Abstract
In many vascular segments, endovascular therapy is the treatment of choice for arteriosclerotic lesions. For the treatment of common femoral artery (CFA) lesions, surgical reconstruction is still considered the gold standard. The purpose of this study is to evaluate the safety and efficacy of stent angioplasty for the treatment of common femoral artery (CFA) lesions in a real-world population during a two-year follow up. This retrospective, single-center study includes 250 patients requiring treatment with stent angioplasty of CFA lesions. The primary end point was the target lesion revascularization (TLR) rate. Secondary end points were the overall procedural complication rate, the rate of ipsilateral CFA punctures during follow-up, changes in the Rutherford–Becker class (RBC) and ankle–brachial index (ABI), primary patency rates, amputation rate, time to and the type of TLR. A total of 236 interventions (94.4%) were successfully defined as a residual stenosis < 30%. Periinterventionally, there were 23 complications (9.1%), 3 of which had to be treated surgically. Median follow up was 21 months (average 19.2 ± 7.8). In total, 41 patients (16.4%) needed a TLR. The primary patency rate was 90.8%, 81.2% and 72% at 6, 12 and 24 months, respectively. ABI and RBC were significantly better at all time points compared to baseline. During follow up, seven amputations (three minor and four major) had to be performed. More than half of the patients (56.0%) were punctured at the stented CFA during the follow up. Multivariate logistic regression analysis showed continued nicotine use and coronary heart disease as predictors for TLR. Stent angioplasty for the treatment of CFA lesions is safe and effective. Further studies are needed to compare this endovascular option with surgical therapy.
1. Introduction
The incidence of peripheral artery disease (PAD) has increased worldwide [1]. Limitation of pain-free walking distance (intermittent claudication), rest pain or tissue ulceration represent the indications for treatment of PAD [2]. Due to lower invasiveness, endovascular therapy has become the treatment of choice over open surgical therapy in many arterial regions [2,3]. This does not yet include treatment of the common femoral artery (CFA). Here, surgical endarterectomy is still considered the therapeutic “gold standard”. The primary 1-year patency rates after surgical endarterectomy reported in the literature are 85–95% [4]. Numerous small studies indicate that endovascular therapy may have the potential to replace open surgery at least for some anatomical characteristics of CFA lesions [5,6,7,8,9]. In a retrospective study, the use of stents was associated with significant lower 1-year restenosis and target lesion revascularization (TLR) rate and was a protective factor against procedure failure [6]. The TECCO trial [10], a prospective, randomized, multicenter study comparing primary stent angioplasty and open surgical reconstruction for CFA lesions, documented comparable reintervention rates at 2 years. Because of remaining concerns about stent implantation in the CFA, the aim of the present retrospective, monocentric study is to evaluate the technical and clinical outcomes of stent angioplasty in CFA lesions in a real-world patient and lesion population.
2. Materials and Methods
Patients treated with atherosclerotic lesions of the CFA between January 2008 and December 2018 were retrospectively selected from a prospective maintained database. The study was approved by the ethics committee of the Albert-Ludwigs University Freiburg, Germany. Approval was given on 2 July 2020. Medical records, angiographies and endovascular procedures as well as duplex ultrasound examinations and ankle–brachial index (ABI) measurements were analyzed. Patients with PAD Rutherford–Becker class (RBC) 2 to 5 with a CFA stenosis ≥ 70% (estimated by duplex ultrasound with a peak systolic velocity ratio of >3.5 and/or visually on angiography) and stent angioplasty of this lesion were included in this analysis. Patients treated with other interventional techniques such as plain balloon angioplasty and atherectomy without final stent placement were excluded. CFA lesions were categorized according to the new classification of Rabellino, et al. [11].
Two-year target lesion revascularization (TLR) rate was the primary end point. Secondary end points were primary patency (restenosis was defined as stenosis with a peak systolic velocity ratio > 2.5 on color-flow duplex ultrasound measuring), overall procedural complication rate, the rate of ipsilateral CFA punctures during follow up, changes in RBC and ankle–brachial index (ABI), amputation rate and predictors on the reintervention rates. The procedural complication rate includes access site complications, target vessel perforation, outflow embolization and compartment syndrome. The time to TLR and the type of TLR (surgery or endovascular procedure) were documented.
According to department standard, follow up visits including physical examination, estimation of the RBC, ABI measurements and duplex ultrasound were scheduled for 6, 12 and 24 months post procedure.
Analyses were performed using SPSS software (version 25.0; SPSS, Chicago, IL, USA). Continuous data are presented as means ± standard deviation; categorical data are given as counts (percentages). TLR-free survival was evaluated using Kaplan–Meier analysis; the survival curves were compared using the Mantel–Cox log-rank test. Univariate logistic regression analysis included the following variables: age, gender, index, smoking status, hypertension, dyslipidemia, diabetes mellitus, initial lesion grade (stenosis versus occlusion), lesion calcification, POBA, DCB or atherectomy use and post procedural residual stenosis. Outcomes of the regression analysis are given as an odds ratio with 95% confident intervals. Significance level was set as p < 0.05.
3. Results
During the study period, 1046 interventions were performed at the CFA level. According to the inclusion criteria, 250 patients could be included in the analysis. The study flow chart is shown in Figure 1.
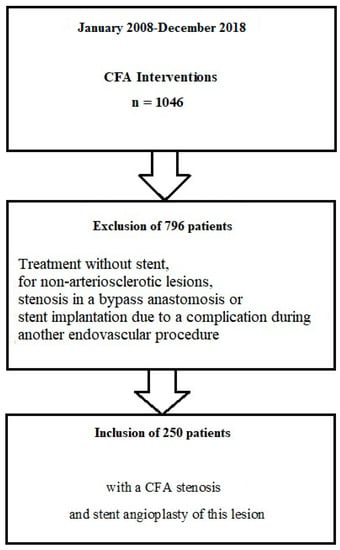
Figure 1.
Study flow chart. CFA—common femoral artery.
Baseline patient characteristics are given in Table 1. The classification of lesions is shown in Table 2. A de novo stenosis was treated in 180 cases (72.0%). In a large number of cases, additional lesions in vessel segments on the ipsilateral or contralateral side were treated. Lesion and interventional details are presented in Table 3. The most frequently implanted stent was the S.M.A.R.TTM stent (Cordis, Miami Lakes, FL, USA). Details of stents used are shown in Table 4.

Table 1.
Baseline Characteristics.

Table 2.
Lesion classification according to Rabellino et al. [11].

Table 3.
Lesion and Index Procedure Characteristics.

Table 4.
Stent Characteristics.
3.1. Acute Outcome
Based on visual estimation, the preinterventional stenosis degree was 86.49 ± 10.7%. A total of 236 interventions (94.4%) were successfully defined as a residual stenosis < 30%. Periinterventionally, there were 23 complications (9.2%), 3 of which had to be treated surgically (2 compartment syndromes, 1 false aneurysm after brachial artery puncture) (Table 5). In 6 of the 10 pseudoaneurysms, no closure device was used.

Table 5.
Procedural Complications.
3.2. Follow Up Outcome
Median follow up was 21 months (average 19.2 ± 7.8). In total, 41 patients (16.4%) underwent a TLR (Table 6). The survival without TLR by Kaplan–Meier analysis is shown in Figure 2.

Table 6.
Clinical and Procedural Outcome.
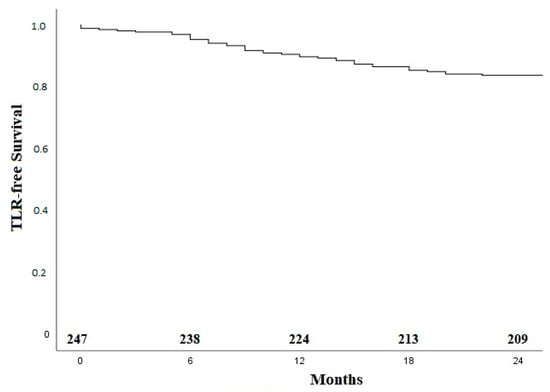
Figure 2.
Kaplan–Meier curve for TLR-free survival. TLR—target lesion revascularization.
A total of 26 of the 41 TLRs (63.4%) occurred within the first 12 months. Figure 3 shows the Kaplan–Meier curve regarding TLR-free survival for the group of claudicants and patients with critical limb-threatening ischemia (CLTI).
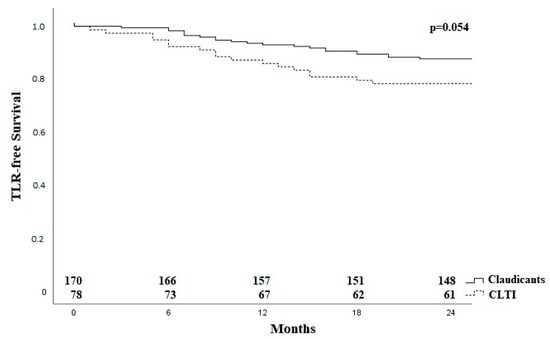
Figure 3.
Kaplan–Meier curve for TLR-free survival for claudicants and patients with critical limb ischaemia. CLTI—critical limb ischaemia; TLR—target lesion revascularization.
Applying the Rabellino classification [11], there was no difference regarding TLR after subdivision into group I/II and the groups with bifurcation involvement (III/IV) as shown in Figure 4.
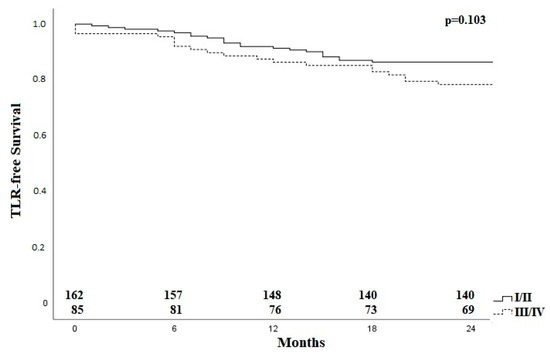
Figure 4.
Kaplan–Meier curve for TLR-free survival for patients with lesions with and without bifurcation involvement. TLR—target lesion revascularization, I–IV; classification according to Rabellinio [11].
Duplexsonographically determined the primary patency rate was 90.8%, 81.2% and 72% at 6, 12 and 24 months, respectively. Compared to the baseline, ABI and RBC were significantly better at all time points during follow up (Table 6). During follow up, seven amputations (three minor and four major) had to be performed. Time to amputation ranged from 1 to 14 months (mean 3.4 months) (Table 6).
More than half of the patients (56.0%) were punctured at the stented CFA during the follow up. Access via the stented vessel was most common for a further peripheral angioplasty (n = 125, 89.3%). However, access via stented CFA was also used for coronary angiography, endovascular aortic repair or transcatheter aortic valve implantation. Ipsilateral puncture had no significant effect on primary patency (p = 0.190) or TLR rates (p = 0.058).
Multivariate logistic regression analysis showed continued nicotine abuse and coronary heart disease as predictors for TLR (Table 7).

Table 7.
Predictors of TLR.
4. Discussion
The CFA is considered a challenging vessel segment for endovascular treatment due to the potential high stress caused by its location in a motion segment. Thus, this vascular segment was traditionally reserved for surgical therapy. However, in recent years, some studies have also shown encouraging results after endovascular therapy, particular the randomized controlled TECCO trial [10]. The present 2-year TLR rate of unselected patients of 16.4% is comparable to both study arms of the TECCO trial (14.4% for the stent cohort and 15.2% for the surgical cohort). However, the proportion of patients with CLTI was lower in the TECCO study than in the present study (30%). In the TECCO study, the proportion was 8% in the surgical group and 18% in the endovascular group. In general, the literature reported TLR rates range from 7 to 20% at 12 months [6,7,8,12,13]. However, in most of these studies, the proportion of patients with CLTI is lower.
In our evaluation, there was no significant difference regarding TLR between the different lesion groups according to Rabellinio et al. [11]; in particular, bifurcation lesions did not result in inferior outcomes as compared to lesions limited to the CFA main stem.
Univariate and multivariate logistic regression analysis showed continued nicotine abuse and coronary heart disease as predictors for TLR. In other studies, age, renal insufficiency, or the presence of CLTI were predictive of reintervention [7,14,15]. Nicotine use has not yet been described as a predictor. On the contrary, one study showed a lower rate of re-intervention in the group of patients smoking 10 or more cigarettes a day [16]. However, this particular study only reports about the course of the disease within the first year after intervention. The presence of coronary artery disease as a predictor for subsequent TLR may be a sign of a more diffuse and progressive kind of atherosclerotic disease in these patients.
The primary patency rate of 81.2% at 12 months is in line with other CFA studies [6,7,8,9]. Slightly higher patency rates are reported after surgical therapy. Kang et al. reported a primary patency rate of 93% at 12 months. Another study found primary patency rates of 97.3% at 6 months and 90.2% at 3 years [4,17].
The complication rate is comparable to those of other studies in this vascular segment [6,8,14] and is driven by access site complications, in particular pseudoaneurysm formation. One pseudoaneurysm of a brachial artery had to be treated surgically; the remaining ones underwent conservative treatment with compression therapy comparable to other studies [6].
At each follow up time point, the present cohort showed persistent significant improvement in ABI and RBC compared to the baseline. This is in line with former trials dealing with endovascular therapy of the CFA [7,18]. One past study showed that the primary sustained clinical improvement was better in patients who underwent stent angioplasty than in patients who underwent angioplasty alone [18]. The major amputation rate of less than 2% is consistent with those of numerous other studies after endovascular therapy of CFA (range 1–3.8%) [6,8,18].
Former concerns that a stent in the CFA would limit future endovascular procedures or surgery were not confirmed in recent studies. Nasr et al. [19] reported unproblematic surgical therapies (aorto–bi–femoral bypasses, iliofemoral bypasses, femoro–popliteal bypasses) involving stented CFA. As in the present work, femoral access was possible for following endovascular procedures. As in other studies, follow up interventions were often repeated endovascular procedures [12,19,20,21]. Depending on stent localization and length, the puncture level can be selected proximal or distal to the stent. Puncture through the stent struts in particular of self-expanding stents is easily possible. It is also feasible to use an occlusion system through an implanted stent.
5. Conclusions
Stent angioplasty of the CFA is a treatment option associated with low TLR rates. Peri-procedural complications can be treated conservatively or endovascularly in the majority of cases. Further comparative studies are needed to compare this endovascular option with surgical therapy in the long term, in particular for identifying potential lesion characteristics that may benefit from one or the other revascularization technique.
Author Contributions
Conceptualization, T.B. and T.Z.; methodology, U.B.; formal analysis, E.N.; data curation, M.A.; writing—original draft preparation, T.B. and E.N.; writing—review and editing, U.B. and T.Z.; visualization, U.B.; supervision, T.Z.; project administration, T.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of the Albert-Ludwigs-University Freiburg, Germany (protocol code: 236/20; date of approval: 2 July 2020).
Informed Consent Statement
There is no informed consent for this retrospective analysis of pseudonymized data. This was accepted by the Ethics Committee.
Data Availability Statement
The data presented in this study are available in the article. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
Elias Noory: Honoraria received from: BARD, Boston Scientific, Abbott, Medtronic. Thomas Zeller: Honoraria received from: Abbott Vascular, BIBA Medical, Biotronik, Boston Scientific Corp., Cook Medical, Efemoral, Philips-Spectranetics, Shockwave, Veryan. Consulted for: CSI, Intact Vascular, Bayer, Vesper Medical. Common stock: QT Medical. The other authors have no conflict of interest.
References
- Fowkes, F.G.R.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.A.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Rooke, T.W.; Hirsch, A.T.; Misra, S.; Sidawy, A.N.; Beckman, J.A.; Findeiss, L.; Golzarian, J.; Gornik, H.L.; Jaff, M.R.; Moneta, G.L.; et al. American College of Cardiology Foundation Task Force; American Heart Association Task Force. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 61, 1555–1570. [Google Scholar] [PubMed] [Green Version]
- Tendera, M.; Aboyans, V.; Bartelink, M.-L.; Baumgartner, I.; Clement, D.L.; Collet, J.-P.; Cremonesi, A.; De Carlo, M.; Erbel, R.; Fowkes, F.G.R.; et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: The Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2011, 32, 2851–2906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.L.; Patel, V.I.; Conrad, M.F.; LaMuraglia, G.M.; Chung, T.; Cambria, R.P. Common femoral artery occlusive disease: Contemporary results following surgical endarterectomy. J. Vasc. Surg. 2008, 48, 872–877.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böhme, T.; Romano, L.; Macharzina, R.-R.; Noory, E.; Beschorner, U.; Jacques, B.; Bürgelin, K.; Flügel, P.-C.; Zeller, T.; Rastan, A. Midterm Results of Directional Atherectomy for the Treatment of Atherosclerotic Common Femoral Artery Disease. EuroIntervention J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2021, 17, 260–266. [Google Scholar]
- Bonvini, R.F.; Rastan, A.; Sixt, S.; Noory, E.; Schwarz, T.; Frank, U.; Roffi, M.; Dorsaz, P.A.; Schwarzwälder, U.; Bürgelin, K.; et al. Endovascular Treatment of Common Femoral Artery Disease: Medium-Term Outcomes of 360 Consecutive Procedures. J. Am. Coll. Cardiol. 2011, 58, 792–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azéma, L.; Davaine, J.; Guyomarch, B.; Chaillou, P.; Costargent, A.; Patra, P.; Gouëffic, Y. Endovascular Repair of Common Femoral Artery and Concomitant Arterial Lesions. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 787–793. [Google Scholar] [CrossRef] [Green Version]
- Stricker, H.; Jacomella, V. Stent-Assisted Angioplasty at the Level of the Common Femoral Artery Bifurcation:Midterm Outcomes. J. Endovasc. Ther. 2004, 11, 281–286. [Google Scholar] [CrossRef]
- Bath, J.; Avgerinos, E. A pooled analysis of common femoral and profunda femoris endovascular interventions. Vascular 2016, 24, 404–413. [Google Scholar] [CrossRef]
- Gouëffic, Y.; Della Schiava, N.; Thaveau, F.; Rosset, E.; Favre, J.-P.; du Mont, L.S.; Alsac, J.-M.; Hassen-Khodja, R.; Reix, T.; Allaire, E.; et al. Stenting or Surgery for De Novo Common Femoral Artery Stenosis. JACC: Cardiovasc. Interv. 2017, 10, 1344–1354. [Google Scholar] [CrossRef]
- Rabellino, M.; Raleigh, J.V.; Chiabrando, J.G.; Di Caro, V.; Chas, J.; Garagoli, F.; Bluro, I. Novel Common Femoral Artery Lesion Classification in Patients Undergoing Endovascular Revascularization. Cardiovasc. Interv. Radiol. 2022, 45, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Cioppa, A.; Stabile, E.; Salemme, L.; Popusoi, G.; Pucciarelli, A.; Iacovelli, F.; Arcari, A.; Coscioni, E.; Trimarco, B.; Esposito, G.; et al. Combined use of directional atherectomy and drug-coated balloon for the endovascular treatment of common femoral artery disease: Immediate and one-year outcomes. EuroIntervention 2017, 12, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Paris, C.L.; White, C.J.; Collins, T.J.; Jenkins, J.S.; Reilly, J.P.; A Grise, M.; McMullan, P.W.; Verma, A.; Ramee, S.R. Catheter-based therapy of common femoral artery atherosclerotic disease. Vasc. Med. 2011, 16, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.S.M.; Adair, W.; Bolia, A.; Fishwick, G.; Sayers, R.D.; McCarthy, M.J. Endovascular Treatment of the Common Femoral Artery for Limb Ischemia. Vasc. Endovasc. Surg. 2013, 47, 639–644. [Google Scholar] [CrossRef]
- Bonvini, R.F.; Rastan, A.; Sixt, S.; Beschorner, U.; Noory, E.; Schwarz, T.; Roffi, M.; Dorsaz, P.-A.; Schwarzwälder, U.; Bürgelin, K.; et al. Angioplasty and Provisional Stent Treatment of Common Femoral Artery Lesions. J. Vasc. Interv. Radiol. 2013, 24, 175–183. [Google Scholar] [CrossRef]
- Schillinger, M.; Exner, M.; Mlekusch, W.; Haumer, M.; Sabeti, S.; Ahmadi, R.; Wagner, O.; Minar, E. Effect of Smoking on Restenosis during the 1st Year after Lower-Limb Endovascular Interventions. Radiology 2004, 231, 831–838. [Google Scholar] [CrossRef]
- Wieker, C.M.; Schönefeld, E.; Osada, N.; Lührs, C.; Beneking, R.; Torsello, G.; Böckler, D. Results of common femoral artery thromboendarterectomy evaluation of a traditional surgical management in the endovascular era. J. Vasc. Surg. 2016, 64, 995–1001. [Google Scholar] [CrossRef] [Green Version]
- Baumann, F.; Ruch, M.; Willenberg, T.; Dick, F.; Do, D.-D.; Keo, H.-H.; Baumgartner, I.; Diehm, N. Endovascular treatment of common femoral artery obstructions. J. Vasc. Surg. 2011, 53, 1000–1006. [Google Scholar] [CrossRef] [Green Version]
- Nasr, B.; Kaladji, A.; Vent, P.-A.; Chaillou, P.; Costargent, A.; Quillard, T.; Gouëffic, Y. Long-Term Outcomes of Common Femoral Artery Stenting. Ann. Vasc. Surg. 2017, 40, 10–18. [Google Scholar] [CrossRef]
- Stavroulakis, K.; Schwindt, A.; Torsello, G.; Beropoulis, E.; Stachmann, A.; Hericks, C.; Bollenberg, L.; Bisdas, T. Directional Atherectomy with Antirestenotic Therapy vs Drug-Coated Balloon Angioplasty Alone for Common Femoral Artery Atherosclerotic Disease. J. Endovasc. Ther. 2017, 25, 92–99. [Google Scholar] [CrossRef]
- Stricker, H.; Spinedi, L.; Limoni, C.; Giovannacci, L. Stent-Assisted Angioplasty (SAA) at the Level of the Common Femoral Artery Bifurcation: Long-Term Outcomes. Cardiovasc. Interv. Radiol. 2020, 43, 541–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).