Abstract
Neuronal ceroid lipofuscinoses type 2 (CLN2), the most common form of Batten disease, is caused by TPP1 loss of function, resulting in tripeptidyl peptidase-1 enzyme deficiency and cerebral accumulation of lipopigments. Clinical hallmarks include epileptic seizures, vision loss, progressive movement disorder, ataxia, and eventually death. Diagnosis is often delayed due to the rarity of the conditions. Results: Here, we report a case presenting with clinical features of CLN2, carrying a homozygous novel nonsense variant in TPP1 (NM_000391:c.C832T, (p.Q278*), rs1352347549). Moreover, we performed a comprehensive literature review regarding previously identified disease-causing TPP1 mutations and genotype-phenotype correlations. Conclusion: Depending on the type of mutation, different phenotypes are observed in patients with CLN2, suggesting that the severity of phenotypes is related to the genotype of the patients.
1. Introduction
Neuronal ceroid lipofuscinoses (NCLs) represent a group of rare clinically and genetically heterogeneous progressive neurodegenerative lysosomal storage disorders (LSD), mainly affecting children aged 2–6 years, caused by loss-of-function mutations in CLN-genes. With the exception of CLN4 which is autosomal-dominantly inherited, all other forms follow an autosomal-recessive mode of inheritance [1,2,3]. Children mostly show normal psychomotoric development until progressive development of seizures, vision loss, intellectual and motor decline, cognitive impairment, and eventually premature death. Despite the rarity of this disease, 300–350 cases of NCLs are reported annually in the US, and its global incidence is one per 100,000 live births (orphaned; ORPHA 79264). The highest incidence rates have been reported in some Nordic countries (one per 14,000 in Iceland) [1,4,5], while incidence is unknown for many other countries, including Iran.
The classification of NCLs is based on the defective gene or protein as well as the age of onset of clinical symptoms. To date, more than 537 disease-causing mutations have been published in 13 different genes [6], https://www.ucl.ac.uk/ncl-disease/, accessed on 20 June 2022). Defective genes encode for lysosomal proteins with enzyme function (Palmitoyl-protein thioesterase 1, (CLN1); Tripeptidyl-peptidase 1 (CLN2), Cathepsin D (CLN10), and Cathepsin F (CLN13)); transmembrane lysosomal proteins (Battenin (CLN3) and ATP13A2 (CLN12); soluble lysosomal proteins (CLN5 and CLN11 (Granulin precursor, GRN)); a transmembrane endosomal protein of the major facilitator superfamily domain-containing protein-8 (CLN7); endoplasmic reticulum (ER) transmembrane proteins (CLN6 and CLN8); as well as soluble cytosolic proteins (CLN4 (DNAJC5) and CLN14 (KCTD7)), Figure 1.
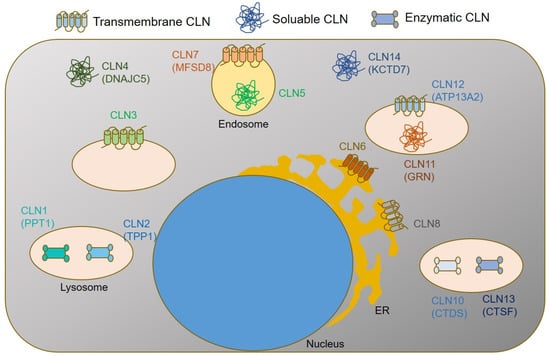
Figure 1.
Simplified graphical summary of subcellular CLN protein localizations and functions.
According to the age of onset, CLNs are classified into six subtypes, including a congenital form (CLN10, MIM610127), an infantile form (CLN1, Santavuori-Haltia disease; MIM256730), a late infantile form (CLN2, Jansky Bielschowsky disease; MIM204500), variable late infantile forms (CLN5, MIM256731; CLN6, MIM601780; CLN7, MIM610951 and CLN8, MIM600143), a juvenile form (CLN3; Spiel-Meyer-Vogt-Sjogren disease; MIM304200), and an adult form (CLN4; Kufs disease; MIM204300) [7,8,9,10,11,12]. Accumulation of mitochondrial ATP synthase subunit C or Saposin A and D (autofluorescent lipopigments) in the lysosomal storage bodies of different cell types, specifically neurons, are the main neuropathological features in NCLs [13,14].
CLN2 (OMIM#607998) is located on the short arm of chromosome 11 (11p15), consisting of 13 exons and 12 introns [5,15,16,17,18]. CLN2 encodes for Tripeptidyl Peptidase 1 (TPP1), a lysosomal aminopeptidase cleaving N-terminal tripeptides from small polypeptides. In humans, this gene encodes a 61-kDa inactive precursor (proenzyme) containing 563 amino acids (aa), including a 368-aa catalytic domain, a 19-aa leader sequence (signal peptide), and a 176-aa prodomain. A signal peptide enables ER-specific localization for cotranslational cleavage in the ER lumen. This is followed by N-glycosylation at the Golgi, enabling proper folding, enhancing protein stability, activity, and intracellular enzyme targeting. Transport to the lysosome is enabled by mannose-6-phosphate receptor binding. At the lysosome, CLN2 is first further processed into a 50 kDa polypeptide followed by cleavage into the mature 368 amino acid (48 kDa) enzyme by lysosomal exoglycosidases such as neuraminidases [19,20,21]. Lysosomal degradation pathways involving TPP1 are summarized in Figure 2.
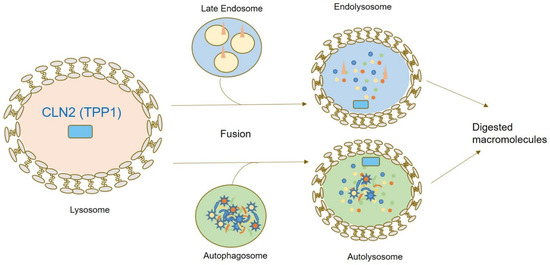
Figure 2.
Graphical summary of CLN2/TPP1 localization and function within the cell. TPP1 represents a peptidase contributing to N-terminal protein degradation. Upon fusion of autophagosomes and late endosomal vesicles with lysosomal vesicles, lysosomal enzymes including TPP1 enable digestion of macromolecules.
Overlapping clinical features and extensive genetic heterogeneity renders next-generation sequencing (NGS) the most efficient and precise genetic diagnostic method if NCL is suspected. In the present study, we describe the identification of a novel homozygous TPP1 variant in an Iranian patient with CLN2 and present an overview of previously published CLN2 disease alleles as well as genotype-phenotype correlations.
2. Materials and Methods
The research ethics committee ethically approved this study of the Mashhad University of medical sciences committee, and all study participants signed the written informed consent (IR.MUMS.REC.1395.534). Genetic diagnostics was performed under the Radboudumc Innovative Genetics Diagnostics program.
2.1. DNA Extraction
Genomic DNA was extracted from the peripheral blood of the proband and his family members using the established salting-out method, and the integrity of the extracted DNA was assessed by gel electrophoresis (1% agarose).
2.2. Whole Exome Sequencing
Two microgrammes of DNA of the proband was subjected to exome capture using Agilent SureSelect Human All Exon V6 Kit (Agilent, Sata Clara, CA, USA) and sequencing performed on an Illumina HiSeq 2500 (Illumina, San Diego, CA, USA) sequencer for an average 50× sequencing depth, resulting in sequences of greater than 100 bases from each end of the fragments (Novogene, Cambridge, UK). GATK-based pipeline [22] was used for variant calling using Burrows–Wheeler alignment [23] to perform sequence alignment to the GRCh37/UCSC hg19 reference genome. SNV were detected using VarScan version 2.2.5, MuTec, Iranome, gnomAD, and Greater Middle East (GME) Variome Project databases were used for population-frequency specific variant filtering.
2.3. Polymerase Chain Reaction (PCR) and Sanger sequencing
We used 1 µL of genomic DNA for PCR reactions. Furthermore, 50 pmol of each forward (5′-GGGATCACTGTGGAGTCAAAG-3′) and reverse (5′-AGACCTGGCTCAGTTCATGC-3′) primers, 10 µL of 2× PCR Master Mix (Sina Clon Cat. No.: MM2062), and 12 µL of distilled water were used in a final volume of 25 µL. PCR reactions were carried out using an initial incubation step at 95 °C for 5 min followed by 35 cycles at 94 °C for 40 s, annealing at 60 °C for 40 s, and extension at 72 °C for 40 s. A final extension step at 72 °C for 10 min was used. We analyzed 5 µL of PCR products using a 2% agarose gel for electrophoresis. Subsequently, the PCR products were purified and sequenced using Sanger sequencing.
2.4. Literature Search
Search terms “TPP1” or “CLN2” were used in PubMed to retrieve relevant human mutation reports and ClinVar and ACMG databases were searched for reported TPP1 alleles.
3. Results
3.1. Clinical Assessment
A 5-year-old patient diagnosed with epilepsy and ataxia was referred to our clinic after a generalized tonic-clonic seizure. The boy was born to a consanguineous Iranian family (parents were first cousins) with an unremarkable family history for neurological disorders; however, the mother experienced two miscarriages due to unknown reasons at 12 and 14 weeks of gestation (Figure 3A).
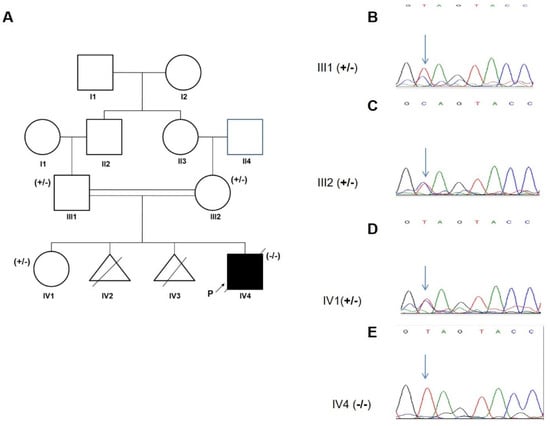
Figure 3.
Family pedigree and segregation pattern. (A) Pedigree of the family; +/−: heterozygous, −/−: homozygous. (B–E) Sanger sequencing chromatograms of the proband, his parents and healthy sister showing the TPP1 c. C832T variant indicated by an arrow.
The patient was born naturally at term with a birth weight of 3.2 kg. Perinatal course and development were normal. Motor and developmental milestones were reached at normal age, for example, free walking at 12 months and talking in sentences at 24 months. At four years of age, a generalized tonic-clonic seizure occurred followed by rapid cognitive and motoric regression, including losing the ability to walk, loss of speech, progressive ataxia, spasticity, and difficulties swallowing. In the last examination, the patient was found to be legally blind. Hematology tests revealed mild anemia (erythroctyes 4.48/µL, hematocrit 32.6%, and hemoglobin 10.6 g/dL). Serum glutamic-oxaloacetic transaminase and alkaline phosphatase were increased (46 mg/dL and 430 U/L, respectively). Magnetic resonance imaging (MRI) findings revealed only mild supratentorial dilation of the ventricular system. There was cerebellar vermian and significant cerebellar hemispheres hypoplasia with prominent 4th ventricle and cistern magna, thinning of superior cerebellar peduncles and cystic lesion of cerebrospinal fluid (CSF) intensity communication with 4th ventricle, and delayed myelinisation of the periventricular white matter and centrum semiovale (Figure 4). The patient underwent chest physiotherapy and received Levetiracetam, Sodium valproate, Nitrazepam, Tetracosactide, Vitamin B6, and Biotin. At the age of 4 years and 8 months, the patient was admitted to the ICU with a GCS of 4.5 and died after 2 months.
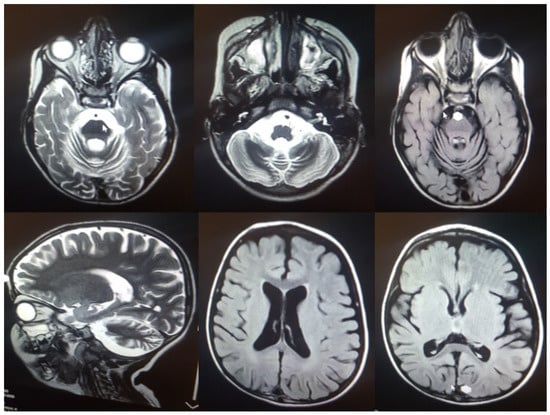
Figure 4.
MRI findings in the index case: Hypoplasia of the cerebellar vermis and cerebellar hemispheres.
3.2. Genetic Analysis
Whole-exome sequencing and analysis revealed a novel homozygous loss of function (LOF) variant in exon 7 of TPP1 (c.C832T: p.Gln278Ter, rs135234754). The allelic frequency of this variant in the gnomAD database is 0.000003977 while no frequency has been reported in ExAC or the 1000 genomes databases. The homozygous variant co-segregated with the disease in family, with both parents and a healthy sibling found to be heterozygous carriers (Figure 3B–E).
4. Discussion
CLN2 is a specific subtype of NCLs caused by TPP1 loss of function, resulting in seizures, vision loss, dementia, cerebellar ataxia, sleep disorders, progressive psychomotor decline, and death in the first decade of life [24]. Early symptoms of CLN2 include seizures and photosensitivity [25]; however, seizures can rarely be absent [3,26]. In line with these findings, our case initially presented with a generalized tonic-clonic seizure. Microcephaly, absent in our case, has additionally been reported in a few cases, including a 9-year-old Australian boy [27] and an 8-year-old boy from Turkey [7], both of whom had non-consanguineous parents. Further common findings include cerebellar atrophy, resulting in ataxia and/or tremor including in our case [7,13,28,29,30,31] (Table 1). Further, vision loss and/or optic atrophy was observed in most patients. Further frequent manifestations include spasticity as well as swallowing and sleeping problems.

Table 1.
Pathogenic TPP1 variants and associated clinical details (literature review).
Based on PubMed publications and submissions in ClinVar, more than 155 potentially disease-associated TPP1 variants have been reported in total (Supplementary Table S1 and Figure 5). TPP1 is required in lysosomes for protein degradation and loss of TPP1 activity results in the accumulation of auto fluorescent lysosomal storage material in various cell types, including neurons [39]. Overall, missense variants are most frequently identified (63, 48%), followed by frameshift (21, 16%), and stop-gain (17, 13%) variants [6,17]. In addition to CLN2, TPP1 dysfunction can also result in autosomal-recessive Spinocerebellar Ataxia 7 (MIM609270) [2,32]. This phenotype is characterized by cerebellar ataxia, tremor, dysarthria, and nystagmus with an age of onset during the first or second life decade. Likely, alleles causing spinocerebellar ataxia result on proteins with some residual function, hence the hypomorphic phenotype in comparison to CLN2 disease alleles with later age of onset and an overall less severe clinical picture [40]. In line with this, missense alleles can be frequently identified in Spinocerebellar Ataxia 7, while approximately 60% of patients with CLN2 carry two common LOF mutations: the splice acceptor site mutation (c.509-1G > C) and the stop-gain mutation in exon 6 (c.622C > T, p.R208X), which may occur homozygously or heterozygously in trans (compound-heterozygously) with other disease alleles [1,8,41]. In the present study, we report a novel homozygous pathogenic stop-gain variant (p.Arg278*) in an Iranian family. This allele results in a truncation within the peptidase S_53 domain. Up to now, 73 TPP1 null variants have been reported in the ClinVar, six of which are found in exon 7.

Figure 5.
Overview of CLN2–causing TPP1 variants at protein levels. Missense mutations are marked in green while red and black indicate nonsense and frameshift mutations, respectively, and blue indicates insertions and deletions. SP: Signal peptide, Ca BS: Ca2+ Binding site.
The role of genetic diagnostics in CLN, especially CLN2, has become more important than ever. In pediatric cases of CLN2, replacement of the dysfunctional TPP1 enzyme with a functional recombinant enzyme (Cerliponase alpha) by intraventricular injection has been reported to effectively delay disease progression and to stabilize the loss of motor and language function [24,41,42]. To achieve the best possible outcomes, an early genetic diagnosis will be essential. For our case, diagnosis came too late for possible interventions.
Further, genetic testing enables prenatal and pre-implantation diagnostics as well as carrier identification, offering novel family planning options.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11216415/s1, Table S1: TPP1 variants identified in CLN2 patients.
Author Contributions
Conceptualization, M.S., D.M.K.-T. and M.N.; data curation, T.B. and M.N.; resources and Investigation, D.M.K.-T. and A.K.; writing, T.B. and M.S.; supervision, M.S., D.M.K.-T. and M.N.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the European Research Council (ERC) (ERC starting grant No. 716344 to MS), the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Project-ID 431984000–SFB 1453 to MS) and under Germany’s Excellence Strategy (CIBSS—EXC-2189—project ID 390939984 to MS).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki DNA samples processed under the Radboudumc Innovative Diagnostics Programme.
Informed Consent Statement
Informed consent was obtained from all subjects or their legal guardians involved in the study.
Data Availability Statement
Sequencing data is available on personal request for reasonable purposes.
Acknowledgments
We are especially grateful to all the participants who took part in this study.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CLN2 | Neuronal Ceroid Lipofuscinoses 2 |
| NMD | Nonsense-Mediated Decay |
| ExAC | Exome Aggregation Consortium |
| ACMG | American College of Medical Genetics and Genomics |
| GCS | Glasgow Coma Scale |
| LOF | Loss of Function |
| PTC | Premature Stop Codon |
References
- Angural, A.; Ponnusamy, K.; Langeh, D.; Kumari, M.; Spolia, A.; Rai, E.; Sharma, S. Missense Variation in TPP1 Gene causes Neuronal Ceroid Lipofuscinosis Type 2 in a Family from Jammu and Kashmir-India. Preprints 2021, 2021070661. [Google Scholar] [CrossRef]
- Chen, Z.-R.; Liu, D.-T.; Meng, H.; Liu, L.; Bian, W.-J.; Liu, X.-R.; Zhu, W.-W.; He, Y.; Wang, J.; Tang, B.; et al. Homozygous missense TPP1 mutation associated with mild late infantile neuronal ceroid lipofuscinosis and the genotype-phenotype correlation. Seizure 2018, 69, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Uygur, O.; Oncel, M.Y.; Gencpinar, P.; Guvenc, M.S.; Dundar, N.O. A Case with Neonatal-onset Type 2 Neuronal Ceroid Lipofuscinosis: A Novel Mutation. J. Coll. Physicians Surg. Pak. 2020, 30, 543–544. [Google Scholar] [CrossRef] [PubMed]
- Haltia, M.; Goebel, H.H. The neuronal ceroid-lipofuscinoses: A historical introduction. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1832, 1795–1800. [Google Scholar] [CrossRef]
- Gardner, E.; Mole, S.E. The Genetic Basis of Phenotypic Heterogeneity in the Neuronal Ceroid Lipofuscinoses. Front. Neurol. 2021, 12, 754045. [Google Scholar] [CrossRef]
- Gardner, E.; Bailey, M.; Schulz, A.; Aristorena, M.; Miller, N.; Mole, S.E. Mutation update: Review of TPP1 gene variants associated with neuronal ceroid lipofuscinosis CLN2 disease. Hum. Mutat. 2019, 40, 1924–1938. [Google Scholar] [CrossRef]
- Kose, M.; Kose, E.; Ünalp, A.; Yılmaz, Ü.; Edizer, S.; Tekin, H.G.; Karaoğlu, P.; Özdemir, T.R.; Er, E.; Onay, H.; et al. Neuronal ceroid lipofuscinosis: Genetic and phenotypic spectrum of 14 patients from Turkey. Neurol. Sci. 2021, 42, 1103–1111. [Google Scholar] [CrossRef]
- Segura, O.E.; Hernández, Z.; Mancilla, N.; Naranjo, R.; Tavera, L. Real world effectiveness of cerliponase alfa in classical and atypical patients. A case series. Mol. Genet. Metab. Rep. 2021, 27, 100718. [Google Scholar] [CrossRef]
- Kurachi, Y.; Oka, A.; Itoh, M.; Mizuguchi, M.; Hayashi, M.; Takashima, S. Distribution and development of CLN2 protein, the late-infantile neuronal ceroid lipofuscinosis gene product. Acta Neuropathol. 2001, 102, 20–26. [Google Scholar] [CrossRef]
- Radwan, A.; Fateen, E.; Gouda, A.; Zaki, M.; Ali, O. Enzyme based diagnosis of type 1 and 2 neuronal ceroid lipofuscinoses. Azhar Int. J. Pharm. Med. Sci. 2021, 1, 40–48. [Google Scholar] [CrossRef]
- Santorelli, F.M.; Garavaglia, B.; Cardona, F.; Nardocci, N.; Bernardina, B.D.; Sartori, S.; Suppiej, A.; Bertini, E.; Claps, D.; Battini, R.; et al. Molecular epidemiology of childhood neuronal ceroid-lipofuscinosis in Italy. Orphanet J. Rare Dis. 2013, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Kohan, R.; Carabelos, M.N.; Xin, W.; Sims, K.; Guelbert, N.; Cismondi, I.A.; Pons, P.; Alonso, G.I.; Troncoso, M.; Witting, S.; et al. Neuronal ceroid lipofuscinosis type CLN2: A new rationale for the construction of phenotypic subgroups based on a survey of 25 cases in South America. Gene 2013, 516, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.-T.; Wang, X.-H.; Ding, C.-H.; Shen, X.; Zhang, H.; Zhang, W.-H.; Li, J.-W.; Ren, C.-H.; Fang, F. Next-Generation Sequencing Analysis Reveals Novel Pathogenic Variants in Four Chinese Siblings with Late-Infantile Neuronal Ceroid Lipofuscinosis. Front. Genet. 2019, 10, 370. [Google Scholar] [CrossRef] [PubMed]
- Kozina, A.A.; Okuneva, E.G.; Baryshnikova, N.V.; Kondakova, O.B.; Nikolaeva, E.A.; Fedoniuk, I.D.; Mikhailova, S.V.; Krasnenko, A.Y.; Stetsenko, I.F.; Plotnikov, N.A.; et al. Neuronal ceroid lipofuscinosis in the Russian population: Two novel mutations and the prevalence of heterozygous carriers. Mol. Genet. Genom. Med. 2020, 8, e1228. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Zhong, R.; Moore, S.; Moroziewicz, D.; Currie, J.R.; Parfrey, P.; Brown, W.T.; Zhong, N. Identification of novel CLN2 mutations shows Canadian specific NCL2 alleles. J. Med. Genet. 2002, 39, 822–825. [Google Scholar] [CrossRef] [PubMed]
- Barisić, N.; Logan, P.; Pikija, S.; Skarpa, D.; Blau, N. R208X mutation in CLN2 gene associated with reduced cerebrospinal fluid pterins in a girl with classic late infantile neuronal ceroid lipofuscinosis. Croat. Med. J. 2003, 44, 489–493. [Google Scholar]
- Goetz, M.; Istrail, S. Alternative Splicing Analysis of Tripeptidyl Peptidase 1 Mutations in CLN2 Batten Disease Models; Presented at the Data Science Initiative; Brown University: Providence, RI, USA, 14 April 2021. [Google Scholar]
- Ma, L.; Prada, A.; Schmidt, M.; Morrow, E.M. Generation of Pathogenic TPP1 Mutations in Human Stem Cells as a Model for CLN2 Disease. Stem Cell Res. 2021, 53, 102323. [Google Scholar] [CrossRef]
- Wujek, P.; Kida, E.; Walus, M.; Wisniewski, K.E.; Golabek, A.A. N-Glycosylation Is Crucial for Folding, Trafficking, and Stability of Human Tripeptidyl-peptidase I. J. Biol. Chem. 2004, 279, 12827–12839. [Google Scholar] [CrossRef]
- Golabek, A.A.; Kida, E. Tripeptidyl-peptidase I in health and disease. Biol. Chem. 2006, 387, 1091–1099. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Chandra, S.; Roy, A.; Dasarathi, S.; Kundu, M.; Pahan, K. Upregulation of tripeptidyl-peptidase 1 by 3-hydroxy-(2, 2)-dimethyl butyrate, a brain endogenous ligand of PPARα: Implications for late-infantile Batten disease therapy. Neurobiol. Dis. 2019, 127, 362–373. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate long-read alignment with Burrows–Wheeler transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, C.M.; Pessoa, A.; Mendes, C.C.; Rivera-Nieto, C.; Vergara, D.; Troncoso, M.; Mole, S.E. Revealing the clinical phenotype of atypical neuronal ceroid lipofuscinosis type 2 disease: Insights from the largest cohort in the world. J. Paediatr. Child Health 2021, 57, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Specchio, N.; Bellusci, M.; Pietrafusa, N.; Trivisano, M.; De Palma, L.; Vigevano, F. Photosensitivity is an early marker of neuronal ceroid lipofuscinosis type 2 disease. Epilepsia 2017, 58, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Liu, X.-M.; Chen, Y.-H.; Zhang, S.-Q.; Wang, K. A novel CLN2/TPP1 mutation in a patient with late infantile neuronal ceroid lipofuscinosis. Neurol. Sci. 2015, 36, 1917–1919. [Google Scholar] [CrossRef] [PubMed]
- Helman, G.; Taylor, L.E.; Walkiewicz, M.; Le Moing, M.; Eggers, S.; Yaplito-Lee, J.; Fuller, M.; Dabscheck, G.; Rodriguez-Casero, V.; White, S.M.; et al. Aberrant splicing and transcriptional activity of TPP1 result in CLN2-like disorder. Eur. J. Med. Genet. 2021, 64, 104259. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.C.; Hsu, C.-J.; Lee, W.-T. Perampanel attenuates myoclonus in a patient with neuronal ceroid lipofuscinoses type 2 disease. Brain Dev. 2019, 41, 817–819. [Google Scholar] [CrossRef]
- Saini, A.G.; Sankhyan, N.; Singhi, P. Chorea in Late-Infantile Neuronal Ceroid Lipofuscinosis: An Atypical Presentation. Pediatr. Neurol. 2016, 60, 75–78. [Google Scholar] [CrossRef]
- Chang, X.; Huang, Y.; Meng, H.; Jiang, Y.; Wu, Y.; Xiong, H.; Wang, S.; Qin, J. Clinical study in Chinese patients with late-infantile form neuronal ceroid lipofuscinoses. Brain Dev. 2012, 34, 739–745. [Google Scholar] [CrossRef]
- Bessa, C.; Teixeira, C.A.; Dias, A.; Alves, M.; Rocha, S.; Lacerda, L.; Ribeiro, M.G. CLN2/TPP1 deficiency: The novel mutation IVS7-10A> G causes intron retention and is associated with a mild disease phenotype. Mol. Genet. Metab. 2008, 93, 66–73. [Google Scholar] [CrossRef]
- Ługowska, A.; Purzycka-Olewiecka, J.K.; Płoski, R.; Truszkowska, G.; Pronicki, M.; Felczak, P.; Bednarska-Makaruk, M. Tripeptidyl Peptidase 1 (TPP1) Deficiency in a 36-Year-Old Patient with Cerebellar-Extrapyramidal Syndrome and Dilated Cardiomyopathy. Life 2021, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.W.; Miu-Kuen Poon, P.; Tong, S.F.; Ko, C.H. Two novel CLN2 gene mutations in a Chinese patient with classical late-infantile neuronal ceroid lipofuscinosis. Am. J. Med. Genet. 2001, 99, 161–163. [Google Scholar] [PubMed]
- Amadori, E.; Scala, M.; Cereda, G.S.; Vari, M.S.; Marchese, F.; Di Pisa, V.; Mancardi, M.M.; Giacomini, T.; Siri, L.; Vercellino, F.; et al. Targeted re-sequencing for early diagnosis of genetic causes of childhood epilepsy: The Italian experience from the ‘beyond epilepsy’ project. Ital. J. Pediatr. 2020, 46, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hartikainen, J.; Ju, W.; Wisniewski, K.E.; Moroziewicz, D.N.; Kaczmarski, A.L.; McLendon, L.; Zhong, D.; Suarez, C.T.; Brown, W.; Zhong, N. Late Infantile Neuronal Ceroid Lipofuscinosis Is Due to Splicing Mutations in the CLN2 Gene. Mol. Genet. Metab. 1999, 67, 162–168. [Google Scholar] [CrossRef]
- Gall, K.; Izzo, E.; Seppälä, E.H.; Alakurtti, K.; Koskinen, L.; Saarinen, I.; Alastalo, T.P. Next-generation sequencing in childhood-onset epilepsies: Diagnostic yield and impact on neuronal ceroid lipofuscinosis type 2 (CLN2) disease diagnosis. PLoS ONE 2021, 16, e0255933. [Google Scholar]
- Elleder, M.; Dvořáková, L.; Stolnaja, L.; Vlášková, H.; Hůlková, H.; Druga, R.; Mikuláštík, J. Atypical CLN2 with later onset and prolonged course: A neuropathologic study showing different sensitivity of neuronal subpopulations to TPP1 deficiency. Acta Neuropathol. 2008, 116, 119–124. [Google Scholar]
- Goldberg-Stern, H.; Halevi, A.; Marom, D.; Straussberg, R.; Bloch, A.M. Late Infantile Neuronal Ceroid Lipofuscinosis: A New Mutation in Arabs. Pediatr. Neurol. 2009, 41, 297–300. [Google Scholar] [CrossRef]
- Johnson, A.M.; Mandelstam, S.; Andrews, I.; Boysen, K.; Yaplito-Lee, J.; Fietz, M.; Ellaway, C. Neuronal ceroid lipofuscinosis type 2: An Australian case series. J. Paediatr. Child Health 2020, 56, 1210–1218. [Google Scholar]
- Sun, Y.; Almomani, R.; Breedveld, G.J.; Santen, G.W.; Aten, E.; Lefeber, D.J.; Maat-Kievit, A.J. Autosomal recessive spinocerebellar ataxia 7 (SCAR 7) is caused by variants in TPP1, the gene involved in classic late-infantile neuronal ceroid lipofuscinosis 2 disease (CLN 2 disease). Hum. Mutat. 2013, 34, 706–713. [Google Scholar] [CrossRef]
- Sheth, J.; Mistri, M.; Bhavsar, R.; Pancholi, D.; Kamate, M.; Gupta, N.; Kabra, M.; Mehta, S.; Nampoothiri, S.; Thakker, A.; et al. Batten disease: Biochemical and molecular characterization revealing novel PPT1 and TPP1 gene mutations in Indian patients. BMC Neurol. 2018, 18, 1. [Google Scholar] [CrossRef]
- Karimi, S.; Rezaei-moghadam, M.; Mosavi, S.A. Juvenile Neuronal Ceroid Lipofuscinosis in a Patient of Iranian Origin. Razavi Int. J. Med. 2018, 6, 42–45. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).