Anticoagulant Treatment in Severe ARDS COVID-19 Patients
Abstract
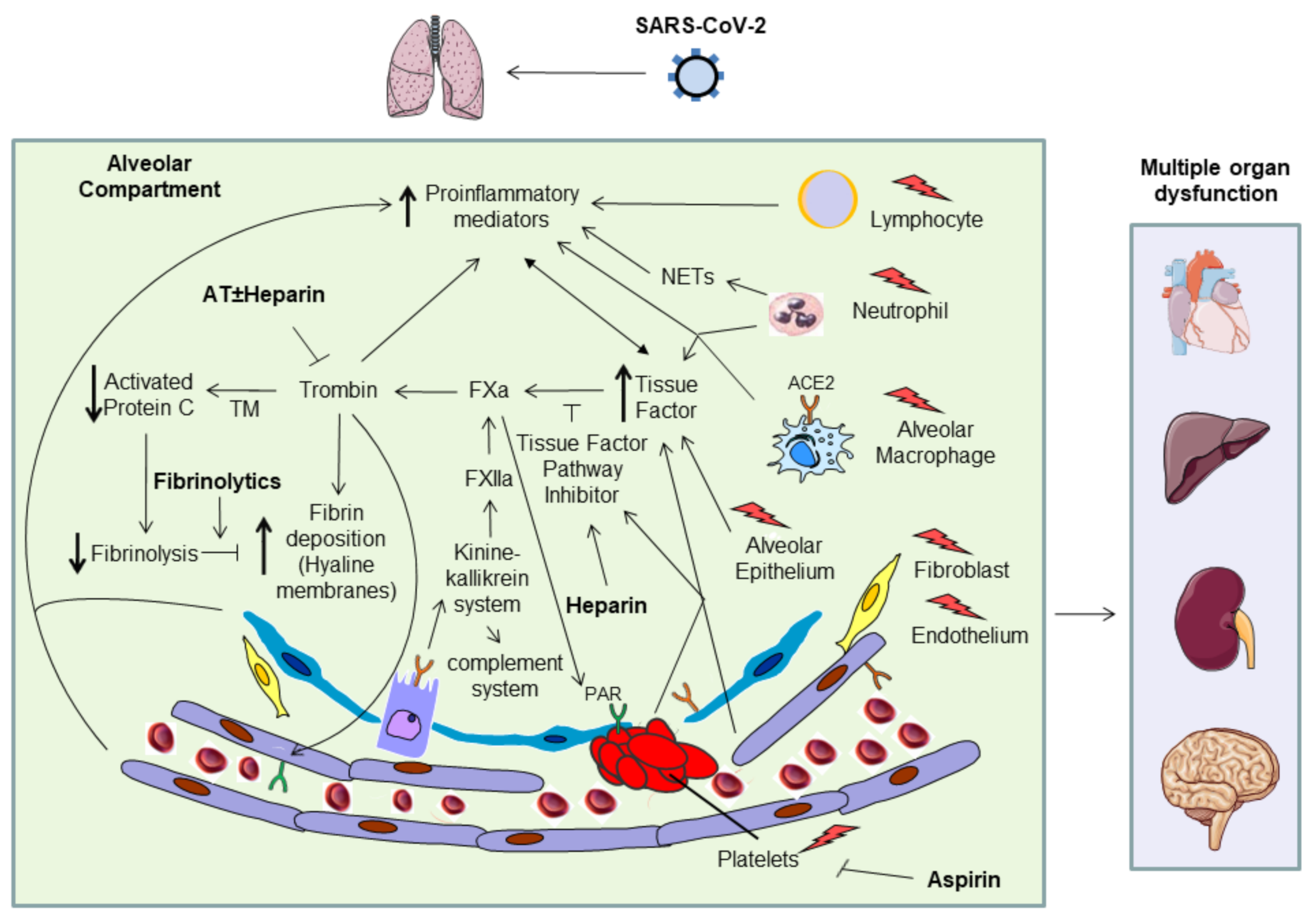
1. Introduction
2. Pathophysiology
3. Thrombotic Events
4. Anticoagulant Treatment
5. Other Antithrombotic Treatments
6. Nebulized Anti-Coagulants
7. Recommended Management
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Nopp, S.; Moik, F.; Jilma, B.; Pabinger, I.; Ay, C. Risk of venous thromboembolism in patients with COVID-19: A systematic review and meta-analysis. Res. Pract. Thromb. Haemost. 2020, 4, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Parasher, A. COVID-19: Current understanding of its pathophysiology, clinical presentation and treatment. Postgrad. Med. J. 2021, 97, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C.; van der Poll, T. Severe Sepsis and Septic Shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Lax, S.F.; Skok, K.; Zechner, P.; Kessler, H.H.; Kaufmann, N.; Koelblinger, C.; Vander, K.; Bargfrieder, U.; Trauner, M. Pulmonary Arterial Thrombosis in COVID-19 with Fatal Outcome: Results from a Prospective, Single-Center, Clinicopathologic Case Series. Ann. Intern. Med. 2020, 173, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Van Haren, F.M.P.; Page, C.; Laffey, J.G.; Artigas, A.; Camprubi-Rimblas, M.; Nunes, Q.; Smith, R.; Shute, J.; Carroll, M.; Tree, J.; et al. Nebulised heparin as a treatment for COVID-19: Scientific rationale and a call for randomised evidence. Crit. Care 2020, 24, 454. [Google Scholar] [CrossRef]
- Goshua, G.; Pine, A.B.; Meizlish, M.L.; Chang, C.-H.; Zhang, H.; Bahel, P.; Baluha, A.; Bar, N.; Bona, R.D.; Burns, A.J.; et al. Endotheliopathy in COVID-19-associated coagulopathy: Evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020, 7, e575–e582. [Google Scholar] [CrossRef]
- Iba, T.; Warkentin, T.E.; Thachil, J.; Levi, M.; Levy, J.H. Proposal of the Definition for COVID-19-Associated Coagulopathy. J. Clin. Med. 2021, 10, 191. [Google Scholar] [CrossRef]
- José, R.J.; Williams, A.; Manuel, A.; Brown, J.S.; Chambers, R.C. Targeting coagulation activation in severe COVID-19 pneumonia: Lessons from bacterial pneumonia and sepsis. Eur. Respir. Rev. 2020, 29, 200240. [Google Scholar] [CrossRef]
- Chi, G.; Lee, J.J.; Jamil, A.; Gunnam, V.; Najafi, H.; Memar Montazerin, S.; Shojaei, F.; Marszalek, J. Venous Thromboembolism among Hospitalized Patients with COVID-19 Undergoing Thromboprophylaxis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 2489. [Google Scholar] [CrossRef] [PubMed]
- Tufano, A.; Rendina, D.; Abate, V.; Casoria, A.; Marra, A.; Buonanno, P.; Galletti, F.; Di Minno, G.; Servillo, G.; Vargas, M. Venous Thromboembolism in COVID-19 Compared to Non-COVID-19 Cohorts: A Systematic Review with Meta-Analysis. J. Clin. Med. 2021, 10, 4925. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.a.M.P.J.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Oba, S.; Hosoya, T.; Amamiya, M.; Mitsumura, T.; Kawata, D.; Sasaki, H.; Kamiya, M.; Yamamoto, A.; Ando, T.; Shimada, S.; et al. Arterial and Venous Thrombosis Complicated in COVID-19: A Retrospective Single Center Analysis in Japan. Front. Cardiovasc. Med. 2021, 8, 767074. [Google Scholar] [CrossRef]
- Indes, J.E.; Koleilat, I.; Hatch, A.N.; Choinski, K.; Jones, D.B.; Aldailami, H.; Billett, H.; Denesopolis, J.M.; Lipsitz, E. Early experience with arterial thromboembolic complications in patients with COVID-19. J. Vasc. Surg. 2021, 73, 381–389.e1. [Google Scholar] [CrossRef]
- Zuin, M.; Engelen, M.M.; Barco, S.; Spyropoulos, A.C.; Vanassche, T.; Hunt, B.J.; Vandenbriele, C.; Verhamme, P.; Kucher, N.; Rashidi, F.; et al. Incidence of venous thromboembolic events in COVID-19 patients after hospital discharge: A systematic review and meta-analysis. Thromb. Res. 2022, 209, 94–98. [Google Scholar] [CrossRef]
- Shah, A.; Donovan, K.; McHugh, A.; Pandey, M.; Aaron, L.; Bradbury, C.A.; Stanworth, S.J.; Alikhan, R.; Von Kier, S.; Maher, K.; et al. Thrombotic and haemorrhagic complications in critically ill patients with COVID-19: A multicentre observational study. Crit. Care 2020, 24, 561. [Google Scholar] [CrossRef]
- Lodigiani, C.; Iapichino, G.; Carenzo, L.; Cecconi, M.; Ferrazzi, P.; Sebastian, T.; Kucher, N.; Studt, J.-D.; Sacco, C.; Bertuzzi, A.; et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020, 191, 9–14. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef]
- Martín-Rojas, R.M.; Pérez-Rus, G.; Delgado-Pinos, V.E.; Domingo-González, A.; Regalado-Artamendi, I.; Alba-Urdiales, N.; Demelo-Rodríguez, P.; Monsalvo, S.; Rodríguez-Macías, G.; Ballesteros, M.; et al. COVID-19 coagulopathy: An in-depth analysis of the coagulation system. Eur. J. Haematol. 2020, 105, 741–750. [Google Scholar] [CrossRef]
- Trigonis, R.A.; Holt, D.B.; Yuan, R.; Siddiqui, A.A.; Craft, M.K.; Khan, B.A.; Kapoor, R.; Rahman, O. Incidence of Venous Thromboembolism in Critically Ill Coronavirus Disease 2019 Patients Receiving Prophylactic Anticoagulation. Crit. Care Med. 2020, 48, e805–e808. [Google Scholar] [CrossRef] [PubMed]
- Mizurini, D.M.; Hottz, E.D.; Bozza, P.T.; Monteiro, R.Q. Fundamentals in COVID-19-Associated Thrombosis: Molecular and Cellular Aspects. Front. Cardiovasc. Med. 2021, 8, 785738. [Google Scholar] [CrossRef] [PubMed]
- Panigada, M.; Bottino, N.; Tagliabue, P.; Grasselli, G.; Novembrino, C.; Chantarangkul, V.; Pesenti, A.; Peyvandi, F.; Tripodi, A. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 2020, 18, 1738–1742. [Google Scholar] [CrossRef] [PubMed]
- Bowles, L.; Platton, S.; Yartey, N.; Dave, M.; Lee, K.; Hart, D.P.; MacDonald, V.; Green, L.; Sivapalaratnam, S.; Pasi, K.J.; et al. Lupus Anticoagulant and Abnormal Coagulation Tests in Patients with COVID-19. N. Engl. J. Med. 2020, 383, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Plebani, M.; Henry, B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin. Chim. Acta 2020, 506, 145–148. [Google Scholar] [CrossRef]
- Giannis, D.; Ziogas, I.A.; Gianni, P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J. Clin. Virol. 2020, 127, 104362. [Google Scholar] [CrossRef]
- Torres, A.; Motos, A.; Riera, J.; Fernández-Barat, L.; Ceccato, A.; Pérez-Arnal, R.; García-Gasulla, D.; Peñuelas, O.; Lorente, J.A.; Rodriguez, A.; et al. The evolution of the ventilatory ratio is a prognostic factor in mechanically ventilated COVID-19 ARDS patients. Crit. Care 2021, 25, 331. [Google Scholar] [CrossRef]
- Chandra, A.; Chakraborty, U.; Ghosh, S.; Dasgupta, S. Anticoagulation in COVID-19: Current concepts and controversies. Postgrad. Med. J. 2022, 98, 395–402. Available online: https://pmj.bmj.com/content/early/2021/04/12/postgradmedj-2021-139923 (accessed on 28 March 2022). [CrossRef]
- Cornet, A.D.; Smit, E.G.M.; Beishuizen, A.; Groeneveld, A.B.J. The role of heparin and allied compounds in the treatment of sepsis. Thromb. Haemost. 2007, 98, 579–586. [Google Scholar] [CrossRef]
- Jackson, C.M. Mechanism of heparin action. Baillieres Clin. Haematol. 1990, 3, 483–504. [Google Scholar] [CrossRef]
- Baglin, T.; Barrowcliffe, T.W.; Cohen, A.; Greaves, M.; British Committee for Standards in Haematology. Guidelines on the use and monitoring of heparin. Br. J. Haematol. 2006, 133, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Camprubí-Rimblas, M.; Tantinyà, N.; Bringué, J.; Guillamat-Prats, R.; Artigas, A. Anticoagulant therapy in acute respiratory distress syndrome. Ann. Transl. Med. 2018, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Rentsch, C.T.; Beckman, J.A.; Tomlinson, L.; Gellad, W.F.; Alcorn, C.; Kidwai-Khan, F.; Skanderson, M.; Brittain, E.; King, J.T.; Ho, Y.-L.; et al. Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: Cohort study. BMJ 2021, 372, n311. [Google Scholar] [CrossRef]
- Al-Samkari, H.; Gupta, S.; Leaf, R.K.; Wang, W.; Rosovsky, R.P.; Brenner, S.K.; Hayek, S.S.; Berlin, H.; Kapoor, R.; Shaefi, S.; et al. Thrombosis, Bleeding, and the Observational Effect of Early Therapeutic Anticoagulation on Survival in Critically Ill Patients with COVID-19. Ann. Intern. Med. 2021, 174, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.O.; Main-Ian, A.; Tebbutt, J.; Thrasher, M.; Waite, A.; Welters, I. Standard- versus intermediate-dose enoxaparin for anti-factor Xa guided thromboprophylaxis in critically ill patients with COVID-19. Thromb. J. 2021, 19, 87. [Google Scholar] [CrossRef] [PubMed]
- INSPIRATION Investigators. Effect of Intermediate-Dose vs. Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial. JAMA 2021, 325, 1620–1630. [Google Scholar]
- Spyropoulos, A.C.; Goldin, M.; Giannis, D.; Diab, W.; Wang, J.; Khanijo, S.; Mignatti, A.; Gianos, E.; Cohen, M.; Sharifova, G.; et al. Efficacy and Safety of Therapeutic-Dose Heparin vs. Standard Prophylactic or Intermediate-Dose Heparins for Thromboprophylaxis in High-risk Hospitalized Patients with COVID-19: The HEP-COVID Randomized Clinical Trial. JAMA Intern. Med. 2021, 181, 1612–1620. [Google Scholar] [CrossRef]
- Sholzberg, M.; Tang, G.H.; Rahhal, H.; AlHamzah, M.; Kreuziger, L.B.; Áinle, F.N.; Alomran, F.; Alayed, K.; Alsheef, M.; AlSumait, F.; et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with COVID-19 admitted to hospital: RAPID randomised clinical trial. BMJ 2021, 375, n2400. [Google Scholar] [CrossRef]
- REMAP-CAP Investigators; ACTIV-4a Investigators; ATTACC Investigators. Therapeutic Anticoagulation with Heparin in Critically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 385, 777–789. [Google Scholar] [CrossRef]
- ATTACC Investigators; ACTIV-4a Investigators; REMAP-CAP Investigators. Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 385, 790–802. [Google Scholar] [CrossRef]
- Lopes, R.D.; de Barros e Silva, P.G.M.; Furtado, R.H.M.; Macedo, A.V.S.; Bronhara, B.; Damiani, L.P.; Barbosa, L.M.; de Aveiro Morata, J.; Ramacciotti, E.; de Aquino Martins, P.; et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): An open-label, multicentre, randomised, controlled trial. Lancet 2021, 397, 2253–2263. [Google Scholar] [CrossRef]
- Kow, C.S.; Ramachandram, D.S.; Hasan, S.S. The effect of higher-intensity dosing of anticoagulation on the clinical outcomes in hospitalized patients with COVID-19: A meta-analysis of randomized controlled trials. J. Infect. Chemother. 2022, 28, 257–265. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8595354/ (accessed on 7 January 2022). [CrossRef] [PubMed]
- Sholzberg, M.; da Costa, B.R.; Tang, G.H.; Rahhal, H.; AlHamzah, M.; Baumann Kreuziger, L.; Ní Áinle, F.; Almarshoodi, M.O.; James, P.D.; Lillicrap, D.; et al. Randomized trials of therapeutic heparin for COVID-19: A meta-analysis. Res. Pract. Thromb. Haemost. 2021, 5, e12638. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Paz, L.; Galli, M.; Capodanno, D.; Franchi, F.; Rollini, F.; Bikdeli, B.; Mehran, R.; Montalescot, G.; Gibson, C.M.; Lopes, R.D.; et al. Safety and efficacy of different prophylactic anticoagulation dosing regimens in critically and non-critically ill patients with COVID-19: A systematic review and meta-analysis of randomized controlled trials. Eur. Heart J. Cardiovasc. Pharmacother. 2021, pvab070. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group. Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2022, 399, 143–151. [Google Scholar] [CrossRef]
- Berger, J.S.; Kornblith, L.Z.; Gong, M.N.; Reynolds, H.R.; Cushman, M.; Cheng, Y.; McVerry, B.J.; Kim, K.S.; Lopes, R.D.; Atassi, B.; et al. Effect of P2Y12 Inhibitors on Survival Free of Organ Support Among Non-Critically Ill Hospitalized Patients with COVID-19: A Randomized Clinical Trial. JAMA 2022, 327, 227–236. [Google Scholar] [CrossRef]
- Barrett, C.D.; Moore, H.B.; Moore, E.E.; Benjamin Christie, D.; Orfanos, S.; Anez-Bustillos, L.; Jhunjhunwala, R.; Hussain, S.; Shaefi, S.; Wang, J.; et al. MUlticenter study of tissue plasminogen activator (alteplase) use in COVID-19 severe respiratory failure (MUST COVID): A retrospective cohort study. Res. Pract. Thromb. Haemost. 2022, 6, e12669. [Google Scholar] [CrossRef]
- Barrett, C.D.; Moore, H.B.; Moore, E.E.; Wang, J.; Hajizadeh, N.; Biffl, W.L.; Lottenberg, L.; Patel, P.R.; Truitt, M.S.; McIntyre, R.C.; et al. Study of Alteplase for Respiratory Failure in SARS-CoV-2 COVID-19: A Vanguard Multicenter, Rapidly Adaptive, Pragmatic, Randomized Controlled Trial. CHEST 2022, 161, 710–727. [Google Scholar] [CrossRef]
- Dixon, B.; Santamaria, J.D.; Campbell, D.J. A phase 1 trial of nebulised heparin in acute lung injury. Crit. Care 2008, 12, R64. [Google Scholar] [CrossRef]
- Dixon, B.; Schultz, M.J.; Hofstra, J.J.; Campbell, D.J.; Santamaria, J.D. Nebulized heparin reduces levels of pulmonary coagulation activation in acute lung injury. Crit. Care 2010, 14, 445. [Google Scholar] [CrossRef]
- Dixon, B.; Schultz, M.J.; Smith, R.; Fink, J.B.; Santamaria, J.D.; Campbell, D.J. Nebulized heparin is associated with fewer days of mechanical ventilation in critically ill patients: A randomized controlled trial. Crit. Care 2010, 14, R180. [Google Scholar] [CrossRef] [PubMed]
- Dixon, B.; Smith, R.J.; Campbell, D.J.; Moran, J.L.; Doig, G.S.; Rechnitzer, T.; MacIsaac, C.M.; Simpson, N.; van Haren, F.M.P.; Ghosh, A.N.; et al. Nebulised heparin for patients with or at risk of acute respiratory distress syndrome: A multicentre, randomised, double-blind, placebo-controlled phase 3 trial. Lancet Respir. Med. 2021, 9, 360–372. [Google Scholar] [CrossRef]
- Glas, G.J.; Muller, J.; Binnekade, J.M.; Cleffken, B.; Colpaert, K.; Dixon, B.; Juffermans, N.P.; Knape, P.; Levi, M.M.; Loef, B.G.; et al. HEPBURN—Investigating the efficacy and safety of nebulized heparin versus placebo in burn patients with inhalation trauma: Study protocol for a multi-center randomized controlled trial. Trials 2014, 15, 91. [Google Scholar] [CrossRef] [PubMed]
- Glas, G.J.; Serpa Neto, A.; Horn, J.; Cochran, A.; Dixon, B.; Elamin, E.M.; Faraklas, I.; Dissanaike, S.; Miller, A.C.; Schultz, M.J. Nebulized heparin for patients under mechanical ventilation: An individual patient data meta-analysis. Ann. Intensive Care 2016, 6, 33. [Google Scholar] [CrossRef]
- Bandeshe, H.; Boots, R.; Dulhunty, J.; Dunlop, R.; Holley, A.; Jarrett, P.; Gomersall, C.D.; Lipman, J.; Lo, T.; O’Donoghue, S.; et al. Is Inhaled Prophylactic Heparin Useful for Prevention and Management of Pneumonia in Ventilated ICU Patients? The IPHIVAP Investigators of the Australian and New Zealand Intensive Care Society Clinical Trials Group. J. Crit. Care 2016, 34, 95–102. [Google Scholar] [CrossRef]
- IPHIVAP Investigators of the Australian and New Zealand Intensive Care Society Clinical Trials Group. Is inhaled prophylactic heparin useful for prevention and management of pneumonia in ventilated ICU patients? J. Crit. Care 2016, 35, 231–239. [Google Scholar] [CrossRef]
- Ghiasi, F.; Sadeghian, M.; Emami, M.; Kiaie, B.A.; Mousavi, S. A Pilot Study of Nebulized Heparin for Prevention of Ventilator Induced Lung Injury: Comparative Effects with an Inhaled Corticosteroid. Indian J. Crit. Care Med. 2017, 21, 634–639. [Google Scholar] [CrossRef]
- Camprubí-Rimblas, M.; Tantinyà, N.; Guillamat-Prats, R.; Bringué, J.; Puig, F.; Gómez, M.N.; Blanch, L.; Artigas, A. Effects of nebulized antithrombin and heparin on inflammatory and coagulation alterations in an acute lung injury model in rats. J. Thromb. Haemost. 2020, 18, 571–583. [Google Scholar] [CrossRef]
- Chimenti, L.; Camprubí-Rimblas, M.; Guillamat-Prats, R.; Gomez, M.N.; Tijero, J.; Blanch, L.; Artigas, A. Nebulized Heparin Attenuates Pulmonary Coagulopathy and Inflammation through Alveolar Macrophages in a Rat Model of Acute Lung Injury. Thromb. Haemost. 2017, 117, 2125–2134. [Google Scholar] [CrossRef]
- Van Haren, F.M.P.; van Loon, L.M.; Steins, A.; Smoot, T.L.; Sas, C.; Staas, S.; Vilaseca, A.B.; Barbera, R.A.; Vidmar, G.; Beccari, H.; et al. Inhaled nebulised unfractionated heparin for the treatment of hospitalised patients with COVID-19: A multicentre case series of 98 patients. Br. J. Clin. Pharmacol. 2022. [Google Scholar] [CrossRef]
- Van Haren, F.M.P.; Laffey, J.G.; Artigas, A.; Page, C.; Schultz, M.J.; Cosgrave, D.; McNicholas, B.; Smoot, T.L.; Nunes, Q.; Richardson, A.; et al. Can nebulised HepArin Reduce morTality and time to Extubation in patients with COVID-19 Requiring invasive ventilation Meta-Trial (CHARTER-MT): Protocol and statistical analysis plan for an investigator-initiated international meta-trial of prospective randomised clinical studies. Br. J. Clin. Pharmacol. 2022. [Google Scholar] [CrossRef]
- Van Haren, F.M.P.; Richardson, A.; Yoon, H.-J.; Artigas, A.; Laffey, J.G.; Dixon, B.; Smith, R.; Vilaseca, A.B.; Barbera, R.A.; Ismail, T.I.; et al. INHALEd nebulised unfractionated HEParin for the treatment of hospitalised patients with COVID-19 (INHALE-HEP): Protocol and statistical analysis plan for an investigator-initiated international metatrial of randomised studies. Br. J. Clin. Pharmacol. 2021, 87, 3075–3091. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, T.; Dixon, R.; Page, C.; Carroll, M.; Griffiths, G.; Ho, L.-P.; De Soyza, A.; Felton, T.; Lewis, K.E.; Phekoo, K.; et al. ACCORD: A Multicentre, Seamless, Phase 2 Adaptive Randomisation Platform Study to Assess the Efficacy and Safety of Multiple Candidate Agents for the Treatment of COVID-19 in Hospitalised Patients: A Structured Summary of a Study Protocol for a Randomised Controlled Trial. Trials 2020, 21, 691. [Google Scholar] [PubMed]
- Ramacciotti, E.; Barile Agati, L.; Calderaro, D.; Aguiar, V.C.R.; Spyropoulos, A.C.; de Oliveira, C.C.C.; dos Santos PharmD, J.L.; Volpiani, G.G.; Sobreira, M.L.; Joviliano, E.E.; et al. Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): An open-label, multicentre, randomised, controlled trial. Lancet 2022, 399, 50–59. [Google Scholar] [CrossRef]

| Treatment | Safety and Benefit |
|---|---|
| Antiplatelet therapy | None benefit on mortality, Aspirin may improve probability of been discharged alive at 28-day. |
| Fibrinolytic | Safety proved in phase 1–2, benefits on oxygenation have been observed. |
| Nebulized anticoagulants | Nebulized heparin showed safety profile. Benefits on oxygenation were observed in patients with moderate COVID-19. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceccato, A.; Camprubí-Rimblas, M.; Campaña-Duel, E.; Areny-Balagueró, A.; Morales-Quinteros, L.; Artigas, A. Anticoagulant Treatment in Severe ARDS COVID-19 Patients. J. Clin. Med. 2022, 11, 2695. https://doi.org/10.3390/jcm11102695
Ceccato A, Camprubí-Rimblas M, Campaña-Duel E, Areny-Balagueró A, Morales-Quinteros L, Artigas A. Anticoagulant Treatment in Severe ARDS COVID-19 Patients. Journal of Clinical Medicine. 2022; 11(10):2695. https://doi.org/10.3390/jcm11102695
Chicago/Turabian StyleCeccato, Adrian, Marta Camprubí-Rimblas, Elena Campaña-Duel, Aina Areny-Balagueró, Luis Morales-Quinteros, and Antonio Artigas. 2022. "Anticoagulant Treatment in Severe ARDS COVID-19 Patients" Journal of Clinical Medicine 11, no. 10: 2695. https://doi.org/10.3390/jcm11102695
APA StyleCeccato, A., Camprubí-Rimblas, M., Campaña-Duel, E., Areny-Balagueró, A., Morales-Quinteros, L., & Artigas, A. (2022). Anticoagulant Treatment in Severe ARDS COVID-19 Patients. Journal of Clinical Medicine, 11(10), 2695. https://doi.org/10.3390/jcm11102695







