van Zanten, J.; Sandoo, A.; Metsios, G.S.; Stavropoulos-Kalinoglou, A.; Ntoumanis, N.; Kitas, G.D. Comparison of the effects of exercise and anti-TNF treatment on cardiovascular health in rheumatoid arthritis: Results from two controlled trials. Rheumatol. Int. 2019, 39, 219–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Juanatey, C.; Llorca, J.; Miranda-Filloy, J.A.; Amigo-Diaz, E.; Testa, A.; Garcia-Porrua, C.; Martin, J.; Gonzalez-Gay, M.A. Endothelial dysfunction in psoriatic arthritis patients without clinically evident cardiovascular disease or classic atherosclerosis risk factors. Arthritis Rheum. 2007, 57, 287–293. [Google Scholar] [CrossRef]
- Kontny, E.B.K.; Gluszko, P. AB0733 Associations of serum osteoprotegerin and il-18 concentrations with cardiovascular risk in ankylosing spondylitis and psoriatic arthritis patients. Ann. Rheum. Dis. 2017, 76, 1310–1311. [Google Scholar]
- Bonek, K.; Kuca-Warnawin, E.; Kontny, E.; Głuszko, P. AB0775 Peripheral joint inflammation is associated with more proatherogenic cardiovascular risk profile in patients with psoriatic arthritis. Ann. Rheum. Dis. 2020, 79, 1685–1686. [Google Scholar] [CrossRef]
- Taleb, S.; Romain, M.; Ramkhelawon, B.; Uyttenhove, C.; Pasterkamp, G.; Herbin, O.; Esposito, B.; Perez, N.; Yasukawa, H.; Van Snick, J.; et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J. Exp. Med. 2009, 206, 2067–2077. [Google Scholar] [CrossRef] [Green Version]
- Liuzzo, G.; Trotta, F.; Pedicino, D. Interleukin-17 in atherosclerosis and cardiovascular disease: The good, the bad, and the unknown. Eur. Heart J. 2013, 34, 556–559. [Google Scholar] [CrossRef] [Green Version]
- Ghoreschi, K.; Laurence, A.; Yang, X.P.; Hirahara, K.; O’Shea, J.J. T helper 17 cell heterogeneity and pathogenicity in autoimmune disease. Trends Immunol. 2011, 32, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Ravelli, A.; Minoia, F.; Davì, S.; Horne, A.; Bovis, F.; Pistorio, A.; Aricò, M.; Avcin, T.; Behrens, E.M.; De Benedetti, F.; et al. 2016 Classification criteria for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: A European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation collaborative initiative. Arthritis Rheumatol. 2016, 68, 566–576. [Google Scholar] [PubMed] [Green Version]
- Alehashemi, S.; Goldbach-Mansky, R. Human autoinflammatory diseases mediated by NLRP3-, pyrin-, NLRP1-, and NLRC4-inflammasome dysregulation updates on diagnosis, treatment, and the respective roles of IL-1 and IL-18. Front. Immunol. 2020, 11, 1840. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.S.; Girard-Guyonvarc’h, C.; Holzinger, D.; de Jesus, A.A.; Tariq, Z.; Picarsic, J.; Schiffrin, E.J.; Foell, D.; Grom, A.A.; Ammann, S.; et al. Interleukin-18 diagnostically distinguishes and pathogenically promotes human and murine macrophage activation syndrome. Blood 2018, 131, 1442–1455. [Google Scholar] [CrossRef]
- Aranda-Valera, I.C.; de la Rosa, I.A.; Roldán-Molina, R.; del Carmen Ábalos-Aguilera, M.; Torres-Granados, C.; Patiño-Trives, A.; Luque-Tevar, M.; Ibáñez-Costa, A.; Guzmán-Ruiz, R.; del Mar Malagón, M.; et al. Subclinical cardiovascular risk signs in adults with juvenile idiopathic arthritis in sustained remission. Pediatric Rheumatol. Online J. 2020, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Bi, Y.; Jin, G.; Gan, H.; Yu, L. High and fluctuating glucose levels increase the expression and secretion of interleukin18 in mouse peritoneal macrophages. Mol. Med. Rep. 2015, 12, 2715–2720. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, H.; Aoyama-Ishikawa, M.; Takahara, M.; Yamauchi, C.; Inoue, T.; Miyoshi, M.; Maeshige, N.; Usami, M.; Nakao, A.; Kotani, J. Endogenous interleukin 18 suppresses hyperglycemia and hyperinsulinemia during the acute phase of endotoxemia in mice. Surg. Infect. 2015, 16, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Troseid, M.; Seljeflot, I.; Hjerkinn, E.M.; Arnesen, H. Interleukin-18 is a strong predictor of cardiovascular events in elderly men with the metabolic syndrome: Synergistic effect of inflammation and hyperglycemia. Diabetes Care 2009, 32, 486–492. [Google Scholar] [CrossRef] [Green Version]
- Ridker, P.M.; MacFadyen, J.G.; Thuren, T.; Libby, P. Residual inflammatory risk associated with interleukin-18 and interleukin-6 after successful interleukin-1beta inhibition with canakinumab: Further rationale for the development of targeted anti-cytokine therapies for the treatment of atherothrombosis. Eur. Heart J. 2020, 41, 2153–2163. [Google Scholar] [CrossRef] [Green Version]

| Measured Parameters | AS n = 94 | PsAtotal n = 61 | axPsA n = 14 | perPsA n= 47 | Normal Ranges | p K–W |
|---|---|---|---|---|---|---|
| Age [years] | 43.5 (IQR 18) | 46.5 (IQR 19) | 43 (IQR 19) | 47 (IQR 18.8) | ns | |
| male | 67.7% | 52.5% | 71.4% | 48.9% | ns | |
| female | 32.3% | 47.5% | 29.6% | 51.1% | ns | |
| Disease duration time [years] | 5 (IQR 10) | 4.5 (IQR 8.3) | 4 (IQR 10) | 4.5 (IQR 9) | ns | |
| Disease activity | ||||||
| BASDAI | 5.8 (IQR 3.1) | 5.8 (IQR 4.1) | ns | |||
| ASDAS CRP | 3.6 (IQR 2.8) | 3.1 (IQR 2.3) | 0.011 | |||
| DAPSA | 21.3 (IQR 16.8) | 18.7 (IQR 39) | 28 (IQR 21.15) | |||
| Biochemical parameters | ||||||
| CRP [mg/L] | 8.5 (IQR 13) | 9 (IQR 12) | 12.4 (IQR 12) | 6 (IQR 13) | 1–5 | ns |
| ESR. [mm/h] | 16 (IQR 22) | 11 (IQR 21) | 14 (IQR 13.5) | 12 (IQR 22) | 1–10 | ns |
| Fasting glucose [mg/dL] | 89.5 (IQR 9) | 94 (IQR 15) | 94 (IQR 16) | 93.5 (16.3) | 60–100 | 0.02 |
| Atherogenic index. TC/HDL-AI | 3.45 (1.2) | 3.7 (IQR 1.7) | 3.2 (IQR 3.14) | 3.8 ( IQR 1.6) | 4.5/4.0 3.5/3 | 0.024 |
| Total Cholesterol [mg/dL] | 185 (IQR 51) | 183 (IQR 62) | 165 (IQR 60) | 183 (IQR 62) | 190 | ns |
| LDL [mg/dL] | 108.5 (IQR 38.8) | 105.8 (IQR 50.7) | 94.2(IQR 50.7) | 110.2 (IQR 51.4) | 110/90/70/50 * | ns |
| Non-HDL cholesterol [mg/dL] | 127 (IQR 47) | 138 (IQR 53.5) | 111 (IQR 64) | 145 (IQR 52.7) | 145/130/100/100 * | ns |
| HDL [mg/dL] | 55.5 (IQR 25) | 49 (IQR 17) | 52 (IQR 45) | 48 (IQR 19) | 40–50 | 0.025 |
| TG [mg/dL] | 98.5 (IQR 52) | 137 (IQR 77) | 121 (IQR 95) | 160 (IQR 91) | 35–150 | <0.001 |
| Uric Acid [mg/dL] | 5.1 (IQR 1.8) | 5.5 (IQR 2) | 4.6 (IQR 2.8) | 5.3 (IQR 1.6) | 4–5 | ns |
| Biometric indices | ||||||
| BMI [kg/m2] | 25.5 (IQR 6) | 29 (IQR 7.6) | 26 (IQR 5.3) | 29 (IQR 8.2) | 18.5–24.9 | 0.02 |
| Waist circumference [cm] | 90 (IQR 15) | 94 (IQR 22) | 94 (IQR 15.8) | 94 (IQR 16) | 0.044 | |
| Hip circumference [cm] | 95 (IQR 13) | 101 (IQR 17) | 95 (IQR 14) | 102 (IQR 19) | 0.007 | |
| Cytokine profile | ||||||
| IL-18 [pg/mL] | 80 (IQR 68) | 140 (IQR 161.9) | 118( IQR165) | 160 (IQR 137) | <0.001 | |
| IL 17 [pg/mL] | 1 (IQR 0.7) | 1.3 (IQR 1.3) | 1 (IQR 0.6) | 1.13 (IQR 0.8) | ns | |
| Treatment | p Chi2 | |||||
| Methotrexate (15–25 mg per week) | 17 (18%) | 38 (62.3%) | 8 (57%) | 30 (63.8%) | <0.001 | |
| Sulphasalazine (2–3 grams/day) | 20 (21.3%) | 21 (34.4%) | 3 (21.4%) | 18 (38.3%) | ns | |
| Cyclosporine (3–5 mg/kg per day) | 0 | 5 (8.5%) | 0 | 5 (10.6%) | ns | |
| Steroids (the equivalent of 5–15 mg prednisone per day) | 6 (6.51%) | 5 (8.5%) | 2(14.3%) | 3 (6.4%) | ns | |
| NSAIDS constantly | 69 (73.4%) | 23 (37.7%) | 7 (50%) | 16 (34%) | <0.001 | |
| NSAIDS “on demand” | 7 (7.54%) | 17 (27.9%) | 8 (57%) | 9 (19%) | ns | |
| Physical therapy | 23 (24.5%) | 11 (18%) | 2 (14.3%) | 9 (19%) | ns | |
| Cardiovascular Risk Factors | AS n = 94 | Axial PsA n = 14 | Peripheral PsA n = 47 | p chi2 | Post Hoc Tests | ||
|---|---|---|---|---|---|---|---|
| p (AS vs. axPsA) | p (AS vs. perPsA) | p (axPsA vs. perPsA) | |||||
| Smoking more than 1 cigarette per day for 30 days | 43 (45.7%) | 3 (21.4%) | 21 (44.7%) | ns | ns | ns | ns |
| Physical activity more than 120 min twice weekly | 21 (22.3%) | 2 (14.3%) | 11 (23.4%) | ns | ns | ns | ns |
| Hyperuricemia | 13 (13.8%) | 5 (35.7%) | 3 (6.4%) | ns | ns | ns | ns |
| Hypertriglyceridemia | 15 (16%) | 2 (14.3%) | 23 (48.9%) | <0.001 | <0.001 | <0.001 | <0.001 |
| Obesity BMI > 30kg/m2 | 16 (17%) | 3 (21.4%) | 20 (42.6%) | ns | ns | ns | ns |
| Dyslipidemia in patients older than 40 years old | 17 (18.1%) | 3 (21.4%) | 7 (14.9%) | ns | ns | ns | ns |
| Dyslipidemia in patients younger than 40 years old | 24 (25.5%) | 4 (28.6%) | 12 (25.5%) | ns | ns | ns | ns |
| Hypertension | 30 (32%) | 5 (35.7%) | 21 (44.78%) | ns | ns | ns | ns |
| Ischaemic heart disease | 9 (9.6%) | 0 (0.00%) | 14 (29.8%) | 0.002 | 0.05 | 0.05 | 0.049 |
| Model | AICc | Delta AICc | Wi | Adjusted R2 |
|---|---|---|---|---|
| Tender joint count | 596.376 | 0 | 0.01 | 0.593 |
| Tender joint count + TG | 589.325 | 7.051 | 0.32 | 0.566 |
| Tender joint count + TG + IL-17 | 587.842 | 8.534 | 0.67 | 0.597 |
| Coefficient of determination from the regression with confidence intervals | ||||
| Coefficient | B | CI | p | |
| Tender joint count | 0.648 | 269.12 (130:408) | <0.001 | |
| TG | 0.247 | 0.946 (0.299:1.592) | <0.001 | |
| IL-17 | 0.105 | 3.193 (−0.151:6.537) | 0.06 | |
| Model | AICc | Delta AICc | Wi | Adjusted R2 |
|---|---|---|---|---|
| IL-18 | 413.589 | 0 | 0.88 | 0.17 |
| IL-18 + BMI | 408.146 | 5.443 | 0.06 | 0.299 |
| IL-18 + BMI + CRP | 408.091 | 5.498 | 0.06 | 0.3 |
| Coefficient of determination from the regression with confidence intervals | ||||
| Coefficient | B | CI | p | |
| BMI | 0.45 | 8.24 (3.03:13.451) | <0.001 | |
| IL-18 | 0.45 | 0.314 (0.116: 0.511) | <0.001 | |
| CRP | −0.1 | −1.12 (−2.36: 0.334) | 0.109 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonek, K.; Kuca-Warnawin, E.; Kornatka, A.; Zielińska, A.; Wisłowska, M.; Kontny, E.; Głuszko, P. Associations of IL-18 with Altered Cardiovascular Risk Profile in Psoriatic Arthritis and Ankylosing Spondylitis. J. Clin. Med. 2022, 11, 766. https://doi.org/10.3390/jcm11030766
Bonek K, Kuca-Warnawin E, Kornatka A, Zielińska A, Wisłowska M, Kontny E, Głuszko P. Associations of IL-18 with Altered Cardiovascular Risk Profile in Psoriatic Arthritis and Ankylosing Spondylitis. Journal of Clinical Medicine. 2022; 11(3):766. https://doi.org/10.3390/jcm11030766
Chicago/Turabian StyleBonek, Krzysztof, Ewa Kuca-Warnawin, Anna Kornatka, Agnieszka Zielińska, Małgorzata Wisłowska, Ewa Kontny, and Piotr Głuszko. 2022. "Associations of IL-18 with Altered Cardiovascular Risk Profile in Psoriatic Arthritis and Ankylosing Spondylitis" Journal of Clinical Medicine 11, no. 3: 766. https://doi.org/10.3390/jcm11030766
APA StyleBonek, K., Kuca-Warnawin, E., Kornatka, A., Zielińska, A., Wisłowska, M., Kontny, E., & Głuszko, P. (2022). Associations of IL-18 with Altered Cardiovascular Risk Profile in Psoriatic Arthritis and Ankylosing Spondylitis. Journal of Clinical Medicine, 11(3), 766. https://doi.org/10.3390/jcm11030766







