Abstract
The diagnostic process for biliary strictures remains challenging in some cases. A broad differential diagnosis exists for indeterminate biliary strictures, including benign or malignant lesions. The diagnosis of indeterminate biliary strictures requires a combination of physical examination, laboratory testing, imaging modalities, and endoscopic procedures. Despite the progress of less invasive imaging modalities such as transabdominal ultrasonography, computed tomography, and magnetic resonance imaging, endoscopy plays an essential role in the accurate diagnosis, including the histological diagnosis. Imaging findings and brush cytology and/or forceps biopsy under fluoroscopic guidance with endoscopic retrograde cholangiopancreatography (ERCP) are widely used as the gold standard for the diagnosis of biliary strictures. However, ERCP cannot provide an intraluminal view of the biliary lesion, and its outcomes are not satisfactory. Recently, peroral cholangioscopy, confocal laser endomicroscopy, endoscopic ultrasound (EUS), and EUS-guided fine-needle aspiration have been reported as useful for indeterminate biliary strictures. Appropriate endoscopic modalities need to be selected according to the patient’s condition, the lesion, and the expertise of the endoscopist. The aim of this review article is to discuss the diagnostic process for indeterminate biliary strictures using endoscopy.
1. Introduction
Biliary strictures can lead to hepatobiliary dysfunction and eventually liver failure. They need to be appropriately treated, for example with biliary drainage and surgery; therefore, a correct diagnosis is necessary before treatment. A broad differential diagnosis exists between benign (including inflammatory) and malignant conditions. Benign biliary strictures are caused by primary sclerosing cholangitis (PSC), IgG4-related sclerosing cholangitis, bile duct stones, infection, ischemia related to surgical interventions, or iatrogenic injury. Malignant biliary strictures are caused by carcinomas involving the intra/extrahepatic bile duct, gallbladder (cystic duct), pancreatic duct, ampulla of Vater, liver, and metastatic cancers. Malignant biliary diseases have a poor prognosis, with an overall five-year survival as low as approximately 10%. The natural prognosis of cholangiocarcinoma without chemotherapy leads to an overall survival of 3.9 months. If palliative chemotherapy is used, median survival can be prolonged to 12–15 months [1,2]. If it can be treated surgically with R0 resection, long-term survival would be expected [3]. Therefore, correct and early diagnoses are required. Staging diagnosis and extension of the lesion are required to select a treatment or resection area. However, differentiating between benign and malignant bile duct strictures remains challenging in some cases. Multidisciplinary approaches such as physical examination, laboratory testing, imaging modalities, and endoscopic procedures are required to make a correct diagnosis. Despite the progress of less invasive imaging modalities such as transabdominal ultrasonography (AUS), computed tomography (CT), and magnetic resonance imaging (MRI), endoscopy plays an essential role in the accurate diagnosis, including the histological diagnosis. In this review, we discuss the diagnostic process using endoscopy for indeterminate biliary strictures.
2. Noninvasive Evaluation
Before performing endoscopic procedures, noninvasive evaluation is the first approach to diagnosing biliary strictures. It is important to take patient’s history in detail. Surgical operation history such as cholecystectomy, hepatectomy, or pancreatoduodenectomy has the potential to cause biliary strictures or anastomotic strictures. These strictures need to be treated by balloon dilation or stent placement [4]. Chronic pancreatitis could cause distal biliary stricture so it is necessary to examine the patient’s pancreatitis history or alcohol intake. He strictures related to PSC can be seen in chronic liver disorder or inflammatory bowel disease, so it is necessary to examine patients’ inflammatory bowel disease history. Moreover, PSC patients could be concurrent with cholangiocarcinoma in 10–15% of cases [5]. Therefore, careful follow-up for PSC patients is required. Besides the patient’s history, it is also important to examine the patient’s symptoms, such as jaundice, abdominal pain, fever, lymphadenopathy, and decreased blood pressure. These symptoms could be detected patients with biliary strictures.
Laboratory testing is performed following taking the patient’s history and examining the patient’s symptoms. Hepatobiliary dysfunction is detected in cases of biliary strictures. The higher the bilirubin level, the more likely that the stricture is malignant [6]. IgG4 levels are examined for autoimmune pancreatitis and IgG4-associated cholangitis but may also be elevated in cholangiocarcinoma and PSC [7,8,9]. Tumor markers provide better insight into the presence of malignancies that cause biliary stricture. Serum carbohydrate antigen (CA) 19-9 is a tumor marker that is useful for pancreatobiliary cancer with a sensitivity of approximately 70% for pancreatic cancer and 50–80% for bile duct cancer [10,11]. However, CA19-9 increases in the presence of jaundice, and false positives may arise in cases of biliary strictures. Moreover, one study reported although CA19-9 had a sensitivity and specificity of 79–81% and 82–90%, respectively, for the diagnosis of cancer, it was not so useful as a screening marker because of its low positive predictive value (0.5–0.9%) [12]. Carcinoembryonic antigen (CEA) has a sensitivity and specificity of 53–84% and 50–79%, respectively, for cholangiocarcinoma [13]. Although tumor markers are good to examine as an initial laboratory test, they are insufficient to make a correct diagnosis so other modalities should be added.
Cross-sectional imaging is performed to detect lesions that cause biliary strictures. AUS is frequently used as a first-line imaging test, and it may suggest the location of a biliary stricture, although a detailed anatomic description is usually difficult. The sensitivities of AUS for detecting hilar bile duct cancer, middle, and distal were 86, 59, and 33%, respectively [14]. Bile duct dilatation on AUS findings is an important sign for the early diagnosis of bile duct cancer. Both CT and MRI have high diagnostic accuracy for the identification and characterization of primary lesions [15,16] and to determine the resectability of malignancies [16,17]. Therefore, multiphasic contrast-enhanced CT or MRI should be considered in the initial evaluation of patients with biliary strictures prior to endoscopic procedures. CT and MRI have the added advantage of potentially detecting distant metastasis. Both CT and MRI have high diagnostic accuracy for the identification and characterization of primary lesions so it is difficult to mention which modality is superior or not. Magnetic resonance cholangiopancreatography (MRCP) generally provides superior visualization of the bile duct, particularly with regard to the intrahepatic ducts, compared to CT [18]. Moreover, MRCP has the benefit of allowing for imaging of the proximal bile duct in cases of severe obstruction that may prevent contrast from traversing the stricture and/or preventing undrained areas during endoscopic retrograde cholangiopancreatography (ERCP) [19].
3. Endoscopic Retrograde Cholangiopancreatography with Cytology and Forceps Biopsy
Since its introduction in 1968, endoscopic retrograde cholangiopancreatography (ERCP) has been an essential and established procedure for the diagnosis and treatment of biliopancreatic diseases. The success rate of the procedure has been reported to be approximately 95% [20,21], and it is still considered the gold standard for biliary imaging. A duodenoscope is advanced to the ampulla, and an ERCP catheter is inserted into the biliary tract over a guidewire. Following cannulation of the biliary tract, the contrast medium is injected for cholangiography. When performing ERCP, the interpretation of the cholangiography findings is the first step. An accurate distinction between benign and malignant biliary strictures is needed. Malignancy is suggested when the cholangiography shows strictures that are longer than 10 mm, asymmetric, and irregular. Benign disease is suggested when cholangiography shows short, regular, and symmetric strictures. Using these criteria, the diagnostic sensitivity and specificity for cholangiography findings were reported to be 74% and 70%, respectively [22]. After cholangiography, intraductal ultrasound (IDUS) is performed to detect the main lesion. Moreover, superficial extension from the main lesion or vascular invasion could be confirmed using IDUS [23,24,25]. A large retrospective study reported a sensitivity of 93.2%, a specificity of 89.5%, and an accuracy of 91.4% for the evaluation of malignant strictures [26]. When inserting the IDUS catheter into the bile duct, some cases are difficult due to the tension in the sphincter of Oddi. In such cases, endoscopic sphincterotomy may be performed. When inserting the IDUS catheter over the stricture, balloon dilation may be performed to pass the stricture. However, it should be limited for mandatory cases where investigation of proximal superficial extension is required because it might damage the main lesion. Figure 1 shows the procedures of cholangiography and IDUS.
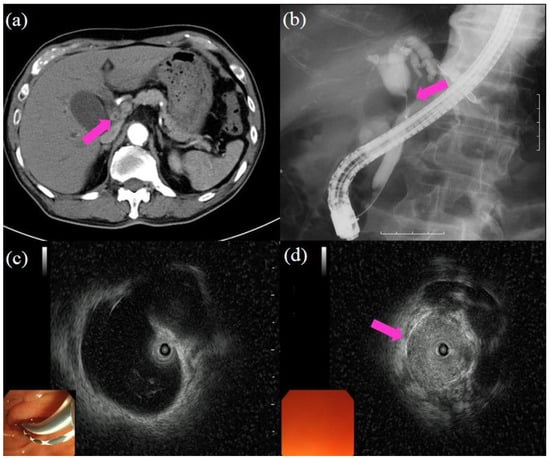
Figure 1.
Procedures of cholangiography and intraductal ultrasound (IDUS): (a) computed tomography showing the wall thickness in the bile duct (pink arrow); (b) cholangiography showing the biliary stricture in the hilar bile duct (pink arrow); the proximal part of the bile duct shows dilatation; (c) IDUS showing dilatation in the proximal part of the bile duct and no lesion; (d) IDUS showing a lesion in the biliary stricture (pink arrow).
Although cholangiography or IDUS findings provide information on whether the biliary stricture is benign or malignant, it is difficult to make a final diagnosis using only these methodologies. To make the final diagnosis, cytology/forceps biopsy under fluoroscopic guidance with ERCP is still the gold standard. Obtaining a specimen of adequate cellularity is essential for the evaluation of any potential malignancy. There are three approaches during ERCP: (1) bile juice aspiration cytology, (2) brush cytology, and (3) forceps biopsy.
Bile juice aspiration cytology is performed after the insertion of a biliary catheter. Although it is the easiest way to obtain a specimen, the sensitivity is extremely low, between 6% and 24% [27,28].
Regarding cytology, brush cytology may be considered a superior method to obtain a specimen compared to bile juice aspiration cytology. Brushing to obtain cytologic material involves advancing a brush with its catheter sheath through the endoscope into the biliary tree, generally over the guidewire. The device is advanced to the proximal part of the stricture, then the brush is advanced from the catheter, withdrawn slightly, and moved back and forth across the stricture approximately 15 times. The brush is then withdrawn into the catheter, and the device is withdrawn from the endoscope. The brush can be smeared onto glass slides, cut off from the device and placed into a fixative solution, or both. Its diagnostic performance has been evaluated in many studies with the sensitivity for malignancy in the range of 21–70% and specificity of 97–100% [29,30,31,32,33,34,35,36,37]. Table 1 summarizes the diagnostic yield of previous studies on brush cytology.

Table 1.
The diagnostic yield of studies on brush cytology.
Forceps biopsy is more time consuming and more technically challenging than brush cytology because it is sometimes difficult to insert thick forceps into the bile duct and grasp a targeted specimen. However, it could provide a sample of bile duct tissue from deep in the epithelium, which is expected to improve diagnostic yield compared with brush cytology. The biopsy forceps are thicker than an ERCP catheter so it could be difficult to insert them into the bile duct. Difficult cannulation has been identified as a risk factor of post-ERCP pancreatitis. Therefore, it may be better to perform sphincterotomy in advance to facilitate biliary cannulation using biopsy forceps to prevent post-ERCP pancreatitis. Under fluoroscopic guidance, the forceps are advanced to the part of the stricture, opened, and then closed to grasp a specimen from the distal aspect of the stricture. Some reports suggested that at least three specimens should be obtained [30,38]. The diagnostic performance of forceps biopsy has also been evaluated in many studies, with the sensitivity for malignancy in the range of 43–81% and specificity, 90–100% [30,33,34,35,36,39,40,41]. Table 2 summarizes the data of previous studies on forceps biopsy under fluoroscopic guidance. A meta-analysis reported that the pooled sensitivity and specificity of the brush cytology for the diagnosis of biliary strictures was 45% (95% confidence interval (CI) (40–50%)) and 99% (95% CI (98–100%)), respectively [42], whereas forceps biopsy had a pooled sensitivity and specificity of 48.1% (95% CI (42.8–53.4%)) and 99.2% (95% CI (97.6–99.8%)), respectively. Although forceps biopsy may have better sensitivity than brush cytology, these results have an insurmountable limit under fluoroscopic guidance.

Table 2.
The diagnostic yield of studies on forceps biopsy under fluoroscopic guidance.
In the recent European Society of Gastrointestinal Endoscopy Guidelines [43], the rates of pancreatitis, cholangitis, and perforation during/post-ERCP have been reported to be 3.5–9.7%, 0.5–3.0%, and 0.08–0.6%, respectively [44,45,46]. Moreover, the mortality rate of post-ERCP pancreatitis has been reported to be 0.1–0.7% [44]. Although ERCP is an essential procedure to assess biliary strictures, we must be mindful that severe and fatal ERCP-related adverse events can occur.
4. Cholangioscopy
As described above, ERCP is the gold standard for diagnosing biliary strictures. However, ERCP does not provide an intraluminal view of biliary strictures. Cholangio/pancreatoscopy overcomes this limitation by allowing direct visualization of the biliary and pancreatic ducts. Moreover, it can perform targeted biopsies of the site of interest. The traditional “mother–daughter” peroral cholangioscopy (POCS) requires two endoscopists, with one operating the cholangioscope, while the second endoscopist controls the duodenoscope [47]. The limitations of this system are the need for two operators, scope fragility, and time consumption. Over the past decade, single-operator cholangioscopy (SOC) (SpyGlass™ Direct Visualization System, Boston Scientific, Marlborough, MA, USA) has been widely utilized with disposable fiberoptic technology [48]. The setup of SOC is easy; only one operator is needed, four-way tip deflection is allowed, and targeted biopsies and therapeutic procedures such as lithotripsy can be performed. Nowadays, the new digital SOC with high-resolution digital technology (SpyGlass DS Direct Visualization System) provides improved image quality and maneuverability of the catheter tip [49]. The system consists of a 10.8-Fr catheter. The POCS is generally advanced over a guidewire into the bile duct through the working channel of a duodenoscope. Before insertion, sphincterotomy is generally performed. The working channel (1.2-mm diameter in SOC) allows the passage of accessory devices and aspiration.
POCS findings are defined as either malignant or benign according to the previous reports (Figure 2) [50,51,52,53]. Malignant findings include: (i) irregular thick tortuous vessels, (ii) oozing, (iii) irregular papillogranular surface, and (iv) a nodular elevated surface such as a submucosal tumor. Benign findings include: (i) a fine network of thin vessels and a flat surface with or without shallow pseudodiverticula; (ii) a lower homogeneous papillogranular surface without primary masses, suggesting hyperplasia; (iii) a bumpy surface with or without pseudodiverticula, suggesting inflammation; and (iv) a white surface with a convergence of folds, suggesting scarring. Table 3 provides the data on the diagnostic yield of POCS visual findings for indeterminate biliary strictures [37,41,48,49,54,55,56,57,58]. The sensitivity for malignancy is in the range of 83–100%, the specificity is 67–96%, and the accuracy is 85–96%. In a systematic review and meta-analysis of the diagnostic yield of POCS visual findings, the pooled sensitivity and specificity for diagnosing malignant biliary strictures were 84.5% (95% CI (79.2–88.9%)) and 82.6% (95% CI (77.1–87.3%)), respectively [59]. Moreover, POCS can detect superficial extension of cholangiocarcinoma in detail. It has been reported that POCS-guided biopsy provides an accurate diagnosis of the superficial extension of the cholangiocarcinoma compared with ERCP alone [60].
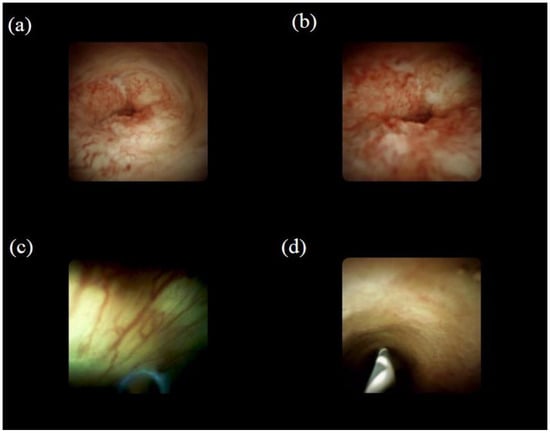
Figure 2.
Visual findings of cholangioscopy: (a) irregular thick tortuous vessels, suggesting malignancy; (b) irregular papillogranular surface, suggesting malignancy; (c) fine network of thin vessels, suggesting a benign lesion; (d) lower homogeneous papillogranular surface without primary masses, suggesting a benign lesion.

Table 3.
The diagnostic yield of studies on POCS visual findings and biopsy under direct view with POCS.
Despite good outcomes, the visual criteria for malignancy are not yet fully established, and there is significant interobserver variation in the interpretation of POCS visual findings. These variations can be misleading and may result in false-positive malignant diagnoses. Therefore, definite pathological confirmation is important for a definitive diagnosis of indeterminate biliary strictures. A prospective study reported that POCS-guided 3Fr mini-forceps tissue sampling has significantly higher accuracy compared with fluoroscopy-guided standard forceps biopsies [61]. Table 3 also provides data on the diagnostic yield of POCS-guided biopsy for indeterminate biliary strictures. The sensitivity for malignancy is in the range of 64–86%, the specificity is 89–100%, and the accuracy is 70–90% [37,41,48,49,54,55,56,57,58]. In a systematic review and meta-analysis of the diagnostic yield of the POCS-guided biopsy, the pooled sensitivity and specificity for diagnosing malignant biliary strictures were 60.1% (95% CI (54.9–65.2%)) and 98.0% (95% CI (96.0–99.0%)), respectively [59].
In a meta-analysis regarding POCS procedures, overall and serious adverse event rates of 7% and 1%, respectively, were reported [62]. When performing POCS, we must be mindful that cholangitis could be caused by an increase in intraductal pressure due to water irrigation during the procedure. Therefore, it is necessary to use antibiotic prophylaxis and perform biliary drainage to prevent cholangitis. Figure 3 highlights the procedure using POCS (SOC) to diagnose biliary strictures.
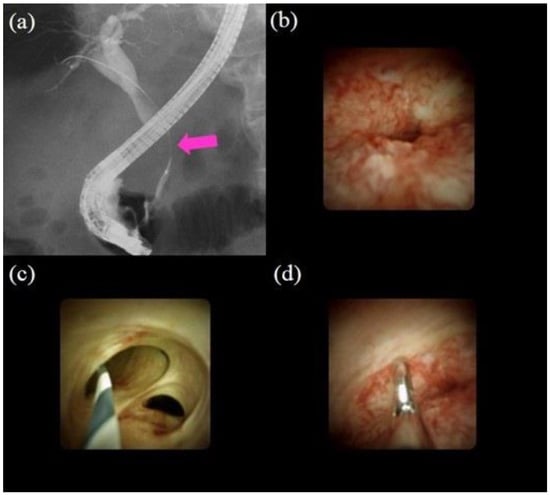
Figure 3.
Procedures of peroral cholangioscopy (POCS) and biopsy under direct view with POCS: (a) cholangiography showing the biliary stricture in the distal bile duct (pink arrow); (b) POCS showing an irregular papillogranular surface at the stricture, suggestive of malignancy; (c) POCS showing a fine network of thin vessels at the hilar bile duct, suggesting no malignancy; (d) forceps biopsy under direct view with POCS; the histological examination revealed adenocarcinoma.
5. Confocal Laser Endomicroscopy
Confocal laser endomicroscopy (CLE) is an endoscopic imaging technique that can provide in vivo histological assessment in real-time, known as “virtual biopsy.” Probe-based CLE (pCLE; CholangioFlex, Cellvizio; Mauna Kea Technologies, Paris, France) has been cited in the recent American Society for Gastrointestinal Endoscopy guidelines for the management of biliary neoplasia as a useful alternative to the existing diagnostic workup [63]. pCLE is performed under fluoroscopy guidance or direct view with POCS during ERCP. The CholangioFlex pCLE probe is designed to obtain in vivo, real-time, microscopic images of the bile duct during ERCP procedures. The probe has a diameter of 0.94 mm, a field of view of 325 µm, and a lateral resolution of 3.5 µm. Each probe provides images from 40–70 µm below the tissue surface. The confocal probe is advanced through the working channel of the POCS and gently applied to the part of interest to carry out confocal imaging at 12 frames per second. Intraductal images are recorded and saved to a computer unit connected to the probe. Although pCLE can be performed both under fluoroscopy guidance or direct view with POCS, the pCLE findings under direct view with POCS can be accurately matched with biopsy tissue. Therefore, these results could be diagnostically more reliable [58,64].
The Miami classification was initially created to differentiate malignant and benign tumors [65] (Figure 4). The criteria for the diagnosis of malignancy are listed as follows: (1) thick white bands (>20 µm), (2) thick dark bands (>40 µm), (3) dark clumps, and (4) epithelium. The criteria for the diagnosis of benign lesions are as follows: (1) a reticular network of thin dark branching bands (<20 µm), (2) a light-gray background, and (3) blood vessels (<20 µm). Subsequently, to solve the problem of false-positive cases (such as inflammation) as a result of using the Miami classification, the Paris classification was created [66].

Figure 4.
Probe-based confocal laser endomicroscopy images for biliary strictures: (a) thick dark bands (>40 µm) (pink arrow) according to the Miami classification; (b) dark clumps (pink arrow) according to the Miami classification; (c) reticular network of thin dark branching bands (<20 µm) according to the Miami classification.
Table 4 provides the data on the diagnostic yield of pCLE for biliary strictures [58,64,65,66,67,68]. The sensitivity for malignancy is in the range of 83–98%, the specificity is 33–93%, and the accuracy is 78–93%. In a systematic review and meta-analysis of the diagnostic yield of pCLE, the pooled sensitivity and specificity for diagnosing malignancy were 90% (95% CI (84–94%)) and 75% (95% CI (66–83%)), respectively [69]. Figure 5 highlights the procedure of pCLE under direct view with POCS to diagnose biliary strictures. pCLE has been shown to have high-performance characteristics in the evaluation of biliary strictures, possibly reducing the need for repeat procedures, thereby decreasing cost. However, CLE requires additional training for interpretation. This variability of interpretation is considered to be the greatest obstacle to the widespread use of CLE.

Table 4.
The diagnostic yield of studies on pCLE.
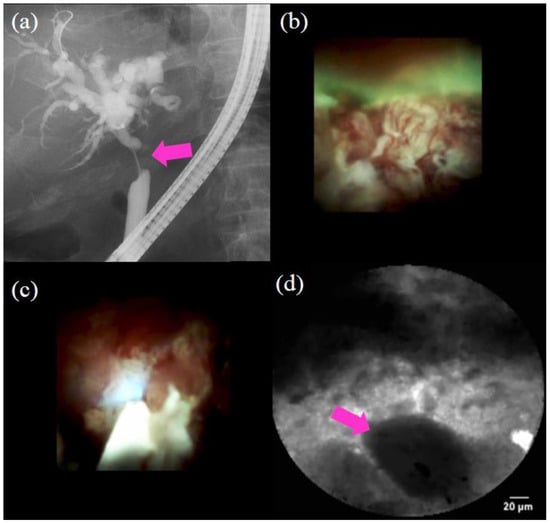
Figure 5.
Procedure of probe-based confocal laser endomicroscopy (pCLE) under direct view with peroral cholangioscopy (POCS) to diagnose biliary strictures: (a) cholangiography showing the biliary stricture in the hilar bile duct (pink arrow); (b) POCS showing irregular thick tortuous vessels at the stricture, suggestive of malignancy; (c,d) pCLE under direct view with POCS showing dark clumps, suggestive of malignancy; the histological examination demonstrated adenocarcinoma.
6. Endoscopic Ultrasound and Endoscopic Ultrasound-Guided Fine-Needle Aspiration
Endoscopic ultrasound (EUS) is an ultrasound technique in which the tip of the endoscope is equipped with a high-frequency transducer. High-resolution images of the biliary tract can be obtained through the stomach and duodenum. Regarding malignant biliary stricture detection, EUS without fine-needle aspiration (FNA) was found to provide a sensitivity of 78% and specificity of 84% [70]. Another study proved that EUS was superior for the detection of malignancies compared to CT and MRI (94, 30, and 42%, respectively) [71]. Regarding adverse events, EUS, especially for observation purposes, can avoid pancreatitis, which is mainly problematic for ERCP.
EUS-FNA is the established diagnostic modality to obtain specimens, particularly of pancreatic tumors [72,73]. EUS-FNA enables the acquisition of histological evidence of cancer when chemotherapy is being considered to distinguish benign or malignant tumors when deciding whether surgery or follow-up is needed, and assessment of the degree of progression of malignant tumors when unexplained lymph node swelling is detected. At present, the most frequently used needle sizes are 22 gauge and 25 gauge. Table 5 provides the data on the diagnostic yield of EUS-FNA of the biliary tract [71,74,75,76,77,78]. The sensitivity for malignancy is in the range of 43–94%, the specificity is 100%, and the accuracy is 70–94%. In a recent meta-analysis, it was reported that the mean sensitivities of ERCP and EUS-FNA for the diagnosis of malignant biliary strictures were 49% and 75%, while specificities were 96% and 100%, respectively [79]. EUS-FNA might offer a safer alternative to ERCP. With the recent progress of needles, the fine-needle biopsy (FNB) device, which was designed primarily to obtain core tissue samples, was introduced to overcome the FNA sampling material limitation [80,81]. In a recent meta-analysis comparing FNA with FNB needles, FNB provided a higher pooled diagnostic accuracy, tissue core rate, and allowed diagnosis with fewer passes in both pancreatic and nonpancreatic lesions [82]. Although there were no reports using FNB needles regarding the biliary tract, FNB needles have the potential to increase the diagnostic accuracy. Hence, studies regarding EUS-FNB use for the biliary tract are warranted. Recently, increasing case reports of needle tract seeding following EUS-FNA or EUS-FNB are emerging. In a recent review regarding needle tract seeding following EUS-FNA or EUS-FNB, 33 patients (27, pancreatic cancer; 6, others) with needle tract seeding following EUS-FNA or EUS-FNB have been reported up to January 2020 [83]. Although there were no reports regarding the biliary tract, needle tract seeding could be caused. Thus, EUS-FNA should not be performed when it does not guide treatment selection [84].

Table 5.
The diagnostic yield of studies on EUS-FNA.
7. Molecular Diagnostics
Next-generation DNA sequencing (NGS) as molecular diagnostics has been the upcoming technology for diagnosing biliary strictures. It allows for the rapid and simultaneous sequencing of genetic material on a single medium or surface [85,86]. One study showed that the combination of a 28-gene panel (BiliSeq) and pathological evaluation increased the sensitivity to 83% and the specificity of 99% in diagnosing biliary strictures [86]. Although further studies are required, it has the potential to diagnose biliary strictures and identify targetable genomic alterations.
8. Percutaneous Transhepatic Cholangiography
Percutaneous Transhepatic Cholangiography (PTC) is a radiologic procedure that directly accesses the biliary tract using an ultrasound-guided percutaneous needle. It was reported that the sensitivity and specificity of PTC were 70.8% and 47.6%, respectively [87]. It is similar diagnostic ability to ERCP. In this study, percutaneous transhepatic cholangioscopy (PTCS) was also useful for diagnosing biliary strictures. Although PTC is a useful option to diagnose biliary strictures, seeding could be caused by PTC. In a recent systematic review comparing the incidence of seeding metastasis between endoscopic biliary drainage (EBD) and PTC reported that the incidence of seeding metastasis in the EBD group was significantly lower than that in the PTBD group (10.5% vs. 22.0% OR = 0.35, 95% CI 0.23~0.53) [88]. Therefore, PTC might be limited to cases in which ERCP-related procedures have failed.
9. Conclusions
We discussed the diagnostic process using endoscopy for indeterminate biliary strictures. Various modalities using endoscopy for the diagnosis of biliary strictures have been reported, and their capabilities have improved. We propose the diagnostic algorithm (Figure 6). First of all, noninvasive evaluation such as taking the patient’s history, examining the patient’s symptoms, hepatobiliary enzymes, and tumor markers should be performed. Second, cross-sectional imaging such as US, CT, and MRI (MRCP) should be performed. EUS imaging is also useful at the same time. Third, an ERCP-related procedure should be performed. As we showed, peroral cholangioscopy (POCS) findings and POCS-guided biopsy/Confocal laser endomicroscopy (CLE) provide better outcomes than ERCP under fluoroscopic guidance. However, as POCS and CLE are too expensive to use in the first instance, ERCP (IDUS) with brush cytology and forceps biopsy should be performed first. If the ERCP with brush cytology and forceps biopsy is positive, surgery should be performed. If the stricture remains indeterminate, ERCP with POCS (POCS-guided biopsy)/CLE should be performed. Although EUS-FNA may be performed at this time, we must take into consideration that seeding could be caused. If the stricture remains indeterminate, repeat consideration should be made for repeat ERCP with brushings, POCS with biopsies, and pCLE. If the stricture remains indeterminate even though repeat procedures were performed and suspicion for malignancy remains high, close follow-up or surgery might be considered. Although progress has been made regarding endoscopic procedures, further improvement is needed.
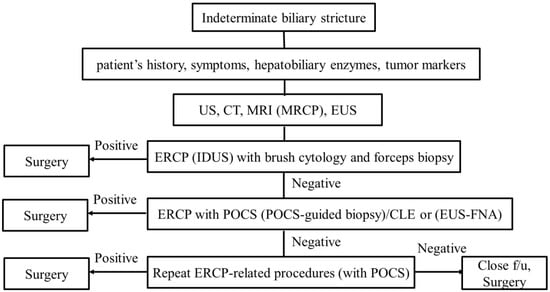
Figure 6.
Diagnostic algorithm for indeterminate biliary strictures.
Author Contributions
The paper was authored Y.T., who designed and drafted the article. M.M., A.F., T.O., M.S., H.K., Y.S., K.M., T.T., Y.M. and S.R. provided critical revision of the article for important intellectual content. Y.T. finally approved the article for submission. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Park, J.; Kim, M.H.; Kim, K.P.; Park, D.H.; Moon, S.H.; Song, T.J.; Eum, J.; Lee, S.S.; Seo, D.W.; Lee, S.K. Natural History and Prognostic Factors of Advanced Cholangiocarcinoma without Surgery, Chemotherapy, or Radiotherapy: A Large-Scale Observational Study. Gut Liver 2009, 3, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- DeOliveira, M.L.; Cunningham, S.C.; Cameron, J.L.; Kamangar, F.; Winter, J.M.; Lillemoe, K.D.; Choti, M.A.; Yeo, C.J.; Schulick, R.D. Cholangiocarcinoma: Thirty-one-year experience with 564 patients at a single institution. Ann. Surg. 2007, 245, 755–762. [Google Scholar] [CrossRef]
- Sano, I.; Katanuma, A.; Kuwatani, M.; Kawakami, H.; Kato, H.; Itoi, T.; Ono, M.; Irisawa, A.; Okabe, Y.; Iwashita, T.; et al. Long-term outcomes after therapeutic endoscopic retrograde cholangiopancreatography using balloon-assisted enteroscopy for anastomotic stenosis of choledochojejunostomy/pancreaticojejunostomy. J. Gastroenterol. Hepatol. 2019, 34, 612–619. [Google Scholar] [CrossRef]
- Prokopič, M.; Beuers, U. Management of primary sclerosing cholangitis and its complications: An algorithmic approach. Hepatol. Int. 2021, 15, 6–20. [Google Scholar] [CrossRef]
- Garcea, G.; Ngu, W.; Neal, C.P.; Dennison, A.R.; Berry, D.P. Bilirubin levels predict malignancy in patients with obstructive jaundice. HPB 2011, 13, 426–430. [Google Scholar] [CrossRef]
- Bowlus, C.L.; Olson, K.A.; Gershwin, M.E. Evaluation of indeterminate biliary strictures. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Oseini, A.M.; Chaiteerakij, R.; Shire, A.M.; Ghazale, A.; Kaiya, J.; Moser, C.D.; Aderca, I.; Mettler, T.A.; Therneau, T.M.; Zhang, L.; et al. Utility of serum immunoglobulin G4 in distinguishing immunoglobulin G4-associated cholangitis from cholangiocarcinoma. Hepatology 2011, 54, 940–948. [Google Scholar] [CrossRef]
- Azeem, N.; Ajmera, V.; Hameed, B.; Mehta, N. Hilar cholangiocarcinoma associated with immunoglobulin G4-positive plasma cells and elevated serum immunoglobulin G4 levels. Hepatol. Commun. 2018, 2, 349–353. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Okusaka, T.; Shimizu, K.; Furuse, J.; Ito, Y.; Hanada, K.; Shimosegawa, T.; Okazaki, K. Committee for Revision of Clinical Guidelines for Pancreatic Cancer of the Japan Pancreas Society. Clinical Practice Guidelines for Pancreatic Cancer 2016 from the Japan Pancreas Society: A Synopsis. Pancreas 2017, 46, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Yoshitomi, H.; Miyakawa, S.; Uesaka, K.; Unno, M.; Endo, I.; Ota, T.; Ohtsuka, M.; Kinoshita, H.; Shimada, K.; et al. Clinical practice guidelines for the management of biliary tract cancers 2015: The 2nd English edition. J. Hepatobiliary Pancreat. Sci. 2015, 22, 249–273. [Google Scholar] [CrossRef]
- Ballehaninna, U.K.; Chamberlain, R.S. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J. Gastrointest. Oncol. 2012, 3, 105–119. [Google Scholar] [PubMed]
- Qin, X.-L.; Wang, Z.-R.; Shi, J.-S.; Lu, M.; Wang, L.; He, Q.-R. Utility of serum CA19-9 in diagnosis of cholangiocarcinoma: In comparison with CEA. World J. Gastroenterol. 2004, 10, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Albu, S.; Tantau, M.; Sparchez, Z.; Branda, H.; Suteu, T.; Badea, R.; Pascu, O. Diagnosis and treatment of extrahepatic cholangiocarcinoma: Results in a series of 124 patients. Rom. J. Gastroenterol. 2005, 14, 33–36. [Google Scholar]
- Takakura, K.; Sumiyama, K.; Munakata, K.; Ashida, H.; Arihiro, S.; Kakutani, H.; Tajiri, H. Clinical usefulness of diffusion-weighted MR imaging for detection of pancreatic cancer: Comparison with enhanced multidetector-row CT. Abdom. Imaging 2011, 36, 457–462. [Google Scholar] [CrossRef]
- Fusari, M.; Maurea, S.; Imbriaco, M.; Mollica, C.; Avitabile, G.; Soscia, F.; Camera, L.; Salvatore, M. Comparison between multislice CT and MR imaging in the diagnostic evaluation of patients with pancreatic masses. Radiol. Med. 2010, 115, 453–466. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Luo, M.; Sun, Y.W.; Xu, Q.; Chen, W.; Zhao, G.; Wu, Z.Y. Computed tomography in diagnosing vascular invasion in pancreatic and periampullary cancers: A systematic review and meta-analysis. Hepatobiliary Pancreat. Dis. Int. 2009, 8, 457–464. [Google Scholar] [PubMed]
- Martinez, N.S.; Trindade, A.J.; Sejpal, D.V. Determining the Indeterminate Biliary Stricture: Cholangioscopy and Beyond. Curr. Gastroenterol. Rep. 2020, 22, 58. [Google Scholar] [CrossRef]
- Al-Dhuhli, H. Role of Magnetic Resonance Cholangiopancreatography in the Evaluation of Biliary Disease. Sultan Qaboos Univ. Med. J. 2009, 9, 341–352. [Google Scholar]
- Freeman, M.L.; Guda, N.M. ERCP cannulation: A review of reported techniques. Gastrointest. Endosc. 2005, 61, 112–125. [Google Scholar] [CrossRef]
- Suissa, A.; Yassin, K.; Lavy, A.; Lachter, J.; Chermech, I.; Karban, A.; Tamir, A.; Eliakim, R. Outcome and early complications of ERCP: A prospective single center study. Hepatogastroenterology 2005, 52, 352–355. [Google Scholar] [PubMed]
- Park, M.S.; Kim, T.K.; Kim, K.W.; Park, S.W.; Lee, J.K.; Kim, J.S.; Lee, J.H.; Kim, K.A.; Kim, A.Y.; Kim, P.N.; et al. Differentiation of extrahepatic bile duct cholangiocarcinoma from benign stricture: Findings at MRCP versus ERCP. Radiology 2004, 233, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Tamada, K.; Ushio, J.; Sugano, K. Endoscopic diagnosis of extrahepatic bile duct carcinoma: Advances and current limitations. World J. Clin. Oncol. 2011, 2, 203–216. [Google Scholar] [CrossRef]
- Farrell, R.J.; Agarwal, B.; Brandwein, S.L.; Underhill, J.; Chuttani, R.; Pleskow, D.K. Intraductal US is a useful adjunct to ERCP for distinguishing malignant from benign biliary strictures. Gastrointest. Endosc. 2002, 56, 681–687. [Google Scholar] [CrossRef]
- Sun, B.; Hu, B. The role of intraductal ultrasonography in pancreatobiliary diseases. Endosc. Ultrasound 2016, 5, 291–299. [Google Scholar] [CrossRef]
- Meister, T.; Heinzow, H.S.; Woestmeyer, C.; Lenz, P.; Menzel, J.; Kucharzik, T.; Domschke, W.; Domagk, D. Intraductal ultrasound substantiates diagnostics of bile duct strictures of uncertain etiology. World J. Gastroenterol. 2013, 19, 874–881. [Google Scholar] [CrossRef]
- Desa, L.A.; Akosa, A.B.; Lazzara, S.; Domizio, P.; Krausz, T.; Benjamin, I.S. Cytodiagnosis in the management of extrahepatic biliary stricture. Gut 1991, 32, 1188–1191. [Google Scholar] [CrossRef] [PubMed]
- Kurzawinski, T.; Deery, A.; Dooley, J.; Dick, R.; Hobbs, K.; Davidson, B. A prospective controlled study comparing brush and bile exfoliative cytology for diagnosing bile duct strictures. Gut 1992, 33, 1675–1677. [Google Scholar] [CrossRef] [PubMed]
- Foutch, P.G.; Kerr, D.M.; Harlan, J.R.; Kummet, T.D. A prospective, controlled analysis of endoscopic cytotechniques for diagnosis of malignant biliary strictures. Am. J. Gastroenterol. 1991, 86, 577–580. [Google Scholar] [PubMed]
- Ponchon, T.; Gagnon, P.; Berger, F.; Labadie, M.; Liaras, A.; Chavaillon, A.; Bory, R. Value of endobiliary brush cytology and biopsies for the diagnosis of malignant bile duct stenosis: Results of a prospective study. Gastrointest. Endosc. 1995, 42, 565–572. [Google Scholar] [CrossRef]
- Pugliese, V.; Conio, M.; Nicolò, G.; Saccomanno, S.; Gatteschi, B. Endoscopic retrograde forceps biopsy and brush cytology of biliary strictures: A prospective study. Gastrointest. Endosc. 1995, 42, 520–526. [Google Scholar] [CrossRef]
- Mansfield, J.C.; Griffin, S.M.; Wadehra, V.; Matthewson, K. A prospective evaluation of cytology from biliary strictures. Gut 1997, 40, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Jailwala, J.; Fogel, E.L.; Sherman, S.; Gottlieb, K.; Flueckiger, J.; Bucksot, L.G.; Lehman, G.A. Triple-tissue sampling at ERCP in malignant biliary obstruction. Gastrointest. Endosc. 2000, 51, 383–390. [Google Scholar] [CrossRef]
- Stewart, C.J.; Mills, P.R.; Carter, R.; O’Donohue, J.; Fullarton, G.; Imrie, C.W.; Murray, W.R. Brush cytology in the assessment of pancreatico-biliary strictures: A review of 406 cases. J. Clin. Pathol. 2001, 54, 449–455. [Google Scholar] [CrossRef]
- Kitajima, Y.; Ohara, H.; Nakazawa, T.; Ando, T.; Hayashi, K.; Takada, H.; Tanaka, H.; Ogawa, K.; Sano, H.; Togawa, S.; et al. Usefulness of transpapillary bile duct brushing cytology and forceps biopsy for improved diagnosis in patients with biliary strictures. J. Gastroenterol. Hepatol. 2007, 22, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Tsuyuguchi, T.; Sakai, Y.; Sugiyama, H.; Tawada, K.; Mikata, R.; Tada, M.; Ishihara, T.; Miyazaki, M.; Yokosuka, O. Factors affecting the accuracy of endoscopic transpapillary sampling methods for bile duct cancer. Dig. Endosc. 2014, 26, 276–281. [Google Scholar] [CrossRef]
- Gerges, C.; Benya, T.; Tang, R.S.Y.; Bahin, F.; Lau, J.Y.W.; Geenen, E.V.; Neuhaus, H.; Reddy, D.N.; Ramchandani, M. Digital single-operator peroral cholangioscopy-guided biopsy sampling versus ERCP-guided brushing for indeterminate biliary strictures: A prospective, randomized, multicenter trial (with video). Gastrointest. Endosc. 2020, 91, 1105–1113. [Google Scholar] [CrossRef]
- Schoefl, R.; Haefner, M.; Wrba, F.; Pfeffel, F.; Stain, C.; Poetzi, R.; Gangl, A. Forceps biopsy and brush cytology during endoscopic retrograde cholangiopancreatography for the diagnosis of biliary stenoses. Scand. J. Gastroenterol. 1997, 32, 363–368. [Google Scholar] [CrossRef]
- Kubota, Y.; Takaoka, M.; Tani, K.; Ogura, M.; Kin, H.; Fujimura, K.; Mizuno, T.; Inoue, K. Endoscopic transpapillary biopsy for diagnosis of patients with pancreaticobiliary ductal strictures. Am. J. Gastroenterol. 1993, 88, 1700–1704. [Google Scholar] [PubMed]
- Sugiyama, M.; Atomi, Y.; Wada, N.; Kuroda, A.; Muto, T. Endoscopic transpapillary bile duct biopsy without sphincterotomy for diagnosing biliary strictures: A prospective comparative study with bile and brush cytology. Am. J. Gastroenterol. 1996, 91, 465–467. [Google Scholar]
- Hartman, D.J.; Slivka, A.; Giusto, D.A.; Krasinskas, A.M. Tissue Yield and Diagnostic Efficacy of Fluoroscopic and Cholangioscopic Techniques to Assess Indeterminate Biliary Strictures. Clin. Gastroenterol. Hepatol. 2012, 10, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, U.; Njei, B.; Venkatesh, P.G.; Lourdusamy, V.; Sanaka, M.R. Endoscopic ultrasound in the diagnosis of cholangiocarcinoma as the etiology of biliary strictures: A systematic review and meta-analysis. Gastroenterol. Rep. 2015, 3, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Dumonceau, J.-M.; Kapral, C.; Aabakken, L.; Papanikolaou, I.S.; Tringali, A.; Vanbiervliet, G.; Beyna, T.; Dinis-Ribeiro, M.; Hritz, I.; Mariani, A.; et al. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2020, 52, 127–149. [Google Scholar] [CrossRef] [PubMed]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef]
- Kiriyama, S.; Kozaka, K.; Takada, T.; Strasberg, S.M.; Pitt, H.A.; Gabata, T.; Hata, J.; Liau, K.-H.; Miura, F.; Horiguchi, A.; et al. Tokyo Guidelines 2018: Diagnostic criteria and severity grading of acute cholangitis (with videos). J. Hepato-Biliary-Pancreat. Sci. 2018, 25, 17–30. [Google Scholar] [CrossRef]
- Cotton, P.B.; Eisen, G.M.; Aabakken, L.; Baron, T.H.; Hutter, M.M.; Jacobson, B.C.; Mergener, K.; Nemcek, A., Jr.; Petersen, B.T.; Petrini, J.L.; et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest. Endosc. 2010, 71, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Akasaka, Y.; Yamaguchi, K.; Fujimoto, S.; Kawai, K. Direct endoscopic visualization of the bile and pancreatic duct systems by peroral cholangiopancreatoscopy (PCPS). Gastrointest. Endosc. 1978, 24, 141–145. [Google Scholar] [CrossRef]
- Chen, Y.K.; Pleskow, D.K. Preclinical characterization of the Spyglass peroral cholangiopancreatoscopy system for direct access, visualization, and biopsy. Gastrointest. Endosc. 2007, 65, 303–311. [Google Scholar] [CrossRef]
- Navaneethan, U.; Hasan, M.K.; Kommaraju, K.; Zhu, X.; Hebert-Magee, S.; Hawes, R.H.; Vargo, J.J.; Varadarajulu, S.; Parsi, M.A. Digital, single-operator cholangiopancreatoscopy in the diagnosis and management of pancreatobiliary disorders: A multicenter clinical experience (with video). Gastrointest. Endosc. 2016, 84, 649–655. [Google Scholar] [CrossRef]
- Seo, D.W.; Lee, S.K.; Yoo, K.S.; Kang, G.H.; Kim, M.H.; Suh, D.-J.; Min, Y.I. Cholangioscopic findings in bile duct tumors. Gastrointest. Endosc. 2000, 52, 630–634. [Google Scholar] [CrossRef]
- Itoi, T.; Neuhaus, H.; Chen, Y.K. Diagnostic Value of Image-Enhanced Video Cholangiopancreatoscopy. Gastrointest. Endosc. Clin. N. Am. 2009, 19, 557–566. [Google Scholar] [CrossRef]
- Itoi, T.; Sofuni, A.; Itokawa, F.; Tsuchiya, T.; Kurihara, T.; Ishii, K.; Tsuji, S.; Moriyasu, F.; Gotoda, T. Peroral cholangioscopic diagnosis of biliary-tract diseases by using narrow-band imaging (with videos). Gastrointest. Endosc. 2007, 66, 730–736. [Google Scholar] [CrossRef]
- Awadallah, N.S.; Chen, Y.K.; Piraka, C.; Antillon, M.R.; Shah, R.J. Is there a role for cholangioscopy in patients with primary sclerosing cholangitis? Am. J. Gastroenterol. 2006, 101, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.S.; Lee, J.K.; Oh, S.H.; Kim, M.J.; Jung, J.G.; Lee, K.H.; Lee, K.T. Role of SpyGlass peroral cholangioscopy in the evaluation of indeterminate biliary lesions. Dig. Dis. Sci. 2014, 59, 2565–2570. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, T.; Yasuda, I.; Isayama, H.; Tsuyuguchi, T.; Yamaguchi, T.; Kawabe, K.; Okabe, Y.; Hanada, K.; Hayashi, T.; Ohtsuka, T.; et al. Diagnostic and therapeutic single-operator cholangiopancreatoscopy in biliopancreatic diseases: Prospective multicenter study in Japan. World J. Gastroenterol. 2016, 22, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Imanishi, M.; Kurisu, Y.; Onda, S.; Sano, T.; Takagi, W.; Okuda, A.; Miyano, A.; Amano, M.; Nishioka, N. Prospective evaluation of digital single-operator cholangioscope for diagnostic and therapeutic procedures (with videos). Dig. Endosc. 2017, 29, 782–789. [Google Scholar] [CrossRef]
- Shah, R.J.; Raijman, I.; Brauer, B.; Gumustop, B.; Pleskow, D.K. Performance of a fully disposable, digital, single-operator cholangiopancreatoscope. Endoscopy 2017, 49, 651–658. [Google Scholar] [CrossRef]
- Tanisaka, Y.; Ryozawa, S.; Nonaka, K.; Yasuda, M.; Fujita, A.; Ogawa, T.; Mizuide, M.; Tashima, T.; Araki, R. Diagnosis of Biliary Strictures Using Probe-Based Confocal Laser Endomicroscopy under the Direct View of Peroral Cholangioscopy: Results of a Prospective Study (with Video). Gastroenterol. Res. Pract. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Navaneethan, U.; Hasan, M.K.; Lourdusamy, V.; Njei, B.; Varadarajulu, S.; Hawes, R.H. Single-operator cholangioscopy and targeted biopsies in the diagnosis of indeterminate biliary strictures: A systematic review. Gastrointest. Endosc. 2015, 82, 608–614. [Google Scholar] [CrossRef]
- Kawakami, H.; Kuwatani, M.; Etoh, K.; Haba, S.; Yamato, H.; Shinada, K.; Nakanishi, Y.; Tanaka, E.; Hirano, S.; Kondo, S.; et al. Endoscopic retrograde cholangiography versus peroral cholangioscopy to evaluate intraepithelial tumor spread in biliary cancer. Endoscopy 2009, 41, 959–964. [Google Scholar] [CrossRef]
- Draganov, P.V.; Chauhan, S.; Wagh, M.S.; Gupte, A.R.; Lin, T.; Hou, W.; Forsmark, C.E. Diagnostic accuracy of conventional and cholangioscopy-guided sampling of indeterminate biliary lesions at the time of ERCP: A prospective, long-term follow-up study. Gastrointest. Endosc. 2012, 75, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Korrapati, P.; Ciolino, J.; Wani, S.; Shah, J.; Watson, R.; Muthusamy, V.R.; Klapman, J.; Komanduri, S. The efficacy of peroral cholangioscopy for difficult bile duct stones and indeterminate strictures: A systematic review and meta-analysis. Endosc. Int. Open 2016, 4, E263–E275. [Google Scholar] [CrossRef] [PubMed]
- American Society for Gastrointestinal Endoscopy (ASGE) Standards of Practice Committee; Anderson, M.A.; Appalaneni, V.; Ben-Menachem, T.; Decker, G.A.; Early, D.S.; Evans, J.A.; Fanelli, R.D.; Fisher, D.A.; Fisher, L.R.; et al. The role of endoscopy in the evaluation and treatment of patients with biliary neoplasia. Gastrointest. Endosc. 2013, 77, 167–174. [Google Scholar] [CrossRef]
- Meining, A.; Chen, Y.K.; Pleskow, D.; Stevens, P.; Shah, R.J.; Chuttani, R.; Michalek, J.; Slivka, A. Direct visualization of indeterminate pancreaticobiliary strictures with probe-based confocal laser endomicroscopy: A multicenter experience. Gastrointest. Endosc. 2011, 74, 961–968. [Google Scholar] [CrossRef]
- Meining, A.; Shah, R.J.; Slivka, A.; Pleskow, D.; Chuttani, R.; Stevens, P.D.; Becker, V.; Chen, Y.K. Classification of probe-based confocal laser endomicroscopy findings in pancreaticobiliary strictures. Endoscopy 2012, 44, 251–257. [Google Scholar] [CrossRef]
- Caillol, F.; Filoche, B.; Gaidhane, M.; Kahaleh, M. Refined Probe-Based Confocal Laser Endomicroscopy Classification for Biliary Strictures: The Paris Classification. Dig. Dis. Sci. 2013, 58, 1784–1789. [Google Scholar] [CrossRef] [PubMed]
- Slivka, A.; Gan, I.; Jamidar, P.; Costamagna, G.; Cesaro, P.; Giovannini, M.; Caillol, F.; Kahaleh, M. Validation of the diagnostic accuracy of probe-based confocal laser endomicroscopy for the characterization of indeterminate biliary strictures: Results of a prospective multicenter international study. Gastrointest. Endosc. 2015, 81, 282–290. [Google Scholar] [CrossRef]
- Dubow, M.; Tatman, P.D.; Shah, R.J. Individual probe based confocal laser endomicroscopy criteria in the analysis of indeterminate biliary strictures. Scand. J. Gastroenterol. 2018, 53, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, Y.; Sun, B.; Zhang, W.-M.; Zhang, Z.-Z.; He, Y.-P.; Yang, X.-J. Probe-based confocal laser endomicroscopy for the diagnosis of undetermined biliary stenoses: A meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2016, 40, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Garrow, D.; Miller, S.; Sinha, D.; Conway, J.; Hoffman, B.J.; Hawes, R.H.; Romagnuolo, J. Endoscopic Ultrasound: A Meta-analysis of Test Performance in Suspected Biliary Obstruction. Clin. Gastroenterol. Hepatol. 2007, 5, 616–623.e1. [Google Scholar] [CrossRef] [PubMed]
- Mohamadnejad, M.; DeWitt, J.M.; Sherman, S.; LeBlanc, J.K.; Pitt, H.A.; House, M.G.; Jones, K.J.; Fogel, E.L.; McHenry, L.; Watkins, J.L.; et al. Role of EUS for preoperative evaluation of cholangiocarcinoma: A large single-center experience. Gastrointest. Endosc. 2011, 73, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Yamao, K.; Sawaki, A.; Mizuno, N.; Shimizu, Y.; Yatabe, Y.; Koshikawa, T. Endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNAB): Past, present, and future. J. Gastroenterol. 2005, 40, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.K.; Hawes, R.H. EUS-Guided FNA of Solid Pancreas Tumors. Gastrointest. Endosc. Clin. N. Am. 2012, 22, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Fritscherravens, A.; Broering, D.C.; Knoefel, W.T.; Rogiers, X.; Swain, P.; Thonke, F.; Bobrowski, C.; Topalidis, T.; Soehendra, N. EUS-Guided Fine-Needle Aspiration of Suspected Hilar Cholangiocarcinoma in Potentially Operable Patients with Negative Brush Cytology. Am. J. Gastroenterol. 2004, 99, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Rösch, T.; Hofrichter, K.; Frimberger, E.; Meining, A.; Born, P.; Weigert, N.; Allescher, H.-D.; Classen, M.; Barbur, M.; Schenck, U.; et al. ERCP or EUS for tissue diagnosis of biliary strictures? A prospective comparative study. Gastrointest. Endosc. 2004, 60, 390–396. [Google Scholar] [CrossRef]
- DeWitt, J.; Misra, V.L.; Leblanc, J.K.; McHenry, L.; Sherman, S. EUS-guided FNA of proximal biliary strictures after negative ERCP brush cytology results. Gastrointest. Endosc. 2006, 64, 325–333. [Google Scholar] [CrossRef]
- Weilert, F.; Bhat, Y.M.; Binmoeller, K.F.; Kane, S.; Jaffee, I.M.; Shaw, R.E.; Cameron, R.; Hashimoto, Y.; Shah, J.N. EUS-FNA is superior to ERCP-based tissue sampling in suspected malignant biliary obstruction: Results of a prospective, single-blind, comparative study. Gastrointest. Endosc. 2014, 80, 97–104. [Google Scholar] [CrossRef]
- Onda, S.; Ogura, T.; Kurisu, Y.; Masuda, D.; Sano, T.; Takagi, W.; Fukunishi, S.; Higuchi, K. EUS-guided FNA for biliary disease as first-line modality to obtain histological evidence. Ther. Adv. Gastroenterol. 2016, 9, 302–312. [Google Scholar] [CrossRef]
- De Moura, D.T.H.; Moura, E.G.H.; Bernardo, W.M.; De Moura, E.T.H.; Baraca, F.I.; Kondo, A.; Matuguma, S.E.; Almeida Artifon, E.L. Endoscopic retrograde cholangiopancreatography versus endoscopic ultrasound for tissue diagnosis of malignant biliary stricture: Systematic review and meta-analysis. Endosc. Ultrasound 2018, 7, 10–19. [Google Scholar] [CrossRef]
- Adler, D.G.; Witt, B.; Chadwick, B.; Wells, J.; Taylor, L.J.; Dimaio, C.; Schmidt, R. Pathologic evaluation of a new endoscopic ultrasound needle designed to obtain core tissue samples: A pilot study. Endosc. Ultrasound 2016, 5, 178–183. [Google Scholar] [CrossRef]
- Bang, J.Y.; Hebert-Magee, S.; Hasan, M.K.; Navaneethan, U.; Hawes, R.; Varadarajulu, S. Endoscopic ultrasonography-guided biopsy using a Franseen needle design: Initial assessment. Dig. Endosc. 2017, 29, 338–346. [Google Scholar] [CrossRef]
- Van Riet, P.A.; Erler, N.S.; Bruno, M.J.; Cahen, D.L. Comparison of fine-needle aspiration and fine-needle biopsy devices for endoscopic ultrasound-guided sampling of solid lesions: A systemic review and meta-analysis. Endoscopy 2020. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.-Y.; Wu, B.-H.; Shen, X.-Y.; Peng, T.-L.; Li, D.-F.; Wei, C.; Yu, Z.-C.; Luo, M.-H.; Xiong, F.; Wang, L.-S.; et al. Overlooked risk for needle tract seeding following endoscopic ultrasound-guided minimally invasive tissue acquisition. World J. Gastroenterol. 2020, 26, 6182–6194. [Google Scholar] [CrossRef] [PubMed]
- Mizuide, M.; Ryozawa, S.; Fujita, A.; Ogawa, T.; Katsuda, H.; Suzuki, M.; Noguchi, T.; Tanisaka, Y. Complications of Endoscopic Ultrasound-Guided Fine Needle Aspiration: A Narrative Review. Diagnostics 2020, 10, 964. [Google Scholar] [CrossRef] [PubMed]
- Alekseyev, Y.O.; Fazeli, R.; Yang, S.; Basran, R.; Maher, T.; Miller, N.S.; Remic, D. A Next-Generation Sequencing Primer—How Does It Work and What Can It Do? Acad. Pathol. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Singhi, A.D.; Nikiforova, M.N.; Chennat, J.; Papachristou, G.I.; Khalid, A.; Rabinovitz, M.; Das, R.; Sarkaria, S.; Ayasso, M.S.; Wald, A.I.; et al. Integrating next-generation sequencing to endoscopic retrograde cholangiopancreatography (ERCP)-obtained biliary specimens improves the detection and management of patients with malignant bile duct strictures. Gut 2020, 69, 52–61. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, H.J.; Oh, H.C.; Lee, K.H.; Jung, J.Y.; Kim, S.; Lee, S.S.; Seo, D.W.; Kim, M.H.; Lee, S.K. The usefulness of percutaneous transhepatic cholangioscopy for identifying malignancies in distal common [corrected] bile duct strictures. J. Korean Med. Sci. 2008, 23, 579–585. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, L.; Lin, N.; Xin, F.; Ke, Q.; Zeng, Y.; Liu, J. A systematic review of the comparison of the incidence of seeding metastasis between endoscopic biliary drainage and percutaneous transhepatic biliary drainage for resectable malignant biliary obstruction. World. J. Surg. Oncol. 2019, 17, 116. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).