Abstract
The purpose of this study was to provide an estimate of the number of current and future patients with polypoidal choroidal vasculopathy (PCV) in Europe. We systematically searched 11 literature databases on 18 May 2022 for studies on the prevalence of PCV among a consecutive and representative group of patients with suspected neovascular age-related macular degeneration (AMD). Prevalence of PCV in patients with suspected neovascular AMD was summarized and included in a prevalence meta-analysis. We then used current population data and population forecasts by Eurostat and the Office for National Statistics to determine current and future number of patients with neovascular AMD in Europe. Then, we calculated the number of patients with PCV with our calculated estimate of the prevalence of PCV among Europeans suspected with neovascular AMD. A total of five eligible studies were identified which included a total of 1359 patients. All these studies used the gold standard of indocyanine green angiography as a routine part of their diagnostic approach. Among patients undergoing detailed retinal examination for suspected neovascular AMD, our meta-analysis calculated the prevalence of PCV to be 8.3% (95% confidence interval: 6.8–9.8%). Our population estimates find that a total of 217,404 patients with PCV exist in Europe in the year 2022, which constitutes 0.04% of the entire population of Europe. This number is estimated to increase to 287,517 patients in the year 2040. Our estimates are important for different healthcare stakeholders, especially when planning and allocating expensive resources.
1. Introduction
Polypoidal choroidal vasculopathy (PCV) was originally described by Yannuzzi in 1982 as a variant of exudative age-related macular degeneration (AMD) [1]. Since then, numerous clinical reports have described this condition in more detail [2,3,4,5]. PCV is a chorioretinal disease with vascular aneurysmal polyp-like lesions with or without an associated branching vascular network, most probably originating from the inner choroid [6]. This vasculopathy leads to protrusion through Bruch’s membrane to the sub-retinal pigment epithelium (RPE) space with exudation into the subretinal space and the neuroretina. The term ‘polyp’ is actually a misnomer, as the polypoidal lesions may be an aneurysmal dilation of the neovascular network [6]. Hence, some authors advocate the use of the term ‘aneurysmal type 1 neovascularization’ [6]. PCV may encompass a spectrum of clinical and pathophysiological subtypes [7,8]. Van Dijk et al. have described three clinical subtypes of PCV: type A PCV (PCV-AMD), which is phenotypically and presumably pathophysiologically more associated with neovascular AMD and drusen; type B PCV, in which PCV is associated with a branching vascular network of non-PCV neovascularization but without drusen (PCV-BVN); and type C PCV, in which patients have a polyp-like lesion without a branching vascular network and without associated signs of AMD such as drusen [7]. Whereas type A PCV, similar to AMD, appears to be associated with a normal to thin choroid, a sizeable subgroup of types B and C may be associated with a normal to thick choroid (pachychoroid) [7]. Many cases also present with subretinal hemorrhage [6]. The gold standard of PCV diagnosis includes indocyanine green angiography (ICGA), which can reveal sub-RPE structures in detail, reveal polyp-like choroidal vascular lesions, and in many cases, an associated branching vascular network [9]. Patients with PCV who do not receive treatment are at risk of fibrovascular scarring and damage to the neuroretina with potentially severe visual impairment [10,11]. Treatment with intravitreal injections of anti-vascular endothelium growth factor (anti-VEGF) medication and photodynamic therapy (PDT) have been found to lead to reduced exudation and closure of polyp-like vascular lesions, which often preserve relatively good vision [12,13,14,15,16].
The pathophysiology of PCV remains incompletely understood. Some forms of PCV have been linked to venous stasis in the choroid with secondary choroidal anastomoses and choroidal vascular remodeling [17]. In line with this hypothesis, some patients with PCV have pachychoroid features upon choroidal imaging and often present without drusen maculopathy [18,19]. This is also supported by studies in which patients with PCV were shown not to have age-related immunological changes that are otherwise associated with AMD [20,21]. On the other hand, another subgroup of PCV patients (e.g., type A PCV according to Van Dijk et al.) may have a pathophysiology that is more similar to that of AMD [22].
Studies on PCV, especially epidemiological population-based and registry-based studies, have been challenged by the lack of a separate International Classification of Diseases (ICD) diagnosis code for the disease. There are currently no good estimates of the prevalence of PCV, which is further challenged by the fact that the disease in Europe is often not recognized. Approximately half of patients with suspected neovascular AMD are diagnosed with PCV in Asians, whereas this is only 8–9% among whites [19,23]. The unknown estimate of the disease burden is a challenge for various reasons, e.g., when planning national health service or when applying for funding for PCV research, as it is not clear how many patients suffer from this disease. Further, the lack of an estimation of the disease burden can also hinder further political attention to the worldwide shortage of verteporfin (Visudyne®, Cheplapharm Arzneimittel GmbH, Greifswald, Germany) which is used for PDT [24], and therefore, to a large extent, currently unavailable for patients with PCV [25].
In this study, we address this issue by providing the first European prevalence estimate of PCV. Since no population-based prevalence estimate of PCV exists, our approach is to systematically review the literature on the prevalence of PCV among European patients suspected of neovascular AMD and calculate a summary estimate using a prevalence meta-analysis. We then apply this estimate to the best current estimate of neovascular AMD in Europe, and to the most likely scenario of population forecast of European countries as provided by the Eurostat and the Office for National Statistics.
2. Materials and Methods
2.1. Study Design
This study consisted of three stages. The first stage was a systematic review and meta-analysis of the prevalence of PCV in patients suspected with neovascular AMD. The second stage was to use age-stratified prevalence estimates of neovascular AMD and apply them to current age-stratified population statistics of countries in Europe and to similar age-stratified estimated future population statistics of countries in Europe. In the third and final stage of this study, we used our calculated prevalence estimate of PCV in patients suspected with neovascular AMD in a European population and applied this estimate on the current and future prevalence estimates of neovascular AMD in Europe. The systematic review was reported according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the protocol was registered a priori in the PROSPERO database (no. CRD42022334049). According to Danish and Dutch law, institutional review board approval is not relevant for systematic reviews nor for forecasting studies of publicly available population data nor future estimates.
2.2. Eligibility Criteria
Eligible studies were defined as those which evaluated the prevalence of PCV in patients suspected with neovascular AMD. The study had to be performed in an European population. We did not restrict diagnostic modalities employed for diagnosing PCV or neovascular AMD, nor restricted diagnostic criteria for PCV or neovascular AMD, but noted the author’s definitions of these aspects. For a study to be eligible, we required that data on PCV should be reported on the patient level. The population had to be representative of the broad population of patients with neovascular AMD and not pre-selected for a certain reason, e.g., poor-responders on anti-VEGF therapy or only type 1 macular neovascularization. Studies were expected to be observational in nature, but we did not restrict on this definition and allowed relevant data from any study design. Single case studies, publications without original data, conference abstracts, or animal studies were not considered eligible. For practical purposes, we only considered studies disseminated in the English language.
2.3. Information Sources, Literature Search, and Study Selection
We searched the literature databases PubMed, EMBASE, Web of Science Core Collection, BIOSIS Previews, Current Contents Connect, Data Citation Index, Derwent Innovations Index, KCI-Korean Journal Database, SciELO Citation Index, and the Cochrane Central. One trained author (Y.S.) conducted the search on 18 May 2022. Details of the search phrases tailored to the individual literature databases are available in Supplementary File S1. One author (Y.S.) examined the title and abstract of all identified records and removed duplicates and those deemed obviously irrelevant. Remaining references were retrieved in full text for evaluation of eligibility. Two authors (J.H. and J.M.E.L.) independently examined these full text studies as well as references from these studies for any additional relevant studies. Disagreements between the authors were discussed and in the lack of consensus, a third author (Y.S.) made the final decision.
2.4. Data Collection Process and Risk of Bias of Individual Studies
Data on study characteristics, population characteristics, methods for diagnosis, and results were extracted from each study using pre-designed data extraction forms. We anticipated that most studies would be cross-sectionally designed and therefore evaluated risk of bias of individual studies using the relevant items from the Agency for Healthcare Research and Quality (AHRQ) checklist for Cross-Sectional Studies (Questions 1–4 and 6), which is the recommended tool for evaluating cross-sectional studies [26]. Two authors (J.H. and J.M.E.L.) independently extracted data and evaluated risk of bias of individual studies. Disagreements between the authors were discussed and if consensus could not be reached, a third author (Y.S.) made the final decision.
2.5. Outcomes and Summary Measures, Synthesis of Results, and Risk of Bias across Studies
The primary outcome measure was the prevalence of PCV in eyes suspected with neovascular AMD. Our unit of analysis was per patient since this is also the unit of analysis for patient prevalence in the following steps of our study. Meta-analysis was performed using MetaXL 5.3 (EpiGear International, Sunrise Beach, QLD, Australia) for Microsoft Excel 2013 (Microsoft, Redmont, WA, USA). The random-effects model was employed to account for potential heterogeneity across studies. Caution must be exercised in prevalence meta-analyses when a number reaches the extremes (i.e., 0% or 100%) since this can result in variance instability and erroneous weighting of studies [27]. To accommodate to this potential issue, all prevalence numbers were transformed for analysis using the double arcsine method and were then back transformed for interpretation [27]. Heterogeneity was evaluated using the Cochran’s Q and I2 [28]. A Funnel plot was used to evaluate any skewed results and publication bias [29]. The final summary measure was the prevalence estimate of PCV. Sensitivity analysis was conducted by removing each study in turn and re-calculating the summary measure to evaluate the magnitude of the change in the results.
2.6. Prevalence Estimation and Forecasting Analysis
Li et al. estimated the prevalence of AMD in Europe in a systematic review and meta-analysis [30]. Based on their meta-analysis on 55,323 European individuals, the authors calculated the prevalence of neovascular AMD to 0.1% (95% confidence interval (CI): 0.1 to 0.3%) for individuals aged ≤64 years, 0.8% (95% CI: 0.6 to 1.0%) for individuals aged 65–74 years, and 3.3% (95% CI: 2.5 to 4.2%) for individuals aged ≥75 years. These prevalence estimates represent, to our knowledge, the best current and highest level of evidence on the prevalence of neovascular AMD in Europe. These estimates were used for the following steps in our study.
We extracted publicly available data on country population statistics and the most likely population project scenario from Eurostat (Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Liechtenstein, Lithuania, Luxembourg, Malta, the Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, Switzerland) and from the Office for National Statistics (United Kingdom defined as the combination of England, Northern Ireland, Wales, and Scotland). Countries included from this approach include almost the entirety of Europe by various geographical and political definitions.
Population data was stratified according to individuals aged ≤64 years, 65–74 years, and ≥75 years and summarized in Supplementary Data S1. We used these age stratified data to calculate the current and estimated future number of patients with neovascular AMD in Europe. Afterwards, we used our calculated prevalence summary estimate to calculate the proportion of these patients with neovascular AMD, which can be assumed to have PCV upon further examination. Then, we could estimate the prevalence of PCV.
3. Results
3.1. Literature Search and Study Selection
Our literature search identified a total of 744 records. We then discarded duplicates (n = 280) and records obviously irrelevant (n = 453). The remaining 11 records were evaluated in full text. Of these, six records were excluded as they did not fulfill our eligibility criteria (Figure 1), and we included five studies for qualitative and quantitative review.
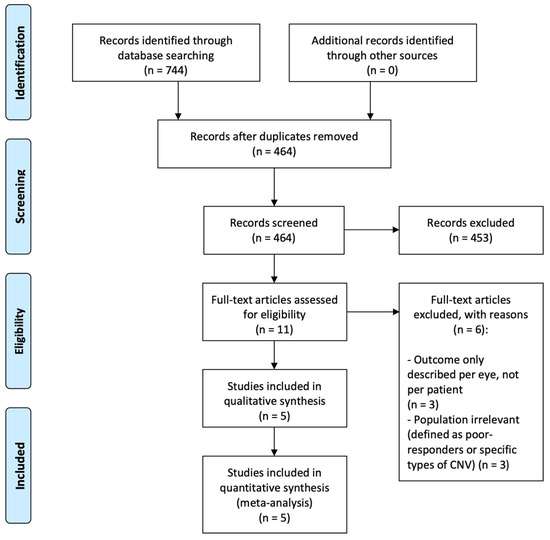
Figure 1.
PRISMA flow diagram of study selection.
3.2. Study and Population Characteristics
The five eligible studies for review included a total of 1359 patients [10,31,32,33,34]. All studies were retrospective in nature, cross-sectionally designed, and performed in a single center. Studies originated from Denmark (n = 2), Greece (n = 1), Italy (n = 1) and the United Kingdom (n = 1). All studies described that the patients underwent fundus examination, fluorescein angiography, and ICGA. Two studies also described optical coherence tomography as part of their examination [10,30]. Details regarding the study characteristics and eligibility criteria are outlined in Table 1.

Table 1.
Study characteristics of eligible studies.
Population demographics were similar across groups as well as across studies (Table 2). The mean age ranged between 70–77 years in patients with PCV and between 73–79 years in patients with neovascular AMD. Females constituted between 41–65% of patients with PCV and 49–68% of patients with neovascular AMD. Diagnostic definitions of PCV were described in detail in four studies [10,32,33,34] and one study simply described classical findings on ICGA and referred to the early studies of Yannuzzi for details [31]. Diagnostic definition of neovascular AMD was only described in one study [10].

Table 2.
Population characteristics of eligible studies.
3.3. Results and Risk of Bias of Individual Studies
Ilginis et al. found that 8% of patients with suspected neovascular AMD were found to actually have PCV upon further examination in a Scandinavian population [31]. Another Scandinavian population was described by Lorentzen et al., who reported that 6% of their patients with suspected neovascular AMD had PCV [10]. This study also reported that the majority of cases were hemorrhagic at presentation [10]. Yadav et al. reported on their findings from United Kingdom, where PCV was identified in 9% of the patients [34]. The authors also reported that PCV was only found in eyes with type 1 macular neovascularization and that PCV constituted 22% of such type 1 macular neovascularization [34]. Ladas et al. and Scassellati-Sforzolini et al. reported the prevalence in Southern European patient populations [32,33]. In Greece, the prevalence of PCV was 8%, was located mostly in the peripapillary area, and fellow eyes were less likely to have large drusen (20%) when compared to eyes diagnosed with neovascular AMD (81%) [32]. In Italy, similar patterns were reported of PCV having a higher incidence of extrafoveal presentation and fewer drusen in the fellow eye when compared to patients with neovascular AMD [33].
Risk of bias evaluation of individual studies showed that all studies clearly defined the source of data and performed consecutive recruitment, and that most studies clearly defined eligibility criteria, time period of participant recruitment/eligibility, and quality assurance protocol. Exclusions were explained clearly in one study, and not clearly in two studies, and not explained in two studies. Details of the risk of bias of individual studies are listed in Table 3.

Table 3.
Risk of bias within individual studies included in the review.
3.4. Synthesis of Results and Risk of Bias across Studies
Synthesis of results using the random-effects model showed a pooled prevalence of PCV in patients suspected with neovascular AMD of 8.3% (95% confidence interval: 6.8–9.8%) (Table 4). Heterogeneity across studies was insignificant and quantified as I2 = 0.2 and Cochran’s Q = 4.0. The Funnel plot did not suggest a significant presence of risk of bias across studies (Supplementary Figure S1). The sensitivity analysis showed robustness of the summary estimate as excluding studies in turn only led to minor changes; the summary estimate only varied between 7.7 and 9.0% (Supplementary Table S1).

Table 4.
Meta-analysis of the prevalence of polypoidal choroidal vasculopathy in patients suspected with neovascular age-related macular degeneration.
3.5. Estimated Current and Future Number of Patients with PCV in Europe
We extracted statistics on country-specific population and forecast of population development for years 2022, 2025, 2030, 2035, and 2040 within an age strata of ≤ 64 years, 65–74 years, and ≥75 years (Supplementary Data S1). These numbers were multiplied with the prevalence of neovascular AMD within each age stratum as estimated by Li et al. [24]. The resulting estimate of current and future numbers of patients with neovascular AMD are summarized in Supplementary Data S2. Briefly, we estimate that 2.6 million individuals in Europe have neovascular AMD in 2022 and that this number is expected to increase gradually to 2.8 million, 3.0 million, 3.2 million, and 3.5 million, respectively, in the years 2025, 2030, 2035, and 2040. Based on these numbers, and our calculated estimate that 8.3% (95% confidence interval: 6.8–9.8%) of patients with neovascular AMD upon further examination will have PCV, we were able to calculate that 217,404 (95% confidence interval: 178,114–256,694) individuals have PCV in Europe in 2022. This number is expected to gradually increase to 287,517 (95% confidence interval 235,556–339,478) in the year 2040. Country-specific and total prevalence estimates of PCV as well as estimated change rates over time are all summarized in Table 5.

Table 5.
Estimated current and future prevalence of polypoidal choroidal vasculopathy in Europe.
4. Discussion
In this systematic review and meta-analysis of a total of 1359 European patients with suspected neovascular AMD, the prevalence of PCV is estimated to be 8.3% (95% confidence interval: 6.8–9.8%). Based on this estimate, and our estimate of neovascular AMD in Europe, we calculate that there are approximately 220,000 patients with PCV in Europe today, a number expected to grow to approximately 300,000 in 2040. Although this number is much smaller than that of neovascular AMD [30], or other highly prevalent ophthalmic diseases in Europe [35], this is still a significant number of patients also in terms of disease and treatment burden, constituting 0.04% of the entire population of 531 million Europeans. These estimates are important for different healthcare stakeholders, especially when planning and allocating resources.
Moreover, the current study addresses the importance on the early diagnosis of PCV, as misdiagnosis can negatively affect the prognosis, and tailored treatment—which usually consists of a combination of PDT and anti-VEGF injections—is therefore required in PCV. Ophthalmologists should have a high index of suspicion and low threshold to perform additional imaging such as combined fluorescein angiography/ICGA in the case of signs suggestive of PCV. Such signs include a pink-orange subretinal nodular lesion on fundoscopy; a peaked RPE elevation on optical coherence tomography scanning, often with a hyperreflective subretinal accumulation beneath it; and either non-response or partial response to anti-VEGF treatment in patients with a neovascularization without evidence of another diagnosis other than AMD [8,36].
Previous studies suggest that white patients with PCV are on average 3.7 years younger than patients with neovascular AMD and that no significant gender difference exists, which could have led to a source of bias in the current study [19]. In particular, the increase in the number of patients with neovascular AMD is largely attributed to the growth of number of elderly individuals in the population [37,38,39]. If PCV pathogenesis is different from neovascular AMD [19,20,21], one can question if the number of patients with PCV will increase in a similar fashion to that of neovascular AMD. This could affect the accuracy of our forecast, also taking the three PCV subtypes into account that have been recently described. The number of type A PCV patients, which present with clinical features that are similar to neovascular AMD, may increase more than numbers of type B PCV and type C PCV patients [7].
Our study has several limitations. First, we rely our estimate on studies of entirely or almost entirely white populations, whereas immigration to Europe leads to an increase in the number of individuals with different ethnicities. This means that as the immigrants grow older, our estimates become less accurate. Second, our estimates are based on the current best forecast from the Eurostat and the Office for National Statistics. Unforeseen developments in economy, politics, immigration, emigration, and death may change the population numbers towards 2040. Thus, the estimates of 2022 can be considered more reliable than those of 2040. Third, we assume that the prevalence of PCV in patients suspected of nAMD will remain constant in future. These are assumptions that may or may not hold true; however, without such assumptions our calculations would not be possible. Finally, our estimates are only as accurate as the studies that we could use for data analysis. Our risk of bias within individual studies did not identify important sources of bias in the study design. Our Funnel plot was not suggestive of a strong risk of bias across studies. However, variation in the definition of PCV, referral pattern differences, poor image quality, and media opacity may all influence the prevalence estimates of the individual studies. Indeed, most of the studies in the meta-analysis include patient cohorts from tertiary referral centers. One can argue that referral pattern differences may be prone to a different prevalence of PCV when examined in tertiary centers. For example, an OCT appearance of type 1 CNV may lead a referral to a tertiary center as the referring ophthalmologist may argue that an ICGA may be needed for further examination. Thus, the proportion of PCV patients may be higher in tertiary referral centers than in the regular population. Calculating a population-wide prevalence from data from tertiary referral center studies possesses the risk of an overestimation of the actual PCV prevalence.
Taken together, we conclude that this study presents the first European prevalence estimate of PCV. Based on our study, we estimate that there are currently approximately 220,000 patients with PCV in Europe, which constitute 0.04% of the entire population of Europe. The number of patients is expected to grow at a rate of 1.6% per year.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11164766/s1, Supplementary File S1: Details of the literature search; Supplementary Data S1: Population statistics and forecasting of current and future number of individuals aged ≤ 64 years, 65–74 years, and ≥ 75 years in European countries; Supplementary Data S2: Estimated current and future number of patients with neovascular age-related macular degeneration in European countries; Supplementary Figure S1: Funnel plot for the evaluation of risk of bias across studies; Supplementary Table S1: Sensitivity analysis of the summary estimate.
Author Contributions
Conceptualization, E.H.C.v.D. and Y.S.; methodology, Y.S.; software, Y.S.; formal analysis, J.K.H., J.M.E.L., and Y.S.; resources, Y.S.; writing—original draft preparation, Y.S.; writing—review and editing, E.H.C.v.D., J.K.H., M.J.S., J.M.E.L., R.M.H.D., R.O.S., C.J.F.B. and Y.S.; supervision, Y.S.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from the Foundation MB.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Author Y.S. declares to have received a speaker’s fee from Bayer and Roche, and to be the inventor of a patent related to biomarkers of polypoidal choroidal vasculopathy (patent no. DK179993B1, https://patents.google.com/patent/DK179993B1/en?oq=DK179993B1, accessed on 12 August 2022), all of which are not directly related to this work. Other authors declare that no potential conflicts of interests exist in relation to this work.
References
- Yannuzzi, L.A. Idiopathic polypoidal choroidal vasculopathy. In Proceedings of the Macula Society Meeting, Miami, FL, USA, 5 February 1982. [Google Scholar]
- Yannuzzi, L.A.; Sorenson, J.; Spaide, R.F.; Lipson, B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina 1990, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yannuzzi, L.A.; Wong, D.W.; Sforzolini, B.S.; Goldbaum, M.; Spaide, R.F.; Freund, K.B.; Slakter, J.S.; Guyer, D.R.; Sorenson, J.A.; Fisher, Y.; et al. Polypoidal choroidal vasculopathy and neovascularized age-related macular degeneration. Arch. Ophthalmol. 1999, 117, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Yannuzzi, L.A.; Ciardella, A.; Spaide, R.F.; Rabb, M.; Freund, K.B.; Orlock, D.A. The expanding clinical spectrum of idiopathic polypoidal choroidal vasculopathy. Arch. Ophthalmol. 1997, 115, 478–485. [Google Scholar] [CrossRef]
- Iijima, H.; Imai, M.; Gohdo, T.; Tsukahara, S. Optical coherence tomography of idiopathic polypoidal choroidal vasculopathy. Am. J. Ophthalmol. 1999, 127, 301–305. [Google Scholar] [CrossRef]
- Dansingani, K.K.; Gal-Or, O.; Sadda, S.R.; Yannuzzi, L.A.; Freund, B.K. Understanding aneurysmal type 1 neovascularization (polypoidal choroidal vasculopathy): A lesson in the taxonomy of ‘expanded spectra’—A review. Clin. Exp. Ophthalmol. 2018, 46, 189–200. [Google Scholar] [CrossRef]
- van Dijk, E.H.C.; Mohabati, D.; Veselinovic, S.; Chung, W.H.; Dijkman, G.; Boon, C.J.F. The spectrum of polypoidal choroidal vasculopathy in Caucasians: Clinical characteristics and proposal of a classification. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 351–361. [Google Scholar] [CrossRef]
- Coscas, G.; Lupidi, M.; Coscas, F.; Benjelloun, F.; Zerbib, J.; Dirani, A.; Semoun, O.; Souied, E.H. Toward a specific classification of polypoidal choroidal vasculopathy: Idiopathic disease or subtype of age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2015, 56, 3187–3195. [Google Scholar] [CrossRef]
- Koh, A.H.; Chen, L.J.; Chen, S.J.; Chen, Y.; Giridhar, A.; Iida, T.; Kim, H.; Lai, T.; Lee, W.K.; Li, X.; et al. Polypoidal choroidal vasculopathy: Evidence-based guidelines for clinical diagnosis and treatment. Retina 2013, 33, 686–716. [Google Scholar] [CrossRef]
- Lorentzen, T.D.; Subhi, Y.; Sørensen, T.L. Presenting characteristics and prevalence of polypoidal choroidal vasculopathy in Scandinavian patients with treatment-naïve exudative age-related macular degeneration. Acta Ophthalmol. 2018, 96, 475–480. [Google Scholar] [CrossRef]
- Cheung, C.M.; Yang, E.; Lee, W.K.; Lee, G.K.; Mathur, R.; Cheng, J.; Wong, D.; Wong, T.Y.; Lai, T.Y. The natural history of polypoidal choroidal vasculopathy: A multi-center series of untreated Asian patients. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 2075–2085. [Google Scholar] [CrossRef]
- Subhi, Y.; Sørensen, T.L. Valsalva-Related Subretinal Hemorrhage as a Presenting Symptom of Polypoidal Choroidal Vasculopathy. Case Rep. Ophthalmol. Med. 2017, 2017, 9650287. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.H.; van Dijk, E.H.; Mohabati, D.; Dijkman, G.; Yzer, S.; de Jong, E.K.; Fauser, S.; Schlingemann, R.O.; Hoyng, C.B.; Boon, C.J. Neovascular age-related macular degeneration without drusen in the fellow eye: Clinical spectrum and therapeutic outcome. Clin. Ophthalmol. 2016, 11, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Gharehbagh, S.S.; Subhi, Y.; Sørensen, T.L. Efficacy of aflibercept for polypoidal choroidal vasculopathy in Caucasians. Acta Ophthalmol. 2018, 96, e94–e95. [Google Scholar] [CrossRef]
- Koh, A.; Lee, W.K.; Chen, L.J.; Chen, S.J.; Hashad, Y.; Kim, H.; Lai, T.Y.; Pilz, S.; Ruamviboonsuk, P.; Tokaji, E.; et al. EVEREST study: Efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina 2012, 32, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.H.; Lai, T.Y.Y.; Takahashi, K.; Wong, T.Y.; Chen, L.J.; Ruamviboonsuk, P.; Tan, C.S.; Lee, W.K.; Cheung, C.M.G.; Ngah, N.F.; et al. Comparison of Ranibizumab With or Without Verteporfin Photodynamic Therapy for Polypoidal Choroidal Vasculopathy: The EVEREST II Randomized Clinical Trial. JAMA Ophthalmol. 2020, 138, 935–942. [Google Scholar] [CrossRef]
- Spaide, R.F.; Gemmy Cheung, C.M.; Matsumoto, H.; Kishi, S.; Boon, C.J.F.; van Dijk, E.H.C.; Mauget-Faysse, M.; Behar-Cohen, F.; Hartnett, M.E.; Sivaprasad, S.; et al. Venous overload choroidopathy: A hypothetical framework for central serous chorioretinopathy and allied disorders. Prog. Retin. Eye Res. 2022, 86, 100973. [Google Scholar] [CrossRef]
- Lee, K.; Park, J.H.; Park, Y.G.; Park, Y.H. Analysis of choroidal thickness and vascularity in patients with unilateral polypoidal choroidal vasculopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 1157–1164. [Google Scholar] [CrossRef]
- Lorentzen, T.D.; Subhi, Y.; Sørensen, T.L. Prevalence of Polypoidal Choroidal Vasculopathy in White Patients with Exudative Age-Related Macular Degeneration: Systematic Review and Meta-Analysis. Retina 2018, 38, 2363–2371. [Google Scholar] [CrossRef]
- Subhi, Y.; Krogh Nielsen, M.; Molbech, C.R.; Oishi, A.; Singh, A.; Nissen, M.H.; Sørensen, T.L. Plasma markers of chronic low-grade inflammation in polypoidal choroidal vasculopathy and neovascular age-related macular degeneration. Acta Ophthalmol. 2019, 97, 99–106. [Google Scholar] [CrossRef]
- Subhi, Y.; Nielsen, M.K.; Molbech, C.R.; Oishi, A.; Singh, A.; Nissen, M.H.; Sørensen, T.L. T-cell differentiation and CD56+ levels in polypoidal choroidal vasculopathy and neovascular age-related macular degeneration. Aging 2017, 9, 2436–2452. [Google Scholar] [CrossRef]
- Yamashiro, K.; Hosoda, Y.; Miyake, M.; Ooto, S.; Tsujikawa, A. Characteristics of Pachychoroid Diseases and Age-Related Macular Degeneration: Multimodal Imaging and Genetic Backgrounds. J. Clin. Med. 2020, 9, 2034. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; Wong, T.Y.; Cheung, C.M. Polypoidal Choroidal Vasculopathy in Asians. J. Clin. Med. 2015, 4, 782–821. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, E.H.C.; van Rijssen, T.J.; Subhi, Y.; Boon, C.J.F. Photodynamic Therapy for Chorioretinal Diseases: A Practical Approach. Ophthalmol. Ther. 2020, 9, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Sirks, M.J.; van Dijk, E.H.C.; Rosenberg, N.; Hollak, C.E.M.; Aslanis, S.; Cheung, C.M.G.; Chowers, I.; Eandi, C.M.; Freund, K.B.; Holz, F.G. Clinical impact of the worldwide shortage of verteporfin (Visudyne®) on ophthalmic care. Acta Ophthalmol. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhang, Y.; Kwong, J.S.; Zhang, C.; Li, S.; Sun, F.; Niu, Y.; Du, L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. J. Evid. Based Med. 2015, 8, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Barendregt, J.J.; Doi, S.A.; Lee, Y.Y.; Norman, R.E.; Vos, T. Meta-analysis of prevalence. J. Epidemiol. Community Health 2013, 67, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Li, J.Q.; Welchowski, T.; Schmid, M.; Mauschitz, M.M.; Holz, F.G.; Finger, R.P. Prevalence and incidence of age-related macular degeneration in Europe: A systematic review and meta-analysis. Br. J. Ophthalmol. 2020, 104, 1077–1084. [Google Scholar] [CrossRef]
- Ilginis, T.; Ottosen, S.; Harbo Bundsgaard, K.; Uggerhøj Andersen, C.; Vorum, H. Polypoidal choroidal vasculopathy in patients diagnosed with neovascular age-related macular degeneration in Denmark. Acta Ophthalmol. 2012, 90, e487–e488. [Google Scholar] [CrossRef]
- Ladas, I.D.; Rouvas, A.A.; Moschos, M.M.; Synodinos, E.E.; Karagiannis, D.A.; Koutsandrea, C.N. Polypoidal choroidal vasculopathy and exudative age-related macular degeneration in Greek population. Eye 2004, 18, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Scassellati-Sforzolini, B.; Mariotti, C.; Bryan, R.; Yannuzzi, L.A.; Giuliani, M.; Giovannini, A. Polypoidal choroidal vasculopathy in Italy. Retina 2001, 21, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Parry, D.G.; Beare, N.A.V.; Pearce, I.A. Polypoidal choroidal vasculopathy: A common type of neovascular age-related macular degeneration in Caucasians. Br. J. Ophthalmol. 2017, 101, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Prokofyeva, E.; Zrenner, E. Epidemiology of major eye diseases leading to blindness in Europe: A literature review. Ophthalmic Res 2012, 47, 171–188. [Google Scholar] [CrossRef]
- Balaratnasingam, C.; Lee, W.K.; Koizumi, H.; Dansingani, K.; Inoue, M.; Freund, K.B. Polypoidal choroidal vasculopathy: A distinct disease or manifestation of many? Retina 2016, 36, 1–8. [Google Scholar] [CrossRef]
- Sedeh, F.B.; Scott, D.A.R.; Subhi, Y.; Sørensen, T.L. Prevalence of neovascular age-related macular degeneration and geographic atrophy in Denmark. Dan. Med. J. 2017, 64, A5422. [Google Scholar]
- Potapenko, I.; la Cour, M. Modelling and prognostication of growth in the number of patients treated for neovascular age-related macular degeneration. Acta Ophthalmol. 2021, 99, e1348–e1353. [Google Scholar] [CrossRef]
- Colijn, J.M.; Buitendijk, G.H.S.; Prokofyeva, E.; Alves, D.; Cachulo, M.L.; Khawaja, A.P.; Cougnard-Gregoire, A.; Merle, B.M.J.; Korb, C.; Erke, M.G.; et al. Prevalence of Age-Related Macular Degeneration in Europe: The Past and the Future. Ophthalmology 2017, 124, 1753–1763. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).