Inflammatory Indexes as Prognostic Factors of Survival in Geriatric Patients with Hepatocellular Carcinoma: A Case Control Study of Eight Slovak Centers
Abstract
1. Introduction
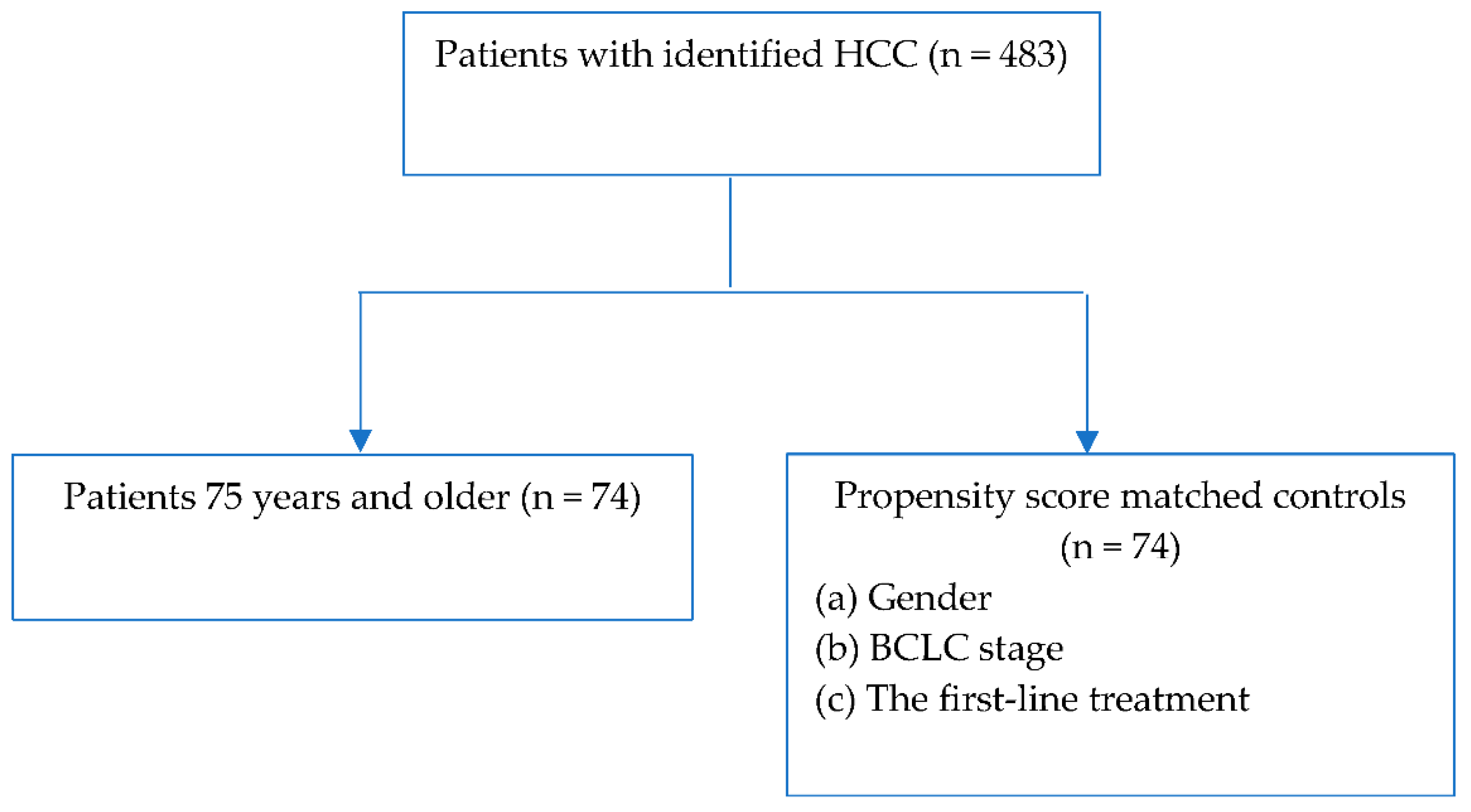
2. Materials and Methods
3. Statistical Analyses
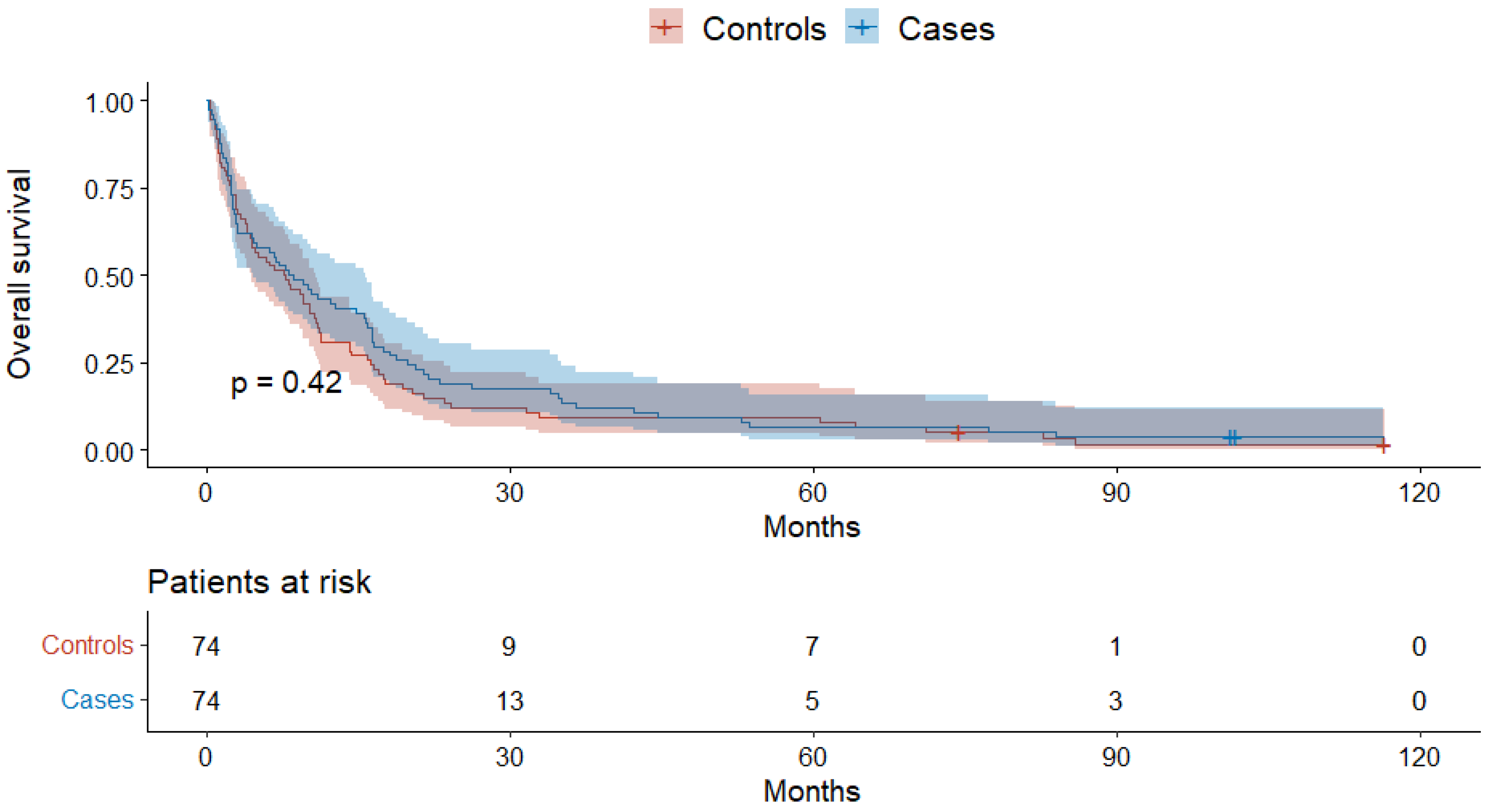
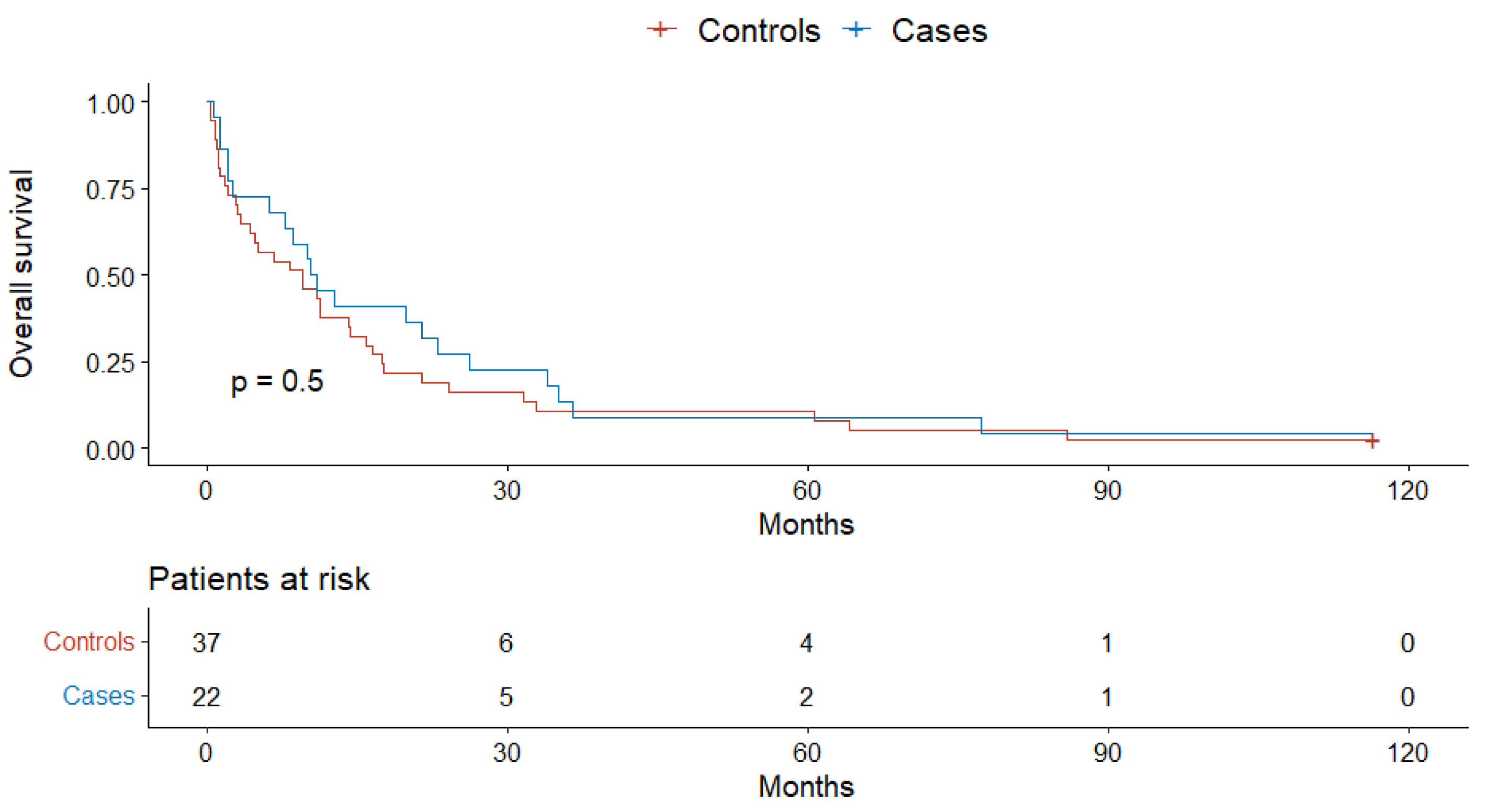
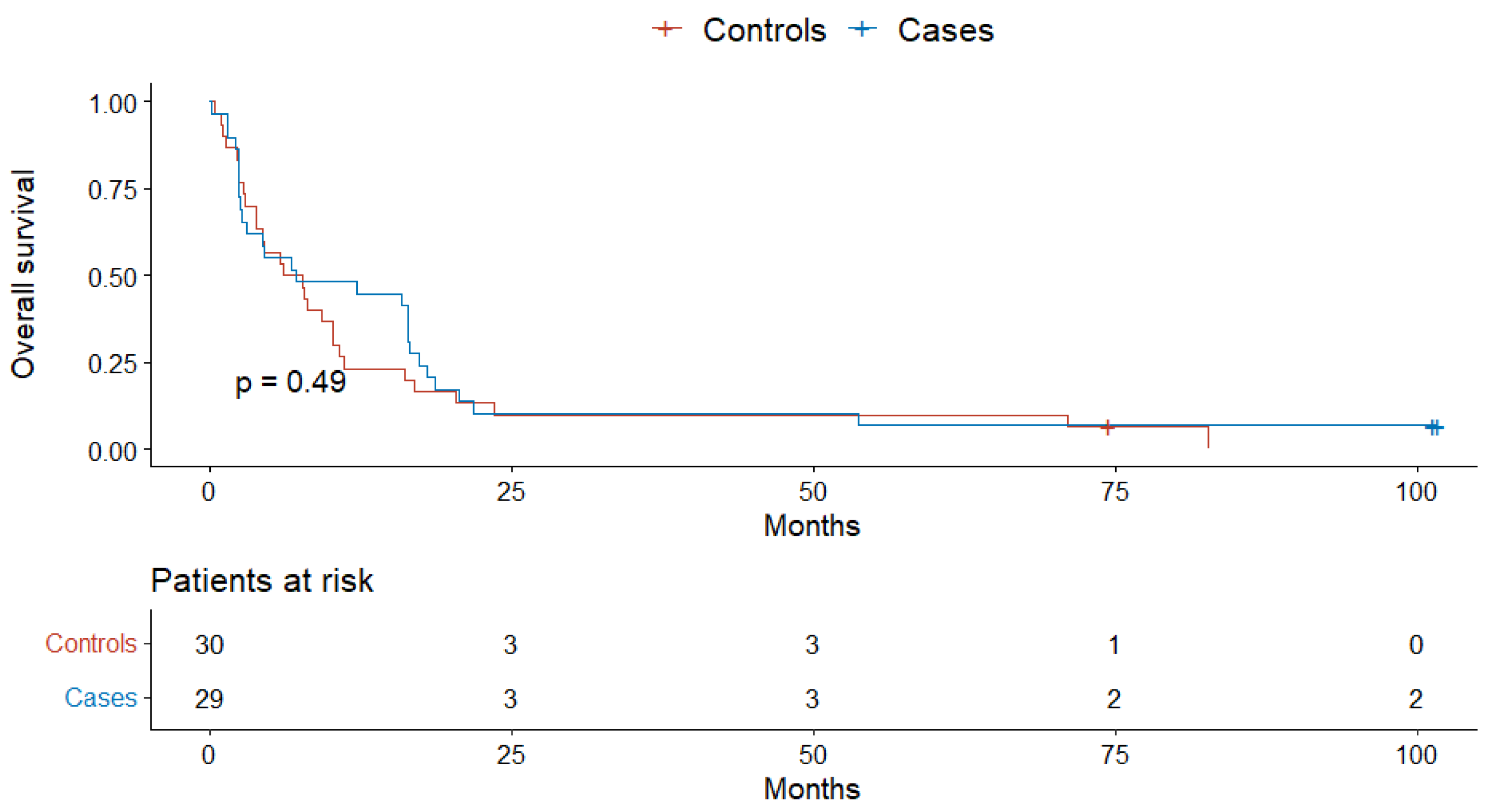
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.-L.; Schirmacher, P.; Vilgrain, V. Easl clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beg, M.S.; Brower, S.T.; Gade, T.P.; Goff, L.; Gupta, S.; Guy, J.; Harris, W.P.; et al. Systemic therapy for advanced hepatocellular carcinoma: Asco guideline. J. Clin. Oncol. 2020, 38, 4317–4345. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the american association for the study of liver diseases. Clin. Liver Dis. 2019, 13, 1. [Google Scholar] [CrossRef]
- Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.C.; Neumann, U.; Ricke, J.; Sangro, B.; et al. Hepatocellular carcinoma: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv238–iv255. [Google Scholar] [CrossRef]
- El–Serag, H.B.; Rudolph, K.L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef]
- Hung, G.-Y.; Horng, J.-L.; Yen, H.-J.; Lee, C.-Y.; Lin, L.-Y. Changing incidence patterns of hepatocellular carcinoma among age groups in taiwan. J. Hepatol. 2015, 63, 1390–1396. [Google Scholar] [CrossRef]
- Li, L.; Yu, R.; Cai, T.; Chen, Z.; Lan, M.; Zou, T.; Wang, B.; Wang, Q.; Zhao, Y.; Cai, Y. Effects of immune cells and cytokines on inflammation and immunosuppression in the tumor microenvironment. Int. Immunopharmacol. 2020, 88, 106939. [Google Scholar] [CrossRef]
- Hu, B.; Yang, X.-R.; Xu, Y.; Sun, Y.-F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.-M.; Qiu, S.-J.; Zhou, J.; et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef]
- Li, X.; Tan, W.; Sun, W.; Zhao, L.; Wang, C.; Zang, A.; Kong, X. Preablation neutrophil-to-lymphocyte ratio as an independent prognostic factor in locally advanced hepatocellular carcinoma patients following radiofrequency ablation. J. Cancer Res. Ther. 2018, 14, 84. [Google Scholar] [CrossRef]
- Otsuka, M.; Li, S.; Feng, X.; Cao, G.; Wang, Q.; Wang, L. Prognostic significance of inflammatory indices in hepatocellular carcinoma treated with transarterial chemoembolization: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0230879. [Google Scholar]
- Shen, Y.; Wang, H.; Chen, X.; Li, W.; Chen, J. Prognostic significance of lymphocyte-to-monocyte ratio and platelet-to-lymphocyte ratio in patients with hepatocellular carcinoma undergoing transcatheter arterial chemoembolization and radiofrequency ablation. OncoTargets Ther. 2019, 12, 7129–7137. [Google Scholar] [CrossRef]
- Wang, D.; Bai, N.; Hu, X.; OuYang, X.W.; Yao, L.; Tao, Y.; Wang, Z. Preoperative inflammatory markers of nlr and plr as indicators of poor prognosis in resectable hcc. PeerJ 2019, 7, e7132. [Google Scholar] [CrossRef]
- Xin, Y.; Yang, Y.; Liu, N.; Chen, Y.; Wang, Y.; Zhang, X.; Li, X.; Zhou, X. Prognostic significance of systemic immune-inflammation index- based nomogram for early stage hepatocellular carcinoma after radiofrequency ablation. J. Gastrointest. Oncol. 2021, 12, 735–750. [Google Scholar] [CrossRef]
- Zahorec, R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl. Lek. Listy 2001, 102, 5–14. [Google Scholar]
- Li, X.; Chen, Z.-H.; Xing, Y.-F.; Wang, T.-T.; Wu, D.-H.; Wen, J.-Y.; Chen, J.; Lin, Q.; Dong, M.; Wei, L.; et al. Platelet-to-lymphocyte ratio acts as a prognostic factor for patients with advanced hepatocellular carcinoma. Tumor Biol. 2014, 36, 2263–2269. [Google Scholar] [CrossRef]
- Ding, P.A.; Yang, P.; Sun, C.; Tian, Y.; Guo, H.; Liu, Y.; Li, Y.; Zhao, Q. Predictive effect of systemic immune-inflammation index combined with prognostic nutrition index score on efficacy and prognosis of neoadjuvant intraperitoneal and systemic paclitaxel combined with apatinib conversion therapy in gastric cancer patients with positive peritoneal lavage cytology: A prospective study. Front. Oncol. 2022, 11, 791912. [Google Scholar]
- Malinchoc, M.; Kamath, P.S.; Gordon, F.D.; Peine, C.J.; Rank, J.; ter Borg, P.C.J. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000, 31, 864–871. [Google Scholar] [CrossRef]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach—the albi grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Di Costanzo, G.G.; Tortora, R.; De Luca, M.; Galeota Lanza, A.; Lampasi, F.; Tartaglione, M.T.; Picciotto, F.P.; Imparato, M.; Mattera, S.; Cordone, G.; et al. Impact of age on toxicity and efficacy of sorafenib-targeted therapy in cirrhotic patients with hepatocellular carcinoma. Med. Oncol. 2013, 30, 446. [Google Scholar] [CrossRef]
- Golfieri, R.; Bilbao, J.I.; Carpanese, L.; Cianni, R.; Gasparini, D.; Ezziddin, S.; Paprottka, P.M.; Fiore, F.; Cappelli, A.; Rodriguez, M.; et al. Comparison of the survival and tolerability of radioembolization in elderly vs. Younger patients with unresectable hepatocellular carcinoma. J. Hepatol. 2013, 59, 753–761. [Google Scholar] [CrossRef]
- Kozyreva, O.N.; Chi, D.; Clark, J.W.; Wang, H.; Theall, K.P.; Ryan, D.P.; Zhu, A.X. A multicenter retrospective study on clinical characteristics, treatment patterns, and outcome in elderly patients with hepatocellular carcinoma. Oncologist 2011, 16, 310–318. [Google Scholar] [CrossRef]
- Mirici-Cappa, F.; Gramenzi, A.; Santi, V.; Zambruni, A.; Di Micoli, A.; Frigerio, M.; Maraldi, F.; Di Nolfo, M.A.; Del Poggio, P.; Benvegnu, L.; et al. Treatments for hepatocellular carcinoma in elderly patients are as effective as in younger patients: A 20-year multicentre experience. Gut 2010, 59, 387–396. [Google Scholar] [CrossRef]
- Nishikawa, H.; Kita, R.; Kimura, T.; Ohara, Y.; Takeda, H.; Sakamoto, A.; Saito, S.; Nishijima, N.; Nasu, A.; Komekado, H.; et al. Transcatheter arterial chemoembolization for intermediate-stage hepatocellular carcinoma: Clinical outcome and safety in elderly patients. J. Cancer 2014, 5, 590–597. [Google Scholar] [CrossRef]
- Wong, H.; Tang, Y.F.; Yao, T.-J.; Chiu, J.; Leung, R.; Chan, P.; Cheung, T.T.; Chan, A.C.; Pang, R.W.; Poon, R.; et al. The outcomes and safety of single-agent sorafenib in the treatment of elderly patients with advanced hepatocellular carcinoma (hcc). Oncologist 2011, 16, 1721–1728. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, G.; Sun, H.; Ding, J.; Xia, F.; Li, X.; Ma, K.; Wang, S.; Bie, P. Radiofrequency ablation of hepatocellular carcinoma in elderly patients fitting the milan criteria: A single centre with 13 years experience. Int. J. Hyperth. 2014, 30, 471–479. [Google Scholar] [CrossRef]
- Murthy, V.H.; Krumholz, H.M.; Gross, C.P. Participation in cancer clinical trials. JAMA 2004, 291, 2720. [Google Scholar] [CrossRef]
- Tajiri, K. Liver physiology and liver diseases in the elderly. World J. Gastroenterol. 2013, 19, 8459. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Kimura, T.; Kita, R.; Osaki, Y. Treatment for hepatocellular carcinoma in elderly patients: A literature review. J. Cancer 2013, 4, 635–643. [Google Scholar] [CrossRef]
- Nishikawa, H.; Arimoto, A.; Wakasa, T.; Kita, R.; Kimura, T.; Osaki, Y. Surgical resection for hepatocellular carcinoma. Eur. J. Gastroenterol. Hepatol. 2013, 25, 912–919. [Google Scholar] [CrossRef]
- Nomi, T.; Hirokawa, F.; Kaibori, M.; Ueno, M.; Tanaka, S.; Hokuto, D.; Noda, T.; Nakai, T.; Ikoma, H.; Iida, H.; et al. Laparoscopic versus open liver resection for hepatocellular carcinoma in elderly patients: A multi-centre propensity score-based analysis. Surg. Endosc. 2019, 34, 658–666. [Google Scholar] [CrossRef]
- Hiraoka, A.; Michitaka, K.; Horiike, N.; Hidaka, S.; Uehara, T.; Ichikawa, S.; Hasebe, A.; Miyamoto, Y.; Ninomiya, T.; Sogabe, I.; et al. Radiofrequency ablation therapy for hepatocellular carcinoma in elderly patients. J. Gastroenterol. Hepatol. 2010, 25, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Mizuta, T.; Kawazoe, S.; Eguchi, Y.; Kawaguchi, Y.; Otuka, T.; Oeda, S.; Ario, K.; Iwane, S.; Akiyama, T.; et al. Efficacy and safety of radiofrequency ablation for elderly hepatocellular carcinoma patients. Hepatol. Res. 2010, 40, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.J. Trans-arterial chemo-embolization is safe and effective for very elderly patients with hepatocellular carcinoma. World J. Gastroenterol. 2013, 19, 2521. [Google Scholar] [CrossRef]
- Cohen, M.J.; Levy, I.; Barak, O.; Bloom, A.I.; Fernández-Ruiz, M.; Di Maio, M.; Perrone, F.; Poon, R.T.; Shouval, D.; Yau, T.; et al. Trans-arterial chemo-embolization is safe and effective for elderly advanced hepatocellular carcinoma patients: Results from an international database. Liver Int. 2014, 34, 1109–1117. [Google Scholar] [CrossRef]
- Hajiev, S.; Allara, E.; Motedayen Aval, L.; Arizumi, T.; Bettinger, D.; Pirisi, M.; Rimassa, L.; Pressiani, T.; Personeni, N.; Giordano, L.; et al. Impact of age on sorafenib outcomes in hepatocellular carcinoma: An international cohort study. Br. J. Cancer 2020, 124, 407–413. [Google Scholar] [CrossRef]
- Nishikawa, H.; Takeda, H.; Tsuchiya, K.; Joko, K.; Ogawa, C.; Taniguchi, H.; Orito, E.; Uchida, Y.; Osaki, Y.; Izumi, N.; et al. Sorafenib therapy for bclc stage b/c hepatocellular carcinoma; clinical outcome and safety in aged patients: A multicenter study in japan. J. Cancer 2014, 5, 499–509. [Google Scholar] [CrossRef][Green Version]
- Ziogas, D.C.; Papadatos-Pastos, D.; Thillai, K.; Korantzis, I.; Chowdhury, R.; Suddle, A.; O’Grady, J.; Al-Khadimi, G.; Allen, N.; Heaton, N.; et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma. Eur. J. Gastroenterol. Hepatol. 2017, 29, 48–55. [Google Scholar] [CrossRef]
- Tada, T.; Kumada, T.; Hiraoka, A.; Michitaka, K.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; Takaguchi, K.; Kariyama, K.; et al. Safety and efficacy of lenvatinib in elderly patients with unresectable hepatocellular carcinoma: A multicenter analysis with propensity score matching. Hepatol. Res. 2019, 50, 75–83. [Google Scholar] [CrossRef]
- Kudo, M.; Galle, P.R.; Llovet, J.M.; Finn, R.S.; Vogel, A.; Motomura, K.; Assenat, E.; Merle, P.; Brandi, G.; Daniele, B.; et al. Ramucirumab in elderly patients with hepatocellular carcinoma and elevated alpha-fetoprotein after sorafenib in reach and reach-2. Liver Int. 2020, 40, 2008–2020. [Google Scholar] [CrossRef]
- Liu, Y.-W.; Yong, C.-C.; Lin, C.-C.; Wang, C.-C.; Chen, C.-L.; Cheng, Y.-F.; Wang, J.-H.; Yen, Y.-H. Liver resection in elderly patients with hepatocellular carcinoma: Age does matter. Updates Surg. 2021, 73, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Mbeunkui, F.; Johann, D.J. Cancer and the tumor microenvironment: A review of an essential relationship. Cancer Chemother. Pharmacol. 2008, 63, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Heymann, F.; Peusquens, J.; Ludwig-Portugall, I.; Kohlhepp, M.; Ergen, C.; Niemietz, P.; Martin, C.; van Rooijen, N.; Ochando, J.C.; Randolph, G.J.; et al. Liver inflammation abrogates immunological tolerance induced by kupffer cells. Hepatology 2015, 62, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Ringelhan, M.; Pfister, D.; O’Connor, T.; Pikarsky, E.; Heikenwalder, M. The immunology of hepatocellular carcinoma. Nat. Immunol. 2018, 19, 222–232. [Google Scholar] [CrossRef]
- Östman, A.; Heldin, C.H. Pdgf receptors as targets in tumor treatment. Adv. Cancer Res. 2007, 97, 247–274. [Google Scholar]
- Salazar-Onfray, F.; López, M.N.; Mendoza-Naranjo, A. Paradoxical effects of cytokines in tumor immune surveillance and tumor immune escape. Cytokine Growth Factor Rev. 2007, 18, 171–182. [Google Scholar] [CrossRef]
- Gan, Y.; Zhou, X.; Niu, X.; Li, J.; Wang, T.; Zhang, H.; Yang, Y.; Liu, Y.; Mao, Q. Neutrophil/lymphocyte ratio is an independent prognostic factor in elderly patients with high-grade gliomas. World Neurosurg. 2019, 127, e261–e267. [Google Scholar] [CrossRef]
- Mandaliya, H.; Jones, M.; Oldmeadow, C.; Nordman, I.I.C. Prognostic biomarkers in stage iv non-small cell lung cancer (nsclc): Neutrophil to lymphocyte ratio (nlr), lymphocyte to monocyte ratio (lmr), platelet to lymphocyte ratio (plr) and advanced lung cancer inflammation index (ali). Transl. Lung Cancer Res. 2019, 8, 886–894. [Google Scholar] [CrossRef]
- Peng, H.; Tan, X. The prognostic significance of sarcopenia and the neutrophil-to-lymphocyte ratio in elderly patients with esophageal squamous cell carcinoma. Cancer Manag. Res. 2021, 13, 3209–3218. [Google Scholar] [CrossRef]
- Hirahara, N.; Tajima, Y.; Matsubara, T.; Fujii, Y.; Kaji, S.; Kawabata, Y.; Hyakudomi, R.; Yamamoto, T.; Uchida, Y.; Taniura, T. Systemic immune-inflammation index predicts overall survival in patients with gastric cancer: A propensity score–matched analysis. J. Gastrointest. Surg. 2020, 25, 1124–1133. [Google Scholar] [CrossRef]
- Saito, H.; Kono, Y.; Murakami, Y.; Shishido, Y.; Kuroda, H.; Matsunaga, T.; Fukumoto, Y.; Osaki, T.; Ashida, K.; Fujiwara, Y. Prognostic significance of platelet-based inflammatory indicators in patients with gastric cancer. World J. Surg. 2018, 42, 2542–2550. [Google Scholar] [CrossRef]
- Ng, S.S.Q.; Chua, G.W.Y.; Kusumawidjaja, G.; Wong, F.Y.; Tham, C.K.; Chua, E.T.; Chua, M.L.K.; Chua, K.L.M. Evaluation of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as prognostic biomarkers for elderly patients with glioblastoma treated with chemoradiation. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, E97. [Google Scholar] [CrossRef][Green Version]
- Zarour, L.R.; Billingsley, K.G.; Walker, B.S.; Enestvedt, C.K.; Orloff, S.L.; Maynard, E.; Mayo, S.C. Hepatic resection of solitary hcc in the elderly: A unique disease in a growing population. Am. J. Surg. 2019, 217, 899–905. [Google Scholar] [CrossRef]
- Riauka, R.; Ignatavicius, P.; Barauskas, G. Preoperative platelet to lymphocyte ratio as a prognostic factor for resectable pancreatic cancer: A systematic review and meta-analysis. Dig. Surg. 2020, 37, 447–455. [Google Scholar] [CrossRef]
- Sanabria, A.; Carvalho, A.L.; Vartanian, J.G.; Magrin, J.; Ikeda, M.K.; Kowalski, L.P. Comorbidity is a prognostic factor in elderly patients with head and neck cancer. Ann. Surg. Oncol. 2007, 14, 1449–1457. [Google Scholar] [CrossRef]
- Lemmens, V.E.P.P.; van Halteren, A.H.; Janssen-Heijnen, M.L.G.; Vreugdenhil, G.; Repelaer van Driel, O.J.; Coebergh, J.W.W. Adjuvant treatment for elderly patients with stage iii colon cancer in the southern netherlands is affected by socioeconomic status, gender, and comorbidity. Ann. Oncol. 2005, 16, 767–772. [Google Scholar] [CrossRef]
- De Boer, A.Z.; Bastiaannet, E.; Putter, H.; Marang-van de Mheen, P.J.; Siesling, S.; de Munck, L.; de Ligt, K.M.; Portielje, J.E.A.; Liefers, G.J.; de Glas, N.A. Prediction of other-cause mortality in older patients with breast cancer using comorbidity. Cancers 2021, 13, 1627. [Google Scholar] [CrossRef]

| Overall, n = 148 1 | <75 years, n = 74 1 | ≥75 years, n = 74 1 | p-Value 2 | |
|---|---|---|---|---|
| Age | 74 (65, 78) | 64 (61, 69) | 78 (76, 82) | <0.001 |
| Sex (male) | 84 (57%) | 42 (57%) | 42 (57%) | >0.9 |
| Etiology (the most common) | 0.2 | |||
| HCV | 24 (16%) | 15 (20%) | 9 (12%) | |
| ALD | 54 (36%) | 29 (39%) | 25 (34%) | |
| NAFLD | 25 (17%) | 12 (16%) | 13 (18%) | |
| BCLC stage | >0.9 | |||
| 0-A | 16 (11%) | 8 (11%) | 8 (11%) | |
| B | 35 (24%) | 18 (24%) | 18 (24%) | |
| C | 62 (42%) | 31 (42%) | 31 (42%) | |
| D | 35 (24%) | 17 (23%) | 17 (23%) | |
| Child–Pugh | >0.9 | |||
| A | 70 (49%) | 34 (48%) | 36 (50%) | |
| B | 61 (43%) | 31 (44%) | 30 (42%) | |
| C | 12 (8.4%) | 6 (8.5%) | 6 (8.3%) | |
| MELD | 6.26 (5.94, 6.64) | 6.25 (5.92, 6.57) | 6.30 (6.06, 6.75) | 0.2 |
| Number of lesions | 1.00 (1.00, 4.00) | 1.00 (1.00, 4.00) | 1.00 (1.00, 4.00) | 0.7 |
| Diameter of the largest lesion | 62 (35, 94) | 56 (32, 92) | 65 (40, 100) | 0.2 |
| AFP | 27 (6, 1105) | 21 (7, 589) | 46 (6, 1624) | 0.8 |
| CRP | 12 (4, 42) | 12 (4, 60) | 14 (3, 37) | 0.3 |
| NLR | 3.6 (2.4, 5.7) | 3.8 (2.4, 6.6) | 3.5 (2.5, 5.4) | 0.6 |
| PLR | 159 (102, 223) | 159 (95, 223) | 158 (109, 221) | >0.9 |
| SII | 641 (312, 1318) | 529 (301, 1399) | 682 (464, 1308) | 0.4 |
| ALBI | −1.90 (−2.39, −1.36) | −1.90 (−2.38, −1.31) | −1.90 (−2.47, −1.46) | 0.7 |
| Albumin | 33 (28, 38) | 33 (28, 38) | 34 (28, 38) | 0.7 |
| Creatinine | 81 (71, 105) | 78 (69, 97) | 87 (71, 112) | 0.08 |
| Total bilirubin | 22 (14, 35) | 22 (15, 38) | 22 (13, 32) | 0.5 |
| Alanine aminotransferase | 0.68 (0.47, 1.15) | 0.68 (0.46, 1.10) | 0.70 (0.48, 1.23) | 0.6 |
| Aspartate aminotransferase | 1.07 (0.62, 2.05) | 1.06 (0.69, 2.00) | 1.07 (0.59, 2.14) | 0.6 |
| Gamma-glutamyl transferase | 2.5 (1.3, 4.7) | 2.4 (1.4, 4.7) | 2.7 (1.2, 4.7) | 0.5 |
| Alkaline phosphatase | 2.17 (1.72, 4.06) | 2.35 (1.77, 4.23) | 2.16 (1.65, 3.73) | 0.3 |
| First-line treatment | 0.9 | |||
| Resection | 10 (6.8%) | 5 (6.8%) | 5 (6.8%) | |
| Radiofrequency ablation | 2 (1.4%) | 1 (1.4%) | 1 (1.4%) | |
| Transarterial chemoembolization | 32 (22%) | 16 (22%) | 16 (22%) | |
| Sorafenib | 49 (33%) | 25 (34%) | 25 (34%) | |
| Best supportive care | 53 (36%) | 26 (35%) | 26 (35%) | |
| Liver transplantation | 2 (1.4%) | 1 (1.4%) | 1 (1.4%) | |
| ECOG Performance Status | 0.5 | |||
| 0 | 5 (3.4%) | 4 (5.4%) | 1 (1.4%) | |
| 1 | 106 (72%) | 52 (70%) | 54 (73%) | |
| 2 | 37 (25%) | 18 (24%) | 19 (26%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safcak, D.; Drazilova, S.; Gazda, J.; Andrasina, I.; Adamcova-Selcanova, S.; Balazova, L.; Barila, R.; Mego, M.; Rac, M.; Skladany, L.; et al. Inflammatory Indexes as Prognostic Factors of Survival in Geriatric Patients with Hepatocellular Carcinoma: A Case Control Study of Eight Slovak Centers. J. Clin. Med. 2022, 11, 4183. https://doi.org/10.3390/jcm11144183
Safcak D, Drazilova S, Gazda J, Andrasina I, Adamcova-Selcanova S, Balazova L, Barila R, Mego M, Rac M, Skladany L, et al. Inflammatory Indexes as Prognostic Factors of Survival in Geriatric Patients with Hepatocellular Carcinoma: A Case Control Study of Eight Slovak Centers. Journal of Clinical Medicine. 2022; 11(14):4183. https://doi.org/10.3390/jcm11144183
Chicago/Turabian StyleSafcak, Dominik, Sylvia Drazilova, Jakub Gazda, Igor Andrasina, Svetlana Adamcova-Selcanova, Lea Balazova, Radovan Barila, Michal Mego, Marek Rac, Lubomir Skladany, and et al. 2022. "Inflammatory Indexes as Prognostic Factors of Survival in Geriatric Patients with Hepatocellular Carcinoma: A Case Control Study of Eight Slovak Centers" Journal of Clinical Medicine 11, no. 14: 4183. https://doi.org/10.3390/jcm11144183
APA StyleSafcak, D., Drazilova, S., Gazda, J., Andrasina, I., Adamcova-Selcanova, S., Balazova, L., Barila, R., Mego, M., Rac, M., Skladany, L., Zigrai, M., Janicko, M., & Jarcuska, P. (2022). Inflammatory Indexes as Prognostic Factors of Survival in Geriatric Patients with Hepatocellular Carcinoma: A Case Control Study of Eight Slovak Centers. Journal of Clinical Medicine, 11(14), 4183. https://doi.org/10.3390/jcm11144183








