Surgical Management of Adrenocortical Carcinoma: A Literature Review
Abstract
1. Introduction
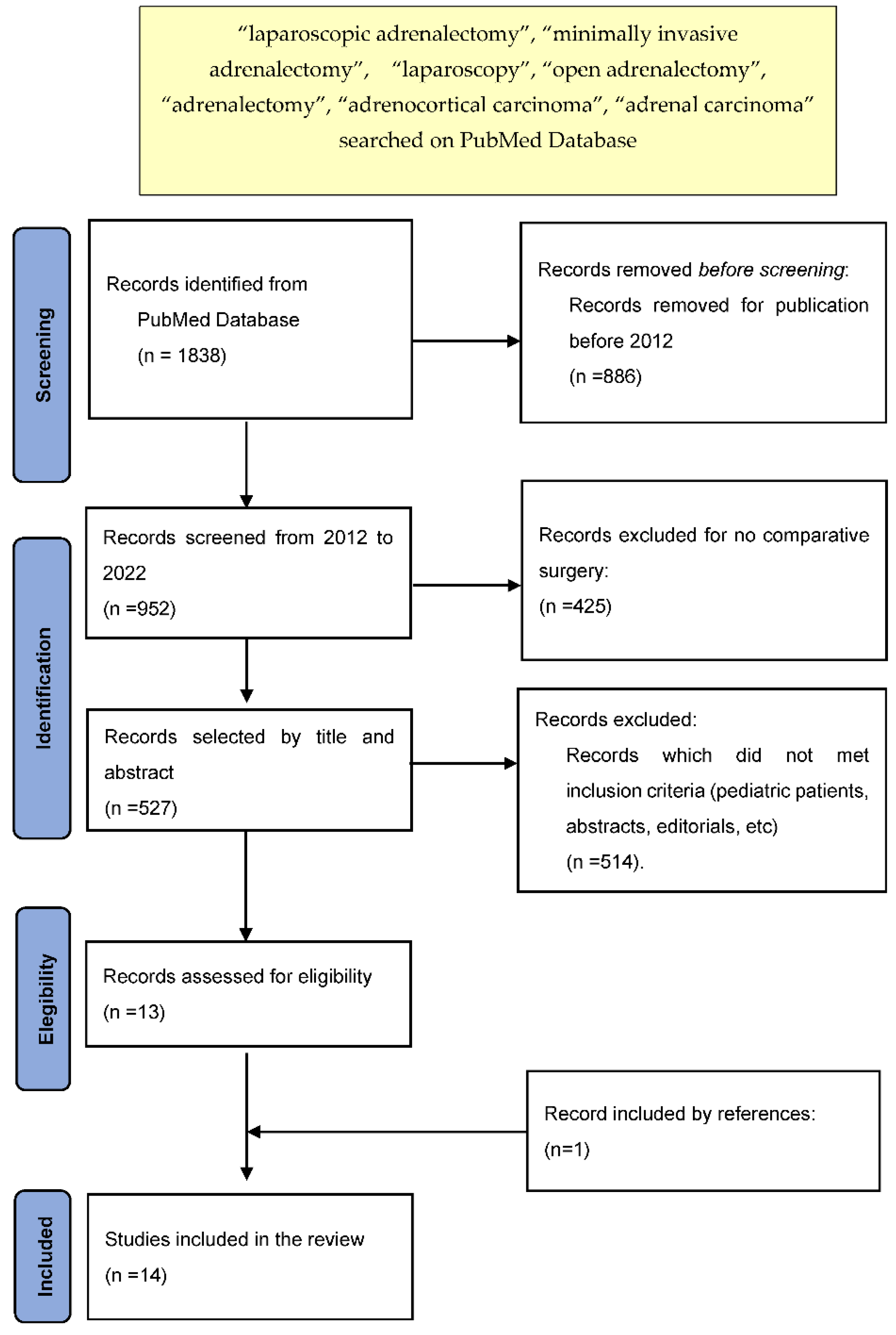
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carnaille, B. Adrenocortical carcinoma: Which surgical approach? Langenbeck’s Arch. Surg. 2012, 397, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Gaujoux, S.; Weinandt, M.; Bonnet, S.; Reslinger, V.; Bertherat, J.; Dousset, B. Surgical treatment of adrenal carcinoma. J. Visc. Surg. 2017, 154, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Winoker, J.S.; Ahlborn, D.T.; Omidele, O.; Fernandez-Ranvier, G.; Derweesh, I.H.; Mehrazin, R. Minimally invasive adrenal surgery: Virtue or vice? Futur. Oncol. 2018, 14, 267–276. [Google Scholar] [CrossRef]
- Gagner, M.; Lacroix, A.; Bolté, E. Laparoscopic Adrenalectomy in Cushing’s Syndrome and Pheochromocytoma. N. Engl. J. Med. 1992, 327, 1033. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, C.P.; Raffaelli, M.; De Crea, C.; Boniardi, M.; De Toma, G.; Marzano, L.A.; Miccoli, P.; Minni, F.; Morino, M.; Pelizzo, M.R.; et al. Open versus endoscopic adrenalectomy in the treatment of localized (stage I/II) adrenocortical carcinoma: Results of a multiinstitutional Italian survey. Surgery 2012, 152, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Donatini, G.; Caïazzo, R.; Cao, C.D.; Aubert, S.; Zerrweck, C.; El-Kathib, Z.; Gauthier, T.; Leteurtre, E.; Wemeau, J.-L.; Vantyghem, M.C.; et al. Long-Term Survival after Adrenalectomy for Stage I/II Adrenocortical Carcinoma (ACC): A Retrospective Comparative Cohort Study of Laparoscopic Versus Open Approach. Ann. Surg. Oncol. 2014, 21, 284–291. [Google Scholar] [CrossRef]
- Wu, K.; Liu, Z.; Liang, J.; Tang, Y.; Zou, Z.; Zhou, C.; Zhang, F.; Lu, Y. Laparoscopic versus open adrenalectomy for localized (stage 1/2) adrenocortical carcinoma: Experience at a single, high-volumecenter. Surgery 2018, 164, 1325–1329. [Google Scholar] [CrossRef]
- Miller, B.S.; Gauger, P.G.; Hammer, G.D.; Doherty, G.M. Resection of adrenocortical carcinoma is less complete and local recurrence occurs sooner and more often after laparoscopic adrenalectomy than after open adrenalectomy. Surgery 2012, 152, 1150–1157. [Google Scholar] [CrossRef]
- Fosså, A.; Røsok, B.I.; Kazaryan, A.M.; Holte, H.J.; Brennhovd, B.; Westerheim, O.; Marangos, I.P.; Edwin, B. Laparoscopic versus open surgery in stage I–III adrenocortical carcinoma–a retrospective comparison of 32 patients. Acta Oncol. 2013, 52, 1771–1777. [Google Scholar] [CrossRef]
- Vanbrugghe, C.; Lowery, A.J.; Golffier, C.; Taieb, D.; Sebag, F. Adrenocortical carcinoma surgery—Surgical extent and approach. Langenbecks Arch. Surg. 2016, 401, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K.T.; Lee, D.Y.; Lau, B.J.; Flaherty, D.C.; Lee, J.; Goldfarb, M. Impact of Laparoscopic Adrenalectomy on Overall Survival in Patients with Nonmetastatic Adrenocortical Carcinoma. J. Am. Coll. Surg. 2016, 223, 485–492. [Google Scholar] [CrossRef]
- Zheng, G.-Y.; Li, H.-Z.; Deng, J.-H.; Zhang, X.-B.; Wu, X.-C. Open adrenalectomy versus laparoscopic adrenalectomy for adrenocortical carcinoma: A retrospective comparative study on short-term oncologic prognosis. OncoTargets Ther. 2018, 11, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Kastelan, D.; Knezevic, N.; Tomsic, K.Z.; Alduk, A.; Kakarigi, L.; Coric, M.; Skoric-Polovina, T.; Solak, M.; Kraljevic, I.; Balasko, A.; et al. Open vs. laparoscopic adrenalectomy for localized adrenocortical carcinoma. Clin. Endocrinol. 2020, 93, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.C.; Klink, J.C.; Guillotreau, J.; Long, J.-A.; Miocinovic, R.; Kaouk, J.H.; Simmons, M.N.; Klein, E.; Krishnamurthi, V.; Campbell, S.C.; et al. Comparative Outcomes of Laparoscopic and Open Adrenalectomy for Adrenocortical Carcinoma: Single, High-Volume Center Experience. Ann. Surg. Oncol. 2013, 20, 1456–1461. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.B.; Habra, M.A.; Grubbs, E.G.; Bednarski, B.K.; Ying, A.K.; Perrier, N.D.; Lee, J.E.; Aloia, T.A. Does laparoscopic adrenalectomy jeopardize oncologic outcomes for patients with adrenocortical carcinoma? Surg. Endosc. 2013, 27, 4026–4032. [Google Scholar] [CrossRef] [PubMed]
- Maurice, M.J.; Bream, M.J.; Kim, S.P.; Abouassaly, R. Surgical quality of minimally invasive adrenalectomy for adrenocortical carcinoma: A contemporary analysis using the National Cancer Database. Br. J. Urol. 2017, 119, 436–443. [Google Scholar] [CrossRef]
- Lee, C.W.; Salem, A.I.; Schneider, D.F.; Leverson, G.E.; Tran, T.B.; Poultsides, G.A.; Postlewait, L.M.; Maithel, S.K.; Wang, T.S.; Hatzaras, I.; et al. Minimally Invasive Resection of Adrenocortical Carcinoma: A Multi-Institutional Study of 201 Patients. J. Gastrointest. Surg. 2017, 21, 352–362. [Google Scholar] [CrossRef]
- Calcatera, N.A.; Hsiung-Wang, C.; Suss, N.R.; Winchester, D.J.; Moo-Young, T.A.; Prinz, R.A. Minimally Invasive Adrenalectomy for Adrenocortical Carcinoma: Five-Year Trends and Predictors of Conversion. World J. Surg. 2018, 42, 473–481. [Google Scholar] [CrossRef]
- Mpaili, E.; Moris, D.; Tsilimigras, D.I.; Oikonomou, D.; Pawlik, T.M.; Schizas, D.; Papalampros, A.; Felekouras, E.; Dimitroulis, D. Laparoscopic Versus Open Adrenalectomy for Localized/Locally Advanced Primary Adrenocortical Carcinoma (ENSAT I–III) in Adults: Is Margin-Free Resection the Key Surgical Factor that Dictates Outcome? A Review of the Literature. J. Laparoendosc. Adv. Surg. Tech. 2018, 28, 408–414. [Google Scholar] [CrossRef]
- Dudley, N.E.; Harrison, B.J. Comparison of open posterior versus transperitoneal laparoscopic adrenalectomy. Br. J. Surg. 1999, 86, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Leboulleux, S.; Déandréis, D.; Al Ghuzlan, A.; Aupérin, A.; Goéré, D.; Dromain, C.; Elias, D.; Caillou, B.; Travagli, J.P.; De Baere, T.; et al. Adrenocortical carcinoma: Is the surgical approach a risk factor of peritoneal carcinomatosis? Eur. J. Endocrinol. 2010, 162, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Autorino, R.; Bove, P.; De Sio, M.; Miano, R.; Micali, S.; Cindolo, L.; Greco, F.; Nicholas, J.; Fiori, C.; Bianchi, G.; et al. Open Versus Laparoscopic Adrenalectomy for Adrenocortical Carcinoma: A Meta-analysis of Surgical and Oncological Outcomes. Ann. Surg. Oncol. 2016, 23, 1195–1202. [Google Scholar] [CrossRef]
- Cavallaro, G.; Tarallo, M.; Chiappini, A.; Crocetti, D.; Polistena, A.; Petramala, L.; Sibio, S.; De Toma, G.; Fiori, E.; Letizia, C. Surgical Management of Adrenocortical Carcinoma: Current Highlights. Biomedicines 2021, 9, 909. [Google Scholar] [CrossRef] [PubMed]
- Buller, D.M.; Hennessey, A.M.; Ristau, B.T. Open versus minimally invasive surgery for suspected adrenocortical carcinoma. Transl. Androl. Urol. 2021, 10, 2246–2263. [Google Scholar] [CrossRef]
- Fassnacht, M.; Dekkers, O.M.; Else, T.; Baudin, E.; Berruti, A.; de Krijger, R.R.; Haak, H.R.; Mihai, R.; Assie, G.; Terzolo, M. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2018, 179, G1–G46. [Google Scholar] [CrossRef]
| Authors | Year of Publication | Patients, n | Surgical Approach, n (%) | ENSAT Stage | Tumor Size (Mean or Median), mm | Conversion, n (%) | R0, % | LND, n (%) | OS, Months or % | DFS, Months |
|---|---|---|---|---|---|---|---|---|---|---|
| Miller et al. [9] | 2012 | 156 | OA 110 (71%) LA 46 (29%) | I–III | OA 120 (range 50–280) MIA 74 (range 32–165) | - | OA 65 MIA 56 | - | II: OA 103 MIA 51 III: OA 44 MIA 28 | - |
| Mir et al. [15] | 2013 | 44 | OA 26 (59%) LA 18 (41%) | I–IV | OA 130 (range 58–218) MIA 70 (range 9–184) | 5 (28%) | OA 61 MIA 61 | OA 14 (54%) MIA 6 (33%) | (OA 54%) (MIA 58%) | OA 14 MIA 10 |
| Cooper et al. [16] | 2013 | 302 | OA 256 (85%) LA 46 (15%) | I–IV | OA 120 (range 40–260) MIA 80 (range 10–150) | 4 (9%) | OA 52 MIA 54 | - | OA 110 MIA 54 | OA 17 MIA 11 |
| Huynh et al. [12] | 2016 | 423 | OA 286 (68%) LA 137 (32%) | I–III | OA 127 (SD ± 71) MIA 80 (SD ± 58) | - | OA 76 MIA 71 | OA 88 (31%) MIA 4 (3%) | - | - |
| Wu et al. [8] | 2018 | 44 | OA 23 (52%) LA 11 (25%) RPSA 10 (23%) | I–II | OA 69 (SD ± 21) MIA 58 (SD ± 19) | 1 (5%) | - | OA 3 (13%) MIA 0 | OA 42 MIA 63 | OA 22 MIA 25 |
| Zheng et al. [13] | 2018 | 42 | OA 22 (52%) LA 20 (48%) | I–III | OA 101 (SD ± 36) MIA 63 (SD ± 22) | 0 | OA 100 MIA 100 | - | - | OA 45 MIA 17 |
| Year of Publication | Patients, n | Surgical Approach n (%) | ENSAT Stage | Tumor Size (Mean or Median), mm | Conversion, n (%) | R0, % | LND, n (%) | OS, Months or % | DFS, Months or % | |
|---|---|---|---|---|---|---|---|---|---|---|
| Lombardi et al. [6] | 2012 | 156 | OA 126 (81%) LA 29 (19%) RPSA 1 (1%) | I–II | OA 90 (SD ± 46) MIA 77 (SD ± 34) | 0 | OA 100 MIA 100 | OA 23 (18%) MIA 1 (3%) | OA 60 MIA 108 | OA 48 MIA 72 |
| Fossa et al. [10] | 2013 | 32 | OA 15 (47%) LA 17 (53%) | I–III | OA 130 (range 60–140) MIA 80 (range 42–160) | 2 (12%) | OA 80 MIA 71 | - | OA 36 MIA 103 | OA 8 MIA 15 |
| Donatini et al. [7] | 2014 | 34 | OA 21 (62%) LA 13 (38%) | I–II | OA 68 (range 45–90) MIA 55 (range 22–80) | 0 | OA 100 MIA 100 | - | (OA 81%) (MIA 85%) | OA 47 MIA 46 |
| Vanbrugghe et al. [11] | 2016 | 25 | OA 9 (36%) LA 16 (64%) | I–III | OA 116 (range 12–200) MIA 62 (range 38–80) | 0 | OA 100 MIA 75 | - | (OA 89%) (MIA 69%) | (OA 63%) (MIA 56%) |
| Maurice et al. [17] | 2017 | 481 | OA 320 (67%) LA 130 (27%) RA 31 (6%) | I–IV | OA 117 (IQR 85–160) MIA 75 (IQR 52–98) | 24 (15%) | OA 83 MIA 80 | OA 42 (13%) MIA 2 (1%) | (OA 62%) (MIA 58%) | - |
| Lee et al. [18] | 2017 | 201 | OA 154 (77%) LA 44 (22%) RPSA 1 (1%) RA 2 (1%) | I–IV | OA 109 (range 37–300) MIA 55 (range 30–160) | 9 (19%) | OA 74 MIA 77 | OA 63 (41%) 0 | OA 54 MIA 91 | OA 10 MIA 14 |
| Calcatera et al. [19] | 2018 | 588 | OA 388 (66%) LA 151 (26%) RA 49 (8%) | I–IV | OA 124 (SD ± 69) MIA 89 (SD ± 62) | 38 (19%) | OA 75 MIA 71 | - | OA 55 MIA 53 | - |
| Kastelan et al. [14] | 2020 | 46 | OA 23 (50%) LA 23 (50%) | I–III | OA 120 (range 70–250) MIA 75 (range 26–110) | 0 | OA 100 MIA 100 | - | OA 149 MIA 109 | OA 129 MIA 109 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, L.; Becucci, C.; Ambrosini, C.E.; Puccini, M.; Vasquez, M.C.; Gjeloshi, B.; Materazzi, G. Surgical Management of Adrenocortical Carcinoma: A Literature Review. J. Clin. Med. 2022, 11, 5754. https://doi.org/10.3390/jcm11195754
Rossi L, Becucci C, Ambrosini CE, Puccini M, Vasquez MC, Gjeloshi B, Materazzi G. Surgical Management of Adrenocortical Carcinoma: A Literature Review. Journal of Clinical Medicine. 2022; 11(19):5754. https://doi.org/10.3390/jcm11195754
Chicago/Turabian StyleRossi, Leonardo, Chiara Becucci, Carlo Enrico Ambrosini, Marco Puccini, Malince Chicas Vasquez, Benard Gjeloshi, and Gabriele Materazzi. 2022. "Surgical Management of Adrenocortical Carcinoma: A Literature Review" Journal of Clinical Medicine 11, no. 19: 5754. https://doi.org/10.3390/jcm11195754
APA StyleRossi, L., Becucci, C., Ambrosini, C. E., Puccini, M., Vasquez, M. C., Gjeloshi, B., & Materazzi, G. (2022). Surgical Management of Adrenocortical Carcinoma: A Literature Review. Journal of Clinical Medicine, 11(19), 5754. https://doi.org/10.3390/jcm11195754






