Abstract
Dystonia diagnosis is based on clinical examination performed by a neurologist with expertise in movement disorders. Clues that indicate the diagnosis of a movement disorder such as dystonia are dystonic movements, dystonic postures, and three additional physical signs (mirror dystonia, overflow dystonia, and geste antagonists/sensory tricks). Despite advances in research, there is no diagnostic test with a high level of accuracy for the dystonia diagnosis. Clinical neurophysiology and genetics might support the clinician in the diagnostic process. Neurophysiology played a role in untangling dystonia pathophysiology, demonstrating characteristic reduction in inhibition of central motor circuits and alterations in the somatosensory system. The neurophysiologic measure with the greatest evidence in identifying patients affected by dystonia is the somatosensory temporal discrimination threshold (STDT). Other parameters need further confirmations and more solid evidence to be considered as support for the dystonia diagnosis. Genetic testing should be guided by characteristics such as age at onset, body distribution, associated features, and coexistence of other movement disorders (parkinsonism, myoclonus, and other hyperkinesia). The aim of the present review is to summarize the state of the art regarding dystonia diagnosis focusing on the role of neurophysiology and genetic testing.
1. Introduction
Dystonia is a term used to identify hyperkinetic movement disorders in which dystonia is the prominent feature. However, dystonia can also be present in other conditions. According to the etiology, dystonia can be distinguished as acquired, inherited, or idiopathic. The diagnosis of dystonia is based on clinical examination conducted by physicians with expertise in movement disorders through a careful examination of the phenomenology of the condition that allows for a classification of dystonia. For the diagnosis of dystonia syndrome, the examiner should follow the definition of dystonia approved in the last expert consensus [1], articulated in three subdefinitions:
- Dystonia is a movement disorder characterized by sustained or intermittent muscle contractions causing abnormal, often repetitive, movements, postures, or both.
- Dystonic movements are typically patterned, twisting, and may be tremulous.
- Dystonia is often initiated or worsened by voluntary action and associated with overflow muscle activation.
The examiner should focus on the classic five physical signs of dystonia syndromes: two main physical signs (dystonic movements and dystonic posture) and three additional physical signs (mirror dystonia, overflow dystonia and geste antagonists/sensory tricks) [2,3].
The role of laboratory analysis, neuroimaging studies, neurophysiology, and genetic tests is to support the etiology definition of the disease, according to the Axis II of Dystonia classification [1,4].
The aim of the present review is to summarize the state of the art regarding dystonia diagnosis focusing on the role of neurophysiology and genetic testing.
2. Clinical Neurophysiology
Clinical neurophysiology techniques such as EMG mapping [2,5] allow clinicians to support the diagnosis of dystonia and to explore the activity of individual muscles which is not always easy to achieve with a clinical inspection alone. In addition, clinical neurophysiology with different techniques, such as transcranial magnetic stimulation (TMS) [6,7], transcranial direct current stimulation (tDCS) [8,9], or the newest transcranial focused ultrasound stimulation (tFUS) [10,11,12], allow clinicians to explore in a non-invasive way the brain functions In recent years, these techniques have been widely used as tools to characterize distinctive features and improve diagnostic accuracy for different movement disorders [13], particularly parkinsonian syndromes [14,15,16], tremor syndromes [17,18,19], myoclonus [20], and dystonia [21]. The literature includes several studies that use different neurophysiological tests to assess dystonia [22] (Table 1). Despite the amount of evidence, most of the studies on dystonia neurophysiology have a small sample size and focus on specific forms of dystonia (e.g., DYT-TOR1A); therefore, results are not always generalizable to all forms of dystonia. Neurophysiology assessment is not formally included in the diagnostic process [1]; however, neurophysiological tests can support the diagnosis.

Table 1.
Main neurophysiological findings in dystonia.
Since the early 1980s, neurophysiology has been used to characterize dystonia pathophysiology. Most studies were performed in focal hand dystonia (FHD) [22]. At first, dystonia was classified as a basal ganglia (BG) disorder; however, in recent years, evidence points to a disorder arising from a complex network system involving the cerebral cortex (motor and sensory area), the basal ganglia, the brainstem, and the cerebellum [43,44], suggesting that is it possible that several structures could be simultaneously involved in the pathogenesis of dystonia subtypes [43,44].
The electromyographic (EMG) pattern observed in dystonia patients records simultaneous activation of agonist and antagonist muscles (co-contraction), prolonged duration of EMG bursts, and involuntary overflow activation of muscles not directly involved in the movement [3,23].
The most relevant neurophysiological feature shared by all dystonia subtypes is the reduced inhibition of central motor circuits [22]. This is demonstrated by characteristics in several structures: (1) at the subcortical level, a reduction of presynaptic inhibition in the spinal cord has been reported in patients with FHD [24]; (2) at the brainstem level, - a reduced inhibition in the blink reflex recovery cycle in blepharospasm patients [25] and an impairment of the trigeminocervical reflex produced by infraorbital nerve stimulation in torticollis patients was noted [26]; and (3) at the motor cortex level, a loss of inhibition was demonstrated with several transcranial magnetic stimulation (TMS) protocols. Several studies reported abnormalities in dystonic patients of paired pulse protocol as short intracortical inhibitions (SICI), that is, an inhibition of motor cortex response produced by a subthreshold conditioning stimulus followed by a supra-threshold stimulus. SICI is reduced in different subtypes of dystonia [27,28,29]. Reduced transcallosal inhibition was also demonstrated in FHD patients with mirror dystonia. In these patients, stimulation of one hemisphere does not suppress motor responses evoked by a stimulus delivered about 10 ms later over the contralateral hemisphere, as observed in normal subjects [30]. Finally, the duration of the cortical silent period (SP), the inhibition of ongoing muscular activity produced by a TMS pulse during muscle contraction, is reduced in dystonic patients [31], and the lack of suppression could be related to some specific tasks [32].
In recent years, the relevance of the cerebellum in dystonia’s pathophysiology has been investigated [45]. The eye blink classic conditioning (EBCC) protocols consist of electric stimulation of the supraorbital nerve. This protocol that involves cerebellar circuits shows impairment in focal dystonia patients [33], while it is normal in inherited dystonia caused by the DYT-TOR1A and DYT-THAP1 gene mutation [34]. A further test evaluates the motor cortex inhibition produced by cerebellar stimulation. In control subjects, stimulation of one cerebellar hemisphere produces a suppression of the contralateral motor cortex at intervals between 5 and 10 ms [46]. Cerebellar inhibition is impaired in dystonic patients [35].
Traditionally, dystonia was referred to as a motor disorder; however, several recent studies have provided evidence on the role of the somatosensory system in dystonia pathogenesis. Several studies suggested that abnormalities in the somatosensory system are present in almost all dystonic patients, and several neurophysiology tests investigated these findings. The most relevant discovery is the abnormality in the somatosensory temporal discrimination threshold (STDT) [37]. STDT represents the shorter interval at which two different stimuli are perceived as separate. Cervical dystonia (CD) patients have abnormally increased STDT, and the effect seems higher in CD patients with tremor. In a validation study, 51 CD were compared to essential tremor (ET) patients and Parkinson’s disease (PD) patients. The authors found that compared to ET patients, if STDT is ≤67 ms, it has 100% sensitivity and 100% negative predictive value, while if STDT is ≥120 ms, it has 100% specificity and 100% positive predictive value to differentiate ET from CD. However, no statistically significant differences were found between the PD and CD groups even though evidence suggests that STDT is normal in the early PD phase and becomes abnormal in later stages, while STDT is abnormally increased from the first stages of dystonia disease. Another important feature in dystonic patients is the somatosensory discrimination threshold tested with a grating orientation task (GOT) that is a measure of spatial tactile discrimination. These parameters results increased in all idiopathic forms of dystonia, while they are normal in inherited disease cases [36]. Proprioception is also altered in dystonic patients as demonstrated by an abnormally increased tonic vibration reflex (TVR) [38]. Moreover, a study demonstrated that dystonic patients have kinanesthesia impairment seen as abnormal perception of the Aristotle’s illusion, suggesting cortical impairment of somatosensory processes [47]. One possible cause of all these abnormalities could be a deficit in the lateral (or surround) inhibition process, as demonstrated by a somatosensory-evoked potential (SEPs) study [48].
Finally, another possible contribution to dystonic pathophysiology is represented by maladaptive plasticity. Abnormal sensory-motor plasticity was demonstrated using a paradigm termed paired associative stimulation (PAS) In this TMS protocol, cortical stimulation is paired with peripheral nerve stimulation at an interstimulus interval of 25 ms resulting in long-term potentiation-like phenomenon (LTP). This form of LTP is pathologically enhanced in FHD [39]. Maladaptive plasticity could be a key factor in the development of dystonic symptoms and a peculiar feature of dystonic patients as suggested by other studies that did not find the same increased plasticity in DYT-TOR1A carrier subjects [49] and in psychogenic dystonia patients [50]. A pronounced increase of PAS-related plasticity was also reported in Costello syndrome, a genetic syndrome characterized by pronounced dystonia [51,52]. Furthermore, evidence of abnormal plasticity in dystonic patients was highlighted with the use of high-frequency repetitive somatosensory stimulation (HF-RSS) [40]. HF-RSS is a repetitive electric stimulation delivered though surface electrodes on the skin that enhances inhibitory sensorimotor processes. In HS, it usually increases inhibition, while in CD patients inhibition is reduced.
Although all this evidence suggests that dystonia is a complex network disorder involving the brainstem, the basal ganglia, the thalamus, the cortex, and the cerebellum [44], originally dystonia was referred to as basal ganglia disease. Several trials point out that electrical modulation of the basal ganglia network through continuous deep brain stimulation (DBS) in internal globus pallidus (GPi) could improve generalized dystonia symptoms [53]. DBS electrodes were also used to invasively record synchronized neuronal activities, pointing out that in line with other movement disorders, pathological basal ganglia oscillatory activities [54] can be found in dystonic patients [41,42]. This invasive recording of local field potentials (LFP) of basal ganglia revealed that GPi and external globus palidus (GPe) have a decreased discharge rate and irregular firing in dystonic patients [55,56]. In addition, LFP studies demonstrated that pallidus nuclei of dystonic patients show excessive synchronized activities in the 4–10 Hz frequency band [42].
The study of oscillatory activities in neurological disorders [54] revealed new pathological biomarkers in recent years. Several authors suggested that these abnormalities could be used as biomarkers to deliver electrical DBS only in response to pathological neuronal oscillation (adaptative DBS-aDBS). This technique was mainly evaluated in Parkinson’s disease patients [57,58,59] in which LFP monitoring could be supported by multiparametric [60] motor symptoms monitoring [61,62,63] with the assistance of artificial intelligence algorithms [64]. It has been suggested that this protocol could be translated to dystonic patients with specific biomarkers, such as GPi LFPs theta-alpha band activity [41,42,53], in combination with dystonic muscle activity monitoring through subcutaneous EMG or wearable accelerometer devices [53].
3. Dystonia Genetics
Dystonia genetics is a wide field with continuous updates. After the first description of DYT-TOR1A, several other genes have been proposed as linked with the dystonia phenotype [65]. As in other fields of genetics, after the first years focused on the genetic marker, the focus is moving on to proteomics, searching the causal link between the protein produced by these genes and the phenotype of dystonia. Camargos and Cardoso [66] proposed a model of the “dystonia cell” linking the dystonic genes to the proteins function (Figure 1), based mainly on the classic DYT nomenclature.
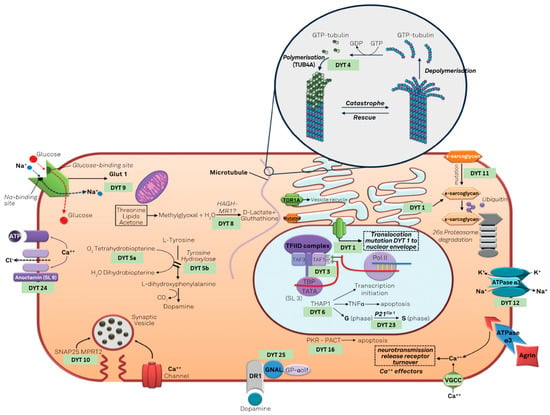
Figure 1.
The “dystonia cell” describe the cellular pathway involved in genetic dystonias (modified under the terms and conditions of the Creative Commons Attribution (CC BY) license from [66]).
The classic DYT nomenclature is based on locus symbols (e.g., DYT 1) and has been used for several years. It is still used in literature and clinical practice [67]. However, the system of locus symbols has been challenged by advances in techniques of genetics research that allow us to define the causative gene, as explained by Marras et al. [68], and the need to renovate the nomenclature system has arisen. The MDS Task Force for the Nomenclature of Genetic Movement Disorders proposed new recommendations, whose use in research and clinical practice is strongly encouraged [69]. This new nomenclature strictly connects the prefix to the predominant phenotype and considers the causative gene rather than the locus symbols (e.g., DYT 1 is now named DYT-TOR1A) [4]. The prefix DYT is used only if dystonia is the prominent disease feature due to a pathogenetic mutation [69]. Otherwise, if another movement disorder is a prominent feature along with dystonia, a double prefix would be assigned (e.g., DYT/PARK-ATP1A3). Indeed, genetic dystonia can be isolated or combined with other movement disorders such as parkinsonism, myoclonus, or other hyperkinesia (Figure 2).
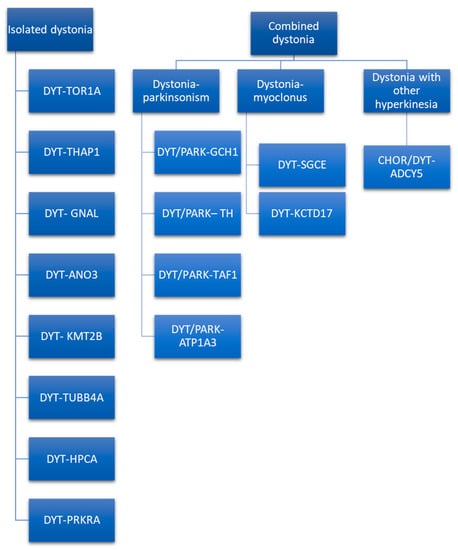
Figure 2.
Isolated and combined genetic forms of dystonia [69].
Moreover, in the proposed nomenclature and in the last consensus update on dystonia, the term complex dystonia is used, referring to conditions in which dystonia predominates the clinical phenotype but occurs in the context of a complex disease including symptoms other than movement disorders [1,69]. For example, Wilson disease is named according to the proposed nomenclature with a DYT prefix (DYT-ATP7B), and the same happens for Lesch–Nyhan syndrome and other infantile and childhood onset disease [69]. Given that most of isolated hereditary dystonia is recognized as an autosomal dominant inheritance, the mode of transmission cannot be used as the only criterion to make a differential diagnosis. To guide the clinician towards a genetic diagnosis of dystonia, at least clinical phenotype and age of onset should be considered (Table 2). If dystonia dominates the clinical picture, one of the isolated dystonias may be considered, and the gene mutations involved may be DYT-TOR1A, DYT-THAP1, DYT-GNAL, DYT-ANO3, DYT-KMT2B, DYT-TUBB4A, DYT-HPCA, and DYT-PRKRA [70]. The last-mentioned dystonia is a controversial classification, as it is considered as combined dystonia by some authors [71] and as isolated dystonia by others [70]. Indeed, despite parkinsonism being described in about half the patients, it seemed to be caused not by true parkinsonian features, but by slow movements of dystonic body parts [70]. The isolated form of dystonia could be distinguished according to the age of onset, body distribution, temporal pattern, associated features, responses to drugs, response to DBS, and brain imaging. Regarding age of onset, in infancy, childhood, and adolescence DYT-TOR1A, DYT-THAP1, DYT-KMT2B, DYT-TUBB4A, DYT-PRKRA, and DYT-HPCA are more probable, while DYT-ANO3 and DYT-GNAL begin in early adulthood. In particular, DYT-ANO3 recognizes two peaks of the age of onset: one in infancy/childhood and one in early-late adulthood [70]. Age at onset may by modified by several aspects, e.g., penetrance as is the case of DYT-TOR1A [72]. Hence, age of onset alone cannot be used as the only criteria to orient the diagnosis. According to body distribution, generalized forms of isolated dystonia are mainly due to DYT-TOR1A, DYT-THAP1, DYT-KMT2B, DYT-HPCA, and DYT-PRKRA. Among these, DYT-TOR1A, DYT-HPCA, and DYT-KMT2B usually begin in the lower limbs asymmetrically with secondary generalization. In contrast, DYT-THAP1 may initiate in the upper part of the body, involving cranio–cervical districts, speech difficulties, and the upper limbs, with successive generalizations [73]. If DYT-TOR1A begins in the upper limbs, it tends to be focal. Focal and segmental isolated dystonia are more likely caused by DYT-GNAL and DYT-ANO3. These two forms of dystonia typically begin at the cervical level and may cause head tremor [70]. DYT-GNAL may be suspected if age at onset is in early-late adulthood. In case of early involvement of craniofacial muscles with laryngeal dystonia and speech difficulties, with secondary generalization involving the arms at younger ages, DYT-ANO3 becomes more probable [70]. Another peculiar form of isolated dystonia with focal distribution involving the cervical district and causing spasmodic dysphonia is caused by DYT-TUBB4A. This focal form may successively evolve into a generalized dystonia [74]. Regarding the temporal pattern, except for the last-mentioned dystonia, all the other isolated dystonia follows a persistent temporal pattern. Associated features may guide the clinician in the differential diagnosis. The presence of additional phenotypic characteristic, such as microcephaly, short stature, intellectual disability, abnormal eye movements, myoclonus, dysmorphisms, and psychiatric symptoms, may be suggestive of DYT-KMT2B [70]. Thin face, body habitus, and hobby horse gait are described in the DYT-TUBB4A [75]. None of the isolated forms of dystonia respond to L-Dopa; DYT-TOR1A, DYT-THAP1, DYT-ANO3, DYT-KMT2B, and DYT-HPCA may respond to anticholinergics [70]. Response to alcohol is described in DYT-GNAL and DYT-TUBB4A. It is important to define the genetic etiology of the dystonia because response to DBS varies according to the genetic conditions, and this is an important prognostic factor to be considered when selecting patients for advanced therapy. Indeed, is well known that DYT-TOR1A, DYT-THAP1, DYT-ANO3, DYT-GNAL, and DYT-KMT2B show a good response to DBS with a target in the GPi, unlike the other forms of isolated dystonia [76,77,78,79]. Brain imaging is not conclusive in distinguishing between the several forms of isolated dystonia, as the sole characteristic described is pallidal hypointensity in DYT-KMT2B [70].

Table 2.
Isolated and combined genetic types of dystonia.
Combined dystonia is characterized by the coexistence of another movement disorder in addition to dystonia. The association of dystonia with parkinsonism defines dystonia–parkinsonism. The monogenic forms of dystonia–parkinsonism are DYT/PARK-GCH1, DYT/PARK-TH, DYT/PARK-TAF1, and DYT/PARK-ATP1A3 [71]. Contrary to what has been observed for isolated dystonia, combined dystonia recognizes a different mode of inheritance: autosomal dominant inheritance is characteristic of DYT/PARK-GCH1 and DYT/PARK-ATP1A3, while autosomal recessive inheritance is typical of DYT/PARK-TH. X-linked transmission characterizes DYT/PARK-TAF1 (also known as Lubag syndrome). Among this, it is of paramount importance to diagnose the dopa-responsive dystonia, DYT/PARK-GCH1. Indeed, patients have excellent and sustained response to L-Dopa [80]. Another form of combined dystonia with response to L-Dopa is DYT/PARK-TH. These two forms of dystonia–parkinsonism may be differentiated according to age of onset, as DYT/PARK-GCH1 begins in infancy/childhood, while DYT/PARK-TH may initiate in infancy. Moreover, diurnal fluctuations of parkinsonian symptoms due to circadian variations in dopamine concentration are more pronounced in DYT/PARK-GCH1 than in DYT/PARK-TH [80]. An adjunctive feature may help in differential diagnosis among the two forms: the presence of hypotonia is suggestive of DYT/PARK-TH, while in DYT/PARK-GCH1 hyperreflexia has been described [81]. The coexistence of non-motor features orients towards the diagnosis of DYT/PARK-GCH1, while a more complex clinical picture, with autonomic disturbances, ptosis, and oculogyric crisis is suggestive of DYT/PARK-TH. In both forms, dystonia begins as focal with subsequent generalization [82,83,84,85].
DYT/PARK TAF1 differs from the previous mentioned strains for the age of onset, body distribution of dystonia, and neuroimaging. This form of combined dystonia begins in early to late adulthood and, contrary to DYT/PARK-GCH1 that begins with foot dystonia and then progress cranially, DYT/PARK TAF1 involves mainly the upper body, with characteristic jaw opening dystonia and bulbar involvement. Another difference with respect to the dopa-responsive dystonia is the absence of diurnal fluctuation. Brain imaging shows striatal atrophy and pallidum volume loss, considered an expression of the neurodegenerative nature of the disease. This form recognizes an X-linked transmission, hence is more frequent in males [86,87,88,89]. Abrupt onset, fluctuating course, psychiatric features, and postural instability may raise suspicion of DYT/PARK-ATP1A3. This disease begins with dystonic spasms, usually following a provoking event (fever, infection, childbirth, alcohol binging, fall, excessive exercise, heat exposure, and psychological stress), with a plateau within 30–60 days of disease onset, with no significant improvement [90]. Dystonia begins in limbs and develops with a characteristic rostrocaudal gradient, cranial symptoms being more severe than upper limbs and lower limbs [91].
Combined dystonia also encompasses dystonia associated with myoclonus and other hyperkinetic disorders. To date, two forms of dystonia–myoclonus have received confirmations: DYT-SGCE and DYT-KCTD17. These diseases have several features in common: age of onset is in the first or second decade of life, myoclonic jerks involve the upper body, and in DYT-SGCE also the neck may be involved. In both diseases, dystonia affects the upper part of the body, with involvement of upper limbs and the cranio-cervical region. If in DYT-SGCE myoclonic jerks dominates the clinical picture, in DYT-KCTD17 dystonia seems to be the prominent feature. Interestingly, DYT-SGCE myoclonic symptoms respond to alcohol, while in DYT-KCTD17 this response is absent [71,92].
Dystonia may coexist with other hyperkinetic disorders, such as chorea, as observed in several forms of complex dystonia. Marras et al. [69] also classify CHOR/DYT-ADCY5 as combined dystonia. This disease is characterized by a plethora of hyperkinetic disorders, such as chorea, dystonia, and myoclonus, beginning in early childhood and with a characteristic fluctuating or paroxysmal course. Interestingly, symptoms do not disappear during sleep, resulting in significant disturbances, and may respond to caffeine [93,94]. Response to DBS is lower than in other form of monogenic dystonia [76].
Genetic Testing and Genetic Counseling
According to the EFNS dystonia guidelines, genetic testing is not sufficient to make a diagnosis of dystonia in the absence of clinical features suggestive of dystonia [95]. Therefore, the clinical picture should orient the decision to carry out genetic testing [96,97,98].
The previously mentioned guidelines recommend, with a level B of evidence, the DYT-TOR1A testing for patients with limb-onset, primary dystonia with onset before age 30 [98], and in those with onset after age 30 if they have an affected relative with early-onset dystonia [98]. Guidelines do not recommend DYT-TOR1A testing in asymptomatic individuals in dystonia families as a good practice point. After exclusion of DYT-TOR1A, in early-onset dystonia or familial dystonia with cranio-cervical predominance, DYT-THAP1 testing is recommended [73]. It is considered a good practice point to conduct a diagnostic levodopa trial in every patient with early-onset dystonia without an alternative diagnosis [99]. Individuals with early-onset myoclonus affecting the arms or neck, particularly if positive for autosomal-dominant inheritance and if triggered by action, should be tested for the DYT-SGCE gene [100].
In clinical practice, genetic testing consists of of using predefined panels for dystonia. The whole-exome sequencing (WES) is also a resource to consider; however, it is expensive and requires a long time. Zech et al. [101] proposed an algorithm to predict diagnostic success rate of WES in individuals with dystonia. This algorithm assigns a score to three clinical characteristics:
- -
- Age at onset (0–20 years: score 2; >21 years: score 0),
- -
- Body distribution (generalized or segmental: score 1; focal: score 0),
- -
- Dystonia category (complex dystonia: score 2; combined dystonia: score 1; isolated dystonia: score 0).
Summary scores range from 0 to 5 and predict the diagnostic success rate of WES in individuals with dystonia. If the score is three, the sensitivity is 96% and the specificity is 62%; if the score is five, the sensitivity is 62% and the specificity is 86%. Hence, if the score is equal to or higher than three, whole-exome sequencing is recommended [101].
An extensive discussion about genetic counseling goes beyond the scope of this review. The main concept to underscore is that genetic counseling depends largely on the determination of the mode of inheritance of a specific cause of an inherited dystonia in an individual (i.e., autosomal dominant, autosomal recessive, mitochondrial, X-linked inheritance). According to the inheritance, Table 3 describes all the possible diseases [4].

Table 3.
Inherited causes of dystonia.
Moreover, penetrance must be considered because of the influence of the phenotypic expression of dystonia [102]. For example, for two hereditary forms of dystonia, mechanisms affecting penetrance have been identified:
- -
- DYT-SGCE dystonia has maternal imprinting of the gene, meaning that the dystonia-myoclonus only manifests when SGCE pathogenic variants are paternally inherited [103].
- -
- DYT-TOR1A has a reduced penetrance of the GAG deletion in TOR1A, from about 35% to 3% in individuals who also have a heterozygous NM_000113.2:646G>C (p.Asp216His) variant in TOR1A on the other allele [72].
Genetic counseling should be offered to the patients and the family by qualified personnel and, according to the EFNS dystonia guideline, is recommended [95].
4. Discussion
The present review summarized the possible contribution of clinical neurophysiology and genetic testing to clinical examination for dystonia diagnosis (Figure 3).
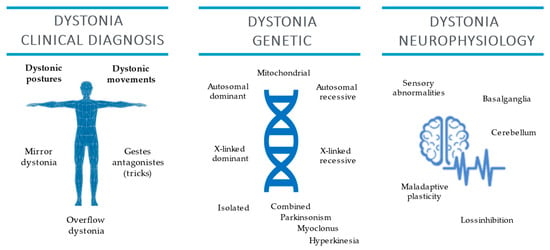
Figure 3.
Dystonia clinical diagnosis and genetic and clinical neurophysiology features.
However, dystonia diagnosis is still based on clinical examination conducted by physicians with expertise in movement disorders. The clinical diagnosis should be based on the observations of two core characteristics and of adjunctive features [1]. According to the EFNS dystonia guidelines, a neurophysiological test may help diagnosis despite low evidence (class IV), hence further and proper studies are needed [95]. However, the role of neurophysiology is not marginal, being an important resource to enlighten the pathophysiology of dystonia (Table 1). Among neurophysiological alterations observed in dystonia, sensitivity, specificity, and positive predictive value have been evaluated only for STDT. That is pathologically increased in patients affected by cervical dystonia compared to patients affected by essential tremor [37]. Neurophysiology also represents an excellent support for the therapy of dystonia in the case of EMG-guided botulinum toxin injection. In future applications, neurophysiology could guide adaptive DBS. Indeed, LFP recorded in GPi could be used as input signals to modulate stimulation parameters as currently used for Parkinson’s disease [104].
Once dystonia has been clinically diagnosed, the definition of the etiology is needed [1]. The etiological diagnosis of dystonia cannot ignore the role of genetics testing. Several genes have been described as causes of isolated, combined, or complex forms of dystonia (see Table 2). Regarding isolated dystonia, age at onset, body distribution (focal, segmental or generalized), and associated features may orient the clinicians towards a specific form of monogenic dystonia. In combined dystonia, the second most represented movement disorder, the clinical picture guides the clinician in the direction of dystonia associated with parkinsonism, myoclonus, or other hyperkinesia. The choice to request WES to reach a diagnosis should be carefully considered when panels for dystonia fail to detect causative mutations. Zech et al. proposed an interesting and feasible algorithm to predict diagnostic success rate of WES, according to dystonia characteristics [101]. The algorithm considers tree items (age at onset, body distribution, dystonia category) and assigns a score to each one. If the summary score is equal or higher than three, WES is recommended because of a high probability to identify causative mutations.
Considering the inheritance mode and the risk of transmission of the disease in the context of the same family, genetic counseling should be offered to the patients and a multidisciplinary approach involving geneticists, psychologist is desirable.
Author Contributions
Conceptualization, L.d.B.; writing—original draft preparation, L.d.B., A.D.S., M.L.C., P.M.P. and S.P.C.; writing—review and editing, L.d.B. and V.D.L.; supervision, V.D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Albanese, A.; Bhatia, K.; Bressman, S.B.; DeLong, M.R.; Fahn, S.; Fung, V.S.; Hallett, M.; Jankovic, J.; Jinnah, H.A.; Klein, C.; et al. Phenomenology and classification of dystonia: A consensus update. Mov. Disord. 2013, 28, 863–873. [Google Scholar] [CrossRef]
- Albanese, A.; Lalli, S. Is this dystonia? Mov. Disord. 2009, 24, 1725–1731. [Google Scholar] [CrossRef]
- Albanese, A.; Di Giovanni, M.; Lalli, S. Dystonia: Diagnosis and management. Eur. J. Neurol. 2018, 26, 5–17. [Google Scholar] [CrossRef]
- Di Biase, L.; Di Santo, A.; Caminiti, M.L.; Pecoraro, P.M.; Di Lazzaro, V. Classification of Dystonia. Life 2022, 12, 206. [Google Scholar] [CrossRef]
- Van Gerpen, J.A.; Matsumoto, J.Y.; Ahlskog, J.E.; Maraganore, D.M.; McManis, P.G. Utility of an EMG mapping study in treating cervical dystonia. Muscle Nerve 2000, 23, 1752–1756. [Google Scholar] [CrossRef]
- Edwards, M.J.; Talelli, P.; Rothwell, J.C. Clinical applications of transcranial magnetic stimulation in patients with movement disorders. Lancet Neurol. 2008, 7, 827–840. [Google Scholar] [CrossRef]
- Chen, R.; Cros, D.; Curra, A.; Di Lazzaro, V.; Lefaucheur, J.-P.; Magistris, M.R.; Mills, K.; Rösler, K.M.; Triggs, W.J.; Ugawa, Y.; et al. The clinical diagnostic utility of transcranial magnetic stimulation: Report of an IFCN committee. Clin. Neurophysiol. 2008, 119, 504–532. [Google Scholar] [CrossRef]
- Fregni, F.; Boggio, P.S.; Santos, M.C.; Lima, M.; Vieira, A.L.; Rigonatti, S.P.; Silva, M.T.A.; Barbosa, E.R.; Nitsche, M.A.; Pascual-Leone, A. Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson’s disease. Mov. Disord. 2006, 21, 1693–1702. [Google Scholar] [CrossRef]
- Ferrucci, R.; Mameli, F.; Ruggiero, F.; Priori, A. Transcranial direct current stimulation as treatment for Parkinson’s disease and other movement disorders. Basal Ganglia 2015, 6, 53–61. [Google Scholar] [CrossRef]
- Darrow, D.P. Focused Ultrasound for Neuromodulation. Neurotherapeutics 2019, 16, 88–99. [Google Scholar] [CrossRef]
- Di Biase, L.; Falato, E.; Di Lazzaro, V. Transcranial Focused Ultrasound (tFUS) and Transcranial Unfocused Ultrasound (tUS) Neuromodulation: From Theoretical Principles to Stimulation Practices. Front. Neurol. 2019, 10, 549. [Google Scholar] [CrossRef]
- Di Biase, L.; Falato, E.; Caminiti, M.L.; Pecoraro, P.M.; Narducci, F.; Di Lazzaro, V. Focused Ultrasound (FUS) for Chronic Pain Management: Approved and Potential Applications. Neurol. Res. Int. 2021, 2021, 1–16. [Google Scholar] [CrossRef]
- Hallett, M. Chapter 1 Movement disorders: Overview. In Handbook of Clinical Neurophysiology; Elsevier: Amsterdam, The Netherlands, 2003; pp. 3–4. [Google Scholar]
- Bologna, M.; Suppa, A.; Di Stasio, F.; Conte, A.; Fabbrini, G.; Berardelli, A. Neurophysiological studies on atypical parkinsonian syndromes. Park. Relat. Disord. 2017, 42, 12–21. [Google Scholar] [CrossRef]
- Valls-Solé, J. Neurophysiological characterization of parkinsonian syndromes. Neurophysiol. Clin. Neurophysiol. 2000, 30, 352–367. [Google Scholar] [CrossRef]
- Valls-Solé, J.; Valldeoriola, F. Neurophysiological correlate of clinical signs in Parkinson’s disease. Clin. Neurophysiol. 2002, 113, 792–805. [Google Scholar] [CrossRef]
- Di Biase, L.; Brittain, J.-S.; Shah, S.A.; Pedrosa, D.; Cagnan, H.; Mathy, A.; Chen, C.C.; Martín-Rodríguez, J.F.; Mir, P.; Timmerman, L.; et al. Tremor stability index: A new tool for differential diagnosis in tremor syndromes. Brain 2017, 140, 1977–1986. [Google Scholar] [CrossRef]
- Di Pino, G.; Formica, D.; Melgari, J.M.; Taffoni, F.; Salomone, G.; di Biase, L.; Caimo, E.; Vernieri, F.; Guglielmelli, E. Neurophysiological bases of tremors and accelerometric parameters analysis. In Proceedings of the 2012 4th IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics (BioRob), Rome, Italy, 24−27 June 2012; pp. 1820–1825. [Google Scholar]
- Deuschl, G.; Krack, P.; Lauk, M.; Timmer, J. Clinical Neurophysiology of Tremor. J. Clin. Neurophysiol. 1996, 13, 110–121. [Google Scholar] [CrossRef]
- Caviness, J.N. Chapter 32 The clinical neurophysiology of myoclonus. In Handbook of Clinical Neurophysiology; Hallett, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 521–548. [Google Scholar]
- Kaji, R. Chapter 28 Dystonia. In Handbook of Clinical Neurophysiology; Hallett, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 451–461. [Google Scholar]
- Hallett, M. Neurophysiology of dystonia: The role of inhibition. Neurobiol. Dis. 2011, 42, 177–184. [Google Scholar] [CrossRef]
- Rothwell, J.C.; Obeso, J.A.; Day, B.L.; Marsden, C.D. Pathophysiology of dystonias. Adv. Neurol. 1983, 39, 851–863. [Google Scholar]
- Nakashima, K.; Rothwell, J.C.; Day, B.L.; Thompson, P.D.; Shannon, K.; Marsden, C.D. Reciprocal inhibition between forearm muscles in patients with writer’s cramp and other occupational cramps, symptomatic hemidystonia and hemiparesis due to stroke. Brain 1989, 112, 681–697. [Google Scholar] [CrossRef]
- Berardelli, A.; Rothwell, J.; Day, B.L.; Marsden, C.D. Pathophysiology of blepharospasm and oromandibular dystonia. Brain 1985, 108, 593–608. [Google Scholar] [CrossRef]
- Quartarone, A.; Girlanda, P.; Di Lazzaro, V.; Majorana, G.; Battaglia, F.; Messina, C. Short latency trigemi-no-sternocleidomastoid response in muscles in patients with spasmodic torticollis and blepharospasm. Clin. Neurophysiol. 2000, 111, 1672–1677. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Rothwell, J.; Lu, C.-S.; Wang, J.-J.; Chen, R.-S. Restoration of motor inhibition through an abnormal premotor-motor connection in dystonia. Mov. Disord. 2010, 25, 696–703. [Google Scholar] [CrossRef]
- Espay, A.; Morgante, F.; Purzner, J.; Gunraj, C.A.; Lang, A.; Chen, R. Cortical and spinal abnormalities in psychogenic dystonia. Ann. Neurol. 2006, 59, 825–834. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Oliviero, A.; Profice, P.; Dileone, M.; Pilato, F.; Insola, A.; Della Marca, G.; Tonali, P.; Mazzone, P. Reduced cerebral cortex inhibition in dystonia: Direct evidence in humans. Clin. Neurophysiol. 2009, 120, 834–839. [Google Scholar] [CrossRef]
- Beck, S.; Shamim, E.A.; Richardson, S.P.; Schubert, M.; Hallett, M. Inter-hemispheric inhibition is impaired in mirror dystonia. Eur. J. Neurosci. 2009, 29, 1634–1640. [Google Scholar] [CrossRef]
- Chen, R.; Wassermann, E.M.; Caños, M.; Hallett, M. Impaired inhibition in writer’s cramp during voluntary muscle activation. Neurology 1997, 49, 1054–1059. [Google Scholar] [CrossRef]
- Tinazzi, M.; Farina, S.; Edwards, M.; Moretto, G.; Restivo, D.; Fiaschi, A.; Berardelli, A. Task−specific impairment of motor cortical excitation and inhibition in patients with writer’s cramp. Neurosci. Lett. 2005, 378, 55–58. [Google Scholar] [CrossRef]
- Kojovic, M.; Pareés, I.; Kassavetis, P.; Palomar, F.J.; Mir, P.; Teo, J.; Cordivari, C.; Rothwell, J.; Bhatia, K.; Edwards, M.J. Secondary and primary dystonia: Pathophysiological differences. Brain 2013, 136, 2038–2049. [Google Scholar] [CrossRef]
- Sadnicka, A.; Teo, J.; Kojovic, M.; Pareés, I.; Saifee, T.A.; Kassavetis, P.; Schwingenschuh, P.; Katschnig−Winter, P.; Stamelou, M.; Mencacci, N.E.; et al. All in the blink of an eye: New insight into cerebellar and brainstem function in DYT1 and DYT6 dystonia. Eur. J. Neurol. 2014, 22, 762–767. [Google Scholar] [CrossRef]
- Brighina, F.; Romano, M.; Giglia, G.; Saia, V.; Puma, A.; Giglia, F.; Fierro, B. Effects of cerebellar TMS on motor cortex of patients with focal dystonia: A preliminary report. Exp. Brain Res. 2008, 192, 651–656. [Google Scholar] [CrossRef]
- Molloy, F.M.; Zeuner, K.E.; Dambrosia, J.M.; Carr, T.D.; Hallett, M. Abnormalities of spatial discrimination in focal and generalized dystonia. Brain 2003, 126, 2175–2182. [Google Scholar] [CrossRef]
- Conte, A.; Ferrazzano, G.; Belvisi, D.; Manzo, N.; Battista, E.; Voti, P.L.; Nardella, A.; Fabbrini, G.; Berardelli, A. Somatosensory temporal discrimination in Parkinson’s disease, dystonia and essential tremor: Pathophysiological and clinical implications. Clin. Neurophysiol. 2018, 129, 1849–1853. [Google Scholar] [CrossRef]
- Grünewald, R.A.; Shipman, J.M.; Sagar, H.J.; Yoneda, Y. Idiopathic focal dystonia: A disorder of muscle spindle afferent processing? Brain 1997, 120, 2179–2185. [Google Scholar] [CrossRef]
- Quartarone, A.; Bagnato, S.; Rizzo, V.; Siebner, H.R.; Dattola, V.; Scalfari, A.; Morgante, F.; Battaglia, F.; Romano, M.; Girlanda, P. Abnormal associative plasticity of the human motor cortex in writer’s cramp. Brain 2003, 126, 2586–2596. [Google Scholar] [CrossRef]
- Erro, R.; Rocchi, L.; Antelmi, E.; Liguori, R.; Tinazzi, M.; Berardelli, A.; Rothwell, J.; Bhatia, K.P. High frequency somatosensory stimulation in dystonia: Evidence fordefective inhibitory plasticity. Mov. Disord. 2018, 33, 1902–1909. [Google Scholar] [CrossRef]
- Silberstein, P.; Kühn, A.A.; Kupsch, A.; Trottenberg, T.; Krauss, J.K.; Wöhrle, J.C.; Mazzone, P.; Insola, A.; Di Lazzaro, V.; Oliviero, A.; et al. Patterning of globus pallidus local field potentials differs between Parkinson’s disease and dystonia. Brain 2003, 126, 2597–2608. [Google Scholar] [CrossRef]
- Chen, C.C.; Kühn, A.A.; Trottenberg, T.; Kupsch, A.; Schneider, G.-H.; Brown, P. Neuronal activity in globus pallidus interna can be synchronized to local field potential activity over 3–12 Hz in patients with dystonia. Exp. Neurol. 2006, 202, 480–486. [Google Scholar] [CrossRef]
- Conte, A.; Rocchi, L.; Latorre, A.; Belvisi, D.; Rothwell, J.C.; Berardelli, A. Ten-Year Reflections on the Neurophysiological Abnormalities of Focal Dystonias in Humans. Mov. Disord. 2019, 34, 1616–1628. [Google Scholar] [CrossRef]
- Latorre, A.; Rocchi, L.; Bhatia, K.P. Delineating the electrophysiological signature of dystonia. Exp. Brain Res. 2020, 238, 1685–1692. [Google Scholar] [CrossRef]
- Bologna, M.; Berardelli, A. The cerebellum and dystonia. Handb. Clin. Neurol. 2018, 155, 259–272. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Molinari, M.; Restuccia, D.; Leggio, M.; Nardone, R.; Fogli, D.; Tonali, P. Cerebro-cerebellar interactions in man: Neurophysiological studies in patients with focal cerebellar lesions. Electroencephalogr. Clin. Neurophysiol. Potentials Sect. 1994, 93, 27–34. [Google Scholar] [CrossRef]
- Tinazzi, M.; Marotta, A.; Fasano, A.; Bove, F.; Bentivoglio, A.R.; Squintani, G.; Pozzer, L.; Fiorio, M. Aristotle’s illusion reveals interdigit functional somatosensory alterations in focal hand dystonia. Brain 2013, 136, 782–789. [Google Scholar] [CrossRef][Green Version]
- Tinazzi, M.; Priori, A.; Bertolasi, L.; Frasson, E.; Mauguière, F.; Fiaschi, A. Abnormal central integration of a dual somatosensory input in dystonia Evidence for sensory overflow. Brain 2000, 123, 42–50. [Google Scholar] [CrossRef]
- Edwards, M.J.; Huang, Y.-Z.; Mir, P.; Rothwell, J.; Bhatia, K.P. Abnormalities in motor cortical plasticity differentiate manifesting and nonmanifesting DYT1 carriers. Mov. Disord. 2006, 21, 2181–2186. [Google Scholar] [CrossRef]
- Quartarone, A.; Rizzo, V.; Terranova, C.; Morgante, F.; Schneider, S.; Ibrahim, N.; Girlanda, P.; Bhatia, K.P.; Rothwell, J.C. Abnormal sensorimotor plasticity in organic but not in psychogenic dystonia. Brain 2009, 132, 2871–2877. [Google Scholar] [CrossRef]
- Dileone, M.; Profice, P.; Pilato, F.; Alfieri, P.; Cesarini, L.; Mercuri, E.; Leoni, C.; Tartaglia, M.; Di Iorio, R.; Zampino, G.; et al. Enhanced human brain associative plasticity in Costello syndrome. J. Physiol. 2010, 588, 3445–3456. [Google Scholar] [CrossRef]
- Dileone, M.; Zampino, G.; Profice, P.; Pilato, F.; Leoni, C.; Ranieri, F.; Capone, F.; Tartaglia, M.; Brown, P.; Di Lazzaro, V. Dystonia in Costello syndrome. Park. Relat. Disord. 2012, 18, 798–800. [Google Scholar] [CrossRef]
- Piña-Fuentes, D.; Beudel, M.; Little, S.; van Zijl, J.; Elting, J.W.; Oterdoom, D.L.M.; van Egmond, M.E.; van Dijk, J.M.C.; Tijssen, M.A.J. Toward adaptive deep brain stimulation for dystonia. Neurosurg. Focus 2018, 45, E3. [Google Scholar] [CrossRef]
- Assenza, G.; Capone, F.; di Biase, L.; Ferreri, F.; Florio, L.; Guerra, A.; Marano, M.; Paolucci, M.; Ranieri, F.; Salomone, G. Oscillatory activities in neurological disorders of elderly: Biomarkers to target for neuromodulation. Front. Aging Neurosci. 2017, 9, 189. [Google Scholar] [CrossRef]
- Starr, P.A.; Rau, G.M.; Davis, V.; Marks, W.J.; Ostrem, J.L.; Simmons, D.; Lindsey, N.; Turner, R. Spontaneous Pallidal Neuronal Activity in Human Dystonia: Comparison With Parkinson’s Disease and Normal Macaque. J. Neurophysiol. 2005, 93, 3165–3176. [Google Scholar] [CrossRef]
- Vitek, J.L.; Delong, M.R.; Starr, P.A.; Hariz, M.I.; Metman, L.V. Intraoperative neurophysiology in DBS for dystonia. Mov. Disord. 2011, 26, S31–S36. [Google Scholar] [CrossRef]
- Little, S.; Pogosyan, A.; Neal, S.; Ba, B.Z.; Zrinzo, L.; Hariz, M.; Foltynie, T.; Limousin, P.; Ashkan, K.; FitzGerald, J.; et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann. Neurol. 2013, 74, 449–457. [Google Scholar] [CrossRef]
- Little, S.; Pogosyan, A.; Neal, S.; Zrinzo, L.; Hariz, M.; Foltynie, T.; Limousin, P.; Brown, P. Controlling Parkinson’s Disease With Adaptive Deep Brain Stimulation. J. Vis. Exp. 2014, 89, e51403. [Google Scholar] [CrossRef]
- Tinkhauser, G.; Pogosyan, A.; Debove, I.; Nowacki, A.; Shah, S.A.; Seidel, K.; Tan, H.; Brittain, J.-S.; Petermann, K.; Di Biase, L.; et al. Directional local field potentials: A tool to optimize deep brain stimulation. Mov. Disord. 2017, 33, 159–164. [Google Scholar] [CrossRef]
- Di Biase, L.; Tinkhauser, G.; Martin Moraud, E.; Caminiti, M.L.; Pecoraro, P.M.; Di Lazzaro, V. Adaptive, personalized closed−loop therapy for Parkinson’s disease: Biochemical, neurophysiological, and wearable sensing systems. Expert Rev. Neurother. 2021, 21, 1371–1388. [Google Scholar] [CrossRef]
- Di Biase, L.; Di Santo, A.; Caminiti, M.L.; De Liso, A.; Shah, S.A.; Ricci, L.; Di Lazzaro, V. Gait Analysis in Parkinson’s Disease: An Overview of the Most Accurate Markers for Diagnosis and Symptoms Monitoring. Sensors 2020, 20, 3529. [Google Scholar] [CrossRef]
- Di Biase, L.; Summa, S.; Tosi, J.; Taffoni, F.; Marano, M.; Cascio Rizzo, A.; Vecchio, F.; Formica, D.; Di Lazzaro, V.; Di Pino, G.; et al. Quantitative Analysis of Bradykinesia and Rigidity in Parkinson’s Disease. Front. Neurol. 2018, 9, 121. [Google Scholar] [CrossRef]
- Raiano, L.; di Pino, G.; di Biase, L.; Tombini, M.; Tagliamonte, N.L.; Formica, D. PDMeter: A Wrist Wearable Device for an at−Home Assessment of the Parkinson’s Disease Rigidity. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1325–1333. [Google Scholar] [CrossRef]
- Di Biase, L.; Raiano, L.; Caminiti, M.L.; Pecoraro, P.M.; Di Lazzaro, V. Artificial intelligence in Parkinson’s disease-symptoms identification and monitoring. In Augmenting Neurological Disorder Prediction and Rehabilitation Using Artifi−cial Intelligence; Elsevier: Amsterdam, The Netherlands, 2022; pp. 35–52. [Google Scholar]
- Bressman, S.B. Dystonia genotypes, phenotypes, and classification. Adv. Neurol. 2004, 94, 101–107. [Google Scholar]
- Camargos, S.; Cardoso, F. Understanding dystonia: Diagnostic issues and how to overcome them. Arq. Neuropsiquiatr. 2016, 74, 921–936. [Google Scholar] [CrossRef] [PubMed]
- Kramer, P.L.; De Leon, D.; Ozelius, L.; Risch, N.; Bressman, S.B.; Brin, M.F.; Schuback, D.E.; Burke, R.E.; Kwiatkowski, D.J.; Shale, H.; et al. Dystonia gene in Ashkenazi Jewish population is located on chromosome 9q32-34. Ann. Neurol. 1990, 27, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Marras, C.; Lohmann, K.; Lang, A.; Klein, C. Fixing the broken system of genetic locus symbols: Parkinson disease and dystonia as examples. Neurology 2012, 78, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Marras, C.; Lang, A.; Van De Warrenburg, B.P.; Sue, C.M.; Tabrizi, S.J.; Bertram, L.; Mercimek-Mahmutoglu, S.; Ebrahimi-Fakhari, D.; Warner, T.; Durr, A.; et al. Nomenclature of genetic movement disorders: Recommendations of the international Parkinson and movement disorder society task force. Mov. Disord. 2016, 31, 436–457. [Google Scholar] [CrossRef]
- Lange, L.M.; Junker, J.; Loens, S.; Baumann, H.; Olschewski, L.; Schaake, S.; Madoev, H.; Petkovic, S.; Kuhnke, N.; Kasten, M.; et al. Genotype–Phenotype Relations for Isolated Dystonia Genes: MDSGene Systematic Review. Mov. Disord. 2021, 36, 1086–1103. [Google Scholar] [CrossRef]
- Weissbach, A.; Saranza, G.; Domingo, A. Combined dystonias: Clinical and genetic updates. J. Neural Transm. 2020, 128, 417–429. [Google Scholar] [CrossRef]
- Risch, N.J.; Bressman, S.B.; Senthil, G.; Ozelius, L.J. Intragenic Cis and Trans Modification of Genetic Susceptibility in DYT1 Torsion Dystonia. Am. J. Hum. Genet. 2007, 80, 1188–1193. [Google Scholar] [CrossRef]
- Djarmati, A.; A Schneider, S.; Lohmann, K.; Winkler, S.; Pawlack, H.; Hagenah, J.; Brüggemann, N.; Zittel, S.; Fuchs, T.; Raković, A.; et al. Mutations in THAP1 (DYT6) and generalised dystonia with prominent spasmodic dysphonia: A genetic screening study. Lancet Neurol. 2009, 8, 447–452. [Google Scholar] [CrossRef]
- Hersheson, J.; Mencacci, N.E.; Davis, M.; Macdonald, N.; Trabzuni, D.; Ryten, M.; Pittman, A.; Paudel, R.; Kara, E.; Fawcett, K.; et al. Mutations in the autoregulatory domain of β-tubulin 4a cause hereditary dystonia. Ann. Neurol. 2012, 73, 546–553. [Google Scholar] [CrossRef]
- Wilcox, R.A.; Winkler, S.; Lohmann, K.; Klein, C. Whispering dysphonia in an Australian family (DYT4): A clinical and genetic reappraisal. Mov. Disord. 2011, 26, 2404–2408. [Google Scholar] [CrossRef]
- Artusi, C.A.; Dwivedi, A.; Romagnolo, A.; Bortolani, S.; Marsili, L.; Imbalzano, G.; Sturchio, A.; Keeling, E.G.; Zibetti, M.; Contarino, M.F.; et al. Differential response to pallidal deep brain stimulation among monogenic dystonias: Systematic review and meta−analysis. J. Neurol. Neurosurg. Psychiatry 2020, 91, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Rajan, R.; Garg, K.; Saini, A.; Radhakrishnan, D.M.; Carecchio, M.; Bk, B.; Singh, M.; Srivastava, A.K. GPi-DBS for KMT2B-Associated Dystonia: Systematic Review and Meta-Analysis. Mov. Disord. Clin. Pract. 2021, 9, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.-T.; Li, L.-X.; Liu, Y.; Zhang, X.-L.; Pan, Y.-G.; Wang, L.; Wan, X.-H.; Jin, L.-J. The expanding clinical and genetic spectrum of ANO3 dystonia. Neurosci. Lett. 2020, 746, 135590. [Google Scholar] [CrossRef] [PubMed]
- Sarva, H.; Trosch, R.; Kiss, Z.H.; Furtado, S.; Luciano, M.S.; Glickman, A.; Ms, D.R.; Ozelius, L.J.; Bressman, S.B.; Saunders-Pullman, R. Deep Brain Stimulation in Isolated Dystonia With a GNAL Mutation. Mov. Disord. 2018, 34, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, H.; Ohye, T.; Takahashi, E.-I.; Seki, N.; Hori, T.-A.; Segawa, M.; Nomura, Y.; Endo, K.; Tanaka, H.; Tsuji, S.; et al. Hereditary progressive dystonia with marked diurnal fluctuation caused by mutations in the GTP cyclohydrolase I gene. Nat. Genet. 1994, 8, 236–242. [Google Scholar] [CrossRef]
- Salles, P.A.; Terán-Jimenez, M.; Vidal-Santoro, A.; Chaná-Cuevas, P.; Kauffman, M.; Espay, A.J. Recognizing Atypical Dopa−Responsive Dystonia and Its Mimics. Neurol. Clin. Pract. 2021, 11, e876–e884. [Google Scholar] [CrossRef]
- Dworniczak, B.; Lüdecke, B.; Bartholomé, K. A point mutation in the tyrosine hydroxylase gene associated with Segawa’s syndrome. Qual. Life Res. 1995, 95, 123–125. [Google Scholar] [CrossRef]
- Wevers, R.A.; Andel, J.F.D.R.-V.; Bräutigam, C.; Geurtz, B.; Heuvel, L.P.W.J.V.D.; Steenbergen-Spanjers, G.C.H.; Smeitink, J.A.M.; Hoffmann, G.F.; Gabreëls, F.J.M. A review of biochemical and molecular genetic aspects of tyrosine hydroxylase deficiency including a novel mutation (291delC). J. Inherit. Metab. Dis. 1999, 22, 364–373. [Google Scholar] [CrossRef]
- Stamelou, M.; Mencacci, N.E.; Cordivari, C.; Batla, A.; Wood, N.W.; Houlden, H.; Hardy, J.; Bhatia, K.P. Myoclonus−dystonia syndrome due to tyrosine hydroxylase deficiency. Neurology 2012, 79, 435–441. [Google Scholar] [CrossRef]
- Weissbach, A.; Pauly, M.G.; Herzog, R.; Hahn, L.; Halmans, S.; Hamami, F.; Bolte, C.; Camargos, S.; Jeon, B.; Kurian, M.A.; et al. Relationship of Genotype, Phenotype, and Treatment in Dopa-Responsive Dystonia: MDSGene Review. Mov. Disord. 2021, 37, 237–252. [Google Scholar] [CrossRef]
- Makino, S.; Kaji, R.; Ando, S.; Tomizawa, M.; Yasuno, K.; Goto, S.; Matsumoto, S.; Tabuena, M.D.; Maranon, E.; Dantes, M.; et al. Reduced Neuron-Specific Expression of the TAF1 Gene Is Associated with X-Linked Dystonia-Parkinsonism. Am. J. Hum. Genet. 2007, 80, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.V.; Pascasio, F.M.; Fuentes, F.D.; Viterbo, G.H. Torsion dystonia in Panay, Philippines. Adv. Neurol. 1976, 14, 137–151. [Google Scholar]
- Brüggemann, N.; Heldmann, M.; Klein, C.; Domingo, A.; Rasche, D.; Tronnier, V.; Rosales, R.L.; Jamora, R.D.G.; Lee, L.V.; Münte, T.F. Neuroanatomical changes extend beyond striatal atrophy in X-linked dystonia parkinsonism. Park. Relat. Disord. 2016, 31, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Song, P.C.; Le Bs, H.; Acuna, P.; De Guzman, J.K.P.; Sharma, N.; Ba, T.N.F.; Dy, M.E.; Go, C.L. Voice and swallowing dysfunction in X-linked dystonia parkinsonism. Laryngoscope 2019, 130, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Brashear, A.; Dobyns, W.; Aguiar, P.D.C.; Borg, M.; Frijns, C.J.M.; Gollamudi, S.; Green, A.; Guimaraes, J.; Haake, B.C.; Klein, C.; et al. The phenotypic spectrum of rapid−onset dystonia−parkinsonism (RDP) and mutations in the ATP1A3 gene. Brain 2007, 130, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, P.D.C.; Sweadner, K.J.; Penniston, J.T.; Zaremba, J.; Liu, L.; Caton, M.; Linazasoro, G.; Borg, M.; Tijssen, M.A.; Bressman, S.B.; et al. Mutations in the Na+/K+−ATPase α3 Gene ATP1A3 Are Associated with Rapid−Onset Dystonia Parkinsonism. Neuron 2004, 43, 169–175. [Google Scholar] [CrossRef]
- Mencacci, N.E.; Rubio-Agusti, I.; Zdebik, A.; Asmus, F.; Ludtmann, M.H.; Ryten, M.; Plagnol, V.; Hauser, A.-K.; Bandres-Ciga, S.; Bettencourt, C.; et al. A Missense Mutation in KCTD17 Causes Autosomal Dominant Myoclonus−Dystonia. Am. J. Hum. Genet. 2015, 96, 938–947. [Google Scholar] [CrossRef]
- Ferrini, A.; Steel, D.; Barwick, K.; Kurian, M.A. An Update on the Phenotype, Genotype and Neurobiology of ADCY5-Related Disease. Mov. Disord. 2021, 36, 1104–1114. [Google Scholar] [CrossRef]
- Méneret, A.; Gras, D.; McGovern, E.; Roze, E. Caffeine and the Dyskinesia Related to Mutations in the ADCY5 Gene. Ann. Intern. Med. 2019, 171, 439. [Google Scholar] [CrossRef]
- Albanese, A.; Asmus, F.; Bhatia, K.; Elia, A.E.; Elibol, B.; Filippini, G.; Gasser, T.; Krauss, J.K.; Nardocci, N.; Newton, A.; et al. EFNS guidelines on diagnosis and treatment of primary dystonias. Eur. J. Neurol. 2010, 18, 5–18. [Google Scholar] [CrossRef]
- Bressman, S.B.; Sabatti, C.; Raymond, D.; de Leon, D.; Klein, C.; Kramer, P.L.; Brin, M.F.; Fahn, S.; Breakefield, X.; Ozelius, L.J.; et al. The DYT1 phenotype and guidelines for diagnostic testing. Neurology 2000, 54, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- American Society of Human Genetics Board of Directors. Points to consider: Ethical, legal, and psychosocial implications of genetic testing in children and adolescents. Am. J. Hum. Genet. 1995, 57, 1233–1241. [Google Scholar]
- Klein, C.; Friedman, J.; Bressman, S.; Vieregge, P.; Brin, M.F.; Pramstaller, P.P.; de Leon, D.; Hagenah, J.; Sieberer, M.; Fleet, C.; et al. Genetic Testing for Early-Onset Torsion Dystonia (DYT1): Introduction of a Simple Screening Method, Experiences from Testing of a Large Patient Cohort, and Ethical Aspects. Genet. Test. 1999, 3, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.; McCarthy, G.T.; Bandmann, O.; Dobbie, M.; Surtees, R.; Wood, N. GTP cyclohydrolase deficiency; intrafamilial variation in clinical phenotype, including levodopa responsiveness. J. Neurol. Neurosurg. Psychiatry 1999, 66, 86–89. [Google Scholar] [CrossRef]
- Valente, E.M.; Edwards, M.J.; Mir, P.; DiGiorgio, A.; Salvi, S.; Davis, M.; Russo, N.; Bozi, M.; Kim, H.-T.; Pennisi, G.; et al. The epsilon−sarcoglycan gene in myoclonic syndromes. Neurology 2005, 64, 737–739. [Google Scholar] [CrossRef]
- Zech, M.; Jech, R.; Boesch, S.; Škorvánek, M.; Weber, S.; Wagner, M.; Zhao, C.; Jochim, A.; Necpál, J.; Dincer, Y.; et al. Monogenic variants in dystonia: An exome−wide sequencing study. Lancet Neurol. 2020, 19, 908–918. [Google Scholar] [CrossRef]
- Klein, C.; Lohmann, K.; Marras, C.; Münchau, A. Hereditary Dystonia Overview. In GeneReviews(®); Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Müller, B.; Hedrich, K.; Kock, N.; Dragasevic, N.; Svetel, M.; Garrels, J.; Landt, O.; Nitschke, M.; Pramstaller, P.P.; Reik, W.; et al. Evidence That Paternal Expression of the ε-Sarcoglycan Gene Accounts for Reduced Penetrance in Myoclonus-Dystonia. Am. J. Hum. Genet. 2002, 71, 1303–1311. [Google Scholar] [CrossRef]
- Arlotti, M.; Colombo, M.; Bonfanti, A.; Mandat, T.; Lanotte, M.M.; Pirola, E.; Borellini, L.; Rampini, P.; Eleopra, R.; Rinaldo, S.; et al. A New Implantable Closed−Loop Clinical Neural Interface: First Application in Parkinson’s Disease. Front. Neurosci. 2021, 15, 763235. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).